Abstract
Chronic stress has a deleterious effect on prefrontal lobe functioning. Empirical evidence suggests elevated vagal tone, indexed by elevated heart rate variability (HRV), mitigates the effect of mental stress on frontal lobe function. Here, the mitigating effect of HRV on stress-related decrements in cognitive performance is assessed based on information processing speed (DSST), word fluency and verbal learning task performance. Artifact free electrocardiogram (ECG) data was analyzed from 1420 Hispanic/Latino adults from the Sociocultural Ancillary of the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). A 12-lead ECG was used to collect shortterm recordings of the root mean square of successive differences in all normal R-peak to R-peak intervals (RMSSD) and the change between adjacent beats and the standard deviation of those intervals (SDNN) as indices of total HRV. As predicted, an interaction emerged for HRV and stress on the task presumed to require the greatest prefrontal lobe involvement, i.e., the DSST. After accounting for sociodemographic factors, chronic stress was associated with better DSST performance amongst individuals at higher quartile of SDNN, but not RMSSD. The paradoxical effect for greater stress exposure on DSST performance may in part be explained by increased speed of information processing and decision making often reported in high-stress cohorts. The nature of this interaction highlights the importance of examining the relationship between stress and cognition across a spectrum of vagal tone.
Keywords: Vagal tone, Information processing speed, Digit symbol substitution test, Autonomic regulation, Spanish-English verbal fluency, Ultra-short HRV
1. Introduction
Variability in the length of successive R-R intervals, i.e., heart rate variability (HRV) is a clinically-relevant index of the balance in sympathetic and parasympathetic nervous system (PNS), i.e., vagal, influence on cardiac pacemaker tissues (Malik, 1996; Shaffer et al., 2014). While the he root-mean square of differences between adjacent normal RR intervals (RMSSD) primarily indexes vagal activity the standard deviation of all normal RR intervals (SDNN) reflects primarily parasympathetic and some SNS influences. The index of elevated HRV that predominately reflects vagal influences is RMSSD (Cardiology, 1996b; Pumprla et al., 2002). This physiological state facilitates afferent feedback to neural structures that supports adaptive allocation of resources to manage internal and environmental demands (Porges, 1995, 2007). When HRV is probed, during emotional perturbation, high vagal tone predicts more flexible regulation of affective responses (Gianaros et al., 2004; Lane et al., 2001; Lane et al., 2008; Lane et al., 2009; Nugent et al., 2008). Importantly, these studies suggest that the flexible allocation of cardioautonomic resources may not only be dependent upon parasympathetic influences, but also the appropriate withdrawal of vagal tone.
Not only is elevated HRV conducive to emotional regulation but it is also shown to be instrumental in buffering the deleterious effect of stress on cognitive task performance (Appelhans and Luecken, 2006). The strongest evidence for the buffering effect of HRV during bouts of elevated stress is evident amongst tasks that probe prefrontal lobe function (Hansen et al., 2004; Thayer et al., 2012; Thayer et al., 2009; Thayer and Lane, 2000; Thayer and Sternberg, 2006). The vast majority of these studies reporting executive performance is sensitive to vagal tone have relied upon spectral indices of high frequency HRV. However, individual time domain indices of HRV have shown divergent effects within time- and between the frequency-domain on measures on executive performance. For example, while lower SDNN, was associated with just performing a more cognitively demanding task prior to, but not after a physiological stressor, i.e., intense bout of exercise (Luft et al., 2009). Another study found individuals with higher RMSSD, but not SDNN showed better performance on executive functioning (EF) tasks (Hansen et al., 2003). Efforts to elucidate the interactive effect of HRV on executive control tasks extend from the laboratory setting and are evident in population data. For example, a population-based study of women comparing time and frequency domain found higher SDNN and RMSSD, but not high frequency HRV were significantly associated with better performance on cognitive tests tapping inhibition, updating, shifting, and psychomotor speed, before adjusting for age (Stenfors et al., 2016). As age has a strong influence on HRV some of the divergent effects of vagal-mediated HRV are seemingly more apparent. More recently, a study of 24-h ECG recording revealed a correlation between SDNN and sympathetic-based frequency parameters of HRV, but not vagal-mediated RMSSD and high frequency, were associated with performance on two measures of global cognitive functioning in elderly outpatients (Dalise et al., 2020).
Chronic exposure to psychological stress negatively impacts structure and function of the neural substrates underpinning several domains of cognitive function (de Souza-Talarico et al., 2011; Juster et al., 2010; McEwen, 2008). In addition to executive function (EF) several other cognitive domains including episodic memory, visuospatial working memory, and attention show decrements with an individual’s cumulative exposure to psychological stress (E. Munoz et al. 2015; Peavy et al., 2009; Tschanz et al., 2013; Turner et al., 2017). It should be noted that African-Americans and Hispanics/Latinos report disparate levels of psychological stress (Jackson et al., 2004; McEwen and Gianaros, 2010) and age-related cognitive decline (Avison et al., 2007; Díaz-Venegas et al., 2016; Mayeda et al., 2016; Thoits, 2010; R. J. Turner and Avison, 2003) compared to their non-Hispanic White counterparts. However, Hispanics/Latinos are largely underrepresented in studies evaluating the role of HRV in stress and cognition. One of the few population-based studies to probe the association between HRV and cognitive function in Hispanics/Latinos, albeit in the absence of self-reported stress, is the Sacramento Area Latino Study on Aging. Within this sample of elderly Central- and Mexican-Americans lower scores on the Mini-Mental State exam were found in those with lower resting HRV than their high vagal tone counterparts (Al Hazzouri et al., 2014). Although compelling, the association between HRV and mental status within this older adult cohort may not generalize to the broader population of Hispanics/Latinos residing in the United States.
Given that the diverse ancestry of Hispanics/Latinos contributes to significant intragroup variability in cardio-autonomic tone (Kerr et al., 2017), the disproportionate exposure of this underrepresented group to psychosocial stress, and work from our group showing cognitive function varies as a function of psychological distress (Camacho et al., 2018), the current study tested the interactive effect of HRV and cumulative stress exposure on three domains of cognitive functioning within a nationally representative of Hispanics/Latinos. Two specific questions were addressed. First, is the self-reported burden of chronic psychological stress associated with performance on tests more dependent upon prefrontal lobe functioning? Second, does HRV, indexed by RMSSD and SDNN, mitigate the association between chronic stress and cognitive task performance such that at higher levels of HRV the effect of stress exposure on cognitive impairment is buffered? Utilizing these two indices of HRV will further delineate whether the buffering effects of HRV on the effect of chronic stress on cognitive function varies predominately as a function of the parasympathetic nervous system.
