ABSTRACT
Phosphatidylinositol(4,5)-bisphosphate (PtdInsP2) is an important modulator of many cellular processes, and its abundance in the plasma membrane is closely regulated. We examined the hypothesis that members of the Dishevelled scaffolding protein family can bind the lipid kinases phosphatidylinositol 4-kinase (PI4K) and phosphatidylinositol 4-phosphate 5-kinase (PIP5K), facilitating synthesis of PtdInsP2 directly from phosphatidylinositol. We used several assays for PtdInsP2 to examine the cooperative function of phosphoinositide kinases and the Dishevelled protein Dvl3 in the context of two receptor signaling cascades. Simultaneous overexpression of PI4KIIIα (also known as PI4KA) and PIP5KIγ (also known as PIP5K1C) had a synergistic effect on PtdInsP2 synthesis that was recapitulated by overexpression of Dvl3. Increasing the activity of Dvl3 by overexpression increased resting plasma membrane PtdInsP2. Knockdown of Dvl3 reduced resting plasma membrane PtdInsP2 and slowed PtdInsP2 resynthesis following receptor activation. We confirm that Dvl3 promotes coupling of PI4KIIIα and PIP5KIγ and show that this interaction is essential for efficient resynthesis of PtdInsP2 following receptor activation.
KEY WORDS: Dishevelled, Dvl3, PtdInsP2, Ror2, Wnt, Kinase, Phosphoinositide
Summary: Efficient synthesis of the membrane phospholipid PtdIns(4,5)P2 from PtdIns depends on expression of the scaffolding protein Dvl3, which dynamically couples lipid kinases PI4KIIIα and PIP5KIγ.
INTRODUCTION
This paper investigates in living cells the organization and cooperative action of phosphoinositide kinases and Dishevelled (Dvl) proteins, a family of scaffolding proteins that organize effectors of Wnt signaling pathways (Sharma et al., 2018). Phosphoinositides are minority membrane lipids with important functions in cellular signaling. The heterogeneous distribution of phosphoinositides gives each cellular membrane a distinct lipid signature, and this distribution is kept in homeostasis by regulation of lipid kinases and phosphatases. Phosphatidylinositol (PtdIns) can be phosphorylated on the 3-, 4-, or 5-hydroxyl positions of its myo-inositol ring to generate seven species of phosphoinositides, including phosphatidylinositol(4,5)-bisphosphate (PtdInsP2), which resides on the inner, cytoplasmic leaflet of the plasma membrane. PtdInsP2 is an important signaling molecule with diverse cellular functions including regulating ion channels and transporters, remodeling the cytoskeleton, and regulating vesicle movement and fusion (Balla, 2013; Mandal, 2020).
Our focus is the generation of PtdInsP2 from PtdIns via phosphorylation by phosphatidylinositol 4-kinase (PI4K) and phosphatidylinositol 4-phosphate 5-kinase (PIP5K). The synthesis of PtdInsP2 from PtdIns is typically considered as two sequential and physically separate reactions catalyzed by PI4K and PIP5K (Fig. 1A, top). A recent kinetic model of phosphoinositide dynamics (Olivença et al., 2018) found it necessary to postulate direct transformation of PtdIns into PtdInsP2 to match experimental rates of PtdInsP2 production, especially under conditions of low phosphatidylinositol (4)-phosphate (PtdInsP). Previous evidence for a ternary complex that includes both kinases and a scaffolding protein (Fig. 1A, bottom) comes from co-immunoprecipitation experiments showing physical association amongst PI4K type IIα (also known as PI4K2A), PIP5K and a Dvl scaffolding protein during Wnt signaling (Qin et al., 2009). We assessed the degree to which a different type of PI4K and PIP5KIγ (also known as PIP5K1C) associate and the functional consequences of this partnership in the context of two receptor signaling cascades.
Fig. 1.
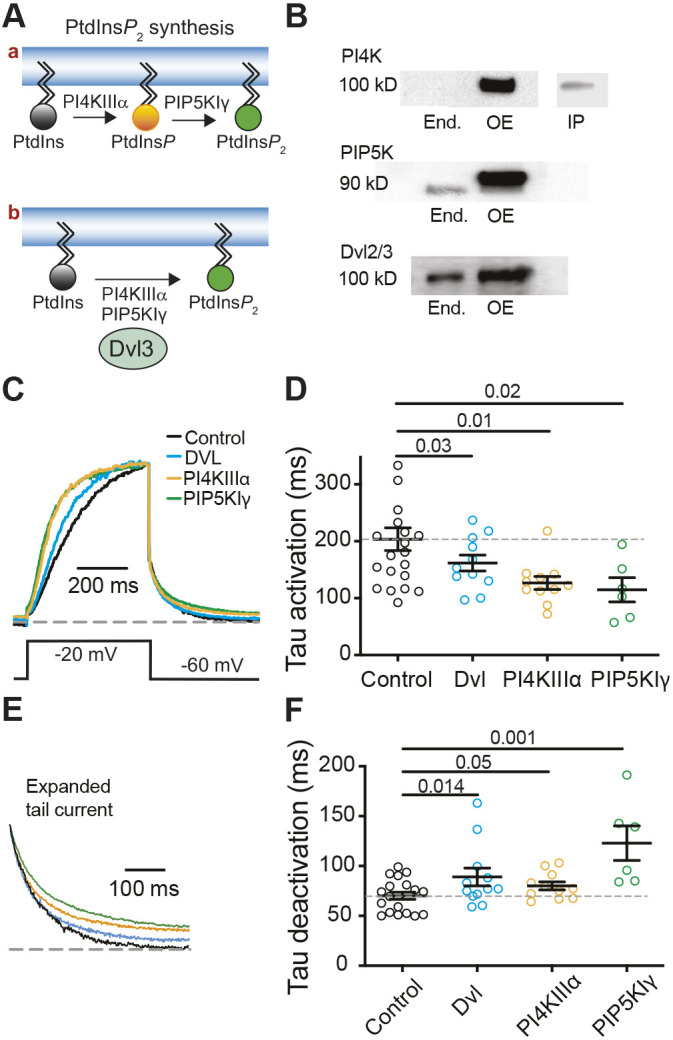
Overexpression of Dvl3 increases baseline PtdInsP2. (A) Schematic representation of PtdInsP2 synthesis as sequential phosphorylation by PI4KIIIα and PIP5KIγ (top) or as coupled and concerted activity of the two phosphoinositide kinases (bottom). (B) Immunoblots showing expression of PI4K, PIP5K, or Dvl2 and Dvl3 (Dvl2/3) in untransfected tsA-201 cells (End.) and in cells overexpressing (OE) PI4K (top), PIP5K (middle) or Dvl3 (bottom). Note that recombinant proteins are tagged with fluorophore; therefore, they run at higher molecular weights (indicated in kilodaltons, kD). Endogenous PI4KIIIα was detected following immunoprecipitation (IP). Blots are representative of six experiments. (C) Normalized voltage-clamp current traces from tsA-201 cells overexpressing KCNQ2 and KCNQ3 channel subunits (control, black) and Dvl3 (DVL, blue), PI4KIIIα–GFP (orange) or PIP5KIγ–GFP (green). (D) The exponential time constant (tau) of KCNQ2/3 activation for conditions as in A. Mean±s.e.m. tau of activation: 204±20 ms (control, n=21), 162±14 ms (Dvl3, n=11), 126.7±11 ms (PI4KIIIα, n=11), 115±21 ms (PIP5KIγ, n=6). (E) Normalized expanded tail current from C to show deactivation. (F) Exponential time constants of KCNQ2/3 deactivation. Mean±s.e.m. tau of deactivation: 70±4 ms (control, n=21), 89±9 ms (Dvl3, n=11), 80±4 ms (PI4KIIIα, n=11), 123±17 ms (PIP5KIγ, n=6). Dashed lines in D and F mark the mean control value. P-values are shown for the comparisons indicated by horizontal bars (ANOVA with Dunnett's multiple comparison test).
Here, we compared signaling from two types of receptors. The M1 muscarinic acetylcholine receptor (CHRM1, referred to here as M1 receptor) couples to Gαq and phospholipase C beta (PLCβ) to hydrolyze PtdInsP2 to inositol (1,4,5)-trisphosphate (InsP3) and diacylglycerol, leading to several downstream events including an increase in Ca2+ concentration, membrane depolarization and regulation of several ion channels (Hille et al., 2015). The Ror2 receptor is a tyrosine kinase-like non-canonical Wnt receptor known to associate with Dvl proteins (Nishita et al., 2010; Witte et al., 2010). The early signaling events initiated by Ror2 are less well defined. In neurons, activation of Ror2 leads to an increase in intracellular Ca2+, membrane depolarization and increased trafficking of NMDA receptors (Cerpa et al., 2011; 2015; McQuate et al., 2017). Dvl proteins regulate the cytoskeleton, modify synaptic activity and shuttle to the nucleus (Ahmad-Annuar et al., 2006; Castro-Piedras et al., 2021; Sharma et al., 2018; Sheldahl et al., 2003). Genetic mutations in DVL1, DVL3, ROR2 or WNT5A cause impaired development, particularly affecting the skeleton, classified as Robinow syndrome (White et al., 2015, 2016, 2018). We tested the role of the Dvl protein Dvl3 in PtdInsP2 metabolism downstream of both receptors and compared it to the role of phosphoinositide kinases.
