ABSTRACT
Polycystins are conserved mechanosensitive channels whose mutations lead to the common human renal disorder autosomal dominant polycystic kidney disease (ADPKD). Previously, we discovered that the plasma membrane-localized fission yeast polycystin homolog Pkd2p is an essential protein required for cytokinesis; however, its role remains unclear. Here, we isolated a novel temperature-sensitive pkd2 mutant, pkd2-B42. Among the strong growth defects of this mutant, the most striking was that many mutant cells often lost a significant portion of their volume in just 5 min followed by a gradual recovery, a process that we termed ‘deflation’. Unlike cell lysis, deflation did not result in plasma membrane rupture and occurred independently of cell cycle progression. The tip extension of pkd2-B42 cells was 80% slower than that of wild-type cells, and their turgor pressure was 50% lower. Both pkd2-B42 and the hypomorphic depletion mutant pkd2-81KD partially rescued mutants of the septation initiation network (SIN), a yeast Hippo-related signaling pathway, by preventing cell lysis, enhancing septum formation and doubling the number of Sid2p and Mob1p molecules at the spindle pole bodies. We conclude that Pkd2p promotes cell size expansion during interphase by regulating turgor pressure and antagonizes the SIN during cytokinesis.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Cell growth, Cytokinesis, Fission yeast, Pkd2, Polycystin, SIN pathway, Turgor pressure
Summary: Mutations of polycystins lead to the genetic disorder ADPKD. We show that the fission yeast polycystin homolog Pkd2p promotes cell expansion during interphase growth and antagonizes the SIN pathway during cytokinesis.
INTRODUCTION
Cytokinesis is the last stage of cell division when two daughter cells separate. It initiates the assembly of an actomyosin contractile ring at the medial division plane. The ring provides the mechanical force required for cleavage furrow ingression, eventually leading to cell separation. Fission yeast, Schizosaccharomyces pombe, represents an excellent model organism to study this evolutionarily conserved cellular process. A large number of genes involved in cytokinesis have been identified in this unicellular eukaryote through genetic screens. Cell biology studies have helped to reveal their functions, most of which are likely conserved in metazoans (for a review see Pollard and Wu, 2010).
In fission yeast cytokinesis, the septation initiation network (SIN), a Hippo-related signaling pathway, plays an essential role. This differs slightly from the metazoan Hippo pathway, which modulates cell growth and organ size (Camargo et al., 2007; Dong et al., 2007). Nevertheless, both pathways consist of a highly conserved kinase cascade (for reviews see Johnson et al., 2012; Simanis, 2015; Yu et al., 2015). At the top of the fission yeast SIN pathway is a GTPase, Spg1p, which is localized at the spindle pole bodies (SPBs) throughout mitosis (Schmidt et al., 1997). Activation of Spg1p propagates the SIN signal through a chain of three kinases, including the essential nuclear Dbf2-related (NDR) family kinase Sid2p, the homolog of human LATS kinases (Balasubramanian et al., 1998). This terminal kinase carries out the SIN functions by targeting many essential cytokinetic proteins through phosphorylation (Bohnert et al., 2013; Hergovich, 2016; Sparks et al., 1999; Willet et al., 2019). It also inhibits the morphogenesis Orb6-related (MOR) pathway that promotes cell growth (Gupta et al., 2013; Ray et al., 2010; Verde et al., 1998).
We recently identified pkd2 as a novel gene required for fission yeast cytokinesis (Morris et al., 2019). It encodes the homolog of polycystins, a family of conserved transient receptor potential (TRP) channels (Palmer et al., 2005). Loss-of-function mutations of polycystins lead to one of the most common human genetic disorders, autosomal dominant polycystic kidney disease (ADPKD), which manifests through the proliferation of numerous liquid-filled cysts in the kidneys (Hughes et al., 1995; Mochizuki et al., 1996). Most polycystins studied so far are Ca2+-permissive cation channels sensitive to membrane tension (Gonzalez-Perrett et al., 2001; Hanaoka et al., 2000; Liu et al., 2018; Nauli et al., 2003; Su et al., 2018), but their cellular functions are surprisingly diverse. While the fruit fly and worm polycystins are essential for male fertility (Barr and Sternberg, 1999; Watnick et al., 2003), that of the green algae Chlamydomonas reinhardtii plays a non-essential structural role in the primary cilia (Huang et al., 2007; Liu et al., 2020; Wood et al., 2012), and the homolog in the social amoeba Dictyostelium discoideum contributes to cell locomotion (Lima et al., 2014). Overall, the mechanism underlying these cellular functions of polycystins remains unclear (for recent reviews see Hardy and Tsiokas, 2020; Ta et al., 2020).
Similar to other unicellular organisms, fission yeast possesses just one polycystin homolog, Pkd2p (Palmer et al., 2005). This putative TRP channel localizes to the plasma membrane. Whereas it concentrates at the cell tips during interphase growth, it moves to the equatorial plane during cytokinesis (Morris et al., 2019). It is an essential protein. The pkd2-null mutant dies either at the single-cell stage or after a couple of rounds of cell division. Depletion of Pkd2p in the mutant pkd2-81KD leads to unusually fast constriction of the contractile ring and delayed or failed cell separation (Morris et al., 2019). The cell wall structure remains normal in these Pkd2p-depleted cells. In addition to their cytokinesis defects, the mutant cells occasionally shrink but quickly recover. However, the molecular processes underlying these defects remain unknown.
Here, we investigated the mechanism of Pkd2p function by characterizing a novel temperature-sensitive pkd2 mutant and examining its genetic interaction with SIN mutants. We found that this mutation, pkd2-B42, led to strong defects in cell morphogenesis. Most unusual was that the mutant cells frequently shrunk rapidly but always recovered in a process typically lasting no more than 20 min. This process did not lead to cell lysis and was independent of septation as well as cell cycle progression. The pkd2-B42 mutation strongly inhibited cell growth by reducing the tip growth rate and blocking the cell volume extension. The turgor pressure of pkd2-B42 cells, modeled through measuring the stiffness of the mutant cells using atomic force microscopy (AFM), was 50% lower than that of the wild-type cells, but their cell wall appeared to be normal. We identified strong epistatic genetic interactions between pkd2-B42 and most SIN mutants. Both of the pkd2 mutations prevented the SIN mutant cells from lysis and increased septation during cytokinesis. Depletion of Pkd2p also increased the localization of SIN proteins to SPBs. We conclude fission yeast Pkd2p promotes cell expansion, most likely by regulating the turgor pressure, and specifically antagonizes the SIN pathway.
RESULTS
A novel temperature-sensitive mutant pkd2-B42
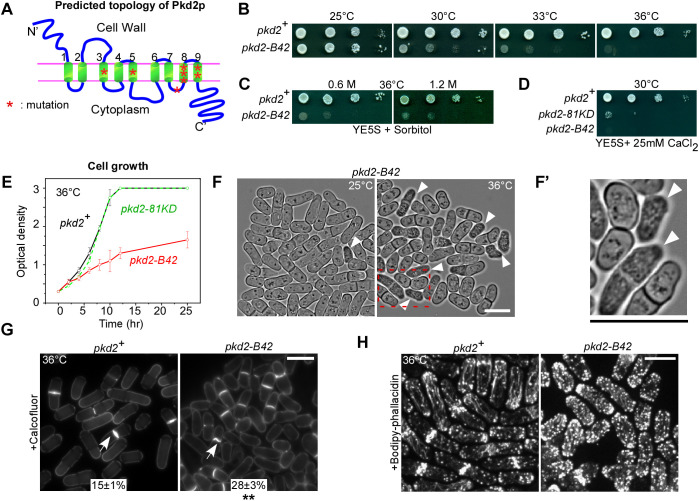
To investigate the essential functions of pkd2, we set out to isolate a loss-of-function mutant allele using random mutagenesis. The only previously available mutant, pkd2-81KD, is a hypomorphic depletion mutant that replaces the endogenous promoter with a weaker one, but the remaining Pkd2p protein levels are 30% of those in wild-type cells (Morris et al., 2019). Through screening, we identified a novel temperature-sensitive mutant, pkd2-B42. It contained eight missense mutations within the sequence encoding the TRP channel domain (Fig. 1A; Table S1). The mutant cells were inviable at 36°C and above (Fig. 1B). To determine whether the mutant could be rescued by osmotic stabilization of its cell wall, we supplemented the medium with sorbitol. Neither 0.6 M nor 1.2 M sorbitol significantly improved the viability of the mutant at 36°C (Fig. 1C). The mutant was hypersensitive to Ca2+, similar to pkd2-81KD (Fig. 1D). The pkd2-B42 cells grew more slowly in liquid medium at the restrictive temperature than either wild-type cells or the pkd2-81KD mutant (Fig. 1E). We concluded that pkd2-B42 is a temperature-sensitive loss-of-function mutant allele.
Fig. 1.
A novel temperature-sensitive pkd2 mutant, pkd2-B42. (A) Schematic of the predicted topology of wild-type Pkd2p. The mutated residues in pkd2-B42 are shown as red asterisks. Numbers denote the nine transmembrane helices. (B–D) Tenfold dilution series of the indicated yeast strains (wild type, pkd2+) on YE5S plates either (B) at different temperatures, (C) in the presence of sorbitol or (D) in the presence of CaCl2. (E) Growth curves of yeast cultures in YE5S liquid medium at 36°C. Data are presented as the mean±s.d. of two biological repeats. (F) Brightfield micrographs of pkd2-B42 cells at either 25°C (left) or 36°C (right). The red dashed rectangle indicates the region shown in F′. (F′) Magnified view of pkd2-B42 cells at 36°C. Arrowheads in F and F′ indicate cells that have shrunk. (G) Micrographs of wild-type and pkd2-b42 cells fixed at 36°C and stained with Calcofluor White to visualize the septum (arrows). The percentage of septated cells (mean±s.d. of two experiments, n>500) is shown for each strain. (H) Fluorescence micrograph of fixed and Bodipy–phallacidin-stained wild-type and pkd2-B42 cells showing the actin cytoskeleton. Data in B–H are representative of at least two independent biological repeats. Scale bars: 10 µm. **P<0.01 (two-tailed unpaired Student’s t-test).
We next examined the morphology of the pkd2-B42 mutant cells at the restrictive temperature. They were significantly shorter and wider than the wild-type cells (Fig. 1F; Fig. S1A,B). Many (20%) appeared to have shrunk and were optically opaque, but were not vacuolated, after being shifted to 36°C for 4 h (Fig. 1F,F′). The fraction of such cells continued to increase with extended incubation at the restrictive temperature. Their septation index was onefold higher than that of the wild-type cells (Fig. 1G). The septum of some mutant cells appeared as either wavy or slightly curved. Compared to the wild-type strain, the actin cytoskeleton of the mutant appeared disorganized. In particular, endocytic actin patches were distributed more evenly throughout the cytoplasm, not restricted to either the cell tip or the equatorial plane (Fig. 1H). We concluded that the pkd2-B42 mutation leads to strong defects in cell morphogenesis.