2. Methods
2.1. Study data
Data were collected as part of a multisite, community-based cohort study, i.e., the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). The study used a 2-stage area probability sample design that includes clustering, stratification, and probability weighting. Detailed sampling methods and discussion of study aims are published elsewhere (LaVange et al., 2010; Sorlie et al., 2010). The time frame for HCHS/SOL data collection was 2008 to 2011 and included sampling from field centers at 4 major U.S. cities (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA), with each site recruiting slightly more than 4000 participants. The HCHS/SOL was approved by institutional review boards at each field center, and all participants provided written consent.
2.2. Sociocultural ancillary study
All HCHS/SOL participants who were eligible and able to attend a second visit within 9 months of their baseline exam (N = 5313) participated in the Sociocultural Ancillary Study (SCAS). Methods for the study and a description of the participants as they relate to the main HCHS/SOL cohort are described elsewhere (Gallo et al., 2014). During the study, participants completed a 1 to 2-hour interview, in their preferred language (either English or Spanish), where psychosocial function was assessed. Institutional Review Board approval was obtained from all study sites for all HCHS/SOL SCAS procedures, and all participants provided written informed consent.
2.3. Exclusion criteria
To ensure the accuracy and quality of the HRV measures, participants without electrocardiogram (ECG) data or with poor quality ECG data, who evidenced a visual pattern of premature beats, and who had Wolff Parkinson-White syndrome, an atrial flutter or a Wenckebach pattern were excluded form analysis in this study. In addition, to be consistent with published recommendations for HRV measurement and interpretation individuals with artificial pacemakers, or individuals who reported taking calcium channel blockers, beta blockers, heart failure medication, antiarrhythmic or abnormal heart rhythm medication were excluded from our analyses (Cardiology, 1996a). Our analyses are based on data from 1420 participants who did not meet exclusion criteria (see Fig. 1).
Fig. 1. Exclusion criteria table.
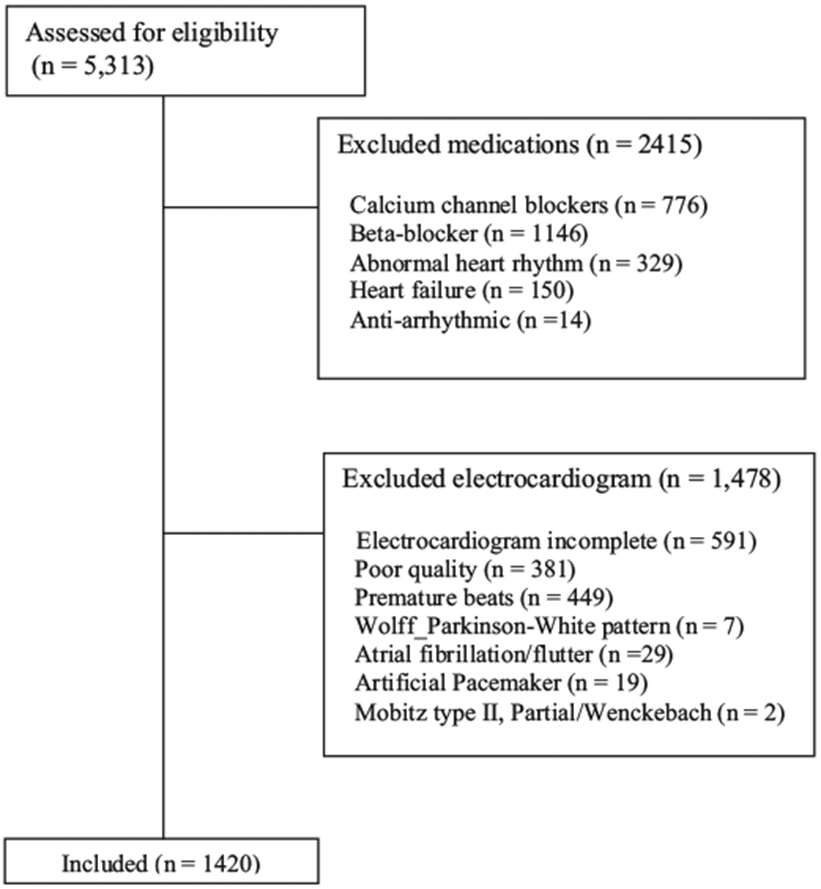
Note: Study flow chart showing the number of cases removed from the initial sample due to medication and electrocardiogram abnormality exclusionary criteria.
2.4. Neurocognitive tests
We use a battery of well-established measures, as described below, to assess cognitive function. The neurocognitive tests were administered in the participants’ preferred language during face-to-face interviews by study staff who were trained and supervised by doctorate-level, licensed, clinical neuropsychologists. With the exception of the B-SEVLT, which was originally developed for both English and Spanish use, the neurocognitive tests were translated from English to Spanish and back-translated from Spanish to English. All tests were administered following published recommendations and procedures (Lezak, 2004). Higher scores on all tests indicate better performance.
Digit Symbol Substitution test (DSST), a subtest of the Wechsler Adult Intelligence Scale-Revised, was administered to assess to measure processing speed and executive processes. Participants were asked to accurately translate digits (i.e., 1–9) into symbols using a key in a 90-second interval. The total number of accurate responses served as the dependent measure (Davis and Pierson, 2012). A host of processes employed in this task (e.g., scanning, matching, switching, and writing operations), are reflective of several higher cognitive functions including encoding and retrieval processes, decision making, as well as transformation of information stored in active memory (Bettcher et al., 2011; Salthouse, 1992). The DSST is shown to have a test-retest reliability from 0.82 to 0.88 (Matarazzo and Herman, 1984).
An abbreviated Controlled Oral Word Association (COWAT or Word Fluency; WF) Test was administered to assess verbal fluency (Lezak, 2004). Participants were instructed to orally generate as many unique words beginning with the letters F and A within 60 s for each letter stimulus. In the original WF task the letter S also serves as a stimulus; however, this letter was omitted because the similar sound of this letter to C in the Spanish language may be a source of language bias. Participants were asked to refrain from responding with conjugated words and proper nouns. The sum of correctly generated words with both letters served as the dependent measure. The WF test is reported to have a test-retest reliability of 0.79 (Cohen and Stanczak, 2000).
An abbreviated version of the Spanish English Verbal Learning Test (B-SEVLT) was administered to assess auditory verbal learning and memory (González et al., 2002; González et al., 2001). Three fixed learning trials of the B-SEVLT were administered (List A) prior to a 15-item distractor (List B) where participants were prompted to repeat aloud each word on the list in order to induce cognitive interference. Immediately following the distractor trial participants performed a short-delayed free-recall trial for List A. The dependent measures were the summed total number of List A items correctly recalled across the three learning trials (B-SEVLT_3Trials) and total from the delayed recall trial (B-SEVLT_Recall).
