Abstract
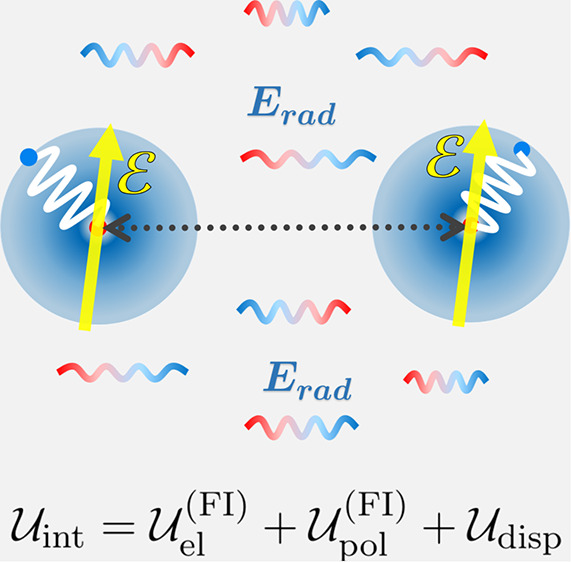
By means of quantum mechanics and quantum electrodynamics applied to coupled harmonic Drude oscillators, we study the interaction between two neutral atoms or molecules subject to a uniform static electric field. Our focus is to understand the interplay between leading contributions to field-induced electrostatics/polarization and dispersion interactions, as considered within the employed Drude model for both non-retarded and retarded regimes. For the first case, we present an exact solution for two coupled oscillators obtained by diagonalizing the corresponding quantum-mechanical Hamiltonian and demonstrate that the external field can control the strength of different intermolecular interactions and relative orientations of the molecules. In the retarded regime described by quantum electrodynamics, our analysis shows that field-induced electrostatic and polarization energies remain unchanged (in isotropic and homogeneous vacuum) compared to the non-retarded case. For interacting species modeled by quantum Drude oscillators, the developed framework based on quantum mechanics and quantum electrodynamics yields the leading contributions to molecular interactions under the combined action of external and vacuum fields.
The stable structure and properties of biomolecules, nanostructured materials, and molecular solids are determined by a delicate balance between different intermolecular forces.1−5 In many realistic systems, molecular interactions are substantially modified by solvents, cell membranes, ionic channels, and other environments.6−9 A proper description of such environments demands robust approaches for modeling both non-retarded and retarded intermolecular interactions under arbitrary fields. Molecular interactions in the presence of static and dynamic electromagnetic and thermal fields have been studied using various approaches,9−24 but a comprehensive understanding is still missing and some results remain controversial. For example, random and inhomogeneous fields have been shown to affect the strength and distance dependence of van der Waals (vdW) interactions or even change their sign.6,17−21 However, similar to the discussion on the textbook dispersion interaction,25,26 there is still an ongoing debate on the interpretation of modified vdW interactions as having either an electrostatic or a quantum-mechanical origin.6,7,27,28 The application of weak static (in)homogeneous fields in the non-retarded regime9,22,23 yields a visible modification of molecular interactions in second and third orders of perturbation theory; however, the retarded regime has not been addressed in these studies. Fiscelli et al.24 used quantum electrodynamics (QED) to propose a dispersion energy, scaling as R–4 (R–3), with respect to the interatomic distance for the retarded (non-retarded) regime, for interacting two-level hydrogen-like atoms in static electric fields. However, it still remains unclear29,30 whether this term corresponds to dispersion or electrostatic interactions. In order to resolve existing controversies and clarify discrepancies in the literature, here we develop a quantum framework for modeling and understanding intermolecular interactions in electric fields based on first-principles of quantum mechanics and QED.
The advances made in this Letter hinge on the usage of two formalisms that enable an accurate modeling and conceptual understanding of non-retarded and retarded interactions for two coupled quantum Drude oscillators (QDOs)31−33 subject to a static electric field: solving Schrödinger’s equation via exact diagonalization and using perturbation theory in QED.34−40 In addition, we employ stochastic electrodynamics (SED)41−48 as a semiclassical formalism that transparently connects molecular interactions to the fields that originate them. The usage of QDOs to accurately and efficiently model the linear response of valence electrons in atoms and molecules is a critical aspect because coupled QDOs enable analytical solutions (with and without an electric field) and have been convincingly demonstrated to provide a reliable quantitative tool to describe response properties of real atoms and molecules subject to external fields or confinement.31−33,49−55 QDOs can quantitatively—within a few percent compared to explicit treatment of electrons—describe polarization and dispersion interactions,33,52,53 capture electron density redistribution induced by these interactions,56 and model intermolecular interactions in electric fields,6,9 among many other response phenomena.3 Our current study benefits from many attractive features of QDOs and demonstrates their applicability to the retarded regime. By means of the developed framework, we derive dominant contributions to the interaction energy of two QDOs in an electric field up to terms ∝ R–6 (R–7) for the non-retarded (retarded) regime. These contributions, corresponding to a linear response to the external field, are interpreted as the field-induced electrostatic and polarization interactions obtained in addition to the conventional leading-order London/Casimir dispersion interaction found to be unchanged in the presence of a static electric field within the QDO model. We note that most previous studies in the QED literature employed the two-level (s and p states) hydrogen-like atom as a model. Unfortunately, there is no known analytical solution for the case of interacting hydrogen atoms under an external electric field,24 causing quite some controversy over the interpretation of field-induced interatomic interactions.29,30,57 Of course, the coupled QDO model employed here also introduces approximations. In particular, the Gaussian form of the QDO wave function does not capture the effect of deformation of an electron cloud by a static field, in contrast to a hydrogen atom, for example. In a homogeneous electric field, the ground-state electron density of a QDO undergoes a rigid displacement and this means that the β and γ hyperpolarizabilities vanish.33 Therefore, field-induced and dispersion-induced changes in the polarizability of interacting species described by these hyperpolarizabilities12−14,16,58−63 are missing within the QDO model.58,64 Hence, some interactions corresponding to the coupling of the field-induced and dispersion-induced changes in polarizabilities will also vanish for coupled QDOs. However, as we discuss below, such terms are either of higher order (β-terms) or smaller magnitude (γ-terms) in comparison to the dispersion- and field-induced electrostatic energies, for weak static electric fields.
