Abstract
The upper respiratory tract is inhabited by diverse range of commensal microbiota which plays a role in protecting the mucosal surface from pathogens. Alterations of the bacterial community from respiratory viral infections could increase the susceptibility to secondary infections and disease severities. We compared the upper respiratory bacterial profiles among Thai patients with influenza or COVID-19 by using 16S rDNA high-throughput sequencing based on MiSeq platform. The Chao1 richness was not significantly different among groups, whereas the Shannon diversity of Flu A and Flu B groups were significantly lower than Non-Flu & COVID-19 group. The beta diversity revealed that the microbial communities of influenza (Flu A and Flu B), COVID-19, and Non-Flu & COVID-19 were significantly different; however, the comparison of the community structure was similar between Flu A and Flu B groups. The bacterial classification revealed that Enterobacteriaceae was predominant in influenza patients, while Staphylococcus and Pseudomonas were significantly enriched in the COVID-19 patients. These implied that respiratory viral infections might be related to alteration of upper respiratory bacterial community and susceptibility to secondary bacterial infections. Moreover, the bacteria that observed in Non-Flu & COVID-19 patients had high abundance of Streptococcus, Prevotella, Veillonella, and Fusobacterium. This study provides the basic knowledge for further investigation of the relationship between upper respiratory microbiota and respiratory disease which might be useful for better understanding the mechanism of viral infectious diseases.
Keywords: Bacterial microbiota, upper respiratory tract, COVID-19, influenza, 16S rDNA, high-throughput sequencing
Impact statement
In this study, the bacterial microbiota in the upper respiratory tract (URT) of Thai patients with influenza or COVID-19 were evaluated. The results revealed the different bacterial communities in the URT between patients with influenza and COVID-19. The common opportunistic pathogens were found in both influenza and COVID-19 patients. Thus, different respiratory viral infections may trigger dysbiosis of the commensal microbiota, which might be associated with more susceptibility to secondary infections and disease progression. However, the change in URT microbiome among the influenza viruses and COVID-19-infected patients has not been clearly investigated. Thus, these findings would be useful for better understanding of the interaction between microbiota and viral infectious diseases.
Introduction
The upper respiratory tract (URT) is inhabited by a diverse range of commensal microbes, contributing beneficial roles in protecting the mucosal surface from pathogens. However, the microbiome could be altered, or undergo dysbiosis, by pathogenic respiratory viruses, resulting in a higher susceptibility to infection and respiratory diseases. 1
The re-emergence of respiratory viruses such as seasonal influenza viruses has a considerable impact on global public health. Seasonal influenza outbreaks are mainly caused by influenza A (H1N1 and H3N2 subtypes) and influenza B (Victoria and Yamagata lineages). Currently, the emergence and rapid transmission of a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-causing Coronavirus Disease 2019 (COVID-19) has become a serious public health problem and also causes significant economic loss. The common symptoms of influenza and COVID-19 are also known as influenza-like illness (ILI). 2 Several studies have reported that healthy individuals and patients with respiratory virus infection have different URT microbiota.3,4 However, the comparison of microbiome alteration caused by different virus infections has not been clearly investigated, especially between influenza and COVID-19, which cause similar ILI symptoms.
The data obtained from previous studies have demonstrated that the differences in respiratory bacterial communities associated with the respiratory infection were dependent on several factors, including environmental factors (geography, pollution, and hygiene), the host factors (race, age, gender, diet, lifestyle, and antibiotic usage), and the microbe–host immune response. 5 Comparison of the bacterial profiles obtained from different studies might be difficult due to the several confounding factors and incompatible techniques used in each study. To reduce these confounding factors, this study aimed to compare the bacterial profiles in the URT of Thai patients with influenza or COVID-19 by using 16S high-throughput sequencing based on the MiSeq platform.
Materials and methods
Study population and setting
A retrospective observational study was carried out to identify the bacterial profile in the URT of patients with influenza viruses or SARS-CoV-2 virus. The study protocols were reviewed and approved by the Institutional Review Board (IRB No. 337/57 for the influenza patient's cohort study and IRB No. 302/63 for the COVID-19 patient's cohort study) and Institutional Biosafety Committee (MDCU-IBC No. 001/2018) of the faculty of Medicine, Chulalongkorn University.