2.5. Chronic stress
Chronic stress burden was evaluated with an 8-item scale that assesses the number of current ongoing problems lasting 6-months or greater in major life domains, including: finance, work, relationship, health problems in self or close other, drug or alcohol problems in close other, caregiving, and other chronic stressors. Each domain was measured with a single item that determined presence, chronicity, and severity of the stressor. Participants indicate whether they experienced ongoing stress and if yes, 1) whether this stress persisted for 6 months or longer and 2) whether the situation was not very stressful (“1”), moderately stressful (“2”), or very stressful (“3”). A summary score (range 0 to 8) is generated that reflects presence, chronicity, and severity of the stress in the last six months’ duration that participants perceived to be moderately or very stressful. The total represents a count of stress in various life domains that may be unrelated. Thus, alpha coefficient estimates are not appropriate. However, this measure has been shown to correspond with indices of psychological distress when used in multi-ethnic cohort studies (Shivpuri et al., 2012; Troxel et al., 2003).
2.6. Heart rate variability
A standard 12-lead ECG was used to record 10 s epoch HRV at rest following a standardized protocol wherein participants were laid supine, asked to breathe freely, and refrain from talking. Electrodes were placed using a chest electrode locator by trained study personnel (Rautaharju et al., 1976). ECG data was recorded using the GE MAC 1200 electrograph (GE, Milwaukee, Wisconsin) with a 10 mm/mV calibration at a speed of 25 mm/s. All ECG data was centrally processed using the GE 12-SL Marquette Version 2001 (GE, Milwaukee, Wisconsin) at the Epidemiology Cardiology Research Center (EPICARE; Wake Forest School of Medicine, Winston Salem, NC). Data collected from all 12 leads were used to derive HRV based on an algorithm for ultra-short term recordings of the mean square of successive differences in all normal R to R intervals (RMSSD) as a marker of the degree RR interval changes between adjacent beats RMSSD (ms) = {[∑nj = 1(RRj + 1 − RRj)2] / n}0.5 and the standard deviation (SDNN) of all normal to normal R to R intervals as an index of total HRV, SDNN = (ms) = {[∑nj = 1(RRmean − RRj)2] / (n − 1)}0.5. The agreement between the gold standard 5-minute calculation of RMSSD and SDNN with ultra-short, i.e., 10-s, 30-s and 120-s recordings suggests high validity of these measures (M.L. Munoz et al., 2015). The correlation between RMSSD and SDNN was positive and significant (r = 0.879, p < .001).
2.7. Covariates
Based upon recommendations for neurocognitive assessment in general epidemiological research (Association, 2012) and further building on previously published neurocognitive research using HCHS/SOL data (González et al., 2014), sociocultural variables that are presumed to be associated with neurocognitive performance were accounted for. Age was measured as a continuous variable. Sex was measured dichotomously (0 = man; 1 = woman). Education was included as a three-category indicator (0 = less than high school; 1 = high school or equivalent degree; 2 = more than high school). Household income was included as a ten-category indicator ranging from <$10,000 to >$100,000. Nativity was included as a two-category indicator characterizing individuals born outside or in the U.S. (0 = U.S.; 1 = Outside the U.S.). For those born outside the U.S., analyses adjusted for years lived in the U.S. The participating center (Bronx, Chicago, Miami, San Diego) was a 4-level categorical variable and Hispanic/Latino background was a seven-level categorical indicator (Mexican, Cuban, Puerto Rican, Dominican, Central American, South American, other or more than one).
2.8. Statistical analysis
Linear regression models were estimated using the SAS SURVEYREG procedure, accounting for the sampling weights and the clustering and stratification design features. Each of four cognitive measures- DSS, WF, B-SEVLT-3Trials, and B-SEVLT-Recall - was specified as the outcome variable in separate models. For each outcome, the model included the aforementioned covariates, chronic stress, one indicator of HRV at a time, and the interaction between chronic stress and the HRV indicator. Unstandardized parameters and confidence intervals were estimated. All analyses were conducted using SAS version 9.4.
3. Results
3.1. Descriptive results
The mean age of our sample was 55 years (SD = 7 years), with 54% of the sample being female, and 42% who reported more than a high school education. The breakdown by background group was as follows: Dominican = 10%, Central American = 7%, Cuban = 27%, Mexican = 32%, Puerto Rican = 15%, and South American = 8%. Only 9% of the sample was born in the U.S and 51% had an annual household income of less than $20,000. The mean number of chronic stressors endorsed by the sample was 8, demonstrating high levels of stress. Finally, the means (SD) for HRV and cognitive function variables in our sample were as follows: RMSSD = 27.61 (19.43); SDNN = 24.07 (16.28); DSS = 35.04 (12.75); WF = 18.95 (7.12); B-SEVLT-3Trials = 22.75 (5.66); B-SEVLT-Recall = 8.27 (2.88).
3.2. Associations of chronic stress, HRV, and cognitive function
In the analytic subsample (n = 1420), several covariates were significantly associated with cognitive function. Surprisingly, chronic stress was not significantly associated with performance on any of the four cognitive function measures (DSST, -SEVLT-3Trials, or -SEVLT-Recall, or WF) in their respective models: DSST (b = 0.26, 95% CI −0.69–1.21, p = .59), B-SEVLT-3Trials (b = 0.30, 95% CI −0.32–0.93, p = .34), B-SEVLT-Recall: (b = 0.21, 95% CI −0.18–0.60, p = .29). A trend was found for WF (b = 0.44, p = .07, 95% CI −0.03–0.91).
With regard to HRV measures, neither RMSSD nor SDNN were significantly associated with DSST or SEVLT-Recall in their respective models: DSST [RMSSD (b = 0.02, 95% CI −0.02–0.06, p = .32); SDNN (b = 0.04, 95% CI −0.01–0.10, p = .11)]; SEVLT-Recall: [RMSSD (b = 0.00005, 95% CI −0.008–0.008, p = .99); SDNN (b = 0.001, 95% CI −0.009–0.01, p = .78)]. In contrast, both HRV measures were significantly associated with WF and B-SEVLT-3Trials in their separate models: WF [RMSSD (b = 0.03, 95% CI 0.01–0.05, p = .01); SDNN (b = 0.05, 95% CI 0.02–0.07, p = .001)]; B-SEVLT-3Trials: RMSSD (b = 0.03, 95% CI 0.01–0.05, p = .01); SDNN (b = 0.04, 95% CI 0.02–0.06, p < .001).