Deflation of pkd2-B42 mutant cells
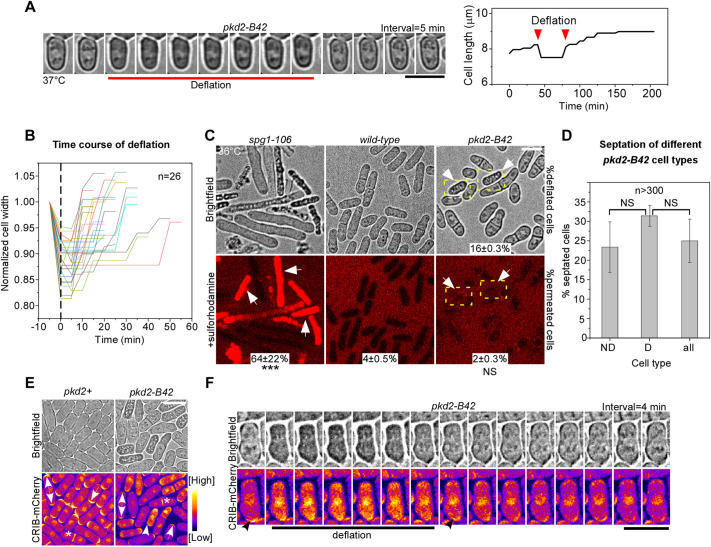
To investigate how the pkd2-B42 mutant cells became shrunk and opaque, we imaged them at the restrictive temperature using time-lapse microscopy. The mutant cells gained such a unique appearance after rapidly reducing their length and width by 6% (6±2%, mean±s.d., n=38) and 11% (11±4%, n=26), respectively (Fig. 2A,B; Fig. S1C). This transformation usually occurred in less than 5 min (Fig. 2B). However, such shrinking was temporary. All the cells that shrank (n =152) gradually regained both their physical dimensions and appearance fully, typically within 15 min (Fig. 2B). Combined, the whole process lasted ∼17 min (17±7 min, mean±s.d.; Fig. 2B). On average, a mutant cell underwent such shrinkage and recovery once every 5 h at 37°C (0.19 events per h per cell, n=229). We termed this process ‘deflation’ due to its similarity to the temporary pressure loss of a bicycle tire.
Fig. 2.
Deflation of pkd2-B42 cells. (A) Deflation of a pkd2-B42 cell at 37°C. Left: time-lapse micrographs. Right: time course of the cell length. (B) Time course of normalized width of 26 pkd2-B42 mutant cells during deflation followed by recovery. Dashed line indicates when the cells started shrinking. (C) Brightfield (top) and fluorescence (bottom) micrographs showing live cells of the indicated strains stained with SRB to visualize lysed cells. Arrows, deflated pkd2-B42 cells. Dashed boxes indicate deflated cells. The percentage of SRB-stained cells (mean±s.d. of two experiments, n>400) is shown for each strain. (D) Percentage of septated cells among the non-deflated (ND), deflated (D) and all pkd2-B42 cells at 36°C. n>300 cells in total. Data are presented as the mean±s.d. of three experiments. (E,F) Polarity in deflated pkd2-B42 cells. (E) Brightfield (top) and fluorescence (bottom) micrographs of wild-type (pkd2+) and pkd2-B42 cells expressing CRIB–mCherry at 36°C. Single-headed arrow, monopolar cell; double-headed arrow, bipolar cell; asterisk, dividing cell; arrowhead, deflated cell with mislocalized CRIB–mCherry. (F) Time-lapse micrographs of a pkd2-B42 mutant cell expressing CRIB–mCherry, taken at 4 min intervals. Data are representative of least two independent biological repeats. Scale bars: 10 µm. ***P<0.001; NS, not significant (two-tailed unpaired Student’s t-test).
Deflation of the pkd2-B42 mutant cells appeared to be similar to cell lysis of some other fission yeast mutants, prompting us to determine whether they are indeed the same in causing membrane rupture. To test this hypothesis, we stained the cells with sulforhodamine B (SRB), an impermeant dye that fluoresces in lysed cells (Lemiere et al., 2021; Skehan et al., 1990). This was confirmed through staining of spg1-106 mutant cells (Fig. 2C), many of which (64%) lysed and became fluorescent at the restrictive temperature. In contrast, very few pkd2-B42 mutant cells (<5%, n>400) were fluorescent in the presence of SRB dye, similar to the wild-type cells (Fig. 2C). We concluded that deflation of pkd2-B42 cells does not induce membrane rupture, unlike cell lysis.
The essential role of Pkd2p in cytokinesis (Morris et al., 2019) also prompted us to determine whether such deflation is linked to cell cycle progression, including cell separation. First, we determined whether cell separation could lead to deflation by quantifying the septation indices of the deflated, non-deflated and total pkd2-B42 mutant cells. We found similar percentages of septated cells among the three groups (Fig. 2D). Therefore, deflation is unlikely to result from cell separation. Second, we determined whether deflation is linked to cell cycle progression by measuring the cell length. The length of fission yeast cells increases continuously during interphase until the cells enter mitosis at 14 µm (Mitchison and Nurse, 1985). We found that the length distribution of the deflated pkd2-B42 mutant cells was similar to that of the whole mutant population (Fig. S1D). Therefore, deflation of pkd2-B42 cells is cell cycle-independent and is not linked to either cell separation or other events in the cell cycle progression.
Lastly, we examined whether deflation is linked to cell polarity. Like most eukaryotes, fission yeast requires the Cdc42-mediated polarity pathway for cell growth and integrity (Miller and Johnson, 1994). We measured the intracellular distribution of active Cdc42p using the fluorescent indicator CRIB–mCherry (Tatebe et al., 2008). Consistent with previous reports, CRIB–mCherry localized to either the cell tip or the division plane of the wild-type cells, depending on their cell cycle stage (Fig. 2E). Such polarized localization of CRIB remained unchanged in most pkd2-B42 mutant cells (Fig. 2E). However, during deflation, the tip and division plane localization of CRIB–mCherry was temporarily lost. Instead, it relocalized to the cytoplasm and nucleus and remained there for ∼20 min (Fig. 2E). Such change followed the initial rapid loss of cell size, but it was gradually reversed as the cells exited deflation (Fig. 2F). We conclude that deflation is correlated with a temporary loss of polarized distribution of active Cdc42p.
Tip and volume expansion of pkd2-B42 mutant cells during interphase growth
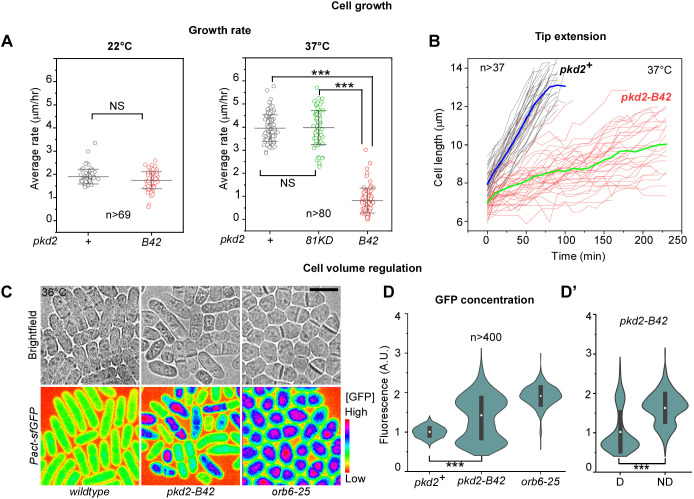
Morphological defects of pkd2-B42 cells, as well as their deflation, suggest a likely growth defect. The rod-shaped fission yeast cells grow by extending their tips while maintaining a constant width. We quantified such tip growth using time-lapse microscopy. The mutant cells grew at a rate similar to wild-type cells at the permissive temperature (Fig. 3A). At the restrictive temperature of 37°C, the wild-type cells maintained their continuous and linear tip extension during interphase growth. In comparison, most mutant cells (66%, n=229) paused their expansion at least once in a 4 h window for an average of 17 min, concurrent with deflation (Fig. 3B; Movie 3). Some cells (6%) paused two or three times. Even between these pauses, the pkd2-B42 mutant cells extended at a rate of 0.6±0.4 µm h−1 (mean±s.d.; n=20), far lower than that of the wild-type cells (∼4 µm h−1). Overall, the mutant cells grew at a rate of less than 0.8 µm h−1 (Fig. 3A,B; Movies 1,2). Consistent with the relatively normal growth of the depletion mutant pkd2-81KD (Fig. 1E), the tip extension rate of this mutant was similar to that of the wild-type strain (Fig. 3A). We concluded that Pkd2p is required for continuous tip extension during cell growth.
Fig. 3.
Pkd2p is required for cell size expansion during interphase growth. (A) Dot plots of the average tip growth rate of the wild-type (pkd2+) and the pkd2 mutant cells at 22°C (left) and 37°C (right). Line and error bars show the mean±s.d. (B) Time courses of the length extension of wild-type and pkd2-B42 cells at 37°C. Thick lines (blue, wild type; green, pkd2-B42) represent the mean time courses, n>37. (C) Brightfield (top) and fluorescence micrographs (bottom, spectrum-colored) of wild-type (left), pkd2-B42 (middle) and orb6-25 (right) cells at 36°C. All strains constitutively expressed super-folder GFP under control of the Pact promoter (Pact-sfGFP). The fluorescence intensity is inversely correlated with the volume expansion rate. Scale bar:10 µm. (D) Violin plots of intracellular GFP concentration in the indicated strains. (D’) The intracellular GFP concentration of the deflated (D) and non-deflated (ND) pkd2-B42 cells, n>400. Black bars indicate s.d. and white dots mark the mean. The data were pooled from at least two independent biological repeats. A.U., arbitrary units. ***P<0.001; NS, not significant (two-tailed unpaired Student's t-test).
The slow growth of pkd2-B42 cells naturally led to the prediction that they will need a much longer growth period to reach the minimum length required for mitotic entry. The wild-type cells initiate mitosis only after reaching a length of ∼14 µm. We tested this hypothesis by staining the cells with DAPI. In an asynchronized population, ∼8% of wild-type cells were mitotic. In comparison, we found less than 1% of mitotic cells among the pkd2-B42 mutant at the restrictive temperature (Fig. S1E). This result confirmed that pkd2-B42 mutant cells grow very slowly during interphase, which delays their mitotic entry.