The localization and regulatory context of phosphoinositide kinases is becoming apparent through structural and functional studies. The substrate of PI4K, PtdIns, is abundant on the endoplasmic reticulum, where colocalization with the PtdInsP 4-phosphatase Sac1 (also known as SACM1L) prevents the accumulation of PtdInsP. PtdIns is transported from the endoplasmic reticulum to the plasma membrane by PtdIns transfer proteins. PI4KIIIα (also known as PI4KA) – which is cytosolic when expressed alone – is anchored to the plasma membrane by cognate proteins, several pairs of which are sufficient plasma membrane tethers: EFR3A/B and TTC7A/B, EFR3A/B and TMEM150A, TTC7A/B and FAM126A (Chung et al., 2015; Lees et al., 2017; Nakatsu et al., 2012). PIP5K localizes to the plasma membrane, and the catalytic activity of PIP5K1A is enhanced by binding the DIX domain of Dvl1 (Hu et al., 2015). We build on this context to show how these proteins work together in living cells. In this paper, we show that Dvl3 organizes PI4K and PIP5K for enzymatic synergy in synthesizing PtdInsP2, that this mechanism is essential for timely PtdInsP2 resynthesis following M1 receptor activation, and that Ror2 increases PtdInsP2, likely via interaction with Dvl3 and PIP5K.
RESULTS
Dvl3 increases baseline PtdInsP2
This study tests the hypothesis that Dvl proteins organize PI4K and PIP5K to facilitate synthesis of PtdInsP2 (Fig. 1A). Immunoblots for PI4KIIIα, PIP5KIγ, and Dvl2 and Dvl3 confirmed that these proteins are expressed endogenously in untransfected human tsA-201 cells and that overexpression increases their levels as expected (Fig. 1B). Endogenous PI4KIIIα was imperceptible in whole-cell lysates but could be detected following immunoprecipitation with an anti-PI4K antibody.
We assessed PtdInsP2 levels by measuring potassium currents upon overexpression of KCNQ2 and KCNQ3 (referred to hereafter as KCNQ2/3). Due to their high sensitivity to plasma membrane PtdInsP2 levels (Li et al., 2005), KCNQ2/3 potassium channels (M current) are specific real-time indicators of plasma membrane PtdInsP2 in living cells. KCNQ2/3 currents are virtually eliminated by strong activation of PLCβ and subsequent depletion of PtdInsP2 (Horowitz et al., 2005), whereas increased levels of PtdInsP2 are correlated with faster activation gating and slower deactivation of the channel (Dai et al., 2016). Changes to baseline PtdInsP2 levels could be ascertained from activation and deactivation kinetics of KCNQ2/3 currents elicited by a depolarizing pulse to −20 mV. Overexpression of the scaffolding protein Dvl3 accelerated activation gating and slowed deactivation compared to control cells, indicating an increase in steady-state levels of plasma membrane PtdInsP2 (Fig. 1C–F, blue traces). As expected, overexpressing either PI4KIIIα or PIP5KIγ also accelerated activation and slowed deactivation (Fig. 1C–F, orange and green traces, respectively).
Dvl3 accelerates PtdInsP2 resynthesis
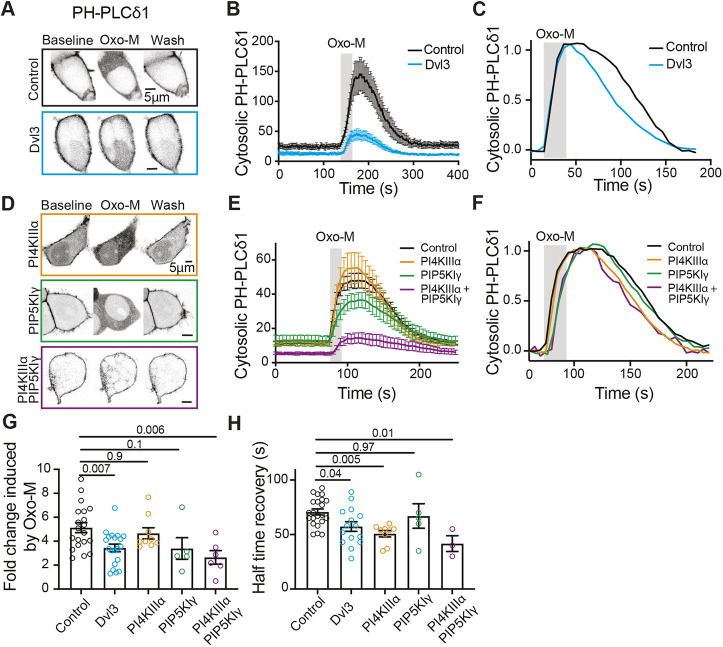
To establish the cellular consequences of Dvl proteins on PtdInsP2, we studied the effects of Dvl3 overexpression on the kinetics of PtdInsP2 depletion and resynthesis in the dynamic environment of receptor activation. Activation of Gq-coupled receptors such as the M1 receptor activates PLCβ, causing hydrolysis of PtdInsP2 (Horowitz et al., 2005). We assessed PtdInsP2 localization using PH-PLCδ1, a fluorophore-tagged probe that serves as a specific visual indicator of PtdInsP2 (Varnai et al., 2006). In control cells, PH-PLCδ1 accumulated primarily at the plasma membrane, as expected, and transiently translocated to the cytosol upon activation of the M1 receptor, reflecting hydrolysis of PtdInsP2 by PLCβ (Fig. 2A–C; control in A, black trace in B and C). Overexpressing Dvl3 reduced the baseline cytosolic intensity of PH-PLCδ1 (Fig. 2B, blue trace), indicating increased resting plasma membrane PtdInsP2. When M1 receptors were activated in cells overexpressing Dvl3, much of the PH-PLCδ1 was retained at the plasma membrane; translocation to the cytosol was reduced (Fig. 2G), and the probe returned to its baseline distribution much faster than in control cells (Fig. 2H). The reduced initial translocation to the cytosol and the faster recovery of the probe to the plasma membrane suggest larger resting pools and accelerated synthesis of PtdInsP2 in the presence of overexpressed Dvl3.
Fig. 2.
Overexpression of Dvl3 accelerates PtdInsP2 resynthesis, mimicking overexpression of PI4KIIIα and PIP5KIγ. (A) Contrast-inverted Airyscan confocal micrographs of tsA-201cells overexpressing M1 receptor and the PtdInsP2 probe PH-PLCδ1–RFP alone (control) or with co-expression of Dvl3–YFP (blue). PH-PLCδ1 localizes to the plasma membrane at rest and relocates to the cytoplasm when Oxo-M (1 μM) activates M1 muscarinic receptors, depleting PtdInsP2. After removal of Oxo-M (wash), PH-PLCδ1 returns to the plasma membrane, indicating resynthesis of PtdInsP2. (B) Time course of cytosolic intensity of PH-PLCδ1 in control and Dvl3-overexpressing cells as in A. Note that an increase in cytosolic PH-PLCδ1 indicates a decrease in plasma membrane PtdInsP2. Mean±s.e.m. baseline cytosolic PH-PLCδ1: 24±4 (control, n=11), 12±2 (Dvl3, n=20). (C) Same mean data as in B, normalized for comparison of kinetics. (D) Contrast-inverted Airyscan confocal micrographs from tsA-201 cells overexpressing M1 receptor, PH-PLCδ1–RFP, and either PI4KIIIα–GFP (orange), PIP5KIγ–GFP (green) or PI4KIIIα–GFP and PIP5KIγ–CFP together (purple), treated as in A. (E) Time course of cytosolic intensity of PH-PLCδ1 in kinase-overexpressing cells as in D. Control, tsA-201 cells overexpressing M1 receptors and PH-PLCδ1–RFP. Mean±s.e.m. baseline cytosolic PH-PLCδ1: 11±1 (control, n=12), 12±1 (PI4KIIIα, n=9), 13±3 (PIP5KIγ, n=5), 6±1 (PI4KIIIα+PIP5KIγ, n=6). (F) Same mean data as in E, normalized for comparison of kinetics. (G) Fold change in peak cytosolic intensity of PH-PLCδ1 following activation of M1 receptors in B and E. Mean±s.e.m. fold change: 5.2±0.4 (control, n=20), 3.4±0.3 (Dvl3, n=20), 4.6±0.5 (PI4KIIIα, n=9), 3.3±0.9 (PIP5KIγ, n=5), 2.6±0.56 (PI4KIIIα+PIP5KIγ, n=6). (H) The half time for recovery of cytosolic PH-PLCδ1–RFP following M1 receptor activation in B and E. Mean±s.e.m. half time of recovery: 71±3 s (control, n=20), 57±4 s (Dvl3, n=20), 51±3 s (PI4KIIIα, n=9), 67±11 s (PIP5KIγ, n=5), 42±7 s (PI4KIIIα+PIP5KIγ, n=3). P-values are shown for the comparisons indicated by horizontal bars (ANOVA with Dunnett's multiple comparison test).