Strength of the association of each IV with performance for each task set was computed using Type II semi-partial correlation that reflects proportion of unique variance controlling for all other predictors in the model. Younger age, female sex, high school or greater education, higher income, U.S. born, and years in the U.S. were all significantly associated with better performance on the DSST (all ps < .05) in models that also included either RMSSD or SDNN. There were also background and center differences, as previously explored (Gallo et al., 2014). The full model, consisting of covariates, an HRV measure, and the chronic stress variable, explained nearly 40% of the variance in DSST scores. In contrast, in the WF models, only education and income were significantly associated with performance on WF (all ps < .05). The full model explained 22% of the variance in WF. In the SEVLT_3Trials model, age, sex, education, and income, were significant predictors (p < .05) and the full model explained 24% of the variance. Lastly, age, sex, education, and income were associated with SEVLT_Recall (p < .05), wherein 20% of the variance was explained. Table 2 displays there were no significant correlations between the continuous control variables and indices of RMSSD and SDNN. Not included in Table 2 were the categorical variables, including: nativity, background group, gender, education, income and center. For these variables, ANOVAs were performed yielding nonsignificant associations (ps > .05).
Table 2.
Correlations between continuous control variables and HRV.
| Variable | RMSSD | SDNN |
|---|---|---|
| Age | −0.10* | −0.05 |
| Years in U.S. | −0.02 | −0.04 |
| Chronic stress | 0.01 | 0.02 |
Note: Non-significant associations between the categorical demographic variables: (nativity, background group, gender, education, income and center), not included.
p < .05.
3.3. Interactions between chronic stress and HRV on cognitive function
With respect to our EF measure (DSST), the interaction between chronic stress and HRV was significant for both measures of HRV (Fig. 2). Estimates for RMSSD and SDNN, respectively, were b = 0.04 (95% CI 0.005–0.067), p = .02 and b = 0.057 (95% CI 0.010–0.104), p = .017. Table 1 shows the simple slopes for the association between chronic stress and DSST performance at each HRV quartile. For individuals at the highest quartile of RMSSD and SDNN the association between chronic stress and DSST performance became positive and significant (p < .001). Amongst those at the lowest HRV quartiles the association between stress and DSST was negative and significant (ps .03 to <.0001). With regard to other cognitive function measures, the interaction between chronic stress and HRV was not significant for WF (ps > .19), B-SEVLT-3Trials (ps > .64), or B-SEVLT-Recall (ps > .98). When analyzing metrics of HRV that are heavily influenced by respiratory sinus arrhythmia methodological considerations should be taken into account that may lead to discrepant findings and misinterpretation of data (Lewis et al., 2012). Most germane to the current study is ensuring that distributions of the measures conform to the assumption of normality. Upon transforming both HRV measures the chronic stress association with performance on the DSST was significantly moderated by the log transform of both SDNN b = 1.67 (95% CI 0.63–2.70), p = .0016 and RMSSD b = 2.13 (95% CI 0.67–3.60), p = .0043.
Fig. 2. Association between chronic stress and DSST performance based on HRV quartile.
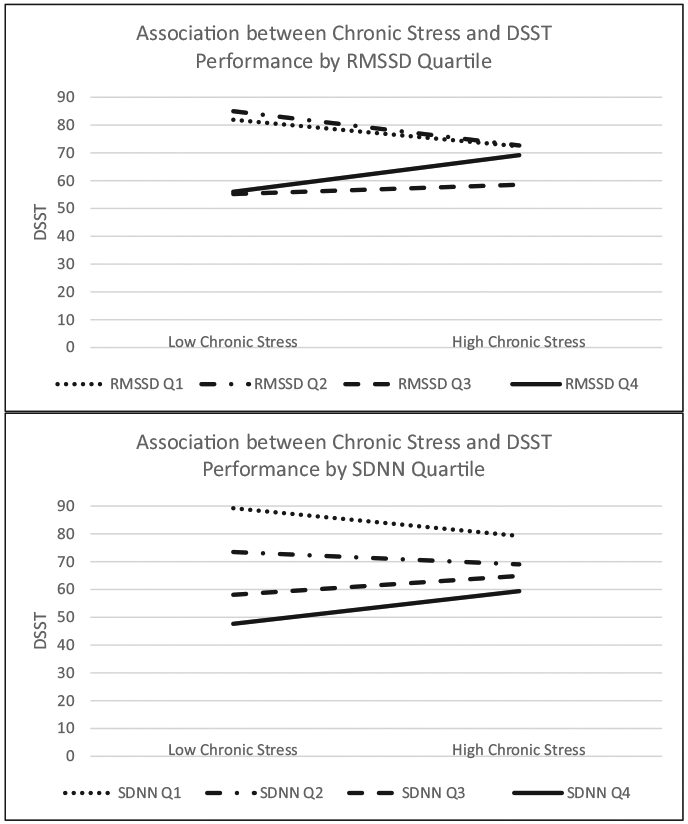
Note: HRV quartile difference in the association between high and low levels of chronic stress and performance on the DSST.
Table 1.
Interaction of chronic stress and performance on the Digit Symbol Substitution Test (DSST) at each HRV quartile.
| HRV quartile |
RMSSD | SDNN |
|---|---|---|
| Q1 | −1.37 (−2.62 to −0.11) p = .03 | −1.43 (−1.89 to −0.96) p <.001 |
| Q2 | −1.76 (−2.34 to −1.18) p <.001 | −0.06 (−1.79 to 0.52) p = .28 |
| Q2 | 0.48 (−0.28 to 1.24) p = .22 | 0.97 (−0.29 to 2.23) p = .97 |
| Q4 | 1.88 (1.08 to 2.68) p < .001 | 1.68 (0.94 to 2.43) p < .001 |
Note: Estimates, 95% confidence intervals and p-values for the interaction with chronic stress on DSST for each HRV quartile (N = 1420). At the highest quartile of RMSSD and SDNN the association between chronic stress and DSST performance became positive and significant. At the lowest HRV quartiles the association between stress and DSST was negative and significant (ps .03 to <.0001).
4. Discussion
In this subsample of Hispanic/Latino participants from the HCHS/SOL, the mitigating effect of HRV was tested for what was expected to be a negative association between stress and performance on a complex attention task requiring executive control. This inverse effect for stress exposure on DSST performance was largely obscured by sociodemographic variables that explained a large percentage of the variance in cognitive performance. These findings are consistent with extant literature in Hispanics/Latinos showing cognitive performance covaries with socioeconomic status (Haan et al., 2011; E. Munoz et al., 2015; Peavy et al., 2009; Tschanz et al., 2013; Turner et al., 2017; Zeki Al Hazzouri et al., 2011) and nativity outside the U.S. (Garcia et al., 2018; Haan et al., 2011; Hill et al., 2012; Sheffield and Peek, 2009; Weden et al., 2017). Nonetheless, a paradoxical effect was observed between stress exposure and DSST performance such that at higher SDNN greater stress was associated with more correct responses suggesting a buffering effect for vagal tone on the effect of life stressors on cognitive performance.