It remained untested whether the volume of pkd2-B42 cells expanded slowly, considering that the mutant cells are wider than the wild-type cells. Since it is technically challenging to directly measure the volume of a fission yeast cell, we used a novel method that does so indirectly. This method quantifies the cellular concentration of a constitutively expressed fluorescent protein, which serves as an indirect volume indicator (Knapp et al., 2019). Because the volume of a fission yeast cell expands proportionally with the biosynthesis of fluorescent proteins such as GFP, the intracellular concentration of the fluorescent protein remains constant throughout the cell cycle. In contrast, a defect in volume expansion would lead to an elevated concentration of the fluorescent protein. Using quantitative fluorescence microscopy, we measured the intracellular concentration of constitutively expressed GFP (super-folder GFP) in both the wild-type and pkd2-B42 mutant cells. Compared to the wild-type cells, the average concentration of GFP in the mutant cells increased by ∼50%, consistent with a defect in volume expansion (Fig. 3C,D). Similarly, the GFP concentration increased by twofold (Fig. 3C,D) in another growth-defective mutant, orb6-25 (Verde et al., 1998). Moreover, in contrast to the wild-type cells, the distribution of the cellular GFP concentration was much more heterogenous among the pkd2-B42 mutant cells (Fig. 3C,D). In particular, the average fluorescence of cells undergoing deflation was lower than that of the other cells (Fig. 3D′). We conclude that Pkd2p is essential for cell volume expansion.
Turgor pressure of pkd2-B42 mutant cells
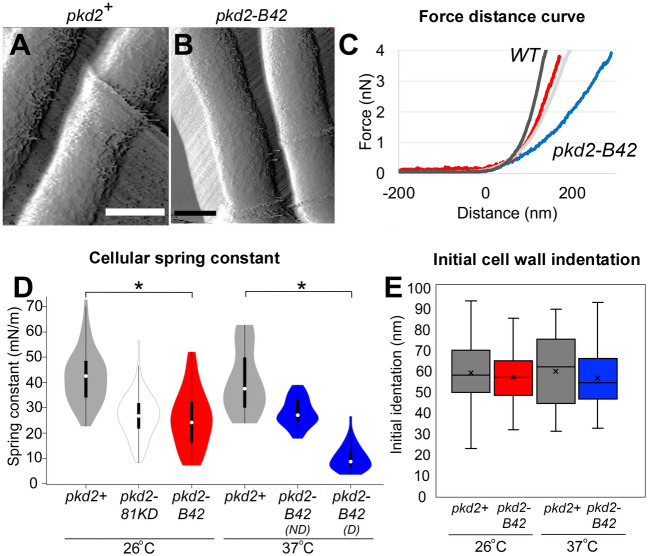
Like other walled cells, fission yeast cells depend on turgor pressure, which results from higher intracellular osmolarity than the environment, to expand. As an indirect measurement of the turgor of the pkd2-B42 mutant, we quantified its stiffness using AFM based on a method that we recently established (Gibbs et al., 2021) (Fig. 4A,B). We quantified force–distance curves of surface indentation and cellular spring constants (Fig. 4C). The spring constant of wild-type cells was 42±10 mN m−1 (mean±s.d.), which is consistent with previously reported values (Gibbs et al., 2021). In comparison, the spring constants of pkd2-B42 and pkd2-81KD mutant cells were 40% lower at the permissive temperature (26±12 mN m−1 and 27±9 mN m−1, respectively) (Fig. 4D). Interestingly, ‘non-deflated’ pkd2-B42 cells at 37°C had a cellular spring constant of 28±6 mN m−1 (Fig. 4D). In comparison, among ‘deflated’ pkd2-B42 cells, the spring constant was further decreased to 10±5 mN m−1 (Fig. 4D). We concluded that the pkd2 mutant cells are much softer than wild-type cells.
Fig. 4.
Cellular stiffness is decreased in pkd2 mutant cells. (A,B) AFM deflection images of yeast cells of the wild-type strain (pkd2+; A) and pkd2-B42 (B) acquired by intermittent contact imaging mode in EMM5S liquid medium. Scale bars: 2 µm. (C) Representative force–extension curves from wild-type (WT, dark gray), pkd2-81KD (light gray) and pkd2-B42 cell surfaces at 26°C (red), and from pkd2-B42 cell surfaces at 37°C (blue). The horizontal linear region of each curve reflects the cantilever approaching the surface, with the zero point on the x-axis indicating the point of contact between the tip and surface. The initial non-linear deflection of the force–extension curve reflects the initial indentation of the cell wall upon initial cantilever contact. The later linear deflection of the force–extension curve indicates the cellular stiffness. (D) Violin plot of cellular spring constants calculated from force–extension curves at 26°C and 37°C for the indicated cells. ND, not deflated; D, deflated. White circles show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5× the interquartile range from the 25th and 75th percentiles; polygons represent density estimates of data and extend to extreme values. n>500 force measurements in total from five cells per condition. (E) Box-and-whisker plot of the initial cell wall indentation from a non-linear region of force–extension curves at 26°C and 37°C. The boxed region indicates the upper and lower quartiles for each data set; the median is indicated by the horizontal line within the box; the mean is indicated by ×; whiskers indicate the range. n>100 force curves evaluated on five cells per condition. *P<1×10−5 (two-way ANOVA and two-tailed, unpaired Student's t-tests).
To estimate the decrease in the turgor pressure of pkd2 mutant cells, we applied the Hertz model (see Eqn 2 in Materials and Methods) to obtain their Young's moduli. AFM indentation analysis has been used previously to calculate the Young's modulus of cellular elasticity and turgor pressure for microbial cells (Arfsten et al., 2010; Gibbs et al., 2021). We modeled the turgor pressure based on the experimentally determined force curves and spring constants. Consistent with previous estimates (Abenza et al., 2015; Atilgan et al., 2015; Davi et al., 2018; Gibbs et al., 2021; Minc et al., 2009), our calculation determined a Young's modulus of 1.3±0.4 MPa (mean±s.d.) for the wild-type cells (Table S2). The turgor pressure decreased by 50% to 0.6±0.3 MPa in pkd2-B42 mutant cells, as well as in pkd2-81KD mutant cells, at 26°C. The Young's modulus of deflated temperature-sensitive pkd2-B42 mutant cells further decreased to 0.3±0.2 MPa at 37°C (Table S2). We concluded that the turgor pressure of the pkd2-B42 mutant is much lower than that of the wild-type strain.
Besides the turgor pressure, another key factor in determining the growth of walled cells is the flexibility of the cell wall (Lew, 2011; Minc et al., 2009). We evaluated this by compressing the yeast cells with the cantilever of the atomic force microscope. We then calculated the indentation within the non-linear region of the force curves (Arfsten et al., 2010; Arnoldi et al., 2000). At either permissive or restrictive temperature, the indentation values observed for both wild-type and pkd2-B42 mutant cells were 60–70 nm (Fig. 4E). This was consistent with the thickness of the outermost galactomannan layer of the cell wall measured by electron microscopy (Cortes et al., 2012; Osumi et al., 1998, 2006). We concluded that the cell wall flexibilities of wild-type and pkd2-B42 cells are similar.
Genetic interaction between pkd2 and SIN mutants
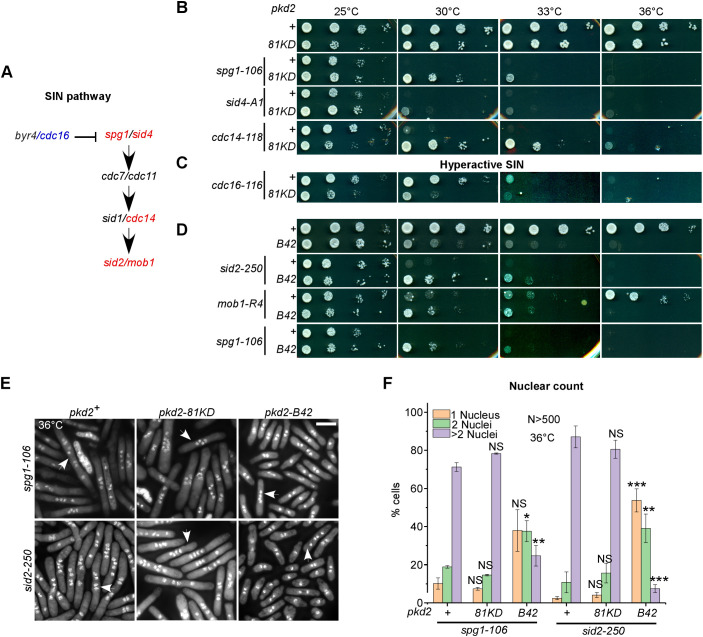
Parallel to the study of the temperature-sensitive pkd2-B42 mutant, we screened genetic interactions between the available hypomorphic pkd2-81KD mutant and a collection of more than thirty cell growth and cytokinesis mutants. We found no genetic interaction with mutants of either the cell wall synthesis pathway (bgs4 or ags1) or the cell wall integrity pathway (rgf1 or rga7). The pkd2-81KD mutant interacted negatively with four cytokinesis mutants, those of myo2, cdc12, rng2 and cdc15. In contrast, two SIN mutants, sid2-250 and mob1-R4, stood out for their strong positive genetic interaction with pkd2-81KD (Table S3; Morris et al., 2019). Further experiments using seven other SIN mutants (Fig. 5A; Table S3) found that three of them – including mutants of spg1, sid4 and cdc14 – interacted similarly with pkd2-81KD, particularly at the semi-permissive temperatures of 30°C and 33°C (Table S3; Fig. 5B). Consistent with these positive genetic interactions, we found negative genetic interactions between pkd2-81KD and MOR mutants including orb6-25 and mor2-282 (Table S3). The MOR pathway is inhibited by the SIN during cytokinesis (Gupta et al., 2013; Ray et al., 2010). As expected, in contrast to the hypomorphic SIN mutants mentioned above, the hypermorphic SIN mutant cdc16-116 (Fankhauser et al., 1993; Furge et al., 1998) interacted negatively with pkd2-81KD (Fig. 5C). Like pkd2-81KD, pkd2-B42 also partially rescued the three SIN mutants that we tested, those of sid2, mob1 or spg1 (Fig. 5D). Our genetic analyses thus suggested an antagonistic relationship between pkd2 and the SIN in cytokinesis.
Fig. 5.
Genetic interactions between pkd2 and SIN mutants. (A) Schematic of the SIN pathway. Gene symbols are colored blue to indicate negative genetic interaction with pkd2-81KD or red to indicate positive genetic interaction with pkd2-81KD. (B–D) Ten-fold dilution series of the indicated yeast strains on YE5S plates. (B,C) Comparison of SIN single mutants and pkd2-81KD SIN double mutants. (D) Comparison of SIN single mutants and pkd2-B42 SIN double mutants. Data in B–D are representative of three experiments. (E,F) Cytokinesis defects of pkd2 SIN double mutants. (E) Fluorescence micrographs of mutant cells (at 36°C) that were fixed and stained with DAPI to visualize the nuclei. Arrows indicate cells with four or more nuclei. Scale bar: 10 µm. (F) Percentage of mono-, bi- and multi-nucleated (>2 nuclei) cells at 36°C. n>500 cells for each strain. Data are presented as mean±s.d. of at least two experiments. *P<0.05; **P<0.01; ***P<0.001; NS, not significant (two-tailed unpaired Student’s t-test).