Sample collection
All clinical samples used in this study were routinely diagnosed for influenza and SARS-CoV-2 from patients with ILI symptoms. The ILI was defined as fever (>38°C) and with respiratory symptoms (cough, runny nose, nasal congestion, and sore throat). Nasopharyngeal swab (NP) samples of the two cohorts were collected in a viral transport medium (MP Biomedicals, USA) at 4°C and then transported to laboratory within 2 h. First, the influenza and Non-Flu & COVID-19 cohort samples were obtained during 2017–2018 (before COVID-19 pandemic) from Bangpakok 9 International Hospital and Chum Phae Hospital. The samples were stored at –80°C freezer until tested. Second, the samples with COVID-19-suspected cases were collected from the Institute for Urban Disease Control and Prevention (IUDC), Thailand during the pandemic of the SARS-CoV-2 virus in 2020. Samples from both cohorts were processed at the same time of the experiment.
Nucleic acid extraction, reverse transcription, and virus detection
Nucleic acids were extracted from 200 µL of NP swab samples using the magLEAD 12gC instrument with a magLEAD Consumable Kit (Precision System Science, Japan) according to the manufacturer's instructions. The extracted RNA was converted into cDNA with the random hexamer primer by using the RevertAid™ First Strand cDNA Synthesis Kit (Thermo Scientific, USA) following the manufacturer's instructions. Detection and subtyping of influenza viruses were performed by using a multiplex real-time reverse-transcription polymerase chain reaction as described previously.6,7 The SARS-CoV-2 viral detection was carried out by real-time PCR with Allplex™ 2019-nCoV Assay (Seegene, Korea) following the manufacturer's instructions. 8
16S amplification and high-throughput sequencing
The DNA library was constructed by amplification of the 16S rDNA within V4 region using Taq DNA Polymerase (New England Biolabs, USA) according to the manufacturer's instructions. Primers, master mix components, and thermo cycles followed the procedure of previous studies.9,10 The DNA libraries (∼430 bp) were subsequently purified by the QIAquick PCR Purification kit (Qiagen, Germany) and quantified by KAPA Library Quantification Kits for Illumina platforms (Kapa Biosystems, USA). The DNA libraries from all samples were pooled with equal concentration. The pooled libraries were paired-end (2 × 250 cycles) sequenced using the Illumina MiSeq platform (Illumina, USA).
Data analysis
The raw sequence datasets were de-multiplexed by MiSeq reporter software (version 2.6.2.3), and the paired-end FASTQ files were processed through the QIIME2 pipeline (version 2019.7). 11 The paired-end reads were quality filtered, merged, and denoised by DADA2 12 plugin implemented in QIIME2. The construction of the Amplicon sequence variant (ASV) and chimeric sequence filtering were subsequently performed based on DADA2 algorithm. Then, the ASVs were classified based on the 16S Greengene database 13 by using the VSEARCH algorithm. The alpha (Shannon and Chao1) and beta (weighted UniFrac distance) diversities were calculated using a plugin implemented in QIIME2. 14 Linear discriminant analysis effect size (LEfSe) was used to identify different taxa among groups. 15 Statistical analyses were performed with the Wilcoxon-matched pairs test using GraphPad Prism version 6.01 software.
Results
In this study, the influenza A, influenza B, and SARS-CoV-2 viruses were detected by real-time PCR and then the samples were divided into four groups, including the patients infected with influenza A virus (Flu A, N = 24), influenza B virus (Flu B, N = 24), SARS-CoV-2 virus (COVID-19, N = 24), and the patients without the influenza viruses and SARS-CoV-2 virus (Non-Flu & COVID-19, N = 24). The age of patients among groups was not statistically different (P = 0.065). The high-throughput sequencing data are shown in Table 1. Briefly, the total reads and pass-filtered reads obtained from each group were sufficient for ASVs classification as demonstrated in the rarefaction curve (Supplementary Figure 1). Moreover, the pass-filtered reads of all groups were efficiently classified as bacterial taxa (more than 98%), indicating that the sequencing data obtained in this study were suitable for 16S rDNA amplicon-based analysis.
Table 1.
