Abstract

Ischemic stroke is a complex systemic disease characterized by high morbidity, disability, and mortality. The activation of the presynaptic adenosine A2A and A1 receptors modifies a variety of brain insults from excitotoxicity to stroke. Therefore, the discovery of dual A2A/A1 adenosine receptor (AR)-targeting therapeutic compounds could be a strategy for the treatment of ischemic stroke. Inspired by two clinical phase III drugs, ASP-5854 (dual A2A/A1 AR antagonist) and preladenant (selective A2A AR antagonist), and using the hybrid medicinal strategy, we characterized novel pyridone-substituted triazolopyrimidine scaffolds as dual A2A/A1 AR antagonists. Among them, compound 1a exerted excellent A2A/A1 AR binding affinity (Ki = 5.58/24.2 nM), an antagonistic effect (IC50 = 5.72/25.9 nM), and good metabolic stability in human liver microsomes, rat liver microsomes, and dog liver microsomes. Importantly, compound 1a demonstrated a dose–effect relationship in the oxygen-glucose deprivation/reperfusion (OGD/R)-treated HT22 cell model. These findings support the development of dual A2A/A1 AR antagonists as a potential treatment for ischemic stroke.
Keywords: Ischemic stroke, dual A2A/A1 AR antagonist, pyridone-substituted triazolopyrimidine
Adenosine, the naturally occurring purine nucleoside, acts as an endogenous modulator in both the central and peripheral nervous systems by interacting with four subtypes of specific G-protein-coupled receptors (GPCRs): A1, A2A, A2B, and A3 adenosine receptors (ARs). The A1 and A3 ARs are negatively coupled to adenylyl cyclase and exert an inhibitory effect on cyclic adenosine monophosphate (cAMP) production by recruiting the Gi protein, whereas the A2A and A2B ARs promote adenylyl cyclase activation and subsequent cAMP production by recruiting the Gs protein.1−4 The A2A and A1 ARs are highly enriched in specific parts of the central nervous system (CNS) and are associated with motor activity, psychiatric behaviors, and neuronal cell death. Many potent selective A2A AR antagonists have been designed as promising candidates for their beneficial effects on Parkinson’s disease (PD),5 ischemia,6 epilepsy,7 Huntington’s disease (HD),8 and Alzheimer’s disease (AD).9
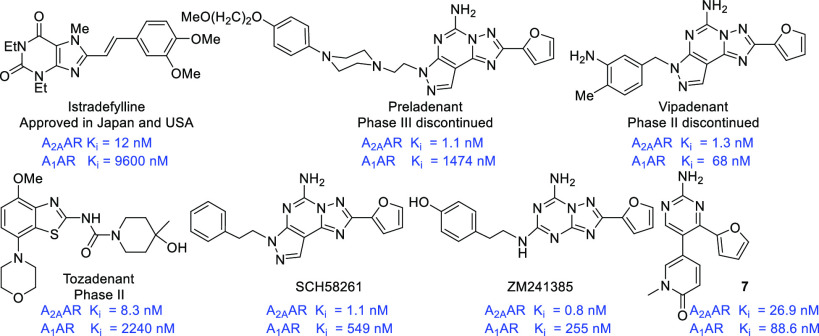
Over the past three decades, the search for novel A2A AR antagonists has been greatly expanded, and a large number of drug candidates have entered clinical trials (Figure 1). Unfortunately, only istradefylline (KW-6002) has been licensed as an antiparkinsonian drug in Japan (2013) and the United States (2019);10 other A2A AR antagonists (preladenant,11 vipadenant,12 tozadenant,13 etc.) were terminated because of a lack of efficacy in vivo or toxicity.14 Recently, the A2A AR emerged as a novel immune checkpoint for the development of a cancer immunotherapy drug in combination with PD-1/PD-L1 or anti-CTLA-4 monoclonal antibody (mAb).15,16 ZM241385 significantly inhibited tumor growth in a lung metastasis model and induced a remarkable delay in tumor growth in melanoma-bearing mice when combined with anti-CTLA-4 mAb.17,18 Moreover, the discovery and development of dual Ax AR therapeutic compounds is an attractive and alternative therapeutic strategy for improving the in vivo efficacy of a single target. For example, AB928, a potent and selective dual A2A/A2B AR antagonist discovered by Arcus Biosciences, is currently undergoing clinical trials in multiple cancer settings.19,20
Figure 1.