To understand why the pkd2 mutations partially rescued the SIN mutants, we characterized pkd2 SIN double-mutant cells after a shift to the restrictive temperature for 4 h. First, we counted the fraction of multinucleated cells among the double-mutant strains. The failure of the contractile ring to constrict in the SIN single mutants led to a large percentage (∼90%) of bi- or tetra-nucleated cells. Presence of the pkd2-81KD mutation did not significantly change that (Fig. 5E,F). In comparison, far more SIN pkd2-B42 double-mutant cells were bi-nucleate (Fig. 5F), likely undergoing just one round instead of two rounds of cell division in 4 h. Nevertheless, ∼60% of SIN pkd2-B42 double-mutant cells were either bi- or tetra-nucleate at 36°C (Fig. 5E,F), far more than were observed for the wild-type strain (8% binucleate). Interestingly, the fraction of mitotic SIN pkd2-B42 double-mutant cells was 5% (Fig. 5E; Fig. S1E), fourfold higher than that of the pkd2-B42 single mutant (1%) and only slightly lower than that of the wild-type strain (8%). Therefore, despite the growth defect of the pkd2-B42 mutant, most SIN pkd2 double mutants can enter mitosis, but their contractile ring constriction largely fails.
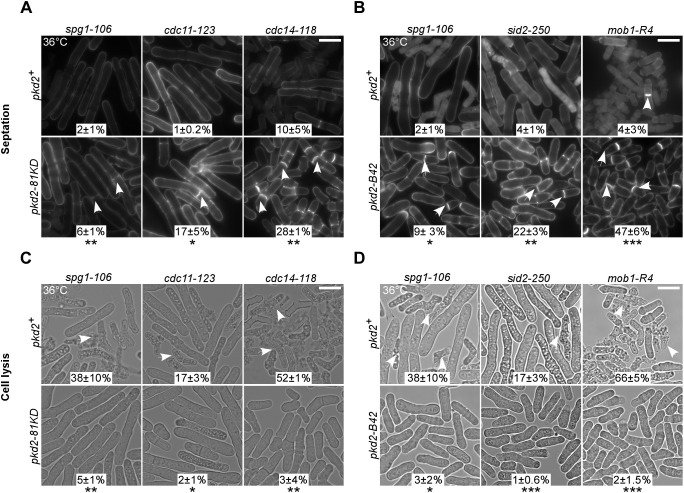
Next, we quantified the septation of the pkd2 SIN double mutants. Most SIN mutants failed to form the septum (Fig. 6A,B; Fig. S2A) (Balasubramanian et al., 1998). We found that both pkd2 mutations partially rescued this defect in the majority of the SIN mutants (Fig. 6A,B; Fig. S2A), with the exceptions of the cdc7 and sid1 mutants (Fig. S2A). The most significant rescue was observed for the cdc11 mutant, where the septation index increased from 1% to 17% in the presence of the pkd2-81KD mutation. Nevertheless, many septa in the double mutants appeared to be thin or only partially assembled. We concluded that the pkd2 mutations significantly promote septum formation in the SIN mutants.
Fig. 6.
Septation and lysis of pkd2 SIN double mutants. (A,B) Micrographs of the indicated SIN mutants (top) and either SIN pkd2-81KD double mutants (A, bottom) or SIN pkd2-B42 double mutants (B, bottom), inoculated at 36°C and fixed and stained with Calcofluor White. Arrows indicate septated cells. (C,D) Lysis of the indicated SIN mutants (top) and either SIN pkd2-81KD double mutants (C, bottom) or SIN pkd2-B42 double mutants (D, bottom), inoculated at 36°C. Arrows indicate lysed cells. The percentage of either septated (A,B) or lysed cells (C,D) is shown for each strain (mean±s.d. of at least two experiments). The data were pooled from at least two independent biological repeats (n>400 cells). Scale bars: 10 µm. *P<0.05; **P<0.01; ***P<0.001 (two-tailed unpaired Student’s t-test).
Lastly, we measured cell lysis among the SIN pkd2 double mutants. Although less understood, the SIN also ensures the integrity of separating cells and prevents them from lysing (Garcia-Cortes and McCollum, 2009; Gupta et al., 2014; Jin et al., 2006). We confirmed that all SIN mutants lysed in varying degrees, ranging from 12% (sid1-125) to 52% (cdc14-118) (Fig. 6C,D; Fig. S2B). Presence of the pkd2 mutations reduced the fraction of lysed SIN mutant cells significantly to less than 5% (Fig. 6C,D; Fig. S2B), with the sid1 mutant being the only exception (Fig. S2B). To determine how specific such rescue was, we tested whether the pkd2 mutations could prevent other fission yeast mutants from lysis. We found that the pkd2-81KD mutation failed to reduce the lysis of rga7Δ cells (Fig. S2B), another cytokinesis mutant that lyses frequently (Liu et al., 2016). Thus, we concluded that the pkd2 mutations specifically prevent the SIN mutant cells from lysing during cytokinesis.
Activity and localization of the SIN in pkd2 mutants
Reduced lysis and increased septation in the pkd2 SIN double-mutant cells suggested that the mutations of pkd2 may have increased SIN activity. To test this hypothesis, we measured the asymmetric localization of Cdc7p. This SIN pathway kinase distributes asymmetrically between two SPBs during cell division in a manner that is dependent on SIN activity (Cerutti and Simanis, 1999; Dey and Pollard, 2018; Feoktistova et al., 2012; Garcia-Cortes and McCollum, 2009; Singh et al., 2011; Sohrmann et al., 1998; Wachowicz et al., 2015). We measured the dwell time of Cdc7p–GFP on the two daughter SPBs (old and new) using time-lapse microscopy. In wild-type cells, Cdc7p–GFP remained at the new SPB for ∼50 min, through the end of telophase (Fig. S2C). In comparison, it dwelled on the old SPB for just ∼16 min until the end of anaphase A (Fig. S2C). Both values are consistent with previous measurements (Dey and Pollard, 2018; Garcia-Cortes and McCollum, 2009; Wachowicz et al., 2015). The ratio of the dwell times of Cdc7p–GFP on the two SPBs (old:new) averaged ∼0.3 for the wild-type cells (Fig. S2D). In comparison, this asymmetry index increased significantly in the pkd2-81KD mutant cells to ∼0.4 (Fig. S2D), despite the duration of mitosis not changing significantly (mean±s.d. of 28±2 min versus 30±4 min for wild type versus pkd2-81KD, n>65). We concluded that depletion of Pkd2p modestly increases SIN activity.
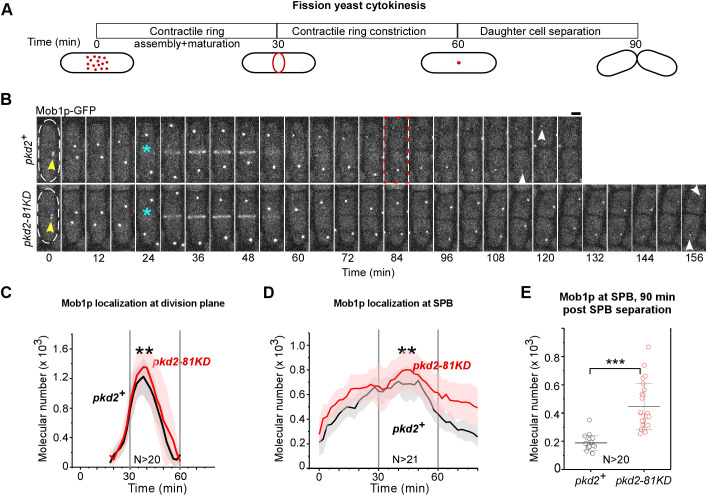
In addition, we tested another hypothesis that the mutations of pkd2 might enhance the cytokinetic localization of SIN proteins. We measured the molecular number of Sid2p kinase and its activator Mob1p using quantitative fluorescence microscopy. Both proteins localize to the SPBs in mitosis and subsequently translocate to the medial division plane during cytokinesis before dissipating into the cytoplasm (Salimova et al., 2000; Sparks et al., 1999). We quantified their localization through three steps of cytokinesis: contractile ring assembly and maturation, ring constriction, and cell separation (Fig. 7A) (Wu et al., 2003). In wild-type cells, each step lasts ∼30 min at room temperature, relative to the start of prometaphase marked by the separation of SPBs (Chen and Pollard, 2011; Morris et al., 2019; Wu et al., 2006) (Fig. 7A). During the first two steps of cytokinesis, the pkd2-81KD mutation did not alter the number of these two SIN molecules substantially at either the SPB or the equatorial plane compared to the numbers observed in wild-type cells (Fig. 7B–D; Fig. S3A–C). However, during the last step of cytokinesis, the numbers of both SIN molecules doubled at the SPBs of the pkd2 mutant cells following their exit from the equatorial plane (Fig. 7B,D,E; Fig. S3A,C,D). We concluded that depletion of Pkd2p significantly increases the localization of SIN proteins at the SPBs.
Fig. 7.
Localization of SIN proteins in pkd2-81KD cells. (A) Schematic of the average time course of fission yeast cytokinesis. Time zero denotes the separation of SPBs during prometaphase. (B) Time-lapse micrographs of either a wild-type (pkd2+, top) or pkd2-81KD (bottom) cell expressing Mob1p–GFP. Time is shown as minutes after SPB separation; time interval, 6 min. Cell outlines are marked with dashed lines at time zero. Arrowheads, SPBs; asterisks, appearance of Mob1p–GFP at the division plane. The red dashed box indicates cell separation. Scale bar: 2 μm. (C,D) Mean time courses of the number of Mob1p–GFP molecules at either the division plane (C) or SPBs (D) of either wild-type (black line) or pkd2-81KD cells (red line). Shaded regions represent the s.d. The three stages of cytokinesis are separated by vertical lines. P-values were calculated by comparing the peak numbers of Mob1p–GFP molecules in either the ring or SPBs. (E) Dot plot of the number of Mob1p–GFP molecules at the SPB 90 min after SPB separation. Data are presented as the mean±s.d. The data were pooled from two independent biological repeats. **P<0.01; ***P<0.001 (two-tailed unpaired Student's t-test).