The DSST is widely regarded as an index of processing speed that requires coordination amongst a wide range of cognitive operations including attention, visuospatial perception and fine motor control (Lezak et al., 2004). Associative learning of stimulus pairings may enhance performance since there is less need to refer to the key to verify accuracy pairings. The decision to learn, hold and update symbol-digit pairings continuously during the task requires executive capabilities such as working memory, planning and strategizing (Jaeger, 2018; Jurado and Rosselli, 2007). In support of the executive bases of DSST performance both functional magnetic resonance imaging and near-infrared spectroscopy studies support the role of frontal lobes in performance speed (Nakahachi et al., 2008; Venkatraman et al., 2010). These studies suggest that although the DSST is widely considered a direct measure of processing speed faster performance on the task can be attributed to associative learning and efficient frontal lobe functioning.
A number of studies support the executive bases of what was a positive trend observed between SDNN and performance on the DSST. Empirical evidence commensurate with a positive effect for vagal-mediated HRV on enhanced working memory, updating, and complex attention is compelling (Hansen et al., 2004; Hansen et al., 2003; Thayer et al., 2009). Findings from a cohort of young adults from Spain showed greater decrements in working memory performance amongst those with lower HRV indexed by RMSSD (Luque-Casado et al., 2016). In a population-based study a positive association for SDNN and RMSSD on a composite measure of EF indexed by response inhibition, updating, set-shifting and psychomotor speed was found before adjusting for sociodemographic variables (Stenfors et al., 2016). Elevated HRV has also been linked to cognitive control on an interference task (Matthews et al., 2004). A cognitive process that might underlie this positive association found between SDNN and DSST observed in the current study is action cascading. Described as the ability to cope with multiple response options when confronted with an assortment of task goals action cascading is integral to performance on complex attention task such as the DSST. Indeed, superior action cascading ability is found in healthy adults with greater HRV (Colzato and Steenbergen, 2017). An alternate explanation of our data would implicate the vagal tone as a primary mediator of frontal lobe perfusion during working memory task performance (Gianaros et al., 2004).
The paradoxical effect of stress exposure on more efficient DSST performance amongst individuals with higher cardiac vagal control is difficult to reconcile considering the immense body of literature supporting a negative effect for stress exposure on cognitive function, particularly in the EF domain (Shields et al., 2016; Ohman et al., 2007). The theory of a non-linear relationship between an individual’s level of stress arousal and their performance on a cognitive task has been documented for over a century and further suggests stressors may induce shifts, lapses and narrowing of attention (Mendl, 1999; Yerkes and Dodson, 1908). Under high levels of stress arousal these adaptations may allow for a decision to be made before all relevant information has been assimilated, thus resulting in increased speed of information processing. Indeed, decision-making processes on the DSST are performed with greater speed amongst individuals reporting elevated levels of accumulated life stress (Friedel et al., 2017; Keinan, 1987). Although this behavioral speeding of the clock is typically error-prone individuals exhibiting greater prefrontal lobe activity during the DSST show less trade-off in speed to accuracy decrement than those showing frontal hypoactivity (Venkatraman et al., 2010; Yuan et al., 2013). In this same vein our findings at the higher levels of HRV can be interpreted as a biomarker of prefrontal lobe function and ultimately resilience to stress (Thayer et al., 2012; Thayer et al., 2009). In other words, amongst individuals with the greatest stress exposure, increased speed of information processing has an interactive effect with vagal-mediated prefrontal lobe function that contributes to a paradoxical effect for chronic stress on DSST performance. Other studies are in support of the interaction between chronic stress exposure, vagal tone and executive processes. A recent study comparing working memory ability to HRV in adults reporting high levels of life stress found better task performance amongst those with elevated vagal tone (Giuliano et al., 2017). Similarly, at higher levels of HRV, a paradoxical effect for psychological stress on inhibitory control was found in children with a history of maltreatment (Giuliano et al., 2018).
In lieu of a direct association between HRV and DSST performance an effect did emerge for word fluency. The finding of an association between HRV and performance on the word fluency as well as the learning & memory domain is novel and conflicts with a previous report comparing the mean circular resultant of R-R intervals with indices of word fluency and learning and memory (Al Hazzouri et al., 2014). Rather, this study of elderly Mexican Americans found a positive association with a global measure of cognitive functioning. Although vagal tone was presumed to most closely align with tasks involving prefrontal lobe functioning, i.e., DSST, other tasks may also tap this substrate when cognitive reserves are sufficiently taxed (Lupien et al., 2009). For example, functional neuroimaging studies suggest frontal lobe activity incurred during an English-Spanish language fluency task is commensurate with that evinced during task switching, cognitive set-shifting and other executive processes (De Baene et al., 2015; Declerck and Philipp, 2015). This effect may be culturally-specific to bilinguals given the absence of an effect for HRV on verbal fluency amongst predominately non-Hispanic Whites from the Whitehall study (Britton et al., 2008).
4.1. Limitations
The decision to exclude individuals based upon the detection of arrhythmias on the electrocardiogram and use of antiarrhythmic drugs (Fig. 1) is based upon standards of measurement, physiological interpretation, and clinical use recommended by the Task Force of The European Society of Cardiology and the North American Society for Pacing and Electrophysiology (Cardiology, 1996a, 1996b). As a result, over 70% of the sample was excluded due to medication or visually flagged issues in the electrocardiograph. Given the utilization of the ultra-short measure of HRV in the absence of the longer (≥5 min) recommended epochs (Cardiology, 1996a) these exclusionary criteria were deemed appropriate. Ultra-short HRV measures are shown to produce valid indices of HRV that are highly reproducible, particularly in the temporal domain (Baek et al., 2015; Bruyne et al., 1999; Salahuddin et al., 2007; Schroeder et al., 2004). Moreover, ultra-short HRV measures are increasingly used to monitor mental stress in mobile settings (Castaldo et al., 2015; Salahuddin et al., 2007). However, a caveat that should be noted is that the SDNN marker does not allow one to fully distinguish whether changes in HRV are incurred due to increased vagal tone of withdrawal of sympathetic influence (Niskanen et al., 2004; Novais et al., 2004). Because our ultrashort recordings of HRV reflect both parasympathetic and sympathetic influences, the specific mechanisms that lead to a buffering effect for stress on cognition remains unclear. With regards to the main outcome measures, the DSST has been considered a measure of controlled attention and processing speed; however, this task is also shown to tap the EF sub-domains of inhibition, shifting and updating, particularly in older adults (Albinet et al., 2012; Baudouin et al., 2009). Although the current study provides strong support for the mitigating effect of HRV on the relationship between stress and cognition, particularly for prefrontal lobe function, it is also possible that HRV is but a proxy for other indices of cardiovascular health (e.g., cardiorespiratory fitness, metabolic and vascular endothelial function) that contribute to cognitive decline in older age (Knopman et al., 2001; Leritz et al., 2011; Müller et al., 2017; Ng et al., 2014). Given the extensive associations observed between age and both the cognitive and autonomic variables future studies should aim to elucidate whether age may mediate the associations of these variables.