Reported selective A2A and dual A2A/A1 AR antagonists.
Ischemic stroke is a complex systemic disease characterized by high morbidity, disability, and mortality.21 Increasing substantial evidence has shown a protective role for A2A AR antagonists in striatal and nigral neurons through the prevention of glutamate-dependent neuronal death, thereby reducing cortical damage in a variety of ischemic stroke models.22−24 In A2A AR knockout (KO) mice, transient focal ischemia causes less neuronal damage compared with that in wild-type (WT) mice. The selective A2A AR antagonist SCH58261 reduced ischemic brain damage in an adult rat model of focal cerebral ischemia.25−27 Meanwhile, the activation of the A1 AR was able to induce ischemic damage protection and the reduction of both reactive and proliferative microglia/macrophages after experimental stroke in rats.28,29 These results demonstrate that the activation of the presynaptic A2A and A1 ARs modifies a variety of brain insults from excitotoxicity to stroke.
Owing to our interest in ARs and the field of ischemic stroke, we set out to design and synthesize novel dual A2A/A1 AR antagonists based on the crystal structures of A2A AR (PDB: 3EML) and A1 AR (PDB: 5EUN) complexes.30,31 We herein report the discovery and characterization of a new chemotype of dual A2A/A1 AR antagonists with a pyridone-substituted triazolopyrimidine scaffold, in which compound 1a demonstrated a remarkable dose–effect relationship in the oxygen-glucose deprivation/reperfusion (OGD/R)-treated HT22 cell model.
Our initial design was inspired by two known clinical phase III drug candidates, preladenant (selective A2A AR antagonist), with a triazolopyrimidine scaffold,11 and ASP-5854 (dual A2A/A1 AR antagonist), with a pyrazine scaffold32 (Figure 2). Using insight from preladenant cocrystal structures with an A2A AR and an A1 AR, we noticed that the primary amide (ring A) and the triazole (ring B) with furan rings in preladenant established two bidentate hydrogen-bonding interactions with Glu169 and Asn253 (Figure 2A,B) with similar binding modes. However, preladenant is just a selective A2A AR antagonist, suggesting that the triazolopyrimidine scaffold was a key pharmacophore as the selective A2A AR antagonist. The primary amide in ASP-5854 also formed a bidentate hydrogen-bonding interaction with Asn253, in which the A2A AR was the key pharmacophore. Moreover, the pyridone in ASP-5854 formed an additional hydrogen bond with His278 in the A2A AR. By contrast, when docked with A1 AR, the pyridone in ASP-5854 formed a hydrogen bond with Thr90 in the A1 AR, which may be a key pharmacophore as the A1 AR antagonist. On the basis of these analyses, we intended to exploit the hybrid drug design approach to access all five aforementioned interactions with the goal of identifying a novel chemical scaffold dual A2A/A1 AR antagonist with better drug-like properties. Therefore, we designed and synthesized a series of compounds with the novel pyridone-substituted triazolopyrimidine chemotype and carried out a systematic study of structure–activity relationships (SARs).
Figure 2.

Design strategy of novel chemotype dual A2A/A1 AR antagonists. (A) Interactions shown between preladenant with A2A AR (A1) and A1 AR (A2). (B) Interactions shown between ASP-5854 with A2A AR (B1) and A1 AR (B2). (C) Hybridization strategy of novel pyridone-substituted triazolopyrimidine scaffold.
As shown in Scheme 1, the synthetic strategy of 1a–1i involved a three-step sequence, including a nucleophilic substitution reaction, Dimroth rearrangement, and a Suzuki coupling process, starting from commercially available aryl formamide derivatives 2. Initially, the nucleophilic substitution of 5-bromo-4-chloropyrimidin-2-amine (3) by aryl formamide 2a–2i at 120 °C in n-butanol proceeded smoothly to deliver compounds 4a–4i. The subsequent Dimroth rearrangement of compounds 4a–4i was conducted in the presence of N,O-bis(trimethylsilyl) acetamide (BSA) and hexamethyldisilazane (HMDS) at 120 °C to give the desired cyclization triazolopyrimidine compounds 5a–5i. Finally, compounds 5a–5i were coupled to pyridone boronic esters (6) to afford the final products 1a–1i in an acceptable yield (4.1–6.5%) over three steps.