DISCUSSION
To understand the function of the fission yeast polycystin Pkd2p in cytokinesis, we adopted two parallel approaches in this study. First, we isolated a novel pkd2 loss-of-function mutant allele. Characterization of pkd2-B42 revealed surprisingly that Pkd2p is essential for the maintenance and expansion of the cell volume during interphase growth in addition to its previously reported role in cytokinesis. Secondly, we examined the genetic interplay between pkd2 and the essential yeast Hippo-related pathway, the SIN. We found that Pkd2p antagonizes both the localization and activity of SIN proteins. Both functions of pkd2 are likely traced to its activity as a putative cation channel that is sensitive to membrane tension and is permissive to Ca2+.
The role of Pkd2p in interphase cell growth
The pkd2 mutant cells frequently lost and regained their volume in a span of less than 20 min. Both pkd2-81KD and pkd2-B42 mutants exhibited such a defect in cell growth (Morris et al., 2019), but it was much more prevalent among cells of the latter strain. This is likely because the depletion mutant pkd2-81KD retains ∼30% of the endogenous protein. In comparison, the temperature-sensitive mutant cells are expected to possess very little Pkd2p at the restrictive temperature.
The rapid loss of cell volume during deflation is not due to plasma membrane rupture. Few pkd2-B42 mutant cells were stained with the SRB dye. It is more likely that deflation is a result of the outflow of water, similar to the shrinking of cells under hyperosmotic shock (Poddar et al., 2021). In both cases, the rapid shrinking suggests that the intracellular osmolarity may not have adapted quickly enough to maintain turgor pressure. As we have shown in our previous study, activation of the mitogen-activated protein kinase (MAPK) Sty1p may have contributed to the graduate recovery observed following the volume loss (Morris et al., 2019; Toda et al., 1996).
Deflation is independent of cell cycle progression. This also differentiates it from the cell lysis that often happens during cell separation in mutants with a defect in septum biosynthesis (Cortes et al., 2002; Muñoz et al., 2013). Interestingly, deflation also occurred during cell division, even though there is no cell growth in this period. This suggests that the regulation of turgor is equally important during mitosis and cytokinesis.
To the best of our knowledge, ‘deflation’ or a similar phenotype has not been described in other fission yeast mutants. To our surprise, it has not been described for the deletion mutant of gpd1, which promotes glycerol biosynthesis for regulation of turgor pressure (Aiba et al., 1995; Minc et al., 2009). Nevertheless, the temporary loss of cell polarity in the deflated pkd2-B42 mutant cells is reminiscent of the phenotype found in gpd1Δ cells under hyperosmotic stress (Haupt et al., 2018). Compared to Pkd2p, Gpd1p may be more important for the regulation of turgor under stress. In contrast to the pkd2-B42 mutants, no such reversible loss of cell volume has been reported in either mutants of the cell wall integrity pathway, such as rgf1Δ and rga7Δ (Garcia et al., 2006; Liu et al., 2016), or those of the cell wall sensors wsc1Δ and mtl2Δ (Cruz et al., 2013). These observations suggest that Pkd2p is likely independent from proteins that sense or repair cell wall damage under stress.
Our data do not support the hypothesis that Pkd2p is required for cell wall biosynthesis. Previously we reported that the cell wall structure of the pkd2-81KD mutant cells is similar to that of the wild-type cells (Morris et al., 2019). Moreover, unlike most mutants of cell wall biosynthesis (Liu et al., 1999), the pkd2-B42 mutant's viability cannot be fully rescued by supplementing the medium with sorbitol. Whereas the mutants of cell wall assembly frequently lyse during cell separation (Cortes et al., 2012; Muñoz et al., 2013), both pkd2-81KD and pkd2-B42 mutant cells rarely do so. Furthermore, we found no genetic interaction between the pkd2 mutants and the mutants of cell wall synthases.
Excluding a direct role for Pkd2p in cell wall assembly or repair, the most likely target for Pkd2p is the regulation of intracellular osmolarity. In fission yeast, as in other fungi, cell expansion depends on the difference between the intracellular and extracellular osmolarity – the turgor pressure (Lew, 2011; Minc et al., 2009). The importance of Pkd2p to turgor pressure is supported by our AFM study showing that the pkd2 mutant cells are much softer than wild-type cells. As a putative mechanosensitive channel, Pkd2p may act as a plasma membrane sensor to regulate intracellular osmolarity and the ensuing turgor pressure.
The role of Pkd2p in cell division
In light of this newly discovered role for Pkd2p in promoting cell growth, it is surprising that pkd2 is also needed for cytokinesis, because fission yeast cells do not expand during cell division. One likely explanation is that Pkd2p continues to regulate intracellular osmolarity to maintain cell size even during cytokinesis. This hypothesis is consistent with two cytokinesis defects of the pkd2 mutant cells (Morris et al., 2019). First, the contractile ring constricts 1.5-times faster in the pkd2-81KD mutant. The softer pkd2 mutant cells may have presented less resistance to the compression force applied by the actomyosin contractile ring during furrow ingression compared to wild-type cells. Second, cells of both the pkd2 mutant strains often failed to separate at the end of cytokinesis. This is also likely due to a failure in increasing turgor pressure at the new ends during the cell separation.
Antagonism between pkd2 and the SIN during cytokinesis
Both pkd2 mutants partially rescued the SIN mutants by preventing cell lysis and promoting septation, suggesting that Pkd2p plays a direct role in cytokinesis in addition to its function in interphase growth. The lack of interphase growth alone or lack of mitotic entry cannot explain this observation. Unlike the temperature-sensitive mutant pkd2-B42, pkd2-81KD has no tip growth defect. Nevertheless, this depletion mutant prevented the SIN mutants from lysis and promoted septum biosynthesis. Furthermore, although pkd2-B42 SIN double-mutant cells exhibited signs of delayed mitotic entry, more than 60% of them still underwent at least one round of cell division without lysis. A likely explanation is that the reduced turgor pressure due to the pkd2 mutations may indirectly prevent the lysis of the SIN mutant cells.
Unlike the SIN mutants, mutants of the contractile ring, such as those of myo2, rng2, cdc12 or cdc15, were not rescued by pkd2 mutation. In contrast, these cytokinesis mutants exhibited negative genetic interactions with pkd2-81KD (Morris et al., 2019). This difference reinforces the idea that the relationship between pkd2 and the SIN during cytokinesis is distinct. It also suggests that reduced turgor pressure due to the pkd2 mutations alone is not sufficient to rescue contractile ring constriction.
Conversely, SIN mutations also allow a much larger fraction of pkd2-B42 mutant cells to progress to mitosis. The mitotic index of pkd2-B42 SIN double mutants is five times higher than that of the pkd2-B42 mutant. Because fission yeast cells need to reach a minimum length of 14 µm to enter mitosis, this observation suggests that the SIN mutations likely promote the tip extension of pkd2-B42 mutant cells. This also lends support to the proposed antagonistic relationship between the SIN and pkd2.
Depletion of Pkd2p also increased the SPB localization of the SIN proteins during cell separation, the last step of cytokinesis. This is despite Pkd2p localizing at the cleavage furrow throughout cytokinesis (Morris et al., 2019). The putative TRP channel Pkd2p might mediate cytokinetic Ca2+ spikes (Poddar et al., 2021) to activate the Ca2+ signaling pathway, moderating the localization of SIN proteins. Further studies will be needed to determine whether Pkd2p modulates the SIN pathway directly. Alternatively, the antagonism between these two could be indirect, acting through the regulation of turgor pressure during cytokinesis.
Activity and function of polycystin channels
To date, only mammalian polycystin channels have had their electrophysiological properties characterized (DeCaen et al., 2013; Delling et al., 2013; Hanaoka et al., 2000). They are permissive to cations, including K+, Na+ and Ca2+ (Liu et al., 2018), after being activated by mechanical stimuli (Nauli et al., 2003). If the fission yeast Pkd2p channel possesses similar ion selectivity, it may regulate both ion homeostasis and Ca2+ signaling. For now, the electrophysiology of the Pkd2p channel remains unknown.
Pkd2p homologs can be found among many fungi species, and they constitute one of seventeen essential fungal protein families (Hsiang and Baillie, 2005). The Neurospora crassa Pkd2p homolog promotes cell growth (Bok et al., 2001; Stephenson et al., 2014) by regulating Ca2+ influx at the cell tip and maintaining a Ca2+ gradient at the expanding hyphae (Silverman-Gavrila and Lew, 2003). The function and mechanism of Pkd2p in cell expansion may be evolutionarily conserved in fungi.
In summary, our study has discovered that the fission yeast polycystin homolog Pkd2p plays a critical role in the maintenance and expansion of cell size during interphase growth and antagonizes the SIN pathway either directly or indirectly during cytokinesis. We propose that this putative TRP channel regulates both intracellular osmolarity and the Ca2+ signaling pathway.
MATERIALS AND METHODS
Yeast genetics
Yeast cell culture and genetics were carried out according to standard methods (Moreno et al., 1991). YE5S medium was used in all experiments unless specified otherwise. SPA5S agar plates were used for genetic crosses and sporulation. A SporePlay+ microscope (Singer, England) was used for tetrad dissection and developing different strains. For drop assays, we inoculated the cells in liquid medium at 25°C overnight before setting up the tenfold dilution series on agar plates. The plates were incubated at designated temperatures for 2–3 d before being scanned using a photo scanner (Epson). Yeast strains used in this study and their sources are listed in Table S5.
Identification of the temperature-sensitive pkd2 mutant
To isolate pkd2 temperature-sensitive mutants, we employed marker reconstitution mutagenesis (Tang et al., 2011) with some modifications. The wild-type strain (MBY6218) and the pH5C and pH5D plasmids were shared by Mohan Balasubramanian (University of Warwick, UK). First, we amplified the DNA fragment encoding the C-terminally truncated His5 (His5CterΔ) and ura4 from the pH5C vector (Tang et al., 2011) by PCR using primers P602 and P603 (Table S4). These primers included nucleotide sequences homologous to the 3′ untranslated region (UTR) of pkd2. The amplified DNA fragment was transformed into yeast using the lithium acetate method. The resulting yeast strain (QCY-999) was selected on EMM plates without uracil and confirmed through PCR and Sanger sequencing. Next, the coding sequence of pkd2 including its 3′ UTR was cloned into the vector pH5D (Tang et al., 2011), which contains the missing coding sequence for the C-terminal fragment of His5 (His5Cter). The resultant vector (QCV-193) was used as the template to perform random mutagenesis of pkd2 through error-prone PCR. The reaction employed Taq DNA polymerase (NEB) in the presence of 200 µM MnCl2 (Sigma). The PCR product consisting of the mutated pkd2 together with the sequence of His5Cter (PCR product) was transformed into QCY-999 to replace the wild-type gene. The transformants were selected on EMM plates without histidine. The positive clones were further screened for their temperature sensitivity through incubation on plates supplemented with Phloxin B at 36°C for 2 d. The temperature-sensitive mutants were isolated and back-crossed with the wild-type strain twice to confirm their phenotype. The open reading frame sequence of the pkd2-B42 mutant was amplified using PCR and sequenced by the Sanger method to identify the point mutations.