First, we consider the non-retarded case, when the distance R between two interacting species is small compared to wavelengths λe of their electronic transitions: R ≪ λe = 2πc/ωe, where c is the speed of light. The system of two interacting QDOs, which represent two atoms or molecules, separated by a distance R along the z axis is shown in Figure 1. In the absence of any field, this system is described by the Hamiltonian
| 1 |
where Vint is the Coulomb potential approximated here by the dipole–dipole coupling
| 2 |
This form of the interaction breaks the initial symmetry between z and x/y parts of the Hamiltonian. However, the symmetry between x and y parts remains. Thus, one needs to obtain just Hx and Hz from the separation H = Hx + Hy + Hz. Introducing the normal-mode coordinates
| 3 |
with 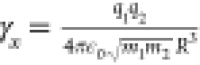 and
and 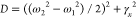 , we transform the x-dependent
part of eq 1 to
, we transform the x-dependent
part of eq 1 to
| 4 |
where 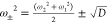 . The Hamiltonian of eq 4 describes two independent QDOs
with unit
masses and the frequencies ω+ and ω– of the two normal modes.
. The Hamiltonian of eq 4 describes two independent QDOs
with unit
masses and the frequencies ω+ and ω– of the two normal modes.
Figure 1.
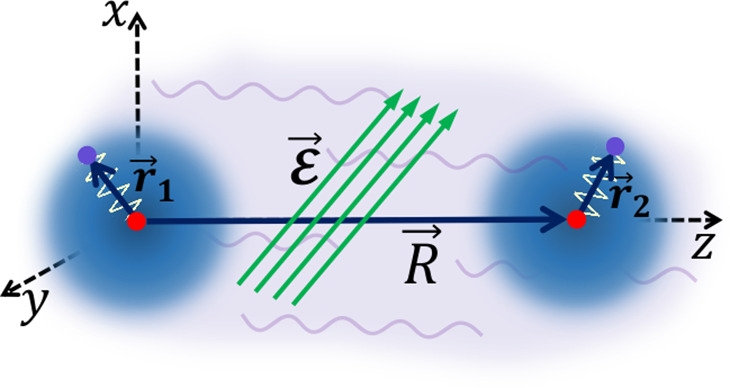
Two interacting quantum Drude oscillators under
the influence of
both the fluctuating vacuum electromagnetic field and an applied uniform
static electric field 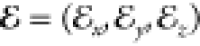 .
.
Now we introduce a uniform electric field with the contribution 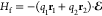 to the Hamiltonian. With the new coordinates,
we obtain
to the Hamiltonian. With the new coordinates,
we obtain
| 5 |
| 6 |
Equation 5 can be rewritten as a quadratic form
| 7 |
which is the Hamiltonian of two one-dimensional
(1D) oscillators with the frequencies ω+ and ω– and the centers shifted by 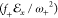 and
and 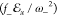 , respectively. The interaction energy of
the two oscillators under an external electric field is given by the
difference between the total energy of the coupled QDOs and the sum
of the total energies of two isolated QDOs in the same field
, respectively. The interaction energy of
the two oscillators under an external electric field is given by the
difference between the total energy of the coupled QDOs and the sum
of the total energies of two isolated QDOs in the same field
| 8 |
where 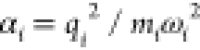 is the static
polarizability of a QDO.
Due to the symmetry,
is the static
polarizability of a QDO.
Due to the symmetry, 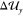 is derived in the same
way as
is derived in the same
way as 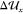 by replacing subscripts x with y. The z-dependent
part of the total Hamiltonian,
by replacing subscripts x with y. The z-dependent
part of the total Hamiltonian, 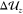 , one can obtain similarly
to
, one can obtain similarly
to 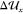 , by replacing γx in eq 3 with
γz = −2γx. For this case, we obtain z± = (γzz1′ + [(a2 – a1) ±
, by replacing γx in eq 3 with
γz = −2γx. For this case, we obtain z± = (γzz1′ + [(a2 – a1) ± 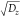 ]z2)/
]z2)/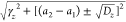 and
and 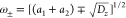 , where
, where 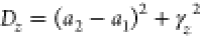 .
.
As shown in ref (65), the resulting expressions for the interaction
energy can be expanded
as an infinite series with respect to small terms proportional to 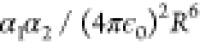 . Retaining all of the leading terms up
to R–6 within the QDO model, we
obtain
. Retaining all of the leading terms up
to R–6 within the QDO model, we
obtain
| 9 |
Here, the first contribution is the
well-known
vdW dispersion energy, stemming from the difference between ℏωi/2 terms in eq 8 as well as the corresponding
expressions for 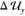 and
and 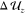 .65 The dispersion
energy is not affected by the static field within the QDO model. The
other two terms in eq 9 are field-induced contributions, which originate from the terms
in eq 8 not containing ℏ. As will be discussed in detail below, these terms
correspond to the field-induced electrostatic and polarization contributions
to the interaction energy, respectively. For real atoms or molecules, eq 9 would contain an additional
term ∝ R–6, which has the
approximate form12,14,63 (for an exact analytical expression, see ref (16))
.65 The dispersion
energy is not affected by the static field within the QDO model. The
other two terms in eq 9 are field-induced contributions, which originate from the terms
in eq 8 not containing ℏ. As will be discussed in detail below, these terms
correspond to the field-induced electrostatic and polarization contributions
to the interaction energy, respectively. For real atoms or molecules, eq 9 would contain an additional
term ∝ R–6, which has the
approximate form12,14,63 (for an exact analytical expression, see ref (16)) 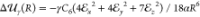 , where
γ is the second hyperpolarizability
and C6 is the dipole–dipole vdW
dispersion coefficient. This contribution is absent within the QDO
model where γ vanishes because of symmetry reasons.58,64 In addition, it is worth mentioning that vdW dispersion interaction
between two atoms or molecules also causes dispersion-induced dipoles15,25,66−73 that vary as R–7. With coupling
to an applied static field, it results in an interaction energy which
is linear in the field and scales as R–7. As first suggested by Hunt,66 such an
interaction energy corresponds to the hyperpolarization of an atom
by the fluctuating field from the neighboring atom in combination
with the applied field, which depends on the B hyperpolarizability
(dipole–dipole–quadrupole hyperpolarizability) and the
static dipole polarizability of the atoms.66 Since a QDO has a nonvanishing B hyperpolarizability,
the dispersion-induced dipole moment can be captured by taking into
account dipole–quadrupole couplings74 in the interaction Hamiltonian of eq 2.
, where
γ is the second hyperpolarizability
and C6 is the dipole–dipole vdW
dispersion coefficient. This contribution is absent within the QDO
model where γ vanishes because of symmetry reasons.58,64 In addition, it is worth mentioning that vdW dispersion interaction
between two atoms or molecules also causes dispersion-induced dipoles15,25,66−73 that vary as R–7. With coupling
to an applied static field, it results in an interaction energy which
is linear in the field and scales as R–7. As first suggested by Hunt,66 such an
interaction energy corresponds to the hyperpolarization of an atom
by the fluctuating field from the neighboring atom in combination
with the applied field, which depends on the B hyperpolarizability
(dipole–dipole–quadrupole hyperpolarizability) and the
static dipole polarizability of the atoms.66 Since a QDO has a nonvanishing B hyperpolarizability,
the dispersion-induced dipole moment can be captured by taking into
account dipole–quadrupole couplings74 in the interaction Hamiltonian of eq 2.