The subject characteristics and sequencing summary of each group.
| Parameters | Flu A (N = 24) | Flu B (N = 24) | COVID-19 (N = 24) | Non-flu and COVID-19 (N = 24) |
|---|---|---|---|---|
| Age | 25.96 ± 20.28 | 26.50 ± 19.30 | 36.09 ± 12.17 | 34.46 ± 9.34 |
| Total reads | 79,425 ± 13,712 | 48,227 ± 11,358 | 79,504 ± 13,480 | 53,092 ± 14,065 |
| Pass-filtered reads | 70,290 ± 13,712 | 41,811 ± 9,514 | 69,663 ± 11,450 | 44,037 ± 12,197 |
| % Classified | 98.58 ± 3.06 | 99.74 ± 0.73 | 99.96 ± 0.14 | 99.86 ± 0.21 |
| Chao1 | 22.46 ± 9.38 | 24.21 ± 8.06 | 24.21 ± 9.86 | 23.13 ± 8.17 |
| Shannon | 1.26 ± 0.83 | 1.28 ± 0.55 | 2.08 ± 1.13 | 2.91 ± 0.93 |
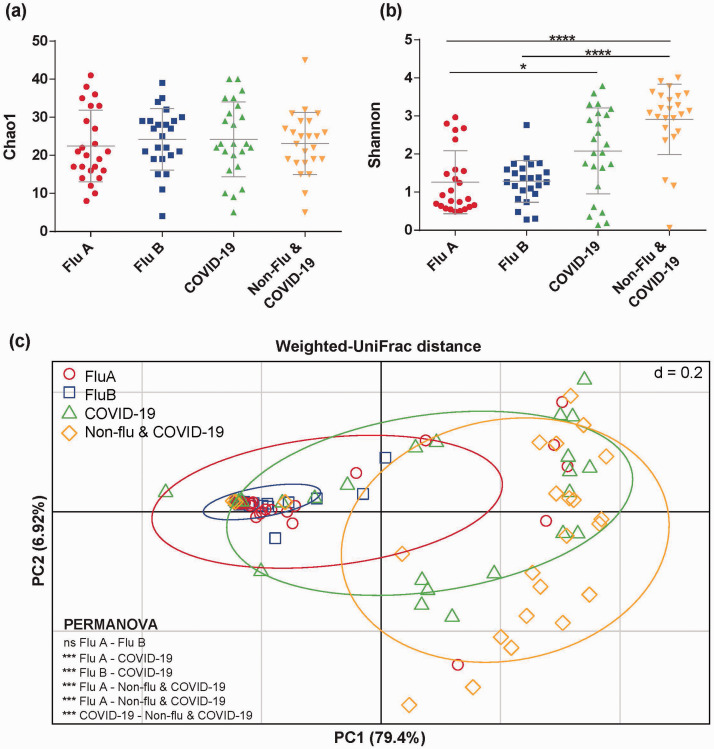
The alpha diversity based on Chao1 analysis revealed that bacterial richness was not different among groups as shown in Figure 1(a). On the other hand, the Shannon diversity of Flu A and Flu B groups were significantly lower than the Non-Flu & COVID-19 group (P < 0.05). Moreover, Flu A group was significantly decreased compared to COVID-19 group (P < 0.05) as demonstrated in Figure 1(b). The beta diversity was analyzed by weighted UniFrac distance to compare the microbial community distance among groups. The result revealed that microbial communities of influenza (Flu A and Flu B) groups were highly similar, but they were significantly distinct from those of the COVID-19 group and Non-Flu & COVID-19 group (PERMANOVA analysis, P < 0.05) as illustrated in Figure 1(c).
Figure 1.
The comparison of the bacterial diversities in Flu A, Flu B, COVID-19, and Non-flu & COVID-19 patients. The bars indicate average diversity of (a) Chao1 richness and (b) Shannon diversity index with standard deviation and significant differences among four groups. (c) Principal-coordinate analysis (PCoA) of the specific microbial communities weighted UniFrac distance colored by sample groups shows the significant difference between groups (tested by PERMANOVA analysis with P < 0.05). *P < 0.05, ***P < 0.001, ****P < 0.0001 and ns (not significant). (A color version of this figure is available in the online journal.)