5. Conclusions
The current findings suggest that at higher levels of HRV, there is a paradoxical association between the incidence of life stress and information processing speed. This supports the transdiagnostic account of cardiovagal tone as a marker of prefrontal lobe function (Beauchaine and Thayer, 2015; Thayer et al., 2009). The inverse effect for stress on processing speed observed at lower HRV quartiles is corresponds with the majority of extent neurocognitive research.. Thus, this study highlights the importance of examining these stress-cognition relationships across the spectrum of HRV. Because this study excluded individuals not identifying as Hispanic/Latino, it is unclear whether this phenomena is unique to this population. U.S. Hispanics/Latinos have very diverse ancestry ranging from European to African (Bryc et al., 2010), thus a tremendous ammount of intragroup variability in racial background is evidenced within this cohort. As to the question of whether genetic ancestry plays a role in these findings, a recent multi-cohort study examining single nucleotide polymorphisms from the HCHS/SOL, Multi-Ethnic Study of Atherosclerosis, and Women’s Health Initiative Hispanic Research project revealed a common variant for RMSSD and SDNN in those Hispanics/Latinos with European ancestry (Kerr et al., 2017). Given the established effects for established cardiometabolic (Meyer et al., 2016) and genetic risk factors (Kerr et al., 2017) in predicting ultrashort indices of HRV amongst Hispanics/Latinos a more integrated allostiatic model is needed to properly understand buffering mechanisms for the effect of chronic stress burden on neurocognitive outcomes in this population.
Acknowledgements
HCHS/SOL support, plus Drs. González and Tarraf are supported by R01AG048642 and RF1AG054548. Dr. McIntosh is supported by K01HL139722.
References
- Al Hazzouri AZ, Haan MN, Deng Y, Neuhaus J, Yaffe K, 2014. Reduced heart rate variability is associated with worse cognitive performance in elderly Mexican Americans. Hypertension 63 (1), 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albinet CT, Boucard G, Bouquet CA, Audiffren M, 2012. Processing speed and executive functions in cognitive aging: how to disentangle their mutual relationship? Brain Cogn. 79 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ, 2006. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol 10 (3), 229. [Google Scholar]
- Association, A. P, 2012. Guidelines for the evaluation of dementia and age-related cognitive change. The American psychologist 67 (1), 1. [DOI] [PubMed] [Google Scholar]
- Avison WR, Ali J, Walters D, 2007. Family structure, stress, and psychological distress: a demonstration of the impact of differential exposure. J. Health Soc. Behav 48 (3), 301–317. [DOI] [PubMed] [Google Scholar]
- Baek HJ, Cho C-H, Cho J, Woo J-M, 2015. Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemedicine and e-Health 21 (5), 404–414. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Clarys D, Vanneste S, Isingrini M, 2009. Executive functioning and processing speed in age-related differences in memory: contribution of a coding task. Brain Cogn. 71 (3), 240–245. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF, 2015. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int. J. Psychophysiol 98 (2), 338–350. [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Libon DJ, Kaplan E, Swenson R, Penney DL, 2011. Digit symbol substitution test. In: Encyclopedia of Clinical Neuropsychology. Springer, pp. 849–853. [Google Scholar]
- Britton A, Singh-Manoux A, Hnatkova K, Malik M, Marmot MG, Shipley M, 2008. The association between heart rate variability and cognitive impairment in middle-aged men and women. Neuroepidemiology 31 (2), 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyne M.C.d., Kors JA, Hoes AW, Klootwijk P, Dekker JM, Hofman A, Grobbee DE, 1999. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. American journal of epidemiology 150 (12), 1282–1288. [DOI] [PubMed] [Google Scholar]
- Bryc K, Velez C, Karafet T, Moreno-Estrada A, Reynolds A, Auton A, Ostrer H, 2010. Genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proceedings of the National Academy of Sciences 107 (Supplement 2), 8954–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A, Tarraf W, Jimenez DE, Gallo LC, Gonzalez P, Kaplan RC, Perreira KM, 2018. Anxious depression and neurocognition among middle-aged and older Hispanic/Latino adults: Hispanic community health study/study of Latinos (HCHS/SOL) results. The American Journal of Geriatric Psychiatry 26 (2), 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiology, T. F. o. t. E. S. o, 1996a. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93, 1043–1065. [PubMed] [Google Scholar]
- Cardiology, T. F. o. t. E. S. o, 1996b. The North American Society of Pacing and Electrophysiology (1996) heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93 (5), 1043–1065. [PubMed] [Google Scholar]
- Castaldo R, Melillo P, Pecchia L, 2015. Acute Mental Stress Detection via Ultra-short Term HRV Analysis. Paper Presented at the World Congress on Medical Physics and Biomedical Engineering, June 7–12, 2015, Toronto, Canada. [Google Scholar]
- Cohen MJ, Stanczak DE, 2000. On the reliability, validity, and cognitive structure of the Thurstone Word Fluency Test. Arch. Clin. Neuropsychol 15 (3), 267–279. [PubMed] [Google Scholar]
- Colzato LS, Steenbergen L, 2017. High vagally mediated resting-state heart rate variability is associated with superior action cascading. Neuropsychologia 106, 1–6. [DOI] [PubMed] [Google Scholar]
- Dalise AM, Prestano R, Fasano R, Gambardella A, Barbieri M, Rizzo MR, 2020. Autonomic nervous system and cognitive impairment in older patients: evidence from long-term heart rate variability in real-life setting. Front. Aging Neurosci 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AS, Pierson EE, 2012. The relationship between the WAIS-III digit symbol coding and executive functioning. Applied Neuropsychology: Adult 19 (3), 192–197. [DOI] [PubMed] [Google Scholar]
- De Baene W, Duyck W, Brass M, Carreiras M, 2015. Brain circuit for cognitive control is shared by task and language switching. J. Cogn. Neurosci 27 (9), 1752–1765. [DOI] [PubMed] [Google Scholar]
- de Souza-Talarico JN, Marin M-F, Sindi S, Lupien SJ, 2011. Effects of stress hormones on the brain and cognition: evidence from normal to pathological aging. Dementia & Neuropsychologia 5 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declerck M, Philipp AM, 2015. A review of control processes and their locus in language switching. Psychon. Bull. Rev 22 (6), 1630–1645. [DOI] [PubMed] [Google Scholar]