Microscopy
For all microscopy experiments, the cells were inoculated first in liquid medium at 25°C for 2 d before being harvested during the exponential growth phase at a density between 5×106 ml−1 and 1.0×107 ml−1. For temperature-sensitive mutants, exponentially growing cell cultures were shifted to the restrictive temperature for 4 h (unless mentioned) before imaging or fixation.
For microscopy of live cells, the cells were harvested by centrifugation at 1500 g for 1 min. Concentrated resuspended cells were deposited on a gelatin pad (25% gelatin in YE5S) on a slide. The samples were sealed under a coverslip with VALAP (a mix of an equal amount of Vaseline, lanolin and paraffin) and imaged.
We used a spinning-disk confocal microscope equipped with an EM-CCD camera for fluorescence microscopy. The Olympus IX71 microscope was equipped with the objective lenses 100× (NA=1.40, oil), 60× (NA=1.40, oil) and 40× (NA=1.30, oil), a confocal spinning-disk unit (CSU-X1, Yokogawa, Japan), a motorized XY stage and a Piezo Z Top plate (ASI, USA). Solid-state lasers of 405 nm, 488 nm or 556 nm were used at a power of no more than 5 mW (<10%). The images were acquired by an Ixon-897 camera controlled by iQ3.0 (Andor, Ireland). We acquired 15 slices at a step size of 0.5 µm or eight slices at a step size of 1 µm for Z series. Most time-lapse microscopy was carried out in a dark room with temperature maintained at ∼23°C. To minimize the temperature variations, we undertook microscopy of both the wild-type and mutants on the same or consecutive days.
To measure the tip growth rate, 20 μl of exponentially growing cell culture was spotted onto a glass coverslip (#1.5) in a 10 mm Petri dish (Cellvis, USA). The coverslip was precoated with 50 μl of 50 μg ml−1 lectin (Sigma, L2380) and allowed to dry overnight at 4°C. The cells were overlaid with a 9 mm diameter YE5S agar block (∼5 mm height) in a covered Petri dish throughout imaging. To measure the growth rate at room temperature, we used the spinning-disk confocal microscope. For imaging at 37°C in the temperature-controlled chamber (Precision Control), we used a wide-field microscope (Olympus IX81) with a 60× oil objective lens (Olympus, NA=1.42) and a CoolSNAP HQ2 camera (Photometrics). Prior to the time-lapse imaging, we shifted the exponentially growing cells to 36°C for inoculation in a water bath for 4 h.
To measure the septation or mitotic indices, Calcofluor White- or DAPI-stained cells were imaged with an Olympus IX81 microscope equipped with a 60× oil lens (NA=1.42) and a digital camera C11440 (Hamamatsu, Japan). Images were acquired using CellSens (Olympus). Brightfield images were illuminated with an LED lamp. For visualizing the cell wall or septum, cells were fixed with 4% paraformaldehyde (Fisher), washed with TEMK buffer (50 mM Tris-HCL pH 7.4, 1 mM MgCl2, 50 mM KCl, 1 mM EGTA pH 8.0), and stained with 1 µg ml−1 of Calcofluor White stain (Sigma). For nuclear staining, cells were fixed with cold 70% ethanol and stained with 1 µg ml−1 DAPI (Sigma). For some measurements of septation indices, we used the spinning-disk confocal microscope combined with a 40× oil objective and 405 nm laser.
To visualize the actin cytoskeleton, cells were fixed with 4% paraformaldehyde (Fisher), washed with TEMK buffer, permeated with 1% Triton X-100 and stained with Bodipy–phallacidin (Invitrogen).
For staining with Sulforhodamine-B (MP Biomedicals LLC # 152479), 1 ml of exponentially growing cells was harvested and resuspended in 1 ml of YE5S supplemented with 6.7 µg/ml of the fluorescent dye. The cells were immediately spun down and resuspended in ∼40 µl of supernatant. We dropped a 6 µl sample of this cell suspension onto a glass slide directly and covered it with a coverslip. The cells were imaged immediately using the spinning-disk confocal microscope with a 40× oil objective and 556 nm laser.
Image analysis
We used Image J (NIH) to process all the microscopy images, with either freely available or customized macros and plug-ins. For quantitative analysis, the fluorescence micrographs were corrected for X–Y drift using StackReg (Thevenaz et al., 1998) and for photo-bleaching using EMBLTools (Rietdorf, EMBL Heidelberg). Unless specified, the average fluorescence of all the Z-slices was used for quantitative measurements. All the measurements were corrected using background subtraction.
The numbers of Sid2p–GFP and Mob1p–GFP molecules were quantified on a calibrated spinning-disk confocal microscope (Morris et al., 2019; Wu and Pollard, 2005). The SPB localization was measured by counting the total fluorescence in a 0.8 µm (8 pixels)-diameter circle. The background fluorescence was the fluorescence intensity in a ring of 1 µm (12 pixels) diameter surrounding the circle. The contractile ring localization of Sid2p–GFP and Mob1p–GFP was quantified by measuring the fluorescence in a 3 or 4 µm by 0.8 µm (30 or 40×8 pixels) rectangle centered on the cell division plane. The background fluorescence was the fluorescence intensity in a 3–4 µm by 0.2 µm rectangle (30–40×2 pixels) adjoined to the equatorial plane. The asymmetry of Cdc7–GFP on the two SPBs were measured similarly without counting the molecule numbers due to the relatively weak fluorescence signal. All the figures were constructed with Canvas X (ACD Systems). The plots were created using either Origin (OriginLab) or KaleidaGraph (Synergy).
AFM imaging and analysis of cells
Yeast strains were cultured in YE5S medium at 25°C in the mid-log phase (OD595<0.6) for 36 h prior to imaging. For restrictive temperature experiments, cells were shifted to 36°C for 8 h. Cells for AFM imaging and analysis were centrifuged at 500 g and washed with EMM5S medium (Moreno et al., 1991) prior to plating on plastic dishes. Plastic dishes were prepared for yeast cell adhesion by plasma cleaning with a PDC-32G plasma cleaner (Harrick Plasma, Ithaca, NY) for 3 min followed by incubation for 48 h at 4°C with 0.8 mg ml−1 CellTak adhesive (Corning Life Sciences, Glendale, AZ) diluted in 0.1 M NaHCO3. Dishes were washed twice with ddH2O to remove excess CellTak prior to plating cells. Yeast cells were added to the dish and centrifuged at 500 g for 20 s using a swinging bucket rotor. Non-adherent cells were removed by three EMM5S washes and aspiration.
Topographical height and deflection AFM images were obtained in contact mode using an MFP-3D atomic force microscope (Asylum Research, Santa Barbara, CA). Silicon Nitride AFM pyramidal tip PNP-TR probes (Nanoworld, Neuchatel, Switzerland) with a nominal spring constant of 0.32 N m−1 were used for all experiments. Cells were maintained in EMM5S medium throughout AFM imaging and analysis to prevent drying out. Two-dimensional images were exported from MFP-3D software, and scale bars were added in Adobe Photoshop. Following topographical imaging, force data was obtained from yeast cells at a deflection setpoint of 4 nN to minimize damage to the cell surface. Ten representative points were selected along the length of each cell, and ten force curves were obtained at each location, for a total of 100 force curves per cell. The extension force curve representing the tip approach and contact with the surface was used to calculate the spring constant for the surface. The spring constants (kcell) were calculated from the linear region of the extension curve using a two-spring model with the equation:
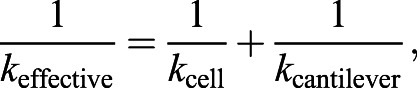 |
(1) |
where kcantilever was experimentally calculated using the thermal tuning method (Hutter and Bechhoefer, 1993), and the slope of the extension force curve linear region is keffective (Volle et al., 2008). Data processing was performed through MFP-3D software in Igor Pro to obtain curve fits and spring constants.
AFM extension force indentation curves were used to calculate Young's elasticity moduli by the Asylum Igor Pro MFP-03D software (WaveMetrics, USA), using the Hertz modeling equation as previously described (Gibbs et al., 2021):
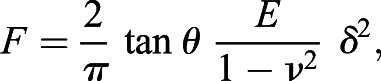 |
(2) |
where F is the applied force, θ is the half-opening angle (35°), E is the Young's modulus, and δ is the indentation depth. A sample Poisson of 0.03 was used in the Hertz modeling.
Supplementary Material
Acknowledgements
We thank Abhishek Poddar and Mamata Malla for their technical support, and Mohan Balasubramanian (University of Warwick, UK), Daniel McCollum (University of Massachusetts Medical School), Jian-Qiu Wu (Ohio State University) and Pilar Perez (University of Salamanca, Spain) for sharing yeast strains and plasmids with us. We acknowledge the National BioResources Project – Yeast Base (Japan) for sending yeast strains. We would like to thank Song-Tao Liu (University of Toledo) for sharing his microscope.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Q.C.; Methodology: D.S., J.G., Q.C.; Software: Q.C.; Validation: D.S., J.G.; Formal analysis: D.S., D.I., E.G., J.G.; Investigation: D.S., D.I., E.G., M.C., J.G., Q.C.; Resources: Q.C.; Data curation: D.S., J.G.; Writing - original draft: D.S., J.G., Q.C.; Writing - review & editing: D.S., J.G., Q.C.; Supervision: J.G., Q.C.; Project administration: Q.C.; Funding acquisition: J.G., Q.C.
Funding
This work was supported by the University of Toledo startup fund (to Q.C.), National Institutes of Health grant R15GM134496 (to Q.C.), the DeArce-Koch Memorial Fund (to Q.C.), the University of Toledo Undergraduate Summer Research and Creative Activities Program (to D.I.), the Wellesley College startup fund (to J.G.) and National Science Foundation grant DBI1528288 (to J.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Deposited in PMC for release after 12 months.