In order to extend our results to the retarded regime, R ≫ c/ωe, we consider the coupling between two QDOs in the framework of QED, where the molecular interactions are mediated by the fluctuating vacuum radiation field. The total system consists of two QDOs, an external static field, and the vacuum field. For a single QDO coupled to the static field, the Hamiltonian is given by
| 10 |
To obtain eigenstates/eigenvalues of 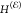 , we diagonalize
it by means of the transformation
, we diagonalize
it by means of the transformation 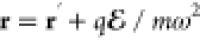 , resulting in
, resulting in 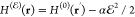 . Thus, the eigenstates
and eigenvalues
of
. Thus, the eigenstates
and eigenvalues
of 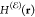 are given by
are given by 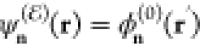 and
and 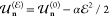 , respectively, where
, respectively, where 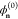 and
and 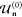 denote the eigenstates
and eigenvalues
of an isolated oscillator.75 Similar to eq 8, the constant energy shift
in
denote the eigenstates
and eigenvalues
of an isolated oscillator.75 Similar to eq 8, the constant energy shift
in 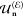 arises
due to the static dipole induced
by the external electric field. The matrix elements of the dipole
moment, μ = qr, are obtained by
arises
due to the static dipole induced
by the external electric field. The matrix elements of the dipole
moment, μ = qr, are obtained by
| 11 |
with 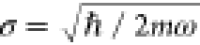 as the QDO characteristic length and the
bras/kets defined such that ⟨x|i⟩ = ψi(x). In a similar way, one obtains μy and μz. The first and second terms
on the r.h.s. of eq 11 correspond to the fluctuating and field-induced static QDO dipoles,
respectively.
as the QDO characteristic length and the
bras/kets defined such that ⟨x|i⟩ = ψi(x). In a similar way, one obtains μy and μz. The first and second terms
on the r.h.s. of eq 11 correspond to the fluctuating and field-induced static QDO dipoles,
respectively.
The Hamiltonian of the total noninteracting system is
| 12 |
where Hrad corresponds to the vacuum radiation field. In the dipole approximation of the multipolar-coupling formalism, the interaction Hamiltonian is given by36
| 13 |
Here, D⊥ is the transverse component of the vacuum displacement field given by
| 14 |
with V as the quantization
volume. The annihilation and creation operators of a mode with the
wave vector k and polarization 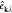 , akλ and
, akλ and 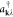 , obey the bosonic
commutation relations.36 The ground state
of H0 is given by |0⟩ = |0, 0, 0⟩1|0, 0, 0⟩2|{0}⟩, where
the
QDO ket states are defined such that
, obey the bosonic
commutation relations.36 The ground state
of H0 is given by |0⟩ = |0, 0, 0⟩1|0, 0, 0⟩2|{0}⟩, where
the
QDO ket states are defined such that 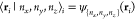 , and |{0}⟩ is the ground state of
the vacuum field. Excited states of the total unperturbed system can
be written similarly. By using these states, we follow standard perturbation
theory to obtain interaction energies distinguishing between different
contributions resulting from coupling of either fluctuating or field-induced
static QDO dipole moments, given by eq 11, to the vacuum field.
, and |{0}⟩ is the ground state of
the vacuum field. Excited states of the total unperturbed system can
be written similarly. By using these states, we follow standard perturbation
theory to obtain interaction energies distinguishing between different
contributions resulting from coupling of either fluctuating or field-induced
static QDO dipole moments, given by eq 11, to the vacuum field.
The first- and third-order
corrections vanish, since in such cases akλ and 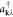 occur between two identical states of the
vacuum field. The second-order perturbation
occur between two identical states of the
vacuum field. The second-order perturbation
| 15 |
yields nonvanishing
interaction terms only
when the vacuum field in |I⟩ is in a single-photon
excitation and both QDOs are in their ground states, namely, |I⟩ = |0, 0, 0⟩1|0, 0, 0⟩2|1kλ⟩.
For this case, after removing the self-energies, 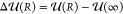 , we obtain an interaction energy
between
the two QDOs given by
, we obtain an interaction energy
between
the two QDOs given by
| 16 |
which is the same as the second term of eq 9. Considering the QDO states in |I⟩, this interaction energy corresponds to the situation when both QDOs couple to the vacuum field via their static field-induced dipoles and exchange one virtual photon, indicating the electrostatic nature of this interaction term. Taking into account the R–3 scaling, this term corresponds to a field-induced (dipole–dipole) electrostatic interaction.
For the fourth-order correction, we have two terms36
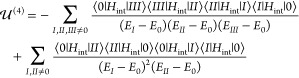 |
17 |
For non-polar species, only the first term of eq 17 is relevant and hence widely used in the literature. The second term becomes relevant for polar atoms/molecules with permanent electrostatic moments, as they can couple to the vacuum field via either fluctuating or static dipoles.36,39 In the latter case, the interacting species can emit/absorb a virtual photon without undergoing a change in their energy eigenstate. In our case, the two QDOs possess static field-induced dipoles due to the applied electric field and the second term in eq 17 plays an important role. Specifically, when each QDO couples to the vacuum field via its static field-induced dipole, both fourth-order terms in eq 16 yield nonvanishing contributions ∝ R–5 of the same magnitude but opposite sign, canceling each other.65 When both atoms couple to the vacuum field via their fluctuating dipoles, the treatment of eq 17 as in refs (36 and 37) delivers65 the known London and Casimir–Polder dispersion energies
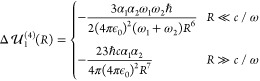 |
18 |
respectively. The former is the first term of eq 9, and the latter is its counterpart for the retarded regime, where the frequencies disappear since at large distances each species effectively “sees” another one as a static object. Finally, when one of the species couples to the vacuum field via its static dipole moment and the other one by its fluctuating dipole, the resulting interaction energy is
| 19 |
which is the third term of eq 9. This interaction stems from the fourth-order correction and has R–6 scaling, similar to the non-retarded dispersion energy, but in contrast to the latter remains unaffected by retardation. These features allow us to identify the term of eq 19 as the field-induced polarization energy. Within the employed QED approach, one can also take into account the influence of a static field on the cloud of virtual photons surrounding each atom.76−80 Although the effect of distortions of such photon clouds on the molecular interactions is usually assumed to be small, it might become important under external fields, similar to the aforementioned effects caused by hyperpolarizabilities. Moreover, the impact of clouds of virtual photons on the molecular interactions can become even more important for atoms at close separation, i.e., when electron exchange effects become crucial. Indeed, the distortion of photon clouds by a static field can change the effective atomic vdW radii, but to investigate such effects in detail,55 one would need to go beyond the perturbative QED approach used here.