- Díaz-Venegas C, Downer B, Langa KM, Wong R, 2016. Racial and ethnic differences in cognitive function among older adults in the USA. International journal of geriatric psychiatry 31 (9), 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel E, Sebold M, Kuitunen-Paul S, Nebe S, Veer IM, Zimmermann US, Walter H, 2017. How accumulated real life stress experience and cognitive speed interact on decision-making processes. Frontiers in human neuroscience 11, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Penedo FJ, Carnethon M, Isasi C, Sotres-Alvarez D, Malcarne VL, Gonzalez P, 2014. The Hispanic community health study/study of Latinos sociocultural ancillary study: Sample, design, and procedures. Ethnicity & disease 24 (1), 77. [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Saenz J, Downer B, Wong R, 2018. The role of education in the association between race/ethnicity/nativity, cognitive impairment, and dementia among older adults in the United States. Demogr. Res 38, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Van der Veen FM, Jennings JR, 2004. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology 41 (4), 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano RJ, Gatzke-Kopp LM, Roos LE, Skowron EA, 2017. Resting sympathetic arousal moderates the association between parasympathetic reactivity and working memory performance in adults reporting high levels of life stress. Psychophysiology 54 (8), 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano RJ, Roos LE, Farrar JD, Skowron EA, 2018. Cumulative risk exposure moderates the association between parasympathetic reactivity and inhibitory control in preschool-age children. Dev. Psychobiol 60 (3), 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González HM, Mungas D, Reed BR, Marshall S, Haan MN, 2001. A new verbal learning and memory test for English-and Spanish-speaking older people. J. Int. Neuropsychol. Soc 7 (5), 544–555. [DOI] [PubMed] [Google Scholar]
- González HM, Mungas D, Haan MN, 2002. A verbal learning and memory test for English-and Spanish-speaking older Mexican-American adults. Clin. Neuropsychol 16 (4), 439–451. [DOI] [PubMed] [Google Scholar]
- González HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, Catellier DJ, 2014. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Archives of Clinical Neuropsychology 30 (1), 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Zeki Al-Hazzouri A, Aiello AE, 2011. Life-span socioeconomic trajectory, nativity, and cognitive aging in Mexican Americans: the Sacramento Area Latino Study on Aging. Journals of Gerontology Series B: Psychological Sciences and Social Sciences 66 (suppl_1), i102–i110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF, 2003. Vagal influence on working memory and attention. Int. J. Psychophysiol 48 (3), 263–274. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Sollers JJ, Stenvik K, Thayer JF, 2004. Heart rate variability and its relation to prefrontal cognitive function: the effects of training and detraining. Eur. J. Appl. Physiol 93 (3), 263–272. [DOI] [PubMed] [Google Scholar]
- Hill TD, Angel JL, Balistreri KS, 2012. Does the “healthy immigrant effect” extend to cognitive aging?. In: Aging, Health, and Longevity in the Mexican-Origin Population. Springer, pp. 19–33. [Google Scholar]
- Jackson JS, Torres M, Caldwell CH, Neighbors HW, Nesse RM, Taylor RJ, Williams DR, 2004. The National Survey of American Life: a study of racial, ethnic and cultural influences on mental disorders and mental health. International journal of methods in psychiatric research 13 (4), 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, 2018. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol 38 (5), 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M, 2007. The elusive nature of executive functions: a review of our current understanding. Neuropsychol. Rev 17 (3), 213–233. [DOI] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, Lupien SJ, 2010. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev 35 (1), 2–16. [DOI] [PubMed] [Google Scholar]
- Keinan G, 1987. Decision making under stress: scanning of alternatives under controllable and uncontrollable threats. J. Pers. Soc. Psychol 52 (3), 639. [DOI] [PubMed] [Google Scholar]
- Kerr KF, Avery CL, Lin HJ, Raffield LM, Zhang QS, Browning BL, Laurie CC, 2017. Genome-wide association study of heart rate and its variability in Hispanic/Latino cohorts. Heart rhythm 14 (11), 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D, Boland L, Mosley T, Howard G, Liao D, Szklo M, Investigators, A. R. i. C. S, 2001. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 56 (1), 42–48. [DOI] [PubMed] [Google Scholar]
- Lane R, Reiman E, Ahern G, Thayer J, 2001. 21. Activity in medial prefrontal cortex correlates with vagal component of heart rate variability during emotion. Brain Cogn. 47 (1–2), 97–100. [Google Scholar]
- Lane R, Weidenbacher H, Fort C, Thayer J, Allen J, 2008. Subgenual anterior cingulate (BA25) activity covaries with changes in cardiac vagal tone during affective set shifting in healthy adults. Psychosomatic medicine 70, A–42. [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF, 2009. Neural correlates of heart rate variability during emotion. Neuroimage 44 (1), 213–222. [DOI] [PubMed] [Google Scholar]
- LaVange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Ryan J, 2010. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Annals of epidemiology 20 (8), 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, McGlinchey RE, Kellison I, Rudolph JL, Milberg WP, 2011. Cardiovascular disease risk factors and cognition in the elderly. Current cardiovascular risk reports 5 (5), 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Furman SA, McCool MF, Porges SW, 2012. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent?. Biol. Psychol 89 (2), 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, 2004. Neuropsychological Assessment: Oxford University Press. USA. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Fischer JS, 2004. Neuropsychological Assessment: Oxford University Press. USA. [Google Scholar]
- Luft CDB, Takase E, Darby D, 2009. Heart rate variability and cognitive function: effects of physical effort. Biol. Psychol 82 (2), 186–191. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci 10 (6), 434. [DOI] [PubMed] [Google Scholar]
- Luque-Casado A, Perales JC, Cárdenas D, Sanabria D, 2016. Heart rate variability and cognitive processing: the autonomic response to task demands. Biol. Psychol 113, 83–90. [DOI] [PubMed] [Google Scholar]
- Malik M, 1996. Task force of the European society of cardiology and the north American society of pacing and electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17, 354–381. [PubMed] [Google Scholar]
- Matarazzo JD, Herman DO, 1984. Base rate data for the WAIS-R: test-retest stability and VIQ-PIQ differences. J. Clin. Exp. Neuropsychol 6 (4), 351–366. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE, 2004. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage 22 (3), 1151–1156. [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA, 2016. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 12 (3), 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 2008. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol 583 (2), 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ, 2010. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci 1186 (1), 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendl M, 1999. Performing under pressure: stress and cognitive function. Appl. Anim. Behav. Sci 65 (3), 221–244. [Google Scholar]
- Meyer ML, Gotman NM, Soliman EZ, Whitsel EA, Arens R, Cai J, Moreiras J, 2016. Association of glucose homeostasis measures with heart rate variability among Hispanic/Latino adults without diabetes: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Cardiovascular diabetology 15 (1), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Chan K, Myers JN, 2017. Association Between Exercise Capacity and Late Onset of Dementia, Alzheimer Disease, and Cognitive Impairment. Paper Presented at the Mayo Clinic Proceedings. [DOI] [PubMed] [Google Scholar]