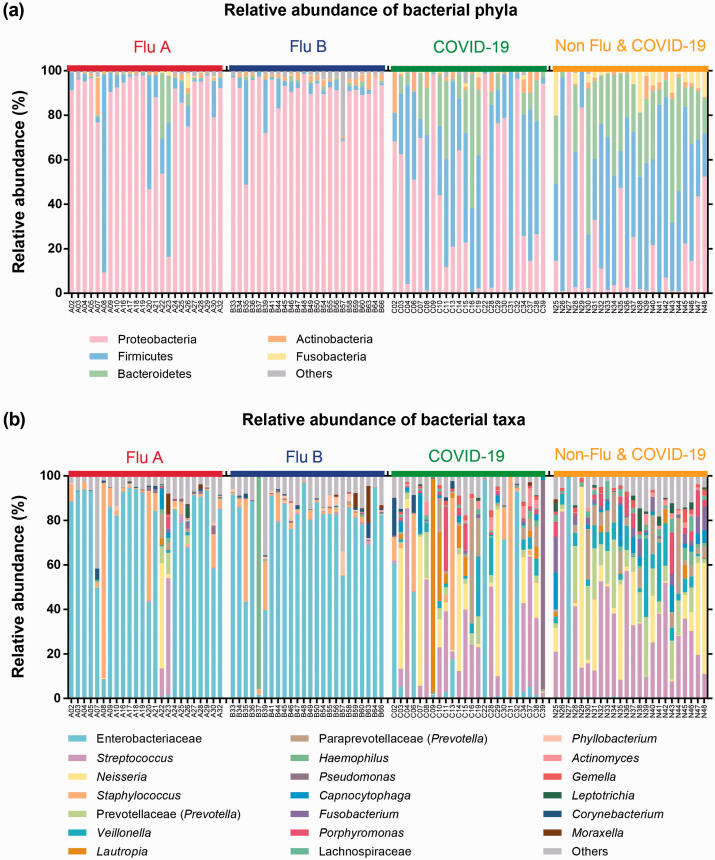
The taxonomic classification demonstrated that the relative abundances of upper respiratory bacteria were different among groups (Figure 2). The influenza (Flu A and Flu B) groups were highly predominated Proteobacteria, while bacterial profile in COVID-19 group was mostly dominated with Firmicutes and Bacteroidetes. Moreover, the Non-Flu & COVID-19 group was enriched by Firmicutes, Bacteroidetes, and Proteobacteria (Figure 2(a)).
Figure 2.
The bar plots illustrate the relative abundance of bacterial phyla (a) and genera (b) in each group (Flu A, Flu B, COVID-19, and Non-Flu & COVID-19) as analyzed by QIIME2 pipeline.
Comparing the relative abundance of bacterial composition at the genus level showed that Enterobacteriaceae had the highest abundance in influenza groups (Flu A and Flu B). Nevertheless, Streptococcus was the most common bacterial composition in COVID-19 and Non-Flu & COVID-19 groups. The differences in bacterial compositions between COVID-19 and Non-Flu & COVID-19 groups were also observed. Enterobacteriaceae, Staphylococcus, Lautropia, Pseudomonas, and Corynebacterium were increased in the COVID-19 group, whereas Prevotella, Veillonella, Capnocytophaga, and Fusobacterium were slightly increased in the Non-Flu & COVID-19 group (Figure 2(b)).
The differentially enriched bacteria among influenza, COVID-19, and Non-Flu & COVID-19 groups were analyzed using the LEfSe method with LDA score >4 and P < 0.05, as shown in Supplementary Figure 2. Subsequently, the characteristics of significant bacterial composition were selected from LEfSe and relative abundance, as demonstrated in Table 2. The results revealed that Enterobacteriaceae was significantly enriched in the influenza group, which contributed approximately 74.51%. Staphylococcus and Pseudomonas genera were significantly dominant in the COVID-19 group, which accounted for 9.83% and 4.01%, respectively. Lastly, the Non-Flu & COVID-19 group showed significant enrichment of Streptococcus, Prevotella, Veillonella, and Fusobacterium, which accounted for 29.85%, 11.28%, 7.21%, and 3.42%, respectively.
Table 2.