In addition
to the two quantum approaches discussed above, we present
a (semi)classical derivation that allows us to transparently connect
different molecular interactions to the fields originating them. To
this end, we employ a SED approach developed by Boyer81,82 based on the theory of classical electrodynamics with a random zero-point
radiation field. Within this picture, the fluctuating vacuum field
induces random polarization of atoms (modeled by classical oscillators)
and couples them to each other through their electromagnetic fields,
as described in classical electrodynamics. For the retarded regime, R ≫ c/ω, only large wavelengths
contribute to the interatomic interaction and the SED equation of
motion for a dipole oscillator reduces to mω2r = qE(r, t). Solving this equation yields μ(r, t) = αE(r, t), with E as the total electric field at point r and time t. The energy of the electric dipole moment induced by E in the same field is known from electrodynamics as 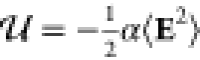 , where the bracket indicates
time averaging.
, where the bracket indicates
time averaging.
Since we apply a static electric field on top
of the random radiation
field, the oscillator dipole has two parts, each related to one of
these fields. According to Figure 1, the first QDO is located at the origin, while we
bring the second QDO to the point r2 = (0, 0, R) from z = +∞.
The difference in the energy of the oscillators for the two configurations, 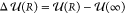 , is the interaction energy. The total electric
field at r2 is given by
, is the interaction energy. The total electric
field at r2 is given by
| 20 |
with E and 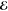 as radiation and static fields, respectively.
Here, the random radiation field is defined by43
as radiation and static fields, respectively.
Here, the random radiation field is defined by43
| 21 |
where 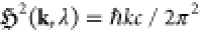 is the energy of each mode of the random
field and the sum runs over the two possible polarizations. The random
phase θ ranges from 0 to 2π and ϵ(k, λ) are orthogonal unit polarization vectors, ϵ(k, λ)·ϵ(k′, λ′) = δλλ′. Then,
is the energy of each mode of the random
field and the sum runs over the two possible polarizations. The random
phase θ ranges from 0 to 2π and ϵ(k, λ) are orthogonal unit polarization vectors, ϵ(k, λ)·ϵ(k′, λ′) = δλλ′. Then, 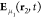 is a time-dependent field radiated by the
oscillating dipole of the first species induced by the random radiation
field. Similarly,
is a time-dependent field radiated by the
oscillating dipole of the first species induced by the random radiation
field. Similarly, 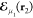 is the electric field of the static dipole
of the first oscillator, which is induced by the uniform external
electric field. The electric field of an oscillating electric dipole
is83
is the electric field of the static dipole
of the first oscillator, which is induced by the uniform external
electric field. The electric field of an oscillating electric dipole
is83
| 22 |
where 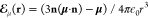 is the electric field
of a static electric
dipole. Therefore, the energy of the second oscillator, with the static
polarizability α2, under the total electric field
given by eq 20 is obtained
as
is the electric field
of a static electric
dipole. Therefore, the energy of the second oscillator, with the static
polarizability α2, under the total electric field
given by eq 20 is obtained
as 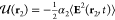 . Then, subtracting energies for z2 = R and z2 = +∞ yields
. Then, subtracting energies for z2 = R and z2 = +∞ yields
| 23 |
where we keep only nonvanishing terms after time and phase averaging.65 The first term in eq 23 gives the interaction energy from the coupling of two fluctuating dipole moments induced by the random field. This point was addressed by Boyer43 who obtained the aforementioned London84,85 and Casimir–Polder81,82,86 results for the non-retarded and retarded regimes, respectively. Here, we focus on other contributions to the interaction energy. The second term in eq 23 is the coupling of the field-induced dipole of the second oscillator with the static field of the field-induced dipole of the first oscillator which gives the electrostatic interaction energy
| 24 |
The third term of eq 23 describes the interaction of the dipole moment of the second oscillator induced by the static field of the first oscillator with the same field and yields the interaction energy
| 25 |
The mechanism responsible for this interaction is similar to the one for intermolecular polarization (induction) interaction between molecules with permanent dipole moments, with the difference that here the static dipoles are induced by the external field and hence the interaction is a field-induced polarization interaction. Adding the term coming from the interaction of the dipole moment of the first oscillator with the static field of the field-induced dipole of the second oscillator yields the full field-induced polarization energy given by eq 19. The polarization energy of eq 25 was originally derived from a pure classical point of view, by considering the coupling of a pair of polarizable objects, interacting and therefore possessing the collisional contribution Δα to the pair polarizability, to an external static field.10−16
Our results, obtained within the QDO model for the linear-response regime, show that the field-induced electrostatic and polarization energies are not influenced by the retardation effects and only the conventional leading-order London/Casimir dispersion energy changes the distance scaling law going from the non-retarded to retarded regime. However, without aforementioned contributions coming from dispersion-induced changes in the polarizability12−14,16,58−64 and dipole moments15,25,66−74 of interacting species as well as field-induced hyperpolarization effects,57,87 the dispersion interaction remains unchanged by the external uniform static field, for both regimes. This fact is in contradiction to the recent results of Fiscelli et al.,24 who obtained a drastic change in the distance dependence of dispersion interaction energy between two atoms after applying a static electric field (R–3/R–4 for the non-retarded/retarded regime). In ref (24), the wave functions of a two-level “hydrogen” atom in a static electric field were obtained from perturbation theory. The interaction energy between two atoms in a field was calculated from QED perturbation theory using the atomic wave functions obtained in the first step. However, since these functions do not form a complete set, employing them as a basis set for the second use of the perturbation theory in ref (24) is questionable. A similar conclusion was recently obtained in ref (57) independently of our work.65
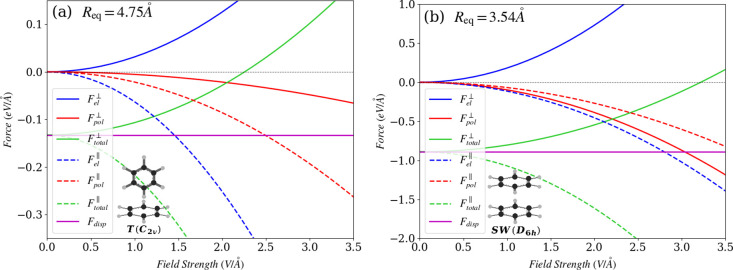
Due to the analytical solutions of the QDOs, the results of eq 9 can be straightforwardly generalized to any number of QDOs, with each of them under their own static field. This provides an opportunity to effectively model internal atom-dependent electric fields present in large molecules. Moreover, the developed framework paves the way for tuning intermolecular interactions by means of external static electric fields, which is important for many practical applications including biophysics.88 This is demonstrated in Figure 2, where the intermolecular forces in a benzene dimer are considered for its two different equilibrium structures, the so-called “T-shape” and “sandwich”.89,90 The interplay between the field-induced forces and the dispersion force can lead either to cooperation or competition. In the case of the “T-shape” structure, the total electrostatic force can overtake the dispersion force, if a static electric field with strength 2.25 V/Å is applied perpendicularly to the dimer. For the “sandwich” configuration, the strength of the perpendicular compensating field is around 3.22 V/Å. Although for the considered molecular dimers at the equilibrium distance these fields are relatively strong, they are still weaker than internal molecular fields experienced by valence electrons. Moreover, as shown in ref (65), the field required for mutual compensation of the intermolecular forces becomes much weaker at larger separations. Furthermore, for large molecules or nanoscale systems with a multiplicity of normal modes contributing to eq 8, a significantly smaller magnitude of the electric field will be required to overcome the dispersion attraction.91 Altogether, this shows that applied static electric fields can be used to influence the stability and dynamics of complex molecular systems.