References
- Abenza, J. F., Couturier, E., Dodgson, J., Dickmann, J., Chessel, A., Dumais, J. and Salas, R. E. C. (2015). Wall mechanics and exocytosis define the shape of growth domains in fission yeast. Nat. Commun. 6, 8400. 10.1038/ncomms9400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba, H., Yamada, H., Ohmiya, R. and Mizuno, T. (1995). The osmo-inducible gpd1+ gene is a target of the signaling pathway involving Wis1 MAP-kinase kinase in fission yeast. FEBS Lett. 376, 199-201. 10.1016/0014-5793(95)01277-4 [DOI] [PubMed] [Google Scholar]
- Arfsten, J., Leupold, S., Bradtmoller, C., Kampen, I. and Kwade, A. (2010). Atomic force microscopy studies on the nanomechanical properties of Saccharomyces cerevisiae. Colloids Surf. B Biointerfaces 79, 284-290. 10.1016/j.colsurfb.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Arnoldi, M., Fritz, M., Bauerlein, E., Radmacher, M., Sackmann, E. and Boulbitch, A. (2000). Bacterial turgor pressure can be measured by atomic force microscopy. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 62, 1034-1044. 10.1103/physreve.62.1034 [DOI] [PubMed] [Google Scholar]
- Atilgan, E., Magidson, V., Khodjakov, A. and Chang, F. (2015). Morphogenesis of the fission yeast cell through cell wall expansion. Curr. Biol. 25, 2150-2157. 10.1016/j.cub.2015.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, M. K., McCollum, D., Chang, L., Wong, K. C., Naqvi, N. I., He, X., Sazer, S. and Gould, K. L. (1998). Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149, 1265-1275. 10.1093/genetics/149.3.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, M. M. and Sternberg, P. W. (1999). A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401, 386-389. 10.1038/43913 [DOI] [PubMed] [Google Scholar]
- Bohnert, K. A., Grzegorzewska, A. P., Willet, A. H., Van der Kooi, C. W., Kovar, D. R. and Gould, K. L. (2013). SIN-dependent phosphoinhibition of formin multimerization controls fission yeast cytokinesis. Genes Dev. 27, 2164-2177. 10.1101/gad.224154.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok, J.-W., Sone, T., Silverman-Gavrila, L. B., Lew, R. R., Bowring, F. J., Catcheside, D. E. and Griffiths, A. J. (2001). Structure and function analysis of the calcium-related gene spray in Neurospora crassa. Fungal Genet. Biol. 32, 145-158. 10.1006/fgbi.2000.1259 [DOI] [PubMed] [Google Scholar]
- Camargo, F. D., Gokhale, S., Johnnidis, J. B., Fu, D., Bell, G. W., Jaenisch, R. and Brummelkamp, T. R. (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054-2060. 10.1016/j.cub.2007.10.039 [DOI] [PubMed] [Google Scholar]
- Cerutti, L. and Simanis, V. (1999). Asymmetry of the spindle pole bodies and spg1p GAP segregation during mitosis in fission yeast. J. Cell Sci. 112, 2313-2321. 10.1242/jcs.112.14.2313 [DOI] [PubMed] [Google Scholar]
- Chen, Q. and Pollard, T. D. (2011). Actin filament severing by cofilin is more important for assembly than constriction of the cytokinetic contractile ring. J. Cell Biol. 195, 485-498. 10.1083/jcb.201103067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes, J. C., Ishiguro, J., Duran, A. and Ribas, J. C. (2002). Localization of the (1,3)β-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 115, 4081-4096. 10.1242/jcs.00085 [DOI] [PubMed] [Google Scholar]
- Cortes, J. C., Sato, M., Muñoz, J., Moreno, M. B., Clemente-Ramos, J. A., Ramos, M., Okada, H., Osumi, M., Duran, A. and Ribas, J. C. (2012). Fission yeast Ags1 confers the essential septum strength needed for safe gradual cell abscission. J. Cell Biol. 198, 637-656. 10.1083/jcb.201202015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, S., Muñoz, S., Manjon, E., Garcia, P. and Sanchez, Y. (2013). The fission yeast cell wall stress sensor-like proteins Mtl2 and Wsc1 act by turning on the GTPase Rho1p but act independently of the cell wall integrity pathway. MicrobiologyOpen 2, 778-794. 10.1002/mbo3.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davi, V., Tanimoto, H., Ershov, D., Haupt, A., De Belly, H., Le Borgne, R., Couturier, E., Boudaoud, A. and Minc, N. (2018). Mechanosensation Dynamically Coordinates Polar Growth and Cell Wall Assembly to Promote Cell Survival. Dev. Cell 45, 170-182. 10.1016/j.devcel.2018.03.022 [DOI] [PubMed] [Google Scholar]
- DeCaen, P. G., Delling, M., Vien, T. N. and Clapham, D. E. (2013). Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504, 315-318. 10.1038/nature12832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling, M., DeCaen, P. G., Doerner, J. F., Febvay, S. and Clapham, D. E. (2013). Primary cilia are specialized calcium signalling organelles. Nature 504, 311-314. 10.1038/nature12833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey, S. K. and Pollard, T. D. (2018). Involvement of the septation initiation network in events during cytokinesis in fission yeast. J. Cell Sci. 131, jcs216895. 10.1242/jcs.216895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J., Feldmann, G., Huang, J., Wu, S., Zhang, N., Comerford, S. A., Gayyed, M. F., Anders, R. A., Maitra, A. and Pan, D. (2007). Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120-1133. 10.1016/j.cell.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C., Marks, J., Reymond, A. and Simanis, V. (1993). The S. pombe cdc16 gene is required both for maintenance of p34cdc2 kinase activity and regulation of septum formation: a link between mitosis and cytokinesis? EMBO J. 12, 2697-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistova, A., Morrell-Falvey, J., Chen, J. S., Singh, N. S., Balasubramanian, M. K. and Gould, K. L. (2012). The fission yeast septation initiation network (SIN) kinase, Sid2, is required for SIN asymmetry and regulates the SIN scaffold, Cdc11. Mol. Biol. Cell 23, 1636-1645. 10.1091/mbc.e11-09-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furge, K. A., Wong, K., Armstrong, J., Balasubramanian, M. and Albright, C. F. (1998). Byr4 and Cdc16 form a two-component GTPase-activating protein for the Spg1 GTPase that controls septation in fission yeast. Curr. Biol. 8, 947-954. 10.1016/S0960-9822(98)70394-X [DOI] [PubMed] [Google Scholar]
- Garcia, P., Tajadura, V., Garcia, I. and Sanchez, Y. (2006). Rgf1p is a specific Rho1-GEF that coordinates cell polarization with cell wall biogenesis in fission yeast. Mol. Biol. Cell 17, 1620-1631. 10.1091/mbc.e05-10-0933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cortes, J. C. and McCollum, D. (2009). Proper timing of cytokinesis is regulated by Schizosaccharomyces pombe Etd1. J. Cell Biol. 186, 739-753. 10.1083/jcb.200902116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, E., Hsu, J., Barth, K. and Goss, J. W. (2021). Characterization of the nanomechanical properties of the fission yeast (Schizosaccharomyces pombe) cell surface by atomic force microscopy. Yeast 38, 480-492. 10.1002/yea.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perrett, S., Kim, K., Ibarra, C., Damiano, A. E., Zotta, E., Batelli, M., Harris, P. C., Reisin, I. L., Arnaout, M. A. and Cantiello, H. F. (2001). Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc. Natl. Acad. Sci. U.S.A. 98, 1182-1187. 10.1073/pnas.98.3.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S., Mana-Capelli, S., McLean, J. R., Chen, C. T., Ray, S., Gould, K. L. and McCollum, D. (2013). Identification of SIN pathway targets reveals mechanisms of crosstalk between NDR kinase pathways. Curr. Biol. 23, 333-338. 10.1016/j.cub.2013.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S., Govindaraghavan, M. and McCollum, D. (2014). Cross talk between NDR kinase pathways coordinates cytokinesis with cell separation in Schizosaccharomyces pombe. Eukaryot. Cell 13, 1104-1112. 10.1128/EC.00129-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka, K., Qian, F., Boletta, A., Bhunia, A. K., Piontek, K., Tsiokas, L., Sukhatme, V. P., Guggino, W. B. and Germino, G. G. (2000). Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408, 990-994. 10.1038/35050128 [DOI] [PubMed] [Google Scholar]
- Hardy, E. and Tsiokas, L. (2020). Polycystins as components of large multiprotein complexes of polycystin interactors. Cell. Signal. 72, 109640. 10.1016/j.cellsig.2020.109640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt, A., Ershov, D. and Minc, N. (2018). A positive feedback between growth and polarity provides directional persistency and flexibility to the process of tip growth. Curr. Biol. 28, 3342-3351. 10.1016/j.cub.2018.09.022 [DOI] [PubMed] [Google Scholar]
- Hergovich, A. (2016). The roles of NDR protein kinases in Hippo signalling. Genes 7, 21. 10.3390/genes7050021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang, T. and Baillie, D. L. (2005). Comparison of the yeast proteome to other fungal genomes to find core fungal genes. J. Mol. Evol 60, 475-483. 10.1007/s00239-004-0218-1 [DOI] [PubMed] [Google Scholar]
- Huang, K., Diener, D. R., Mitchell, A., Pazour, G. J., Witman, G. B. and Rosenbaum, J. L. (2007). Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 179, 501-514. 10.1083/jcb.200704069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, J., Ward, C. J., Peral, B., Aspinwall, R., Clark, K., San Millan, J. L., Gamble, V. and Harris, P. C. (1995). The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151-160. 10.1038/ng0695-151 [DOI] [PubMed] [Google Scholar]
- Hutter, J. L. and Bechhoefer, J. (1993). Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 64, 1868-1873. 10.1063/1.1143970 [DOI] [Google Scholar]
- Jin, Q.-W., Zhou, M., Bimbo, A., Balasubramanian, M. K. and McCollum, D. (2006). A role for the septation initiation network in septum assembly revealed by genetic analysis of sid2-250 suppressors. Genetics 172, 2101-2112. 10.1534/genetics.105.050955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, A. E., McCollum, D. and Gould, K. L. (2012). Polar opposites: Fine-tuning cytokinesis through SIN asymmetry. Cytoskeleton (Hoboken) 69, 686-699. 10.1002/cm.21044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp, B. D., Odermatt, P., Rojas, E. R., Cheng, W., He, X., Huang, K. C. and Chang, F. (2019). Decoupling of Rates of Protein Synthesis from Cell Expansion Leads to Supergrowth. Cell Syst 9, 434-445. 10.1016/j.cels.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemiere, J., Ren, Y. and Berro, J. (2021). Rapid adaptation of endocytosis, exocytosis and eisosomes after an acute increase in membrane tension in yeast cells. eLife 10, e62084 [Epub]. 10.7554/eLife.62084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, R. R. (2011). How does a hypha grow? The biophysics of pressurized growth in fungi. Nat. Rev. Microbiol. 9, 509-518. 10.1038/nrmicro2591 [DOI] [PubMed] [Google Scholar]