The characteristics of significantly enriched bacteria in each group.
| Group | Bacterial composition | Gram strain | Commonly found | Previous reports |
|---|---|---|---|---|
| Influenza | Enterobacteriaceae (74.51%) | Negative | Soil, water, and gastrointestinal tract | 16 |
| COVID-19 | Staphylococcus (9.83%) | Positive | Skin, nasal vestibule, and hospital | 17 |
| Pseudomonas (4.01%) | Negative | Soil, water, and vegetation | 18 | |
| Non-Flu & COVID-19 | Streptococcus (29.85%) | Positive | Throat, skin, ear, and sinus | 19,20 |
| Prevotella (11.28%) | Negative | Human intestine, rumen of cattle, and sheep | 20,21 | |
| Veillonella (7.21%) | Negative | Mammal and human intestines | 21 | |
| Fusobacterium (3.42%) | Negative | Upper respiratory and gastrointestinal tracts | 22 |
Note: The parenthesis indicates the percentage of the relative abundance belonged to the family or genus.
Discussion
The respiratory microbiota has been investigated in several previous studies. The bacterial profiles associated with the healthy respiratory status included Prevotella, Veillonella, Streptococcus, Staphylococcus, and Moraxella. On the other hand, dysbiotic microbiota related with lower microbial diversity and increased amount of specific bacteria such as Streptococcus, Pseudomonas, and Neisseria in patients with respiratory virus infections. 23
This study explored the bacterial compositions in the URT of Thai patients infected with influenza viruses, SARS-CoV-2 virus, and the patients without either influenza viruses or SARS-CoV-2 virus. The data obtained from the present study showed that different viral infections (influenza or SARS-CoV-2) might be related to the alteration in bacterial diversity and community composition of the URT. From our observation, the most abundant bacterial phylum in the influenza group was Proteobacteria, while the COVID-19 and Non-Flu & COVID-19 groups were dominated by Firmicutes. The results correlated with previous studies, in which Proteobacteria and Firmicutes were classified as the normal flora in URT. 24
For other lower respiratory viruses such as respiratory syncytial virus and rhinoviruses, previous study revealed that the Proteobacteria was highly dominated in the infected patients. Indeed, the predominated genera were belonged to the Moraxella and Streptococcus. 25 Similarly, our study also found the Streptococcus which was highly contributed in the COVID-19 and Non-flu & COVID-19 groups. Moreover, the abundance of Acinetobacter and Klebsiella genera in Proteobacteria phylum were higher in the fatal cases than those in non-fatal cases of the MERS-CoV-infected patients in Saudi Arabia. 26
Our finding shows that the Enterobacteriaceae family was predominant in influenza-infected patients, which is similar to the previous study of Chinese patients with H1N1 influenza A virus infection resulting in hypothermia and pneumonia. 16 The Staphylococcus genus is significantly enriched in the Thai patients with COVID-19 in the present study, and this corresponds to the recent finding that Staphylococcus was significantly increased in the Chinese patients with severe COVID-19 illness. 17 Staphylococcus is considered to be one of the causative agents in hospital-acquired infections. 27 Moreover, the secondary infection with Staphylococcus aureus bacteremia was associated with high mortality rates in hospitalized COVID-19. 28 The Pseudomonas genus was also found in COVID-19 patients of our study. This is similar to the previous report, which found that Pseudomonas was significantly increased in both the URT and the gut of children with COVID-19 infection. 18 Interestingly, viral infections such as influenza viruses or SARS-CoV-2 might modulate the bacterial communities which potentially favor the expansion of opportunistic pathogens. These pathogens can cause hospital-acquired infections involving multiple antibiotic resistances associated with pneumonia and high mortality rates. These data suggested that the bacterial composition of the URT can impact host susceptibility and disease outcomes after respiratory infection.
The previous studies showed that microbiota in healthy individuals were enriched with commensal bacteria in the nasopharynx, such as Streptococcus and Fusobacterium, while in the oropharyngeal cavity, Prevotella and Veillonella predominated.19–22 Our data revealed that Streptococcus, Prevotella, Veillonella, and Fusobacterium were significantly dominant in the patients without the influenza viruses and SARS-CoV-2 infections, indicating that bacterial communities in these patients might be closely related to healthy individuals.