PI4K and PIP5K work synergistically to generate PtdInsP2
To understand the relationship of Dishevelled proteins with PI4KIIIα and PIP5KIγ, we compared the overexpression of Dvl3 with overexpression of the phosphoinositide kinases alone or together. We found that neither PI4KIIIα nor PIP5KIγ overexpressed alone changed the baseline cytosolic distribution of PH-PLCδ1, but that in combination they increased resting plasma membrane PtdInsP2, as reflected by decreased baseline cytosolic intensity of PH-PLCδ1 (Fig. 2E, purple trace). Activation of M1 receptors in cells overexpressing either PI4KIIIα or PIP5KIγ depleted PtdInsP2 from the plasma membrane, seen as a transient increase in cytosolic PH-PLCδ1 (Fig. 2D,E). Overexpression of either kinase altered the kinetics of PH-PLCδ1 movement in response to activation of M1 receptors, with PIP5KIγ delaying the release of PH-PLCδ1 from the plasma membrane during M1 receptor activation and PI4KIIIα accelerating the return of PH-PLCδ1 to the plasma membrane during resynthesis of PtdInsP2 (Fig. 2H). When both kinases were co-expressed, translocation of PH-PLCδ1 to the cytosol was suppressed by 62%, similar to the effect of overexpression of Dvl3 (Fig. 2G). In the few cells with evident translocation of PH-PLCδ1, kinetic rates matched those for overexpression of PIP5KIγ during PtdInsP2 hydrolysis and those for PI4KIIIα during PtdInsP2 resynthesis (Fig. 2F,H). These data suggest that overexpression of Dvl3 recapitulates the co-overexpression of PI4KIIIα and PIP5KIγ and support the idea that Dishevelled proteins coordinate PI4KIIIα and PIP5KIγ to act synergistically to synthesize PtdInsP2. Our data also suggest that PI4KIIIα is rate limiting in PtdInsP2 synthesis, in agreement with our previous work (Falkenburger et al., 2010).
Interpretation of these results must be tempered with acknowledgement of the constraints of the probe used. Although PH-PLCδ1 allows us to observe net changes in plasma membrane PtdInsP2, it may not be a fully linear indicator of PtdInsP2 (Myeong et al., 2020). PH-PLCδ1 mobility is likely saturated at high PtdInsP2 levels (such that no further movement to the plasma membrane is possible even with increasing PtdInsP2), for instance when Dvl3 is overexpressed or both kinases are overexpressed together.
PI4K, PIP5K and Dvl3 mutually reinforce localization to the plasma membrane
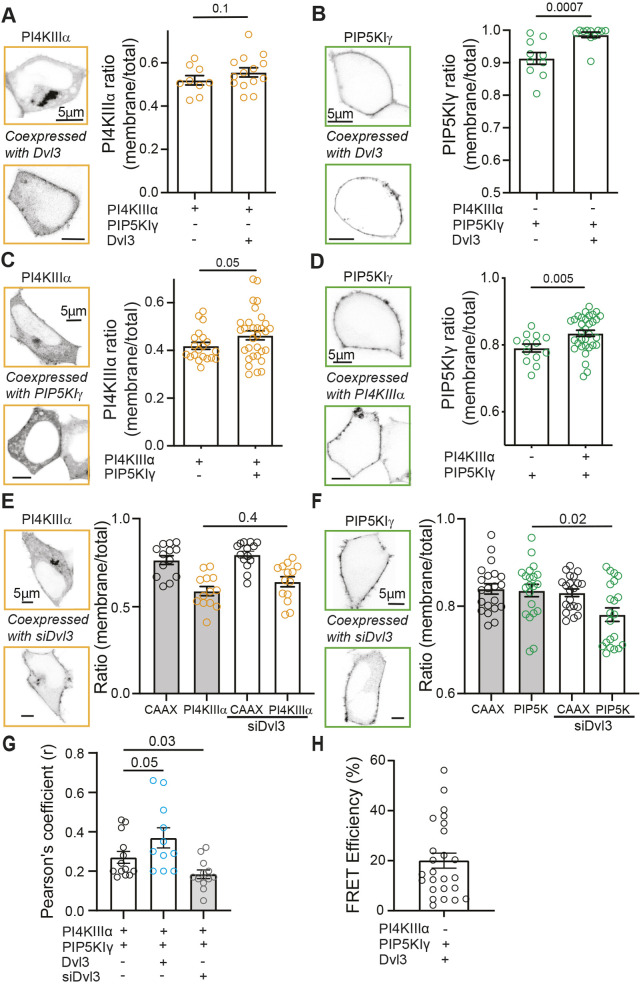
Since PI4KIIIα and PIP5KIγ appear to work synergistically to synthesize PtdInsP2 and overexpressed Dvl3 recapitulates the effects of PI4K and PIP5K co-expression, we hypothesized that these proteins might colocalize. The localization of PI4K, PIP5K and Dvl3 was probed using high-resolution confocal microscopy and fluorescence resonance energy transfer (FRET). Overexpressed PI4KIIIα was found in the cytosol, plasma membrane and a Golgi-like structure (Fig. 3A), whereas PIP5KIγ was localized primarily in the plasma membrane (Fig. 3B). To facilitate plasma membrane docking of PI4KIIIα, throughout this paper this kinase was always co-expressed with the complementary proteins TTC7B and EFR3B (Chung et al., 2015). Notably, overexpressing Dvl3 increased the plasma membrane component of PIP5KIγ (Fig. 3B) but not of PI4KIIIα (Fig. 3A). Overexpressing PI4KIIIα also increased the plasma membrane component of PIP5KIγ (Fig. 3D), whereas PIP5KIγ only marginally increased the proportion of PI4KIIIα at the plasma membrane (Fig. 3C). Knocking down Dvl3 decreased the plasma membrane component of PIP5KIγ (Fig. 3F) but had no effect on PI4KIIIα (Fig. 3E). To quantify the magnitude of interaction between overexpressed PI4KIIIα–GFP and PIP5KIγ–CFP, we calculated the Pearson's r coefficient from confocal micrographs of control cells, those additionally overexpressing Dvl3, and those with knockdown of Dvl3 using siRNA. Co-expression of Dvl3 increased the Pearson's coefficient (mean Pearson's r value=0.37) compared to control cells (mean Pearson's r value=0.27), and knocking down Dvl3 reduced colocalization (mean Pearson's r value=0.18; Fig. 3G). Measurements of FRET between PIP5KIγ–CFP and Dvl3–YFP support the hypothesis that Dvl3 organizes PIP5KIγ via physical interaction (Fig. 3H).
Fig. 3.
Dvl3 relocalizes PIP5KIγ to the plasma membrane and promotes colocalization of PI4KIIIα and PIP5KIγ. (A) Left: contrast-inverted confocal micrographs of PI4KIIIα–GFP in tsA-201 cells overexpressing PI4KIIIα–GFP alone (top) or with co-expression of Dvl3–HcRed (bottom). Right: fraction of PI4KIIIα–GFP that distributes to the plasma membrane when the kinase is expressed individually or with Dvl3. Mean±s.e.m. plasma membrane fraction PI4KIIIα–GFP: 0.52±0.03 (n=9, PI4KIIIα alone), 0.56±0.02 (n=14, PI4KIIIα+Dvl3). For PI4KIIIα, the plasma membrane was delineated by co-expression of CFP–CAAX (pixels positive for CFP–CAAX were used to create a plasma membrane ‘mask’ to delineate the plasma membrane in the PI4KIIIα–GFP channel). (B) Left: contrast-inverted confocal micrographs of PIP5KIγ–CFP in tsA-201 cells overexpressing PIP5KIγ–CFP alone (top) or with co-expression of Dvl3–HcRed (bottom). Right: mean±s.e.m. plasma membrane fraction of PIP5KIγ–CFP: 0.89±0.02 (n=10, PIP5KIγ alone), 0.98±0.01 (n=11, PIP5KIγ+Dvl3). (C) Left: contrast-inverted confocal micrographs of PI4KIIIα–GFP in tsA-201 cells overexpressing PI4KIIIα–GFP alone (top) or with co-expression of PIP5KIγ–CFP (bottom). Right: fraction of PI4KIIIα–GFP that distributes to the plasma membrane when expressed alone or with co-expression of PIP5KIγ–CFP. Mean±s.e.m. plasma membrane fraction of PI4KIIIα–GFP: 0.42±0.01 (n=20, PI4KIIIα alone), 0.47±0.02 (n=31, PI4KIIIα+PIP5KIγ). (D) Left: contrast-inverted confocal micrographs of PIP5KIγ–CFP in tsA-201 cells overexpressing PIP5KIγ–CFP alone (top) or with co-expession of PI4KIIIα–GFP (bottom). Right: fraction of PIP5KIγ–CFP that distributes to the plasma membrane alone or with co-expression of PI4KIIIα–GFP. Mean±s.e.m. plasma membrane fraction PIP5KIγ–CFP: 0.79±0.01 (n=13, PIP5KIγ alone), 0.83±0.01 (n=31, PIP5KIγ+PI4KIIIα). (E,F) Left: contrast-inverted confocal micrographs of tsA-201 cells overexpressing PI4KIIIα–GFP (E) or PIP5KIγ–CFP (F) alone (top) or with co-expression of siRNA for Dvl3 (siDvl3, bottom). Right: fraction of PI4KIIIα–GFP (E) or PIP5KIγ–CFP (F) that distributes to the plasma membrane alone or with co-expression of siDvl3. CAAX localization was used as a mask to delineate the plasma membrane and as a negative control for the effect of siDvl3. Mean±s.e.m. plasma membrane fraction of PI4KIIIα–GFP (E): 0.59±0.03 (n=13, PI4KIIIα alone), 0.64±0.03 (n=14, PI4KIIIα+siDvl3). Mean±s.e.m. plasma membrane fraction of PIP5KIγ–CFP (F): 0.84±0.01 (n=21, PIP5KIγ alone), 0.78±0.02 (n=21, PIP5KIγ+siDvl3). (G) Pearson's coefficient of colocalization derived from confocal micrographs of cells expressing PI4KIIIα–GFP and PIP5KIγ–CFP alone or with co-expression of Dvl3–HcRed or siDvl3. Mean±s.e.m. Pearson's r value: 0.27±0.03 (control, n=13), 0.37±0.05 (Dvl3, n=11), 0.18±0.02 (siDvl3, n=12). (H) FRET efficiency between PIP5KIγ–CFP and Dvl3–YFP. Mean±s.e.m. FRET Efficiency: 20.0±3.0% (n=25). P-values are shown in A–G for the comparisons indicated by horizontal bars (two-tailed unpaired Student's t-test in A–D, ANOVA with Dunnett's multiple comparison test in E–G).