- Munoz E, Sliwinski MJ, Scott SB, Hofer S, 2015a. Global perceived stress predicts cognitive change among older adults. Psychol. Aging 30 (3), 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz ML, van Roon A, Riese H, Thio C, Oostenbroek E, Westrik I, Nolte IM, 2015b. Validity of (ultra-) short recordings for heart rate variability measurements. PLoS One 10 (9), e0138921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahachi T, Ishii R, Iwase M, Canuet L, Takahashi H, Kurimoto R, Honaga E, 2008. Frontal activity during the digit symbol substitution test determined by multichannel near-infrared spectroscopy. Neuropsychobiology 57 (4), 151–158. [DOI] [PubMed] [Google Scholar]
- Ng T, Cheung YT, Ng QS, Ho HK, Chan A, 2014. Vascular endothelial growth factor inhibitors and cognitive impairment: evidence and controversies. Expert Opin. Drug Saf 13 (1), 83–92. [DOI] [PubMed] [Google Scholar]
- Niskanen J-P, Tarvainen MP, Ranta-Aho PO, Karjalainen PA, 2004. Software for advanced HRV analysis. Comput. Methods Prog. Biomed 76 (1), 73–81. [DOI] [PubMed] [Google Scholar]
- Novais L, Sakabe D, Takahashi A, Gongora H, Taciro C, Martins L, Catai A, 2004. Avaliação da variabilidade da frequencia cardíaca em repouso de homens saudáveis sedentários e de hipertensos e coronariopatias em treinamento físico. Braz. j. phys. ther.(Impr.) 207–213. [Google Scholar]
- Nugent AC, Bain EE, Thayer JF, Sollers JJ, Drevets WC, 2008. Alterations in neural correlates of autonomic control in females with major depressive disorder. Int. J. Psychophysiol 69 (3), 195. [Google Scholar]
- Ohman L, Nordin S, Bergdahl J, Slunga LB, Stigsdotter AN, 2007. Cognitive function in outpatients with perceived chronic stress. Scand. J. Work Environ. Health 33 (3), 223–232. [DOI] [PubMed] [Google Scholar]
- Peavy GM, Salmon DP, Jacobson MW, Hervey A, Gamst AC, Wolfson T, Khandrika S, 2009. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. American Journal of Psychiatry 166 (12), 1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, 1995. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology 32 (4), 301–318. [DOI] [PubMed] [Google Scholar]
- Porges SW, 2007. The polyvagal perspective. Biol. Psychol 74 (2), 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumprla J, Howorka K, Groves D, Chester M, Nolan J, 2002. Functional assessment of heart rate variability: physiological basis and practical applications. Int. J. Cardiol 84 (1), 1–14. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM, Wolf HK, Eifler WJ, Blackburn H, 1976. A simple procedure for positioning precordial ECG and VCG electrodes using an electrode locator. J. Electrocardiol 9 (1), 35–40. [DOI] [PubMed] [Google Scholar]
- Salahuddin L, Cho J, Jeong MG, Kim D, 2007. Ultra Short Term Analysis of Heart Rate Variability for Monitoring Mental Stress in Mobile Settings. Paper Presented at the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, 1992. What do adult age differences in the Digit Symbol Substitution Test reflect? J. Gerontol 47 (3), P121–P128. [DOI] [PubMed] [Google Scholar]
- Schroeder EB, Whitsel EA, Evans GW, Prineas RJ, Chambless LE, Heiss G, 2004. Repeatability of heart rate variability measures. J. Electrocardiol 37 (3), 163–172. [DOI] [PubMed] [Google Scholar]
- Shaffer F, McCraty R, Zerr CL, 2014. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front. Psychol 5, 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield KM, Peek MK, 2009. Neighborhood context and cognitive decline in older Mexican Americans: results from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. Am. J. Epidemiol 169 (9), 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, Yonelinas AP, 2016. The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev 68, 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivpuri S, Gallo LC, Crouse JR, Allison MA, 2012. The association between chronic stress type and C-reactive protein in the multi-ethnic study of atherosclerosis: does gender make a difference? J. Behav. Med 35 (1), 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Allison M, 2010. Design and implementation of the Hispanic community health study/study of Latinos. Annals of epidemiology 20 (8), 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfors CU, Hanson LM, Theorell T, Osika WS, 2016. Executive cognitive functioning and cardiovascular autonomic regulation in a population-based sample of working adults. Frontiers in Psychology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord 61 (3), 201–216. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E, 2006. Beyond heart rate variability. Ann. N. Y. Acad. Sci 1088 (1), 361–372. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH, 2009. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med 37 (2), 141–153. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ III, Wager TD, 2012. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev 36 (2), 747–756. [DOI] [PubMed] [Google Scholar]
- Thoits PA, 2010. Stress and health: major findings and policy implications. Journal of Health and Social Behavior 51 (1_suppl), S41–S53. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K, 2003. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 22 (3), 300. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Pfister R, Wanzek J, Corcoran C, Smith K, Tschanz BT, Norton MC, 2013. Stressful life events and cognitive decline in late life: moderation by education and age. The Cache County Study. International journal of geriatric psychiatry 28 (8), 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Avison WR, 2003. Status variations in stress exposure: implications for the interpretation of research on race, socioeconomic status, and gender. J. Health Soc. Behav 488–505. [PubMed] [Google Scholar]
- Turner AD, James BD, Capuano AW, Aggarwal NT, Barnes LL, 2017. Perceived stress and cognitive decline in different cognitive domains in a cohort of older African Americans. Am. J. Geriatr. Psychiatry 25 (1), 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman VK, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, Williamson J, 2010. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage 49 (4), 3436–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weden MM, Miles JN, Friedman E, Escarce JJ, Peterson C, Langa KM, Shih RA, 2017. The Hispanic paradox: race/ethnicity and nativity, immigrant enclave residence and cognitive impairment among older US adults. J. Am. Geriatr. Soc 65 (5), 1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD, 1908. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol 18 (5), 459–482. [Google Scholar]
- Yuan R, Di X, Kim EH, Barik S, Rypma B, Biswal BB, 2013. Regional homogeneity of resting-state fMRJ contributes to both neurovascular and task activation variations. Magn. Reson. Imaging 31 (9), 1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A, Haan MN, Galea S, Aiello AE, 2011. Life-course exposure to early socioeconomic environment, education in relation to late-life cognitive function among older Mexicans and Mexican Americans. Journal of aging and health 23 (7), 1027–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]