Scheme 1.
Reagents and conditions: (a) n-BuOH, 120 °C, 8 h; (b) HMDS, BSA, 120 °C, 8 h; (c) Pd(dppf)Cl2, K2CO3, dioxane, H2O, 90 °C, 8 h, 4.1–6.5%, three steps.
The binding affinity of the synthesized pyridone-substituted triazolopyrimidine derivatives (1a–1i) toward the A2A and A1 ARs, along with A2B and A3 ARs, was evaluated in competitive binding experiments using membrane preparation of the human recombinants A1, A2A and A3, and the A2B AR overexpressed from CHO, HeLa, and HEK-293 cells, respectively. [3H] DPCPX (A1), [3H]ZM241385 (A2A), [3H]DPCPX (A2B), and [3H]NECA (A3) were used as radioligands.33 The binding affinity data of synthesized compounds are listed in Table 1, with pyrazine antagonist compound 7 chosen as the reference. Among them, compound 1a with a furan ring as the Ar group exhibited the most excellent binding affinity with a Ki value of 5.58 nM against the A2A AR and 24.2 nM against the A1 AR and a high degree of selectivity for the A2B AR (A2B/A2A 88-fold) and the A3 AR (A3/A2A 1575-fold), respectively. Compounds 1b (pyridine as the Ar group) and 1c (thiazole as the Ar group) showed moderate binding affinity against the A2A AR (Ki = 62.4 nM) and comparable binding activity against the A1 AR (Ki = 84.6 nM), along with good selectivity over the A2B and A3 ARs. Interestingly, compound 1d (5-methylthiazole as the Ar group) displayed the most potent binding affinity data, with a Ki value of 21.9 nM against the A1 AR and two-fold selectivity over the A2A AR, which can be used as a lead for the further optimization of selective A1 AR antagonists. However, compounds 1e–1i showed less binding affinity against A1 to A3 ARs compared with compounds 1a–1d because of the introduction of a methyl group beside the heteroatom, which may increase the steric hindrance and affect the binding to the target cavity of A1 to A3 ARs. These results suggested that the introduction of heteroatoms in the Ar group was crucial for binding to the A2A AR and increased its affinity, and furan as the Ar group was the most potent. Conversely, the introduction of a methyl group beside the heteroatom on the Ar group was fatal for binding to the target due to the steric hindrance.
Table 1. Binding Affinity (Ki, nM) of Compounds 1a–1i at the Adenosine Receptors.
| binding
affinity (Ki, nM) |
||||
|---|---|---|---|---|
| compound | A1 AR | A2A AR | A2B AR | A3 AR |
| 1a | 24.2 ± 5.49 | 5.58 ± 0.11 | 491 ± 4.45 | 8790 ± 3.27 |
| 1b | 37.6 ± 4.34 | 62.4 ± 3.43 | >10000 | >10000 |
| 1c | 78.0 ± 6.75 | 84.6 ± 4.73 | 1586 ± 4.14 | >10000 |
| 1d | 21.9 ± 4.53 | 43.9 ± 2.75 | 9919 ± 6.56 | 2934 ± 4.11 |
| 1e | 88.4 ± 6.36 | 448 ± 4.42 | >10000 | 2964 ± 4.55 |
| 1f | 926 ± 3.70 | 1032 ± 2.46 | >10000 | >10000 |
| 1g | 722 ± 9.90 | 3427 ± 4.20 | >10000 | >10000 |
| 1h | 1719 ± 6.12 | 3767 ± 2.41 | >10000 | >10000 |
| 1i | 2973 ± 4.65 | 797 ± 2.44 | >10000 | >10000 |
| 7 | 88.6 ± 5.56 | 26.9 ± 2.71 | 58.9 ± 3.33 | >10000 |
Furthermore, the calcium flux functional experiments were carried out to assess the antagonistic/agonistic activity of the most potent compound 1a at the A2A and A1 ARs, along with A2B and A3 ARs.34 The functional assay data IC50 for 1a shown in Figure 3 indicated that the excellent antagonist activity of 1a was consistent with its binding affinity, whereas the agonist activity of 1a was negligible.
Figure 3.

Antagonistic effect (IC50, nM) of compound 1a against A1, A2A, A2B, and A3 ARs.