- Lima, W. C., Vinet, A., Pieters, J. and Cosson, P. (2014). Role of PKD2 in rheotaxis in Dictyostelium. PLoS One 9, e88682. 10.1371/journal.pone.0088682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Wang, H., McCollum, D. and Balasubramanian, M. K. (1999). Drc1p/Cps1p, a 1,3-β-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics 153, 1193-1203. 10.1093/genetics/153.3.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Lee, I. J., Sun, M., Lower, C. A., Runge, K. W., Ma, J. and Wu, J. Q. (2016). Roles of the novel coiled-coil protein Rng10 in septum formation during fission yeast cytokinesis. Mol. Biol. Cell 27, 2528-2541. 10.1091/mbc.e16-03-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Vien, T., Duan, J., Sheu, S. H., DeCaen, P. G. and Clapham, D. E. (2018). Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife 7, e33183. 10.7554/eLife.33183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., Lou, X., Wingfield, J. L., Lin, J., Nicastro, D. and Lechtreck, K. (2020). Chlamydomonas PKD2 organizes mastigonemes, hair-like glycoprotein polymers on cilia. J. Cell Biol. 219, e202001122. 10.1083/jcb.202001122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, P. J. and Johnson, D. I. (1994). Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol. Cell. Biol. 14, 1075-1083. 10.1128/mcb.14.2.1075-1083.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minc, N., Boudaoud, A. and Chang, F. (2009). Mechanical forces of fission yeast growth. Curr. Biol. 19, 1096-1101. 10.1016/j.cub.2009.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison, J. M. and Nurse, P. (1985). Growth in cell length in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 75, 357-376. 10.1242/jcs.75.1.357 [DOI] [PubMed] [Google Scholar]
- Mochizuki, T., Wu, G., Hayashi, T., Xenophontos, S. L., Veldhuisen, B., Saris, J. J., Reynolds, D. M., Cai, Y., Gabow, P. A., Pierides, A.et al. (1996). PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339-1342. 10.1126/science.272.5266.1339 [DOI] [PubMed] [Google Scholar]
- Moreno, S., Klar, A. and Nurse, P. (1991). Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Meth. Enzymol. 194, 795-823. 10.1016/0076-6879(91)94059-l [DOI] [PubMed] [Google Scholar]
- Morris, Z., Sinha, D., Poddar, A., Morris, B. and Chen, Q. (2019). Fission yeast TRP channel Pkd2p localizes to the cleavage furrow and regulates cell separation during cytokinesis. Mol. Biol. Cell 30, 1791-1804. 10.1091/mbc.E18-04-0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, J., Cortes, J. C., Sipiczki, M., Ramos, M., Clemente-Ramos, J. A., Moreno, M. B., Martins, I. M., Perez, P. and Ribas, J. C. (2013). Extracellular cell wall β(1,3)glucan is required to couple septation to actomyosin ring contraction. J. Cell Biol. 203, 265-282. 10.1083/jcb.201304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli, S. M., Alenghat, F. J., Luo, Y., Williams, E., Vassilev, P., Li, X., Elia, A. E., Lu, W., Brown, E. M., Quinn, S. J.et al. (2003). Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129-137. 10.1038/ng1076 [DOI] [PubMed] [Google Scholar]
- Osumi, M., Konomi, M., Sugawara, T., Takagi, T. and Baba, M. (2006). High-pressure freezing is a powerful tool for visualization of Schizosaccharomyces pombe cells: ultra-low temperature and low-voltage scanning electron microscopy and immunoelectron microscopy. J. Electron Microsc. 55, 75-88. 10.1093/jmicro/dfl014 [DOI] [PubMed] [Google Scholar]
- Osumi, M., Sato, M., Ishijima, S. A., Konomi, M., Takagi, T. and Yaguchi, H. (1998). Dynamics of cell wall formation in fission yeast, Schizosaccharomyces pombe. Fungal Genet. Biol. 24, 178-206. 10.1006/fgbi.1998.1067 [DOI] [PubMed] [Google Scholar]
- Palmer, C. P., Aydar, E. and Djamgoz, M. B. (2005). A microbial TRP-like polycystic-kidney-disease-related ion channel gene. Biochem. J. 387, 211-219. 10.1042/BJ20041710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar, A., Sidibe, O., Ray, A. and Chen, Q. (2021). Calcium spikes accompany cleavage furrow ingression and cell separation during fission yeast cytokinesis. Mol. Biol. Cell 32, 15-27. 10.1091/mbc.E20-09-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, T. D. and Wu, J.-Q. (2010). Understanding cytokinesis: lessons from fission yeast. Nature Reviews 11, 149-155. 10.1038/nrm2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, S., Kume, K., Gupta, S., Ge, W., Balasubramanian, M., Hirata, D. and McCollum, D. (2010). The mitosis-to-interphase transition is coordinated by cross talk between the SIN and MOR pathways in Schizosaccharomyces pombe. J. Cell Biol. 190, 793-805. 10.1083/jcb.201002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimova, E., Sohrmann, M., Fournier, N. and Simanis, V. (2000). The S. pombe orthologue of the S. cerevisiae mob1 gene is essential and functions in signalling the onset of septum formation. J. Cell Sci. 113, 1695-1704. [DOI] [PubMed] [Google Scholar]
- Schmidt, S., Sohrmann, M., Hofmann, K., Woollard, A. and Simanis, V. (1997). The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes Dev. 11, 1519-1534. 10.1101/gad.11.12.1519 [DOI] [PubMed] [Google Scholar]
- Silverman-Gavrila, L. B. and Lew, R. R. (2003). Calcium gradient dependence of Neurospora crassa hyphal growth. Microbiology (Read.) 149, 2475-2485. 10.1099/mic.0.26302-0 [DOI] [PubMed] [Google Scholar]
- Simanis, V. (2015). Pombe's thirteen – control of fission yeast cell division by the septation initiation network. J. Cell Sci. 128, 1465-1474. 10.1242/jcs.094821 [DOI] [PubMed] [Google Scholar]
- Singh, N. S., Shao, N., McLean, J. R., Sevugan, M., Ren, L., Chew, T. G., Bimbo, A., Sharma, R., Tang, X., Gould, K. L.et al. (2011). SIN-inhibitory phosphatase complex promotes Cdc11p dephosphorylation and propagates SIN asymmetry in fission yeast. Curr. Biol. 21, 1968-1978. 10.1016/j.cub.2011.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J. T., Bokesch, H., Kenney, S. and Boyd, M. R. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82, 1107-1112. 10.1093/jnci/82.13.1107 [DOI] [PubMed] [Google Scholar]
- Sohrmann, M., Schmidt, S., Hagan, I. and Simanis, V. (1998). Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev. 12, 84-94. 10.1101/gad.12.1.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks, C. A., Morphew, M. and McCollum, D. (1999). Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis. J. Cell Biol. 146, 777-790. 10.1083/jcb.146.4.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson, K. S., Gow, N. A., Davidson, F. A. and Gadd, G. M. (2014). Regulation of vectorial supply of vesicles to the hyphal tip determines thigmotropism in Neurospora crassa. Fungal Biol 118, 287-294. 10.1016/j.funbio.2013.12.007 [DOI] [PubMed] [Google Scholar]
- Su, Q., Hu, F., Ge, X., Lei, J., Yu, S., Wang, T., Zhou, Q., Mei, C. and Shi, Y. (2018). Structure of the human PKD1-PKD2 complex. Science 361, eaat9819. 10.1126/science.aat9819 [DOI] [PubMed] [Google Scholar]
- Ta, C. M., Vien, T. N., Ng, L. C. T. and DeCaen, P. G. (2020). Structure and function of polycystin channels in primary cilia. Cell. Signal. 72, 109626. 10.1016/j.cellsig.2020.109626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., Huang, J., Padmanabhan, A., Bakka, K., Bao, Y., Tan, B. Y., Cande, W. Z. and Balasubramanian, M. K. (2011). Marker reconstitution mutagenesis: a simple and efficient reverse genetic approach. Yeast 28, 205-212. 10.1002/yea.1831 [DOI] [PubMed] [Google Scholar]
- Tatebe, H., Nakano, K., Maximo, R. and Shiozaki, K. (2008). Pom1 DYRK regulates localization of the Rga4 GAP to ensure bipolar activation of Cdc42 in fission yeast. Curr. Biol. 18, 322-330. 10.1016/j.cub.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz, P., Ruttimann, U. E. and Unser, M. (1998). A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27-41. 10.1109/83.650848 [DOI] [PubMed] [Google Scholar]
- Toda, T., Niwa, H., Nemoto, T., Dhut, S., Eddison, M., Matsusaka, T., Yanagida, M. and Hirata, D. (1996). The fission yeast sts5+ gene is required for maintenance of growth polarity and functionally interacts with protein kinase C and an osmosensing MAP-kinase pathway. J. Cell Sci. 109, 2331-2342. 10.1242/jcs.109.9.2331 [DOI] [PubMed] [Google Scholar]
- Verde, F., Wiley, D. J. and Nurse, P. (1998). Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc. Natl. Acad. Sci. U.S.A. 95, 7526-7531. 10.1073/pnas.95.13.7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volle, C. B., Ferguson, M. A., Aidala, K. E., Spain, E. M. and Nunez, M. E. (2008). Spring constants and adhesive properties of native bacterial biofilm cells measured by atomic force microscopy. Colloids Surf. B Biointerfaces 67, 32-40. 10.1016/j.colsurfb.2008.07.021 [DOI] [PubMed] [Google Scholar]
- Wachowicz, P., Chasapi, A., Krapp, A., Cano del Rosario, E., Schmitter, D., Sage, D., Unser, M., Xenarios, I., Rougemont, J. and Simanis, V. (2015). Analysis of S. pombe SIN protein association to the SPB reveals two genetically separable states of the SIN. J. Cell Sci. 128, 741-754. 10.1242/jcs.160150 [DOI] [PubMed] [Google Scholar]
- Watnick, T. J., Jin, Y., Matunis, E., Kernan, M. J. and Montell, C. (2003). A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr. Biol. 13, 2179-2184. 10.1016/j.cub.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Willet, A. H., DeWitt, A. K., Beckley, J. R., Clifford, D. M. and Gould, K. L. (2019). NDR Kinase Sid2 Drives Anillin-like Mid1 from the Membrane to Promote Cytokinesis and Medial Division Site Placement. Curr. Biol. 29, 1055-1063. 10.1016/j.cub.2019.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, C. R., Wang, Z., Diener, D., Zones, J. M., Rosenbaum, J. and Umen, J. G. (2012). IFT proteins accumulate during cell division and localize to the cleavage furrow in Chlamydomonas. PLoS One 7, e30729. 10.1371/journal.pone.0030729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J.-Q. and Pollard, T. D. (2005). Counting cytokinesis proteins globally and locally in fission yeast. Science (New York, NY) 310, 310-314. 10.1126/science.1113230 [DOI] [PubMed] [Google Scholar]
- Wu, J.-Q., Kuhn, J. R., Kovar, D. R. and Pollard, T. D. (2003). Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5, 723-734. 10.1016/S1534-5807(03)00324-1 [DOI] [PubMed] [Google Scholar]
- Wu, J.-Q., Sirotkin, V., Kovar, D. R., Lord, M., Beltzner, C. C., Kuhn, J. R. and Pollard, T. D. (2006). Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J. Cell Biol. 174, 391-402. 10.1083/jcb.200602032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, F.-X., Zhao, B. and Guan, K.-L. (2015). Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811-828. 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.