In summary, this study provides a comparison of the upper respiratory bacterial communities of Thai patients with either influenza or COVID-19. The common opportunistic pathogens such as family Enterobacteriaceae were found in patients with influenza illness, whereas Staphylococcus and Pseudomonas were observed in patients with COVID-19, indicating that respiratory viral infections might be associated with an altered upper respiratory bacterial community and a differential induction of secondary bacterial infections. However, there are several limitations to this study, including small sample sizes, unmatched ages/genders, unknown severity/symptoms, and different periods of samples collection among groups. Therefore, further studies with larger cohorts and matched demographic data should be performed to confirm the bacterial communities which are associated with the respiratory viral infections. Finally, the findings of this study provide the basic knowledge for further investigation of the relationship between upper respiratory microbiota and respiratory disease.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211057473 for Bacterial microbiota in upper respiratory tract of COVID-19 and influenza patients by Somruthai Rattanaburi, Vorthon Sawaswong, Suwalak Chitcharoen, Pavaret Sivapornnukul, Pattaraporn Nimsamer, Nungruthai Suntronwong, Jiratchaya Puenpa, Yong Poovorawan and Sunchai Payungporn in Experimental Biology and Medicine
ACKNOWLEDGMENTS
We would like to thank the staffs from Bangpakok 9 International Hospital, Chum Phae Hospital and Institute for Urban Disease Control and Prevention (IUDC), Thailand for specimen collections.
AUTHORS’ CONTRIBUTIONS: SR carried out the experiment and interpretation of the results. VS, SC, and PN performed data analysis. SR and PS prepared the draft article. NS, JP, and YP processed and provided clinical samples. SP designed the study, revised the article, and coordinated the project.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICAL APPROVAL: The study protocols were approved by the Institutional Review Board (IRB No. 337/57 and IRB No. 302/63) and Institutional Biosafety Committee (MDCU-IBC No. 001/2018) from the faculty of Medicine, Chulalongkorn University.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided in part by the Royal Golden Jubilee (RGJ) Ph.D. Programme scholarship (PHD/0150/2558); National Research Council of Thailand (NRCT) [2564NRCT321520] and Thailand Research Fund (TRF).
ORCID iD: Sunchai Payungporn https://orcid.org/0000-0003-2668-110X
SUPPLEMENTAL MATERIAL: Supplemental material for this article is available online.
References
- 1.de Steenhuijsen Piters WA, Sanders EA, Bogaert D. The role of the local microbial ecosystem in respiratory health and disease. Phil Trans R Soc B 2015; 370:20140294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong W-H, Li Y, Peng M-W, Kong D-G, Yang X-B, Wang L, Liu M-Q. SARS-CoV-2 detection in patients with influenza-like illness. Nat Microbiol 2020; 5:675–8 [DOI] [PubMed] [Google Scholar]
- 3.Kaul D, Rathnasinghe R, Ferres M, Tan GS, Barrera A, Pickett BE, Methe BA, Das SR, Budnik I, Halpin RA. Microbiome disturbance and resilience dynamics of the upper respiratory tract during influenza a virus infection. Nature Commun 2020; 11:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korten I, Mika M, Klenja S, Kieninger E, Mack I, Barbani MT, Gorgievski M, Frey U, Hilty M, Latzin P. Interactions of respiratory viruses and the nasal microbiota during the first year of life in healthy infants. Msphere 2016; 1:e00312–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol 2017; 8:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suwannakarn K, Payungporn S, Chieochansin T, Samransamruajkit R, Amonsin A, Songserm T, Chaisingh A, Chamnanpood P, Chutinimitkul S, Theamboonlers A. Typing (a/B) and subtyping (H1/H3/H5) of influenza a viruses by multiplex real-time RT-PCR assays. J Virol Methods 2008; 152:25–31 [DOI] [PubMed] [Google Scholar]