Many studies have proven that neuron apoptosis is involved in the pathological process of ischemia injury. Thus the OGD/R model (in vitro ischemic model) was used to damage HT22 cells to simulate ischemic injury to investigate the effect of A2A/A1 AR antagonist compound 1a on HT22 cell damage.35 First, we investigated the effect of compound 1a on cell apoptosis induced by OGD/R. The results demonstrated that OGD/R significantly induced apoptosis of HT22 cells, and compound 1a reversed, in a concentration-dependent manner, the up-regulation of pro-apoptotic genes such as cleaved caspase-3, cleaved caspase-9, cleaved PARP1, p53, and Bax in OGD/R-treated HT22 cells (Figure 4A). Notably, immunofluorescence analyses revealed that the antiapoptotic gene Bcl-2 staining was enhanced by compound 1a (Figure 4B). Likewise, the mRNA expression in the apoptosis markers (p53, Bax, Bcl-2) was consistent with the protein expression (Figure 4C). These results provided support that compound 1a protected against cell apoptosis.
Figure 4.

Compound 1a prevented HT22 cell apoptosis after oxygen-glucose deprivation (OGD). (A) HT22 cells were pretreated with or without compound 1a (1–10 μM) for 4 h and then were treated with OG, then 4 and 24 h of reoxygenation. Cell lysates were prepared and blotted with antibodies to Bax, p53, cleaved PARP1, cleaved caspase-3, and cleaved caspase-9; ***p < 0.001, all data are presented as the mean ± SD of three independent experiments. (B) HT22 cells were pretreated with or without 10 μM compound 1a for 4 h and then were treated with OGD, then 4 and 24 h of reoxygenation. Cells were immunostained with antibody Bcl-2 (magnification, 200×). (C) HT22 cells were pretreated with or without 10 μM compound 1a for 4 h and then were treated with OGD, then 4 and 24 h of reoxygenation. mRNA levels of Bax, p53, and Bcl2 were quantified using qRT-PCR. *p < 0.05, ***p < 0.001. All data are presented as the mean ± SD of three independent experiments.
Currently, there is strong evidence that inflammatory processes may contribute to secondary brain damage after ischemic stroke. Indeed, inflammation modulators including iNOS, COX-2, and VCAM-1 were induced by OGD/R; on the contrary, increased inflammation mediators were significantly inhibited by compound 1a treatment (Figure 5A). Consistent with these findings, immunofluorescence staining revealed a lack of COX-2 in compound-1a-treated HT22 cells as compared with that in OGD/R-treated HT22 cells (Figure 5B). Recent findings identified that NLRP3 inflammasomes play a major role in neuronal cell death in stroke and further suggested that targeted inflammasome assembly and activity may ameliorate ischemic injury.36,37 We found that OGD/R robustly induced the expression of inflammasome protein caspase-1 (p20) and mature pro-inflammatory cytokines IL-18 and IL-1β in HT22 cells; in turn, compound 1a reduced caspase-1 (p20), IL-18, and IL-1β expression (Figure 5C). Likewise, the mRNA expression in inflammation markers Nos2, Vcam-1, and II1b was consistent with the protein expression (Figure 5D). These results provide further support that compound 1a protected against neuron OGD/R injury.
Figure 5.

Compound 1a reduced the inflammation response in OGD/R-treated HT22 cells. (A) HT22 cells were pretreated with or without compound 1a (1–10 μM) for 4 h and then were treated with OGD, then 4 and 24 h of reoxygenation. Cell lysates were prepared and blotted with antibodies to iNOS, VCAM-1, and COX-2. ***p < 0.001. All data are presented as the mean ± SD of three independent experiments. (B) HT22 cells were pretreated with or without 10 μM compound 1a for 4 h and then were treated with OGD, then 4 and 24 h of reoxygenation. Cells were immunostained with antibody COX-2 (magnification, 200×). (C) HT22 cells were pretreated with or without compound 1a (1–10 μM) for 4 h and then were treated with OGD, then 4 and 24 h of reoxygenation. Cell lysates were prepared and blotted with antibodies to IL-1β, IL-18, and caspase-1 (p20). ***p < 0.001. All data are presented as the mean ± SD of three independent experiments. (D) HT22 cells were pretreated with or without 10 μM compound 1a for 4 h and then were treated with OGD, then 4 and 24 h of reoxygenation. mRNA levels of Nos2, Vcam-1, and II1b were quantified using qRT-PCR. *p < 0.05, ***p < 0.001. All data are presented as the mean ± SD of three independent experiments.