Dvl3 is necessary for PtdInsP2 resynthesis
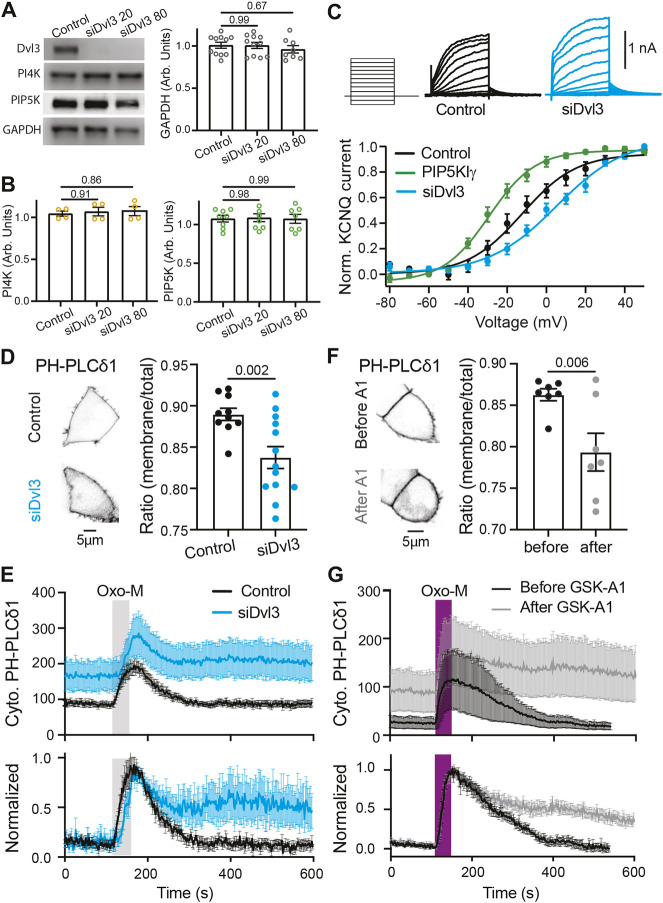
To determine the necessity of Dvl3 for PtdInsP2 resynthesis, we tested the effects of Dvl3 loss of function on steady-state PtdInsP2 levels and on the kinetics of PtdInsP2 resynthesis. An siRNA against Dvl3 (siDvl3) effectively eliminated Dvl3 expression (Fig. 4A). Quantification of PI4KIIIα and PIP5KIγ showed that knocking down Dvl3 had no effect on the endogenous expression of PI4KIIIα or PIP5KIγ (Fig. 4B). Since the voltage activation of KCNQ2/3 channels is strongly sensitive to PtdInsP2 levels (Kim et al., 2016), we measured the current–voltage relationship in cells with and without expression of siDvl3. With siDvl3, KCNQ2/3 currents required more depolarized potentials to activate, consistent with a decrease in PtdInsP2 (Fig. 4C). An increase in PtdInsP2 induced by overexpression of PIP5KIγ had the opposite effect (Fig. 4C), permitting channel opening at less depolarized potentials. Tail currents elicited at a range of voltages were fitted with sigmoid curves yielding a mid-point voltage V1/2 of −11.3 mV for control cells, −28.1 mV for cells overexpressing PIP5KIγ and +5.0 mV for cells with siDvl3 (Fig. 4C).
Fig. 4.
Downregulation of Dvl3 reduces plasma membrane pools of PtdInsP2 and prevents a full recovery. (A) Left: immunoblot showing expression of endogenous Dvl3, PI4KIIIα, PIP5KIγ and GAPDH in tsA-201 cells transfected with two different concentrations of siRNA (second column, 20 pmol siDvl3; third column, 80 pmol siDvl3) for Dvl3. Right: quantification of expression of GAPDH. Data are presented as mean±s.e.m. of 11 experiments. (B) Quantification of expression of PI4KIIIα (left, n=4) and PIP5KIγ (right, n=7) as in A. Data are presented as mean±s.e.m. (C) Top: representative voltage-clamp currents for tsA-201 cells transfected with KCNQ2 and KCNQ3 (control, black) and those with siRNA against Dvl3 (siDvl3, blue). Bottom: normalized tail current–voltage relationship for cells as in B, and for those overexpressing PIP5KIγ–CFP (green). Mean±s.e.m. half-maximal voltage: −11.3±4.0 mV (n=12, control), −28.1±3.0 mV (n=7, PIP5KIγ), +5.0±3.9 mV (n=6, siDvl3). (D) Left: contrast-inverted confocal micrographs of PH-PLCδ1–YFP in tsA-201 cells expressing PH-PLCδ1–YFP alone (control) or with overexpression of siDvl3. TYE 563 dye fluorescence was used to confirm siDvl3 transfection. Right: membrane fraction of PH-PLCδ1–YFP alone or with co-expression of siDvl3. Mean±s.e.m. plasma membrane fraction PH-PLCδ1: 0.89±0.007 (n=10, control), 0.83±0.132 (n=13, siDvl3). (E) Time course of cytosolic PH-PLCδ1 alone (control, black) or with overexpression of siRNA for Dvl3 (siDvl3, blue). Oxo-M treatment (1 µM) is indicated by the gray bar. Data are presented as mean±s.e.m. of n=6 (control) and n=6 (siDvl3). (F) Contrast-inverted confocal micrographs of tsA-201 cells expressing PH-PLCδ1–YFP before and after treatment with PI4KIIIa inhibitor GSK-A1 (100 nM for 15 min) with a graph showing the fraction of PH probe residing in the membrane. Mean±s.e.m. plasma membrane fraction PH-PLCδ1: before GSK-A1 treatment, 0.86±0.007 (n=7); after GSK-A1 treatment, 0.79±0.022 (n=7). (G) Time course of cytosolic PH-PLCδ1 in control cells before (black) or after (gray) treatment with GSK-A1. Oxo-M treatment (1 µM) is indicated by the magenta bar. Data are presented as mean±s.e.m. of n=6 (before GSK-A1) and n=6 (after GSK-A1). P-values are shown in A,B,D and F for the comparisons indicated by horizontal bars (ANOVA with Dunnett's multiple comparison test in A,B; two-tailed unpaired Student's t-test in D,F).
Consistent with a decrease in steady-state plasma membrane PtdInsP2, siDvl3 reduced the resting fraction of PH-PLCδ1 at the plasma membrane (Fig. 4D). In addition, PH-PLCδ1 returned only partially to the plasma membrane following activation of M1 receptors (Fig. 4E), indicating a compromised capacity for PtdInsP2 resynthesis. Treating cells for 15 min with GSK-A1, an inhibitor of PI4KIIIα, elicited a strikingly similar reduction of plasma membrane PtdInsP2 at rest (Fig. 4F) and impaired resynthesis after receptor activation (Fig. 4G). These data indicate that both PI4KIIIα and Dvl3 are necessary for efficient resynthesis of PtdInsP2.
Dvl3 and PIP5K move to the plasma membrane in response to Wnt5a
Ror2 is a non-canonical Wnt receptor activated by Wnt5a whose partially characterized downstream signaling includes Dvl proteins. We overexpressed Ror2 receptors and tested whether application of Wnt5a changes the localization of Dvl3 or PIP5KIγ. Using total internal reflection fluorescence (TIRF) microscopy, we found that Wnt5a induced a small and transient translocation of Dvl3 to the plasma membrane, peaking ∼30 s after agonist application and decreasing before agonist was removed (Fig. 5A,B). During this time, the Ror2 receptor remained stable in the plasma membrane. Likewise, confocal microscopy showed a brief recruitment of PIP5KIγ to the plasma membrane, followed by formation of cytosolic bodies labeled for PIP5KIγ (Fig. 5C,D). Thus, addition of Wnt5a to cells overexpressing Ror2 receptors was sufficient to recruit Dvl3 and PIP5KIγ.
Fig. 5.