- 7.Mayuramart O, Nimsamer P, Rattanaburi S, Chantaravisoot N, Khongnomnan K, Chansaenroj J, Puenpa J, Suntronwong N, Vichaiwattana P, Poovorawan Y. Detection of severe acute respiratory syndrome coronavirus 2 and influenza viruses based on CRISPR-Cas12a. Exp Biol Med (Maywood) 2021; 246:400–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farfour E, Lesprit P, Visseaux B, Pascreau T, Jolly E, Houhou N, Mazaux L, Asso-Bonnet M, Vasse M, Group S-C. FHs: the allplex 2019-nCoV (seegene) assay: which performances are for SARS-CoV-2 infection diagnosis? Eur J Clin Microbiol Infect Dis 2020; 39:1997–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawaswong V, Praianantathavorn K, Chanchaem P, Khamwut A, Kemthong T, Hamada Y, Malaivijitnond S, Payungporn S. Comparative analysis of oral-gut microbiota between captive and wild long-tailed macaque in Thailand. Sci Rep 2021; 11:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirivongrangson P, Kulvichit W, Payungporn S, Pisitkun T, Chindamporn A, Peerapornratana S, Pisitkun P, Chitcharoen S, Sawaswong V, Worasilchai N. Endotoxemia and circulating bacteriome in severe COVID-19 patients. ICMx 2020; 8:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019;37:852–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods 2016; 13:581–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006; 72:5069–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. Isme J 2011; 5:169–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 2011; 12:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Ding J, Xiao Y, Xu B, He W, Yang Y, Yang L, Su M, Hao X, Ma Y. 16S rDNA sequencing analysis of upper respiratory tract flora in patients with influenza H1N1 virus infection. Front Lab Med 2017; 1:16–26 [Google Scholar]
- 17.Zhong H, Wang Y, Shi Z, Zhang L, Ren H, He W, Zhang Z, Zhu A, Zhao J, Xiao F. Characterization of respiratory microbial dysbiosis in hospitalized COVID-19 patients. Cell Discov 2021; 7:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu R, Liu P, Zhang T, Wu Q, Zeng M, Ma Y, Jin X, Xu J, Zhang Z, Zhang C. Progressive deterioration of the upper respiratory tract and the gut microbiomes in children during the early infection stages of COVID-19. J Genet Genom 2021;48:803–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol 2019; 17:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 2011; 184:957–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenck LP, Surette MG, Bowdish DM. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett 2016; 590:3705–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Boeck I, Wittouck S, Wuyts S, Oerlemans EF, van den Broek MF, Vandenheuvel D, Vanderveken O, Lebeer S. Comparing the healthy nose and nasopharynx microbiota reveals continuity as well as niche-specificity. Front Microbiol 2017; 8:2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porto BN, Moraes TJ. The triad: respiratory microbiome–virus–immune response in the pathophysiology of pulmonary viral infections. Expert Rev Respir Med 2021; 15:635–48 [DOI] [PubMed] [Google Scholar]
- 24.Lemon KP, Klepac-Ceraj V, Schiffer HK, Brodie EL, Lynch SV, Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. MBio 2010; 1:e00129–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lysholm F, Wetterbom A, Lindau C, Darban H, Bjerkner A, Fahlander K, Lindberg AM, Persson B, Allander T, Andersson B. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PloS One 2012; 7:e30875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aljabr W, Alruwaili M, Penrice-Randal R, Alrezaihi A, Harrison AJ, Ryan Y, Bentley E, Jones B, Alhatlani BY, AlShahrani D. Amplicon and metagenomic analysis of middle east respiratory syndrome (MERS) coronavirus and the microbiome in patients with severe MERS. Msphere 2021; 6:e00219–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dandagi GL. Nosocomial pneumonia in critically ill patients. Lung India 2010; 27:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cusumano JA, Dupper AC, Malik Y, Gavioli EM, Banga J, Berbel Caban A, Nadkarni D, Obla A, Vasa CV, Mazo D. Staphylococcus aureus bacteremia in patients infected with COVID-19: a case series. Open Forum Infectious Diseases. Oxford: Oxford University Press US, 2020, p.ofaa518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211057473 for Bacterial microbiota in upper respiratory tract of COVID-19 and influenza patients by Somruthai Rattanaburi, Vorthon Sawaswong, Suwalak Chitcharoen, Pavaret Sivapornnukul, Pattaraporn Nimsamer, Nungruthai Suntronwong, Jiratchaya Puenpa, Yong Poovorawan and Sunchai Payungporn in Experimental Biology and Medicine