The metabolic stability is a prime consideration when developing a candidate (Table 2). The in vitro metabolic stability of compound 1a was measured using human liver microsomes (HLMs), rat liver microsomes (RLMs), mouse liver microsomes (MsLMs), dog liver microsomes (DLMs), and monkey liver microsomes (MkLMs). Compound 1a displayed good metabolic stability with a half life of 77.4, 56.0, and 83.9 min along with an intrinsic clearance (CL) of 16.1, 44.5, and 23.8 mL/min/kg in the HLMs, RLMs, and DLMs, respectively. After 60 min, 58.3, 48.6, and 60.6% of compound 1a remained in the HLMs, RLMs, and DLMs, respectively. However, compound 1a displayed less metabolic stability with a half life of 3.4 and 14.6 min along with an intrinsic clearance (CL) of 1601.7 and 128 mL/min/kg in the MsLMs and MkLMs, respectively. In addition, compound 1a showed moderate brain penetration (B/P ratio = 0.22) (Table S3), which is suitable for the lead compound of ischemic stroke.
Table 2. In Vitro Metabolic Stability of Compound 1a in the Presence of Different Microsomes.
| parameters | T1/2 (min) | CLint(mic) (μL/min/mg) | CLint(liver) (mL/min/kg) | unchanged (T = 1 h, %) |
|---|---|---|---|---|
| HLMsa | 77.4 | 17.9 | 16.1 | 58.3 |
| RLMsb | 56.0 | 24.7 | 44.5 | 48.6 |
| MsLMsc | 3.4 | 404.5 | 1601.7 | 0.00 |
| DLMsd | 83.9 | 16.5 | 23.8 | 60.6 |
| MkLMse | 14.6 | 94.8 | 128.0 | 5.9 |
Human liver microsomes.
Rat liver microsomes.
Mouse liver microsomes.
Dog liver microsomes.
Monkey liver microsomes.
Molecular docking modeling was performed to interpret the dual A2A/A1 AR binding affinity of compound 1a at the molecular level. The binding modes of compound 1a at the A2A and A1 AR cavities were analyzed by docking simulations using the Autodock software package, with the crystal structures of the A2A and A1 AR complexes as templates, respectively. The docking results (Figure 6) revealed that compound 1a adopted the general binding mode at both the A2A and A1 AR binding sites. In this binding mode, the pyridone-substituted triazolopyrimidine scaffold was positioned in the depth of the binding pocket and underwent a p–p interaction with the Phe residue (Phe168 in the A2A AR, Phe171 in the A1 AR). In addition, compound 1a formed five hydrogen bonds with the A2A AR (Glu169, Asn253, and His278), whereas it formed only two hydrogen bonds with the A1 AR (Asn253). Therefore, compound 1a adopted a much more favorable binding pose at the A2A AR cavity than at the A1 AR cavity. Moreover, the Autodock docking results also indicated that the binding pose was associated with a better docking score at the A2A AR (−9.02 kcal/mol, Ki = 0.24 μM) than at the A1 AR (−7.79 kcal/mol, Ki = 1.94 μM). Hence, the molecular docking results explained the A2A AR affinity and the slight selectivity over the A1 AR (4.4-fold) of compound 1a.
Figure 6.

Schematic description of the ligand–target interaction between compound 1a and (A) the A2A AR and (B) the A1 AR.