Figure 2.
Dispersion force (Fdisp) as well as field-induced electrostatic (Fel) and polarization (Fpol) forces for two interacting benzene molecules separated by the corresponding equilibrium distance Req = 4.75 Å and Req = 3.54 Å within (a) T-shape and (b) sandwich structures, respectively. The results are shown for two different alignments of the external field and the molecules: the field is either parallel (∥) or perpendicular (⊥) to the line connecting the molecule centers.
Before summarizing our results, it is important to emphasize some of the remaining limitations of the employed coupled QDO model, whose resolution would be needed to form a complete physical picture of intermolecular interactions under a static electric field. As was exhaustively discussed in ref (33), a QDO does possess multipole hyperpolarizabilities starting from the dipole–dipole-quadrupole one, but the first (β) and second (γ) hyperpolarizabilities vanish due to the spherical symmetry and Gaussian wave function, respectively. Therefore, a single QDO does not fully capture the contributions to the intermolecular interaction energy which are related to hyperpolarization effects of interacting atoms and molecules in static fields. For a pair of two-level hydrogen atoms, it is known57 that the energy contribution from the field-induced β hyperpolarizabilities of the atoms scales as R–11. Therefore, this contribution can be neglected in comparison to the dispersion- and field-induced electrostatic and polarization interactions. It has been shown that dispersion interactions modify the polarizability of an interacting pair where the leading-order correction scales as R–6 and depends on the γ hyperpolarizability of atoms or molecules.12−14,16,58−64 In a static electric field, the dispersion-induced polarizability yields an additional interaction energy (quadratic in the applied field) that scales as R–6 being comparable14,63 to the field-induced polarization energy in eq 9. To capture a complete picture of the dipolar interactions in a static field, one could use more than one QDO to represent atoms or molecules, which brings anharmonicity to the system and breaks the spherical symmetry of the model when interacting with external fields. Such a multi-QDO model would exhibit β and γ hyperpolarizabilities and thus provide more realistic response properties in comparison to single QDOs. The dipolar interactions between two (or many) multi-QDO systems can be expressed as a coupled-QDO problem, which is still exactly solvable.
In addition, the considered contributions to molecular interactions between atoms or molecules represented by QDOs were derived assuming large interspecies distances, effectively neglecting exchange and overlap effects. Within the QDO model, an inclusion of the exchange interaction is possible with a further generalization of the existing formalism valid for two identical QDOs54,92 to the case of heteronuclear species. The overlap effects can also be included following the work of refs (59 and 93).
In summary, we presented dominant contributions to the interaction energy between two closed-shell atoms or molecules in a uniform static electric field for both short (non-retarded regime) and long (retarded regime) separation distances. Based on first principles of quantum mechanics and QED and employing the QDO model as a reliable tool for modeling atomic/molecular responses, our framework admits many generalizations. In addition to external fields, the internal field from atomic charges and dipoles within a molecule can be treated as well, leading to an efficient many-body model of different types of intramolecular and intermolecular interactions on equal footing. More general time-dependent fields can also be included. A particularly interesting and novel research direction is to develop and apply a QED treatment to many-body states for a set of many interacting QDOs. This could generalize the transition from the London to Casimir regime for an assembly of atoms or molecules beyond the well-known two-body case. All in all, we are confident that our framework barely scratches the surface of possible developments and applications in the field of molecular interactions under the combined action of external and vacuum fields.
Acknowledgments
The authors acknowledge the financial support from the Luxembourg National Research Fund through the FNR CORE projects “QUANTION(C16/MS/11360857, GrNum:11360857)” and “ PINTA(C17/MS/11686718)” as well as from the European Research Council via ERC Consolidator Grant “BeStMo(GA n725291)”.
The authors declare no competing financial interest.
References
- Kaplan I. G.Intermolecular interactions: physical picture, computational methods and model potentials; John Wiley & Sons: Sussex, U.K., 2006. [Google Scholar]
- Stone A. J.The theory of intermolecular forces; Oxford University Press: Oxford, U.K., 2013. [Google Scholar]
- Tkatchenko A. Current understanding of van der Waals effects in realistic materials. Adv. Funct. Mater. 2015, 25, 2054–2061. 10.1002/adfm.201403029. [DOI] [Google Scholar]
- Langbein D.Theory of van der Waals attraction; Springer tracts in modern physics, Vol. 72; Springer-Verlag: Berlin, 1974. [Google Scholar]
- Ángyán J.; Dobson J.; Jansen G.; Gould T.. London dispersion forces in molecules, solids and nano-structures: an introduction to physical models and computational methods; Royal Society of Chemistry: Cambridge, U.K., 2020. [Google Scholar]
- Sadhukhan M.; Tkatchenko A. Long-range repulsion between spatially confined van der Waals dimers. Phys. Rev. Lett. 2017, 118, 210402. 10.1103/PhysRevLett.118.210402. [DOI] [PubMed] [Google Scholar]