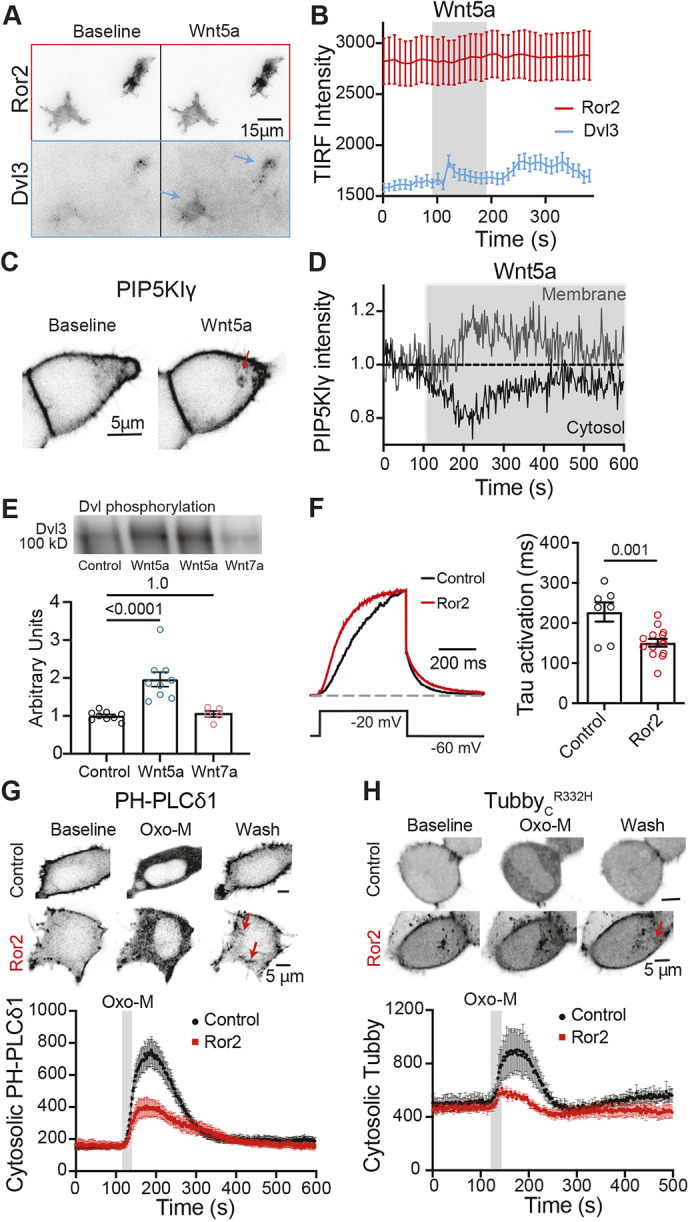
Wnt5a recruits Dvl3 and PIP5KIγ to the plasma membrane in cells overexpressing Ror2, and Ror2 overexpression increases PtdInsP2. (A) Contrast-inverted TIRF micrographs of two tsA-201cells overexpressing Ror2–CFP and Dvl3–dsRed at rest (baseline) and in the presence of 125 ng/ml Wnt5a. Under baseline conditions, Dvl3 is minimally present within the evanescent field. After Wnt5a application, Dvl3 appears, and puncta are visible (blue arrows). (B) Time course of Ror2–CFP and Dvl3–dsRed intensity as in A. Addition of Wnt5a is marked by the gray bar. Data are mean±s.e.m. of nine cells. (C) Contrast-inverted confocal micrographs of PIP5KIγ–GFP in tsA-201 cells overexpressing Ror2–mCherry and PIP5KIγ–GFP before and after application of 125 ng/ml Wnt5a. Red arrow indicates intracellular regions containing PIP5KIγ. Images are representative of five cells. (D) Time course of normalized intensity of PIP5KIγ–GFP in the plasma membrane and the cytosol. Application of Wnt5a is indicated by the gray box. Data are representative of five cells. (E) Top: immunoblot showing phosphorylation of Dvl3 without agonist (control) or with exposure to 125 ng/ml Wnt5a or Wnt7a. Bottom: quantification of phosphorylated Dvl3. Mean±s.e.m. Dvl3 phosphorylation (arbitrary units) control, 1.00±0.04 (n=9); Wnt5a, 1.96±0.20 (n=9); Wnt7a, 1.05±0.08 (n=5). (F) Left: representative voltage-clamp current traces from tsA-201 cells overexpressing KCNQ2 and KCNQ3 channel subunits alone (black) or with Ror2–mCherry (red). Current traces were fitted with single exponentials to determine the time constants (tau) of activation and deactivation (right). Mean±s.e.m. tau activation: control, 220±24 ms (n=7); Ror2, 151±10 ms (n=14). (G,H) Contrast-inverted confocal micrographs (above) and time courses (below) of cytosolic intensity of PtdInsP2 probes PH-PLCδ1–YFP (G) or TubbyCR332H–YFP (H) in tsA-201 cells overexpressing M1 receptor and PtdInsP2 probes alone (control, black) or with overexpression of Ror2 (red). Red arrows indicate intracellular regions containing PtdInsP2. Oxo-M treatment is indicated by gray bars in the time courses. Wash, steady state after removal of Oxo-M. Data are mean±s.e.m. of six cells. P-values are shown in E and F for the comparisons indicated by horizontal bars (ANOVA with Dunnett's multiple comparison test in E; two-tailed unpaired Student's t-test in F).
Wnt5a increases phosphorylation of Dvl3
We performed immunoblots on cell homogenates from cells overexpressing Ror2 to establish whether Dvl3 is phosphorylated upon application of Wnt5a. Phosphorylation of Dvl proteins is thought to be part of their mechanism of activation (Nishita et al., 2010; Witte et al., 2010). Dvl3 phosphorylation was quantified from immunoblots using an anti-phosphotyrosine antibody, and blot intensity was normalized to control non-stimulated cells (Fig. 5E). We found that stimulation with Wnt5a increased Dvl3 tyrosine phosphorylation, whereas Wnt7a, a canonical agonist that fails to activate Ror2 (Cerpa et al., 2015), had no effect (Fig. 5E).
Ror2 overexpression increases the plasma membrane pool of PtdInsP2
Overexpressing Ror2 receptors accelerated activation gating of KCNQ2/3 current (Fig. 5F) and slowed down the deactivation of the channel, consistent with an increase in resting plasma membrane PtdInsP2. Ror2 receptor overexpression also blunted the translocation of PH-PLCδ1 to the cytosol in response to M1 receptor activation (Fig. 5G), reflective of increased PtdInsP2 at the plasma membrane and/or accelerated PtdInsP2 resynthesis. Interestingly, the recovery of PH-PLCδ1 to the plasma membrane appears to be biphasic when Ror2 receptor is overexpressed (Fig. 5G). Confocal micrographs of PH-PLCδ1 taken in the same period show rod-shaped regions adjacent to the plasma membrane (denoted with red arrows in Fig. 5G). It will be interesting to see whether these possibly intracellular pools of PtdInsP2 contribute to the slow, second phase of plasma membrane PtdInsP2 recovery in these cells. This experiment was repeated with TubbyCR332H, another optical probe for PtdInsP2. Similar kinetics for PtdInsP2 depletion and recovery upon M1 receptor activation were observed (Fig. 5H). Even in the absence of exogenous agonist, Ror2 overexpression seems to increase the plasma membrane pool of PtdInsP2.
Total PtdInsP declines in cells overexpressing PI4KIIIα
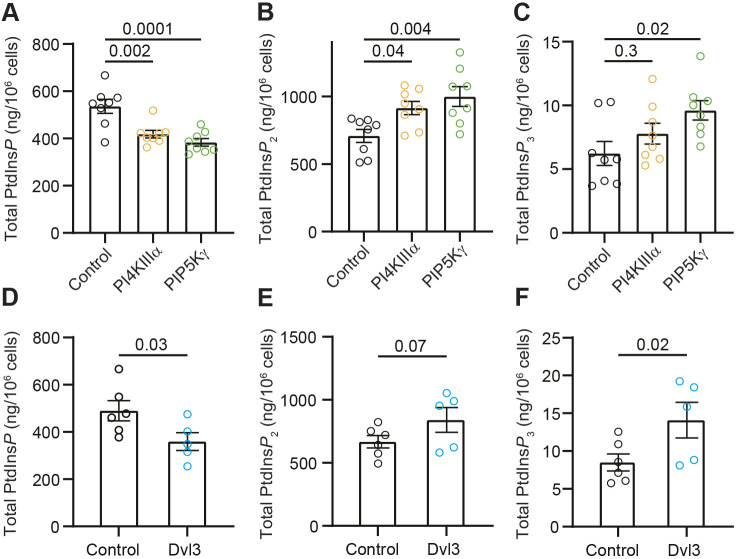
We conclude with a quantitative assay for phosphoinositides. Mass spectrometry was used to measure levels of PtdInsP, PtdInsP2, and PtdInsP3. The conditions of transient transfection matched those for which protein expression was detected in Fig. 1B. In cell populations overexpressing PIP5KIγ, we found as expected that PtdInsP, the substrate of the PIP5K, declined by 29% and PtdInsP2, the product, increased by 41% compared to levels in control cells. However, we were surprised to find that overexpression of PI4KIIIα reduced the prevalence of its immediate product, PtdInsP, by 22% while still increasing PtdInsP2 by 29% (Fig. 6A,B). What could account for the diminution of PtdInsP when PI4K is overexpressed? We hypothesize that an interaction with PI4KIIIα increased the enzymatic activity of PIP5K. Efficient phosphorylation, without the accumulation of intermediate PtdInsP, could be accomplished by coupling of PI4K and PIP5K. PIP5KIγ also increases the prevalence of PtdInsP3 by 54% (Fig. 6C).