In the present study, we designed and synthesized a novel pyridone-substituted triazolopyrimidine scaffold dual A2A/A1 AR antagonist using a computer-aided rational drug design approach along with a hybrid medicinal strategy, inspired by two phase III drugs, ASP-5854 (dual A2A/A1 AR antagonist) and preladenant (selective A2A AR atagonist). Invitro evaluations of the A1, A2A, A2B, and A3 AR binding assays for the synthesized compounds showed promising results. Among them, compared with ASP-5854, the most potent compound 1a showed a better clog P and comparable A2A/A1 AR binding affinity (Ki = 5.58/24.15 nM). In addition, compound 1a showed excellent solubility at pH 1.2 (1.99 mg/mL), which is suitable for oral administration after some rational modification. Moreover, compound 1a showed an excellent antagonistic effect (IC50 = 5.72/25.93 nM), moderate brain penetration (B/P ratio = 0.22), and good metabolic stability with T1/2HLMs = 77.4 min, T1/2RLMs = 56 min and T1/2DLMs = 83.9 min. Importantly, compound 1a demonstrated a remarkable dose–effect relationship in the OGD/R-treated HT22 cell model, including the reduction of HT22 cells apoptosis and an alleviation of inflammatory modulator (iNOS, COX-2, and VCAM-1) and inflammatory cytokine (p20, IL-18, IL-1β, Nos2, Vcam-1, and II1b) release. With these encouraging results, we anticipate that this novel pyridone-substituted triazolopyrimidine scaffold could be an excellent starting point for the further development of dual A2A/A1 AR antagonists to benefit the field of ischemic stroke. The current effort is focused on further improving the potency along with good pharmacokinetics and pharmacodynamics both invivo and invitro, and these findings will be reported in due course.
Acknowledgments
We also thank Prof. Xin Xie (Shanghai Institute of Materia Medica, Chinese Academy of Science) for the calcium flux functional experiments.
Glossary
Abbreviations
- AR
adenosine receptor
- OGD/R
oxygen-glucose deprivation/reperfusion
- iNOS
inducible nitric oxide synthase
- COX-2
cyclooxygenase 2
- VCAM-1
vascular cell adhesion molecule 1
- p20
protein 20
- IL-18
interleukin-18
- IL-1β
interleukin-1β
- Nos2
nitric oxide synthase 2
- GPCR
G-protein-coupled receptor
- cAMP
cyclic adenosine monophosphate
- PD
Parkinson’s disease
- HD
Huntington’s disease
- AD
Alzheimer’s disease
- KO
knockout
- WT
wild-type
- SAR
structure–activity relationship
- BSA
N,O-bis(trimethylsilyl) acetamide
- HMDS
hexamethyldisilazane
- NLRP3
NLR family pyrin domain containing 3
- HLM
human liver microsome
- RLM
rat liver microsome
- MsLM
mouse liver microsome
- DLM
dog liver microsome
- MkLM
monkey liver microsome
- CL
clearance
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00599.
Experimental procedures and characterization for all final compounds and descriptions of in vitro studies (PDF)
Author Contributions
§ M.-L.T., Z.-H.W., and J.-H.W. contributed equally.
This project was supported financially by the National Natural Science Foundation of China (81803605, 81903422) and the Science and Technology Commission of Shanghai Municipality (18431900600).
The authors declare no competing financial interest.
Supplementary Material
References
- Layland J.; Carrick D.; Lee M.; Oldroyd K.; Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc. Interv. 2014, 7, 581–591. 10.1016/j.jcin.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Hou X.; Lee J. H.; Nayak A.; Alexander V.; Sharma P. K.; Chang H.; Phan K.; Gao Z. G.; Jacobson K. A.; Choi S.; Jeong L. S. Subtle Chemical Changes Cross the Boundary between Agonist and Antagonist: New A(3) Adenosine Receptor Homology Models and Structural Network Analysis Can Predict This Boundary. J. Med. Chem. 2021, 64, 12525–12536. 10.1021/acs.jmedchem.1c00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. J.; Chen J.; Li X.; Zhou X.; Hu Y. M.; Chu S. F.; Peng Y.; Chen N. H. Research progress on adenosine in central nervous system diseases. CNS Neurosci. Ther. 2019, 25, 899–910. 10.1111/cns.