- Stöhr M.; Sadhukhan M.; Al-Hamdani Y. S.; Hermann J.; Tkatchenko A. Coulomb interactions between dipolar quantum fluctuations in van der Waals bound molecules and materials. Nat. Commun. 2021, 12, 137. 10.1038/s41467-020-20473-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr M.; Tkatchenko A. Quantum mechanics of proteins in explicit water: The role of plasmon-like solute-solvent interactions. Sci. Adv. 2019, 5, eaax0024 10.1126/sciadv.aax0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleshchonok A.; Tkatchenko A. Tailoring van der Waals dispersion interactions with external electric charges. Nat. Commun. 2018, 9, 3017. 10.1038/s41467-018-05407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein L. Molecular refractivity and atomic interaction. Philos. Mag. 1917, 33, 92–128. 10.1080/14786440108635618. [DOI] [Google Scholar]
- Silberstein L. Molecular refractivity and atomic interaction II. Philos. Mag. 1917, 33, 521–533. 10.1080/14786440608635666. [DOI] [Google Scholar]
- Buckingham A. D. The polarizability of a pair of interacting atoms. Trans. Faraday Soc. 1956, 52, 1035–1041. 10.1039/tf9565201035. [DOI] [Google Scholar]
- Buckingham A. D.; Martin P. H.; Watts R. S. The polarizability of a pair of hydrogen atoms at long range. Chem. Phys. Lett. 1973, 21, 186–190. 10.1016/0009-2614(73)80045-4. [DOI] [Google Scholar]
- Buckingham A. D.; Clarke K. L. Long-range effects of molecular interactions on the polarizability of atoms. Chem. Phys. Lett. 1978, 57, 321–325. 10.1016/0009-2614(78)85517-1. [DOI] [Google Scholar]
- Hunt K. L. C.; Bohr J. E. Effects of van der Waals interactions on molecular dipole moments: The role of field-induced fluctuation correlations. J. Chem. Phys. 1985, 83, 5198–5202. 10.1063/1.449732. [DOI] [Google Scholar]
- Hunt K. L. C.; Bohr J. E. Field-induced fluctuation correlations and the effects of van der Waals interactions on molecular polarizabilities. J. Chem. Phys. 1986, 84, 6141–6150. 10.1063/1.450755. [DOI] [Google Scholar]
- Thirunamachandran T. Intermolecular interactions in the presence of an intense radiation field. Mol. Phys. 1980, 40, 393–399. 10.1080/00268978000101561. [DOI] [Google Scholar]
- Haugland T. S.; Schäfer C.; Ronca E.; Rubio A.; Koch H. Intermolecular interactions in optical cavities: An ab initio QED study. J. Chem. Phys. 2021, 154, 094113. 10.1063/5.0039256. [DOI] [PubMed] [Google Scholar]
- Milonni P. W.; Smith A. van der Waals dispersion forces in electromagnetic fields. Phys. Rev. A 1996, 53, 3484–3489. 10.1103/PhysRevA.53.3484. [DOI] [PubMed] [Google Scholar]
- Sukhov S.; Douglass K. M.; Dogariu A. Dipole–dipole interaction in random electromagnetic fields. Opt. Lett. 2013, 38, 2385–2387. 10.1364/OL.38.002385. [DOI] [PubMed] [Google Scholar]
- Brügger G.; Froufe-Pérez L. S.; Scheffold F.; Sáenz J. J. Controlling dispersion forces between small particles with artificially created random light fields. Nat. Commun. 2015, 6, 7460. 10.1038/ncomms8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinescu M.; You L. Controlling atom-atom interaction at ultralow temperatures by dc electric fields. Phys. Rev. Lett. 1998, 81, 4596–4599. 10.1103/PhysRevLett.81.4596. [DOI] [Google Scholar]
- Muruganathan M.; Sun J.; Imamura T.; Mizuta H. Electrically tunable van der Waals interaction in graphene molecule complex. Nano Lett. 2015, 15, 8176–8180. 10.1021/acs.nanolett.5b03653. [DOI] [PubMed] [Google Scholar]
- Fiscelli G.; Rizzuto L.; Passante R. Dispersion interaction between two hydrogen atoms in a static electric field. Phys. Rev. Lett. 2020, 124, 013604. 10.1103/PhysRevLett.124.013604. [DOI] [PubMed] [Google Scholar]
- Feynman R. P. Forces in Molecules. Phys. Rev. 1939, 56, 340. 10.1103/PhysRev.56.340. [DOI] [Google Scholar]
- Hunt K. L. C. Dispersion dipoles and dispersion forces: Proof of Feynman’s “conjecture” and generalization to interacting molecules of arbitrary symmetry. J. Chem. Phys. 1990, 92, 1180. 10.1063/1.458126. [DOI] [Google Scholar]
- Podeszwa R.; Jansen G. Comment on “Long-range repulsion between spatially confined van der Waals dimers. Phys. Rev. Lett. 2018, 120, 258901. 10.1103/PhysRevLett.120.258901. [DOI] [PubMed] [Google Scholar]
- Sadhukhan M.; Tkatchenko A. Sadhukhan and Tkatchenko reply. Phys. Rev. Lett. 2018, 120, 258902. 10.1103/PhysRevLett.120.258902. [DOI] [PubMed] [Google Scholar]
- Abrantes P. P.; Pessanha V.; Farina C.; de Melo e Souza R. Comment on “Dispersion interaction between two hydrogen atoms in a static electric field. Phys. Rev. Lett. 2021, 126, 109301. 10.1103/PhysRevLett.126.109301. [DOI] [PubMed] [Google Scholar]
- Fiscelli G.; Rizzuto L.; Passante R. Reply to “Comment on ‘Dispersion interaction between two hydrogen atoms in a static electric field. Phys. Rev. Lett. 2021, 126, 109302. 10.1103/PhysRevLett.126.109302. [DOI] [PubMed] [Google Scholar]
- Wang F.; Jordan K. D. A Drude-model approach to dispersion interactions in dipole-bound anions. J. Chem. Phys. 2001, 114, 10717–10724. 10.1063/1.1376630. [DOI] [Google Scholar]
- Sommerfeld T.; Jordan K. D. Quantum Drude oscillator model for describing the interaction of excess electrons with water clusters: an application to (H20)13–. J. Phys. Chem. A 2005, 109, 11531–11538. 10.1021/jp053768k. [DOI] [PubMed] [Google Scholar]
- Jones A. P.; Crain J.; Sokhan V. P.; Whitfield T. W.; Martyna G. J. Quantum Drude oscillator model of atoms and molecules: many-body polarization and dispersion interactions for atomistic simulation. Phys. Rev. B 2013, 87, 144103. 10.1103/PhysRevB.87.144103. [DOI] [Google Scholar]
- Cohen-Tannoudji C.; Dupont-Roc J.; Grynberg G.. Photons and atoms-introduction to quantum electrodynamics; Wiley: New York, 1997. [DOI] [PubMed] [Google Scholar]
- Milonni P. W.The quantum vacuum: an introduction to quantum electrodynamics; Academic Press: San Diego, CA, 1994. [Google Scholar]