Fig. 6.
Overexpression of PI4KIIIα, PIP5KIγ or Dvl3 increases PtdInsP2. (A) Total PtdInsP as measured by mass spectrometry experiments with control tsA201 cells (black), and cells overexpressing PI4KIIIα (orange) or PIP5KIγ (green). Mean±s.e.m. PtdInsP: control, 536±30 ng/million cells; PI4KIIIα, 418±16 ng/million cells; PIP5KIγ, 383±16 ng/million cells; n=8 dishes. (B) Total PtdInsP2 as measured by mass spectrometry with cells as in A. Mean±s.e.m. PtdInsP2: control, 708±48 ng/million cells; PI4KIIIα, 914±49 ng/million cells; PIP5KIγ, 998±73 ng/million cells; n=8 dishes. (C) Total PtdInsP3 as measured by mass spectrometry with cells as in A. Mean±s.e.m. PtdInsP3: control, 6.3±0.9 ng/million cells; PI4KIIIα, 7.9±0.8 ng/million cells; PIP5KIγ, 9.7±0.8 ng/million cells; n=8 dishes. Data in A–C summarize three experiments, two performed in triplicate and one in duplicate. (D) Total PtdInsP as measured by mass spectrometry experiments with control tsA201 cells (black), and cells overexpressing Dvl3 (blue). Mean±s.e.m. PtdInsP: control, 489±43 ng/million cells (n=6 dishes); Dvl3, 359±38 ng/million cells (n=5 dishes). (E) Total PtdInsP2 as measured by mass spectrometry with cells as in D. Mean±s.e.m. PtdInsP2: control, 668±49 ng/million cells (n=6 dishes); Dvl3, 840±99 ng/million cells (n=5 dishes). (F) Total PtdInsP3 as measured by mass spectrometry with cells as in D. Mean±s.e.m. PtdInsP3: control, 8.5±1.1 ng/million cells (n=6 dishes); Dvl3, 14.1±2.4 ng/million cells (n=5 dishes). Data in D–F summarize two experiments, one performed in triplicate and one in duplicate. P-values are shown for the comparisons indicated by horizontal bars (ANOVA with Dunnett's multiple comparison test).
Dvl3 decreases PtdInsP and increases PtdInsP2 and PtdInsP3
We repeated these mass spectrometry measurements in cells overexpressing Dvl3 and found a 27% decrease in PtdInsP (Fig. 6D), a 21% increase in PtdInsP2 (Fig. 6E) and a 66% increase in PtdInsP3 (Fig. 6F). For all three phosphoinositides, overexpression of Dvl3 reproduced the effect of overexpressing PI4KIIIα or PIP5KIγ. Dvl3 seems able to mobilize endogenous PI4K and PIP5K in a manner similar to overexpression of those kinases. Given the increase in PtdInsP3, it could be worth investigating whether the activity of phosphatidylinositol 3-kinases also depends on Dvl3. Our functional and quantitative assays show that Dvl3 increases baseline PtdInsP2, is required for efficient synthesis of PtdInsP2, localizes PIP5KIγ to the plasma membrane and promotes colocalization of PI4KIIIα and PIP5KIγ.
DISCUSSION
This report shows that PI4K and PIP5K act in coordination to synthesize PtdInsP2, and that the Dishevelled protein Dvl3 is necessary to organize the two kinases for this task (Fig. 1A). This coordination is important both for maintenance of basal levels of PtdInsP2 and for replacement of PtdInsP2 following receptor-induced depletion.
Dvl3 organizes the synergistic activity of PI4KIIIα and PIP5KIγ. Overexpression of Dvl3 in the presence of endogenous phosphoinositide kinases was sufficient to reproduce the changes in PtdInsP2 observed upon simultaneous overexpression of PI4KIIIα and PIP5KIγ. Dvl3 overexpression recapitulated the increase in resting PtdInsP2 (Figs 1,6) and acceleration of PtdInsP2 resynthesis (Fig. 2) present with overexpression of PI4KIIIα and PIP5KIγ. By simply organizing the low-abundance endogenous kinases, Dvl3 is sufficient to increase their efficacy substantially. Indeed, when endogenous Dvl3 was knocked down, resting plasma membrane PtdInsP2 was reduced and recovery from receptor-induced PtdInsP2 hydrolysis was incomplete (Fig. 4). Blocking PI4KIIIα in control cells had a markedly similar effect on both resting PtdInsP2 and PtdInsP2 resynthesis (Fig. 4G) as Dvl3 knockdown, underscoring both the importance of Dvl and the notion that PI4K is rate limiting in these cells.
Although previous studies have suggested that PI4K is rate limiting for resynthesis of PtdInsP2 (Falkenburger et al., 2010; Myeong et al., 2020), we provide new details about the specific roles of two kinases. In cells overexpressing PIP5KIγ (but not PI4KIIIα), the translocation of PH-PLCδ1 in response to PLCβ activation is smaller and slower than in control cells. This is presumably because PIP5KIγ continues to generate PtdInsP2 from PtdInsP, counteracting the ongoing PtdInsP2 hydrolysis and slowing down release of PH-PLCδ1 from the plasma membrane. However, PtdInsP is eventually depleted and becomes rate limiting during PtdInsP2 resynthesis, as evidenced by PI4KIIIα overexpression advancing the start of PtdInsP2 resynthesis by 10 s relative to that in control cells (Fig. 2F, orange trace versus black trace after Oxo-M). PIP5KIγ appears to be rate limiting for PtdInsP2 synthesis during PLCβ activation, and PI4KIIIα is rate limiting for PtdInsP2 resynthesis after agonist withdrawal. Interestingly, once begun, the rate of resynthesis is similar among control cells and those overexpressing either PI4KIIIα or PIP5KIγ.
What evidence do we have that PI4KIIIα, PIP5KIγ and Dvl3 associate in a physical complex? First, both Dvl3 and PI4KIIIα increase the presence of PIP5KIγ at the plasma membrane (Fig. 3). Second, overexpressing Dvl3 increases colocalization of PI4KIIIα and PIP5KIγ, whereas knocking down Dvl3 decreases PI4K–PIP5K colocalization (Fig. 3G). Third, FRET between PIP5KIγ and Dvl3 shows proximity at a molecular level between these proteins (Fig. 3H). Future biochemical and structural experiments will be important in clarifying how these proteins interact.
Following application of Wnt5a in cells overexpressing Ror2, we observed sequential and transient recruitment of Dvl3 and PIP5KIγ to the plasma membrane and increased phosphorylation of Dvl3 (Fig. 5A–E). In addition, Ror2 overexpression increases basal PtdInsP2 and blunts the translocation of PH-PLCδ1 (Fig. 5G) comparably to overexpression of PIP5KIγ (compare Fig. 1C, Fig. 2D–H). These effects may be due to Ror2 interacting with Dvl3 and recruiting PI4K and PIP5K to the plasma membrane. It will be interesting to investigate these signaling events in neurons, where the depolarization and Ca2+ concentration increase downstream of Wnt5a appear to depend on Gαq and PLC (McQuate et al., 2017). For instance, does Ror2 coordinate PI4K and PIP5K activity in hippocampal neurons, and how does that impact events downstream of PLC? Ror2 signaling appears to be context specific depending on co-receptors and accessory proteins (Green et al., 2014), so signaling pathways may vary depending on cell type and receptor expression.
In conclusion, we have shown that Dvl3 organizes PI4KIIIα and PIP5KIγ to synthesize PtdInsP2 efficiently, especially in response to receptor-induced PtdInsP2 depletion. Ror2 overexpression increases plasma membrane PtdInsP2, and Wnt5a stimulates Dvl3 phosphorylation and plasma membrane recruitment of Dvl3 and PIP5KIγ. It will be interesting to determine whether kinase organization plays a role in the etiology of Robinow syndrome arising from mutations in Dvl3.
MATERIALS AND METHODS
Materials
Oxotremorine-M (Oxo-M), GSK-A1, sodium formate, HCl and trimethylsilyl-diazomethane (TMS-DM; 2.0 M in diethyl ether or hexanes) were from Sigma-Aldrich, and Wnt5a was from BD Biosciences. Mass spectrometry-grade methanol, chloroform, dichloromethane and acetonitrile were from Fisher Scientific.