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effendi W. I.; Nagano T.; Kobayashi K.; Nishimura Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. 10.3390/cells9030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shook B. C.; Jackson P. F. Adenosine A(2A) Receptor Antagonists and Parkinson’s Disease. ACS Chem. Neurosci. 2011, 2, 555–567. 10.1021/cn2000537. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zheng J.; Zhang X.; Zhen X. Development of Adenosine A(2A) Receptor Antagonists for the Treatment of Parkinson’s Disease: A Recent Update and Challenge. ACS Chem. Neurosci. 2019, 10, 783–791. 10.1021/acschemneuro.8b00313. [DOI] [PubMed] [Google Scholar]; c Hagenow S.; Affini A.; Pioli E. Y.; Hinz S.; Zhao Y.; Porras G.; Namasivayam V.; Müller C. E.; Lin J. S.; Bezard E.; Stark H. Adenosine A(2A)R/A(1)R Antagonists Enabling Additional H(3)R Antagonism for the Treatment of Parkinson’s Disease. J. Med. Chem. 2021, 64, 8246–8262. 10.1021/acs.jmedchem.0c00914. [DOI] [PubMed] [Google Scholar]; d Kim A.; Lalonde K.; Truesdell A.; Gomes Welter P.; Brocardo P. S.; Rosenstock T. R.; Gil-Mohapel J. New Avenues for the Treatment of Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 8363. 10.3390/ijms22168363. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Basu S.; Barawkar D. A.; Ramdas V.; Naykodi M.; Shejul Y. D.; Patel M.; Thorat S.; Panmand A.; Kashinath K.; Bonagiri R.; Prasad V.; Bhat G.; Quraishi A.; Chaudhary S.; Magdum A.; Meru A. V.; Ghosh I.; Bhamidipati R. K.; Raje A. A.; Madgula V. L. M.; De S.; Rouduri S. R.; Palle V. P.; Chugh A.; Hariharan N.; Mookhtiar K. A. Discovery of Potent and Selective A(2A) Antagonists with Efficacy in Animal Models of Parkinson’s Disease and Depression. ACS Med. Chem. Lett. 2017, 8, 835–840. 10.1021/acsmedchemlett.7b00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ganesana M.; Venton B. J. Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PLoS One 2018, 13, e0196932 10.1371/journal.pone.0196932. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang Y.; Venton B. J. Caffeine Modulates Spontaneous Adenosine and Oxygen Changes during Ischemia and Reperfusion. ACS. Chem. Neurosci. 2019, 10, 1941–1949. 10.1021/acschemneuro.8b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Boison D.; Rho J. M. Epigenetics and epilepsy prevention: The therapeutic potential of adenosine and metabolic therapies. Neuropharmacology 2020, 167, 107741. 10.1016/j.neuropharm.2019.107741. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tescarollo F. C.; Rombo D. M.; DeLiberto L. K.; Fedele D. E.; Alharfoush E.; Tomé R.; Cunha R. A.; Sebastião A. M.; Boison D. Role of Adenosine in Epilepsy and Seizures. J. Caffeine Adenosine Res. 2020, 10, 45–60. 10.1089/caff.2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Blum D.; Chern Y.; Domenici M. R.; Buée L.; Lin C. Y.; Rea W.; Ferré S.; Popoli P. The Role of Adenosine Tone and Adenosine Receptors in Huntington’s Disease. J. Caffeine Adenosine Res. 2018, 8, 43–58. 10.1089/caff.2018.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huang N. K.; Lin J. H.; Lin J. T.; Lin C. I.; Liu E. M.; Lin C. J.; Chen W. P.; Shen Y. C.; Chen H. M.; Chen J. B.; Lai H. L.; Yang C. W.; Chiang M. C.; Wu Y. S.; Chang C.; Chen J. F.; Fang J. M.; Lin Y. L.; Chern Y. A new drug design targeting the adenosinergic system for Huntington’s disease. PLoS One 2011, 6, e20934 10.1371/journal.pone.0020934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Franco R.; Rivas-Santisteban R.; Casanovas M.; Lillo A.; Saura C. A.; Navarro G. Adenosine A(2A) Receptor Antagonists Affects NMDA Glutamate Receptor Function. Potential to Address Neurodegeneration in Alzheimer’s Disease. Cells 2020, 9, 1075. 10.3390/cells9051075. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gessi S.; Poloni T. E.; Negro G.; Varani K.; Pasquini S.; Vincenzi F.; Borea P. A.; Merighi S. A(2A) Adenosine Receptor as a Potential Biomarker and a Possible Therapeutic Target in Alzheimer’s Disease. Cells 2021, 10, 2344. 