- Craig D. P.; Thirunamachandran T.. Molecular quantum electrodynamics: an introduction to radiation-molecule interactions; Academic Press: London, 1994. [Google Scholar]
- Salam A.Molecular quantum electrodynamics: long-range intermolecular interactions; Wiley: Hoboken, NJ, 2009. [Google Scholar]
- Buhmann S. Y.Dispersion forces I: macroscopic quantum electrodynamics and ground-state Casimir, Casimir–Polder and van der Waals forces; Springer: London, 2012. [Google Scholar]
- Passante R. Dispersion interactions between neutral atoms and the quantum electrodynamical vacuum. Symmetry 2018, 10, 735. 10.3390/sym10120735. [DOI] [Google Scholar]
- Schäfer C.; Ruggenthaler M.; Rubio A. Ab initio nonrelativistic quantum electrodynamics: Bridging quantum chemistry and quantum optics from weak to strong coupling. Phys. Rev. A 2018, 98, 043801. 10.1103/PhysRevA.98.043801. [DOI] [Google Scholar]
- Marshall T. W. Random electrodynamics. Proc. R. Soc. London A 1963, 276, 475–491. 10.1098/rspa.1963.0220. [DOI] [Google Scholar]
- Marshall T. W. Statistical electrodynamics. Math. Proc. Cambridge Philos. Soc. 1965, 61, 537–546. 10.1017/S0305004100004114. [DOI] [Google Scholar]
- Boyer T. H. Random electrodynamics: the theory of classical electrodynamics with classical electromagnetic zero-point radiation. Phys. Rev. D 1975, 11, 790–808. 10.1103/PhysRevD.11.790. [DOI] [Google Scholar]
- de la Pena-Auerbach L.; Cetto A. M. Quantum mechanics derived from stochastic electrodynamics. Found. Phys. 1978, 8, 191–210. 10.1007/BF00715207. [DOI] [Google Scholar]
- Boyer T. H.A brief survey of stochastic electrodynamics in “Foundations of radiation theory and quantum electrodynamics”; Springer: New York, 1980. [Google Scholar]
- de la Peña L.; Cetto A. M.. The quantum dice: an introduction to stochastic electrodynamics; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Nieuwenhuizen T. M. Stochastic electrodynamics: lessons from regularizing the harmonic oscillator. Atoms 2019, 7, 59. 10.3390/atoms7020059. [DOI] [Google Scholar]
- Boyer T. H. Stochastic electrodynamics: the closest classical approximation to quantum theory. Atoms 2019, 7, 29. 10.3390/atoms7010029. [DOI] [Google Scholar]
- Tkatchenko A.; DiStasio R. A.; Car R.; Scheffler M. Accurate and efficient method for many-body van der Waals interactions. Phys. Rev. Lett. 2012, 108, 236402. 10.1103/PhysRevLett.108.236402. [DOI] [PubMed] [Google Scholar]
- Reilly A. M.; Tkatchenko A. van der Waals dispersion interactions in molecular materials: beyond pairwise additivity. Chem. Sci. 2015, 6, 3289–3301. 10.1039/C5SC00410A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobre V. V.Efficient modelling of linear electronic polarization in materials using atomic response functions. Ph.D. thesis, Fritz Haber Institute Berlin, 2016. [Google Scholar]
- Sadhukhan M.; Manby F. R. Quantum mechanics of Drude oscillators with full Coulomb interaction. Phys. Rev. B 2016, 94, 115106. 10.1103/PhysRevB.94.115106. [DOI] [Google Scholar]
- Hermann J.; DiStasio R. A.; Tkatchenko A. First-principles models for van der Waals interactions in molecules and materials: concepts, theory, and applications. Chem. Rev. 2017, 117, 4714–4758. 10.1021/acs.chemrev.6b00446. [DOI] [PubMed] [Google Scholar]
- Fedorov D. V.; Sadhukhan M.; Stöhr M.; Tkatchenko A. Quantum-mechanical relation between atomic dipole polarizability and the van der Waals radius. Phys. Rev. Lett. 2018, 121, 183401. 10.1103/PhysRevLett.121.183401. [DOI] [PubMed] [Google Scholar]
- Tkatchenko A.; Fedorov D. V.; Gori M. Fine-structure constant connects electronic polarizability and geometric van-der-Waals radius of atoms. J. Phys. Chem. Lett. 2021, 12, 9488–9492. 10.1021/acs.jpclett.1c02461. [DOI] [PubMed] [Google Scholar]
- Hermann J.; Alfè D.; Tkatchenko A. Nanoscale π–π stacked molecules are bound by collective charge fluctuations. Nat. Commun. 2017, 8, 14052. 10.1038/ncomms14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Hu J.; Yu H. Quantum corrections to the classical electrostatic interaction between induced dipoles. Phys. Rev. A 2021, 103, 042803. 10.1103/PhysRevA.103.042803. [DOI] [Google Scholar]
- Jansen L.; Mazur P. On the theory of molecular polarization in gases. 1. Effect of molecular interactions on the polarizability of spherical nonpolar molecules. Physica 1954, 21, 193–207. 10.1016/S0031-8914(54)91188-X. [DOI] [Google Scholar]
- Hunt K. L. C.; Buckingham A. D. The polarizability of H2 in the triplet state. J. Chem. Phys. 1980, 72, 2832–2840. 10.1063/1.439431. [DOI] [Google Scholar]
- Li X.; Hunt K. L. C. Transient changes in polarizability for centrosymmetric linear molecules interacting at long range: Theory and numerical results for H2··· H2, H2··· N2, and N2··· N2. J. Chem. Phys. 1994, 100, 7875–7889. 10.1063/1.466834. [DOI] [Google Scholar]
- Fowler P. W.; Hunt K. L. C.; Kelly H. M.; Sadlej A. J. Multipole polarizabilities of the helium atom and collision-induced polarizabilities of pairs containing He or H atoms. J. Chem. Phys. 1994, 100, 2932–2935. 10.1063/1.467248. [DOI] [Google Scholar]
- Certain P. R.; Fortune P. J. Long-Range Polarizability of the Helium Diatom. J. Chem. Phys. 1971, 55, 5818–5821. 10.1063/1.1675752. [DOI] [Google Scholar]
- Joslin C. G.; Goddard J. D.; Goldman S. The pair polarizability of argon. Mol. Phys. 1996, 89, 791–808. 10.1080/00268979609482507. [DOI] [Google Scholar]
- Hunt K. L. C.; Zilles B. A.; Bohr J. E. Effects of van der Waals interactions on the polarizability of atoms, oscillators, and dipolar rotors at long range. J. Chem. Phys. 1981, 75, 3079–3086. 10.1063/1.442404. [DOI] [Google Scholar]