Cell culture and transfection
Cells of the tsA-201 cell line (RRID:CVCL_2737, Sigma-Aldrich), originally derived from HEK-293 human embryonic kidney cells and certified by the vendor by short tandem repeat profiling, were cultivated in DMEM (Fisher Scientific) with 10% fetal bovine serum (Sigma-Aldrich) and 2% penicillin-streptomycin (Gibco), and were used between passages 15 and 50. Cells were transfected at 75–85% confluency using Lipofectamine 2000 or 3000 (Invitrogen) with the following cDNA plasmids: Ror2–mCherry (Cerpa et al., 2015); PI4KIIIa–GFP, EFR3B and TTC7B (from Pietro de Camilli, Yale University, USA); human EGFP–PIP5KIγ_i2 and CFP–PIP5KIγ_i2 (from Rosa Ana Lacalle and Santos Mañes, Centro Nacional de Biotecnología/Consejo Superior de Investigaciones Científicas, Madrid, Spain; Lacalle et al., 2015); human Dvl3–EYFP and Dvl3–HcRed (from Randall Moon, University of Washington, USA); mouse M1 muscarinic receptor (from Neil Nathanson, University of Washington, USA); human eCFP–PH-PLCδ1, eYFP–PH-PLCδ1 and CFP–CAAX (from Kees Jalink, the Netherlands Cancer Institute, Amsterdam, The Netherlands); RFP–PH-PLCδ (from Ken Mackie, Indiana University, USA), TubbyCR332H–YFP (from Andrew Tinker, University College London, UK); human KCNQ2 and rat KCNQ3 (from David McKinnon, SUNY Stony Brook, USA); and siRNA for Dvl3 (Santa Cruz Biotechnology). TYE 563 dye (Integrated DNA Technologies) was included with siRNA transfection to screen for successfully transfected cells.
Mass spectrometry
Mass spectrometry was performed as previously described (de la Cruz et al., 2020). In brief, lipids were precipitated from pelleted cells treated with trichloroacetic acid and extracted with chloroform–methanol. Dried extracts were methylated with TMS-DM and quantified by targeted analysis as described previously (Traynor-Kaplan et al., 2017). For each phosphoinositide type, peaks were analyzed for the three most predominant species: 38:4, 36:2 and 36:1. Peak areas were converted to mg per 106 cells as described using 37:4 lipid internal standards (Avanti Lipids) and DNA-per-sample measurements.
Electrophysiology
KCNQ2/3 current was recorded by patch clamp in whole-cell configuration at room temperature with a HEKA EPC 9 amplifier (HEKA Elektronik). The resistance of the borosilicate glass pipettes was 3–5 MΩ. Series resistance was ≤10 MΩ and was compensated ≥70%. The bath solution consisted of 160 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 8 mM glucose adjusted to pH 7.4 with NaOH. The pipette solution consisted of 175 mM KCl, 5 mM MgCl2, 5 mM HEPES, 0.1 mM BAPTA K4 (Invitrogen), 3 mM Na2ATP and 0.1 mM Na3GTP adjusted to pH 7.4 with KOH.
High-resolution microscopy
Confocal images were obtained with an inverted confocal system (Zeiss LSM 880) run by ZEN black v2.3. A Plan Apochromat 63×/1.40 NA oil immersion objective was used. Fluorescent proteins were excited with three lasers tuned to 405 nm, 458–514 nm and 561 nm. Emission light was detected using an Airyscan 32 GaAsP detector and appropriate emission filter sets. The point spread functions were calculated using ZEN black software using 0.1 μm fluorescent microspheres. After deconvolution, the point spread functions were: 124 nm in x–y and 216 nm in z (for 488 nm excitation); 168 nm in x–y and 212 nm in z (for 594 nm excitation). Temperature inside the microscope housing was 27–30°C. Time series were taken with an interval of 2–5 s. Drug perfusion while imaging used a gravity system set to flow at 2 ml min−1. Images were analyzed with ImageJ (NIH, Bethesda, MD, USA). Colocalization analysis was performed using the Fiji (https://fiji.sc/) plugin Coloc 2 to measure the Pearson correlation coefficient.
TIRF microscopy
TIRF microscopy was performed on cells plated on #0 glass coverslips fitted to a recording chamber. TIRF footprints were acquired using a Nikon Eclipse Ti-E microscope equipped with a 60×/1.25 oil-immersion objective and Photometrics QuantEM EMCCD camera (Nikon). Time-series images were taken every 10 s at 23°C and analyzed with ImageJ.
FRET
Three-cube FRET between transfected CFP- and YFP-tagged fluorescent proteins was performed as described previously (Myeong et al., 2020). Briefly, cells were illuminated with 440 nm and 500 nm excitation light from a TILL monochromator (Polychrome IV). Light passed through a three-color dichroic cube (CFP, YFP, mCherry; 89006bs; Chroma Technology Corp.) and a 60×/1.40 oil-immersion objective. An adjustable viewfinder was used to collect emission light from single cells. Emission bands were detected using a beamsplitter, emission filters ET480/24 (CFP) and ET535/30 (YFP; Chroma Technology Co.), photodiodes and a TILL FDU-2 detection unit. Recordings were controlled by an EPC9 amplifier with Patchmaster 2.35 software (HEKA). Photometeric measurements were taken at 23°C. Data were corrected for background and bleed-through, and FRET efficiency was determined as follows:
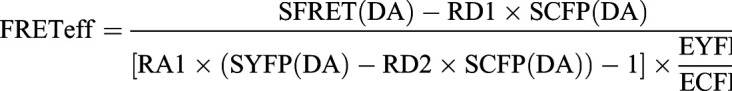 |
where RD1, RD2 and RA1 are bleed-through constants measured with single fluorophores; SCFP(DA), SYFP(DA) and SFRET(DA) are intensity measurements with the indicated fluorescent cube; and ECFP and EYFP are molar extinction coefficients (Erickson et al., 2001).
Immunoblotting
Cells were homogenized in 500 µl of lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1 mM EGTA, 0.5% protease inhibitor (Millipore Sigma), 1% Triton X-100 and 1% (w/v) deoxycholate] and centrifuged at 11,200 g for 10 min. Supernatant was extracted, proteins were quantified using the BCA method, and 20 μg of protein was run on a 7.5% polyacrylamide gel. Proteins were transferred to nitrocellulose membrane, blocked with 5% milk for 2 h, and incubated with the primary antibody: rabbit anti-PI4K antibody (#4902; Cell Signaling Technology), mouse anti-PIP5KIγ antibody (H-9, sc-377061; Santa Cruz Biotechnology) or rabbit anti-Dvl3 antibody (#3218; Cell Signaling Technology) for PI4KIIIΑ, PIP5KI and Dvl3, respectively.
Samples were incubated in primary antibody (1:5000 dilution) overnight and in secondary antibody (1:10,000 dilution) for 2 h [mouse anti-rabbit IgG–HRP (sc-2357, Santa Cruz Biotechnology) or m-IgGκ BP–HRP (sc-516102, Santa Cruz Biotechnology)]. Antibody complexes were detected by chemiluminescence using SuperSignal West Femto Kit (Thermo Fisher Scientific, USA). Chemiluminescence was captured using C-DiGit® Blot Scanner (LI-COR Biosciences, USA).
Immunoprecipitation assay
Cells from three 35 mm dishes were lysed using 500 µl of lysis buffer and centrifuged at 11,200 g for 10 min. The supernatant was extracted for immunoprecipitation by incubation with 0.5 μg of the rabbit anti-PI4K antibody (#4902; Cell Signaling Technology) and 20 µl Protein A–Sepharose™ 4B (Invitrogen) at 4°C for 2 h. The immune complexes obtained were precipitated for 1 min at 11,200 g and washed with 1× phosphate-buffered saline (PBS) three times before being dissolved in 15 µl 6× SDS loading buffer (Thermo Fisher Scientific, USA). The sample was run in denaturing electrophoresis with standard running buffer containing SDS and transferred to nitrocellulose membrane, blocked with 5% milk for 2 h, and incubated with the primary antibody (1:5000, rabbit anti-PI4K; #4902, Cell Signaling Technology) at 4°C overnight. A secondary incubation with m-IgGκ BP–HRP (sc-516102; Santa Cruz Biotechnology) at 1:10,000 was performed, and the antibody complexes were detected by chemiluminescence using SuperSignal West Femto Kit (Thermo Fisher Scientific, USA). Chemiluminescence was captured using a C-DiGit® Blot Scanner (LI-COR Biosciences, USA).
Dvl phosphorylation assay
Immunoprecipitation of cells was performed as above, except that supernatant was extracted by incubation with anti-Dvl3 antibody (1:2000; #3218; Cell Signaling Technology) and membranes were incubated with 1:5000 mouse anti-phosphotyrosine primary antibody (#05-321; EMD Milipore Corp.).
Statistics
Mean values in text and figures are given with s.e.m. calculated using GraphPad Prism. P-values were determined using two-tailed unpaired Student's t-test to compare two groups or ANOVA with Dunnett's multiple comparisons test for three or more groups.
Supplementary Material
Acknowledgements
We thank Lea Miller for administrative support, Alexis Traynor-Kaplan for assistance with mass spectrometry experiments, and Bertil Hille for invaluable mentorship, critical discussion and feedback on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: L.d.l.C., O.V., A.B., J.B.J.; Methodology: L.d.l.C., R.R., A.B., J.B.J.; Formal analysis: L.d.l.C., R.R., O.V., J.B.J.; Investigation: L.d.l.C., R.R., J.B.J.; Writing - original draft: L.d.l.C., J.B.J.; Writing - review & editing: L.d.l.C., O.V., A.B., J.B.J.; Supervision: O.V., A.B.; Project administration: A.B., J.B.J.; Funding acquisition: O.V., A.B.