- Karimpour M. R.; Fedorov D. V.; Tkatchenko A. Quantum framework for describing retarded and nonretarded molecular interactions in external electric fields. Phys. Rev. Research 2022, 4, 013011. 10.1103/PhysRevResearch.4.013011. [DOI] [Google Scholar]
- Hunt K. L. C. Long-range dipoles, quadrupoles, and hyperpolarizabilities of interacting inert-gas atoms. Chem. Phys. Lett. 1980, 70, 336–342. 10.1016/0009-2614(80)85346-2. [DOI] [Google Scholar]
- Galatry L.; Gharbi T. The long-range dipole moment of two interacting spherical systems. Chem. Phys. Lett. 1980, 75, 427–433. 10.1016/0009-2614(80)80548-3. [DOI] [Google Scholar]
- Galatry L.; Hardisson A. The dispersive collision dipole in a pair of polyatomic molecules. J. Chem. Phys. 1983, 79, 1758–1765. 10.1063/1.446020. [DOI] [Google Scholar]
- Craig D. P.; Thirunamachandran T. Elementary derivation of long-range moments of two coupled centrosymmetric systems. Chem. Phys. Lett. 1981, 80, 14–17. 10.1016/0009-2614(81)80047-4. [DOI] [Google Scholar]
- Bohr J. E.; Hunt K. L. C. Dipoles induced by van der Waals interactions during collisions of atoms with heteroatoms or with centrosymmetric linear molecules. J. Chem. Phys. 1987, 86, 5441–5448. 10.1063/1.452568. [DOI] [Google Scholar]
- Bohr J. E.; Hunt K. L. C. Dipoles induced by long-range interactions between centrosymmetric linear molecules: Theory and numerical results for H2··· H2, H2··· N2, and N2··· N2. J. Chem. Phys. 1987, 87, 3821–3832. 10.1063/1.453698. [DOI] [Google Scholar]
- Fowler P. W. Dispersion dipoles, quadrupoles and electric-field gradients. Chem. Phys. 1990, 143, 447–457. 10.1016/0301-0104(90)87024-6. [DOI] [Google Scholar]
- Vigoureux J.; Galatry L. Very-long-range static dipole moment of two coupled systems. Chem. Phys. Lett. 1983, 98, 324–328. 10.1016/0009-2614(83)80216-4. [DOI] [Google Scholar]
- Odbadrakh T. T.; Jordan K. D. Dispersion dipoles for coupled Drude oscillators. J. Chem. Phys. 2016, 144, 034111. 10.1063/1.4940217. [DOI] [PubMed] [Google Scholar]
- Atkins P.; Friedman R.. Molecular quantum mechanics; Oxford University Press: Oxford, U.K., 2005. [Google Scholar]
- Passante R.; Compagno G.; Persico F. Cloud of virtual photons in the ground state of the hydrogen atom. Phys. Rev. A 1985, 31, 2827–2841. 10.1103/PhysRevA.31.2827. [DOI] [PubMed] [Google Scholar]
- Compagno G.; Persico F.; Passante R. Interference in the virtual photon clouds of two hydrogen atoms. Phys. Lett. 1985, 112A, 215–219. 10.1016/0375-9601(85)90506-7. [DOI] [Google Scholar]
- Passante R.; Power E. A. Electromagnetic-energy-density distribution around a ground-state hydrogen atom and connection with van der Waals forces. Phys. Rev. A 1987, 35, 188–197. 10.1103/PhysRevA.35.188. [DOI] [PubMed] [Google Scholar]
- Compagno G.; Palma M. G. Distortion of the virtual photon cloud due to a static electric field. Phys. Rev. A 1988, 37, 2979–2984. 10.1103/PhysRevA.37.2979. [DOI] [PubMed] [Google Scholar]
- Compagno G.; Vivirito S.; Persico F. Cloud of virtual photons surrounding a two-level atom driven by an external field. Phys. Rev. A 1992, 46, 7303–7306. 10.1103/PhysRevA.46.7303. [DOI] [PubMed] [Google Scholar]
- Boyer T. H. Recalculations of long-range van der Waals potentials. Phys. Rev. 1969, 180, 19–24. 10.1103/PhysRev.180.19. [DOI] [Google Scholar]
- Boyer T. H. Asymptotic retarded van der Waals forces derived from classical electrodynamics with classical electromagnetic zero-point radiation. Phys. Rev. A 1972, 5, 1799–1802. 10.1103/PhysRevA.5.1799. [DOI] [Google Scholar]
- Jackson J. D.Classical electrodynamics; Wiley: Hoboken, NJ, 1998. [Google Scholar]
- Boyer T. H. Unretarded London-van der Waals forces derived from classical electrodynamics with classical electromagnetic zero-point radiation. Phys. Rev. A 1972, 6, 314–319. 10.1103/PhysRevA.6.314. [DOI] [Google Scholar]
- Boyer T. H. Contrasting interactions between dipole oscillators in classical and quantum theories: illustrations of unretarded van der Waals forces. Eur. J. Phys. 2018, 39, 045401. 10.1088/1361-6404/aaadf5. [DOI] [Google Scholar]
- Boyer T. H. Retarded van der Waals forces at all distances derived from classical electrodynamics with classical electromagnetic zero-point radiation. Phys. Rev. A 1973, 7, 1832–1840. 10.1103/PhysRevA.7.1832. [DOI] [Google Scholar]
- Salam A. Asymptotic limits of the retarded interaction energy between two hyperpolarizable molecules. Phys. Rev. A 1997, 55, 804–807. 10.1103/PhysRevA.55.804. [DOI] [Google Scholar]
- Martin J. M.; Li W.; Delaney K. T.; Fredrickson G. H. Statistical field theory description of inhomogeneous polarizable soft matter. J. Chem. Phys. 2016, 145, 154104. 10.1063/1.4964680. [DOI] [PubMed] [Google Scholar]
- Dinadayalane T. C.; Leszczynski J. Geometries and stabilities of various configurations of benzene dimer: details of novel V-shaped structure revealed. Struct. Chem. 2009, 20, 11–20. 10.1007/s11224-009-9411-6. [DOI] [Google Scholar]
- Archibong E. F.; Thakkar A. J. Polarizabilities of aromatic six-membered rings: azines and ‘inorganic benzenes. Mol. Phys. 1994, 81, 557–567. 10.1080/00268979400100371. [DOI] [Google Scholar]
- Ambrosetti A.; Ferri N.; DiStasio R. A.; Tkatchenko A. Wavelike charge density fluctuations and van der Waals interactions at the nanoscale. Science 2016, 351, 1171–1176. 10.1126/science.aae0509. [DOI] [PubMed] [Google Scholar]
- Vaccarelli O.; Fedorov D. V.; Stöhr M.; Tkatchenko A. Quantum-mechanical force balance between multipolar dispersion and Pauli repulsion in atomic van der Waals dimers. Phys. Rev. Research 2021, 3, 033181. 10.1103/PhysRevResearch.3.033181. [DOI] [Google Scholar]
- Hunt K. L. C. Nonlocal polarizability densities and the effects of short range interactions on molecular dipoles, quadrupoles, and polarizabilities. J. Chem. Phys. 1984, 80, 393–407. 10.1063/1.446461. [DOI] [Google Scholar]