Funding
This work was supported by National Institutes of Health grants R37-NS08174 to Bertil Hille and MIRA R35-GM142690 to O.V., and a National Science Foundation grant IOS-1755004 to A.B. Deposited in PMC for release after 12 months.
Data availability
The mass spectrometry dataset is available online: https://doi.org/10.6084/m9.figshare.18739265.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.259145.
References
- Ahmad-Annuar, A., Ciani, L., Simeonidis, I., Herreros, J., Fredj, N. B., Rosso, S. B., Hall, A., Brickley, S. and Salinas, P. C. (2006). Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J. Cell Biol. 174: 127-139. 10.1083/jcb.200511054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla, T. (2013). Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019-1137. 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Piedras, I., Sharma, M., Brelsfoard, J., Vartak, D., Martinez, E. G., Rivera, C., Molehin, D., Bright, R. K., Fokar, M., Guindon, J.et al. (2021). Nuclear Dishevelled targets gene regulatory regions and promotes tumor growth. EMBO Rep. 22, e50600. 10.15252/embr.202050600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa, W., Gambrill, A., Inestrosa, N. C. and Barria, A. (2011). Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J. Neurosci. 31: 9466-9471. 10.1523/JNEUROSCI.6311-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa, W., Latorre-Esteves, E. and Barria, A. (2015). RoR2 functions as a noncanonical Wnt receptor that regulates NMDAR-mediated synaptic transmission. Proc. Natl. Acad. Sci. U.S.A. 112, 4797-4802. 10.1073/pnas.1417053112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, J., Nakatsu, F., Baskin, J. M. and De Camilli, P. (2015). Plasticity of PI4KIIIα interactions at the plasma membrane. EMBO Rep. 16: 312-320. 10.15252/embr.201439151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, G., Yu, H., Kruse, M., Traynor-Kaplan, A. and Hille, B. (2016). Osmoregulatory inositol transporter SMIT1 modulates electrical activity by adjusting PI(4,5)P2 levels. Proc. Natl. Acad. Sci. U.S.A. 113, E3290-E3299. 10.1073/pnas.1606348113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz, L., Traynor-Kaplan, A., Vivas, O., Hille, B. and Jensen, J. B. (2020). Plasma membrane processes are differentially regulated by type I phosphatidylinositol phosphate 5-kinases and RASSF4. J. Cell Sci. 133, jcs233254. 10.1242/jcs.233254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, M. G., Alseikhan, B. A., Peterson, B. Z. and Yue, D. T. (2001). Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron 31: 973-985. 10.1016/S0896-6273(01)00438-X [DOI] [PubMed] [Google Scholar]
- Falkenburger, B. H., Jensen, J. B. and Hille, B. (2010). Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 135, 99-114. 10.1085/jgp.200910345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J., Nusse, R. and van Amerongen, R. (2014). The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 6, a009175. 10.1101/cshperspect.a009175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B., Dickson, E. J., Kruse, M., Vivas, O. and Suh, B. C. (2015). Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851: 844-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz, L. F., Hirdes, W., Suh, B. C., Hilgemann, D. W., Mackie, K. and Hille, B. (2005). Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J. Gen. Physiol. 126: 243-262. 10.1085/jgp.200509309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J., Yuan, Q., Kang, X., Qin, Y., Li, L., Ha, Y. and Wu, D. (2015). Resolution of structure of PIP5K1A reveals molecular mechanism for its regulation by dimerization and dishevelled. Nat. Commun. 6: 8205. 10.1038/ncomms9205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. S., Duignan, K. M., Hawryluk, J. M., Soh, H. and Tzingounis, A. V. (2016). The voltage activation of cortical KCNQ channels depends on global PIP2 levels. Biophys. J. 110: 1089-1098. 10.1016/j.bpj.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacalle, R. A., de Karam, J. C., Martinez-Munoz, L., Artetxe, I., Peregil, R. M., Sot, J., Rojas, A. M., Goni, F. M., Mellado, M. and Manes, S. (2015). Type I phosphatidylinositol 4-phosphate 5-kinase homo- and heterodimerization determines its membrane localization and activity. FASEB J. 29: 2371-2385. 10.1096/fj.14-264606 [DOI] [PubMed] [Google Scholar]
- Lees, J. A., Zhang, Y., Oh, M. S., Schauder, C. M., Yu, X., Baskin, J. M., Dobbs, K., Notarangelo, L. D., De Camilli, P., Walz, T.et al. (2017). Architecture of the human PI4KIIIα lipid kinase complex. Proc. Natl. Acad. Sci. U.S.A. 114: 13720-13725. 10.1073/pnas.1718471115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Gamper, N., Hilgemann, D. W. and Shapiro, M. S. (2005). Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25: 9825-9835. 10.1523/JNEUROSCI.2597-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal, K. (2020). Review of PIP2 in cellular signaling, functions and diseases. Int. J. Mol. Sci. 21, 8342. 10.3390/ijms21218342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuate, A., Latorre-Esteves, E. and Barria, A. (2017). A Wnt/Calcium signaling cascade regulates neuronal excitability and trafficking of NMDARs. Cell Rep. 21, 60-69. 10.1016/j.celrep.2017.09.023 [DOI] [PubMed] [Google Scholar]
- Myeong, J., de la Cruz, L., Jung, S. R., Yeon, J. H., Suh, B. C., Koh, D. S. and Hille, B. (2020). Phosphatidylinositol 4,5-bisphosphate is regenerated by speeding of the PI 4-kinase pathway during long PLC activation. J. Gen. Physiol. 152, e202012627. 10.1085/jgp.202012627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu, F., Baskin, J. M., Chung, J., Tanner, L. B., Shui, G., Lee, S. Y., Pirruccello, M., Hao, M., Ingolia, N. T., Wenk, M. R.et al. (2012). PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J. Cell Biol. 199: 1003-1016. 10.1083/jcb.201206095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita, M., Itsukushima, S., Nomachi, A., Endo, M., Wang, Z., Inaba, D., Qiao, S., Takada, S., Kikuchi, A. and Minami, Y. (2010). Ror2/frizzled complex mediates Wnt5a-induced AP-1 activation by regulating dishevelled polymerization. Mol. Cell. Biol. 30: 3610-3619. 10.1128/MCB.00177-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivença, D. V., Uliyakina, I., Fonseca, L. L., Amaral, M. D., Voit, E. O. and Pinto, F. R. (2018). A mathematical model of the phosphoinositide pathway. Sci. Rep. 8: 3904. 10.1038/s41598-018-22226-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Y., Li, L., Pan, W. and Wu, D. (2009). Regulation of phosphatidylinositol kinases and metabolism by Wnt3a and Dvl. J. Biol. Chem. 284: 22544-22548. 10.1074/jbc.M109.014399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M., Castro-Piedras, I., Simmons, G. E., Jr. and Pruitt, K. (2018). Dishevelled: A masterful conductor of complex Wnt signals. Cell. Signal. 47: 52-64. 10.1016/j.cellsig.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl, L. C., Slusarski, D. C., Pandur, P., Miller, J. R., Kuhl, M. and Moon, R. T. (2003). Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol. 161: 769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor-Kaplan, A., Kruse, M., Dickson, E. J., Dai, G., Vivas, O., Yu, H., Whittington, D. and Hille, B. (2017). Fatty-acyl chain profiles of cellular phosphoinositides. Biochim Biophys Acta Mol Cell Biol Lipids 1862: 513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai, P., Thyagarajan, B., Rohacs, T. and Balla, T. (2006). Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 175: 377-382. 10.1083/jcb.200607116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J., Mazzeu, J. F., Hoischen, A., Jhangiani, S. N., Gambin, T., Alcino, M. C., Penney, S., Saraiva, J. M., Hove, H., Skovby, F.et al. (2015). DVL1 frameshift mutations clustering in the penultimate exon cause autosomal-dominant Robinow syndrome. Am. J. Hum. Genet. 96: 612-622. 10.1016/j.ajhg.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. J., Mazzeu, J. F., Hoischen, A., Bayram, Y., Withers, M., Gezdirici, A., Kimonis, V., Steehouwer, M., Jhangiani, S. N., Muzny, D. M.et al. (2016). DVL3 alleles resulting in a -1 frameshift of the last exon mediate autosomal-dominant robinow syndrome. Am. J. Hum. Genet. 98: 553-561. 10.1016/j.ajhg.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. J., Mazzeu, J. F., Coban-Akdemir, Z., Bayram, Y., Bahrambeigi, V., Hoischen, A., van Bon, B. W. M., Gezdirici, A., Gulec, E. Y., Ramond, F.et al. (2018). WNT signaling perturbations underlie the genetic heterogeneity of robinow syndrome. Am. J. Hum. Genet. 102: 27-43. 10.1016/j.ajhg.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte, F., Bernatik, O., Kirchner, K., Masek, J., Mahl, A., Krejci, P., Mundlos, S., Schambony, A., Bryja, V. and Stricker, S. (2010). Negative regulation of Wnt signaling mediated by CK1-phosphorylated Dishevelled via Ror2. FASEB J. 24: 2417-2426. 10.1096/fj.09-150615 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.