10.3390/cells10092344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ishibashi K.; Miura Y.; Wagatsuma K.; Toyohara J.; Ishiwata K.; Ishii K. Adenosine A(2A) Receptor Occupancy by Long-Term Istradefylline Administration in Parkinson’s Disease. Mov. Disord. 2021, 36, 268–269. 10.1002/mds.28378. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hattori N.; Kitabayashi H.; Kanda T.; Nomura T.; Toyama K.; Mori A. A Pooled Analysis From Phase 2b and 3 Studies in Japan of Istradefylline in Parkinson’s Disease. Mov. Disord. 2020, 35, 1481–1487. 10.1002/mds.28095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G.; Jankins T. C.; Patrick C. G. Jr.; Philbrook P.; Sears O.; Hatfield S.; Sitkovsky M.; Vasdev N.; Liang S. H.; Ondrechen M. J.; Pollastri M. P.; Jones G. B. Fluorinated Adenosine A(2A) Receptor Antagonists Inspired by Preladenant as Potential Cancer Immunotherapeutics. Int. J. Med. Chem. 2017, 2017, 1–8. 10.1155/2017/4852537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. H.; Park M. H.; Byeon J. J.; Lee B. I.; Park Y.; Kim N.; Choi J.; Shin Y. G. Analysis of Vipadenant and Its In Vitro and In Vivo Metabolites via Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometry. Pharmaceutics 2018, 10, 260. 10.3390/pharmaceutics10040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. I.; Park M. H.; Shin S. H.; Byeon J. J.; Park Y.; Kim N.; Choi J.; Shin Y. G. Quantitative Analysis of Tozadenant Using Liquid Chromatography-Mass Spectrometric Method in Rat Plasma and Its Human Pharmacokinetics Prediction Using Physiologically Based Pharmacokinetic Modeling. Molecules 2019, 24, 1295. 10.3390/molecules24071295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah U.; Hodgson R. Recent progress in the discovery of adenosine A(2A) receptor antagonists for the treatment of Parkinson’s disease. Curr. Opin. Drug Discovery Dev. 2010, 13, 466–480. [PubMed] [Google Scholar]
- a Reddy G. L.; Sarma R.; Liu S.; Huang W.; Lei J.; Fu J.; Hu W. Design, synthesis and biological evaluation of novel scaffold benzo[4,5]imidazo [1,2-a]pyrazin-1-amine: Towards adenosine A(2A) receptor (A(2A) AR) antagonist. Eur. J. Med. Chem. 2021, 210, 113040. 10.1016/j.ejmech.2020.113040. [DOI] [PubMed] [Google Scholar]; b Vigano S.; Alatzoglou D.; Irving M.; Ménétrier-Caux C.; Caux C.; Romero P.; Coukos G. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front. Immunol. 2019, 10, 925. 10.3389/fimmu.2019.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi S.; Pommey S.; Haibe-Kains B.; Beavis P. A.; Darcy P. K.; Smyth M. J.; Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 11091–11096. 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A.; Gorelik E.; Prasad S. J.; Ronchese F.; Lukashev D.; Wong M. K.; Huang X.; Caldwell S.; Liu K.; Smith P.; Chen J. F.; Jackson E. K.; Apasov S.; Abrams S.; Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 13132–13137. 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone R.; Miele L.; Maiolino P.; Pinto A.; Morello S. Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. Am. J. Cancer. Res. 2014, 4, 172–181. [PMC free article] [PubMed] [Google Scholar]
- Seitz L.; Jin L.; Leleti M.; Ashok D.; Jeffrey J.; Rieger A.; Tiessen R. G.; Arold G.; Tan J. B. L.; Powers J. P.; Walters M. J.; Karakunnel J. Safety, tolerability, and pharmacology of AB928, a novel dual adenosine receptor antagonist, in a randomized, phase 1 study in healthy volunteers. Invest New Drugs 2019, 37, 711–721. 10.1007/s10637-018-0706-6. [DOI] [PubMed] [Google Scholar]
- Rosen B. R.; Ul Sharif E.; Miles D. H.; Chan N. S.; Leleti M. R.; Powers J. P. Improved synthesis of sterically encumbered heteroaromatic biaryls from aromatic b-keto esters. Tetrahedron Lett. 2020, 61, 151855. 10.1016/j.tetlet.2020.151855. [DOI] [Google Scholar]
- Hankey G. J. Secondary stroke prevention. Lancet Neurol 2014, 13, 178–94. 10.1016/S1474-4422(13)70255-2. [DOI] [PubMed] [Google Scholar]
- Gaudry M.; Vairo D.; Marlinge M.; Gaubert M.; Guiol C.; Mottola G.; Gariboldi V.; Deharo P.; Sadrin S.; Maixent J. M.; Fenouillet E.; Ruf J.; Guieu R.; Paganelli F. Adenosine and Its Receptors: An Expected Tool for the Diagnosis and Treatment of Coronary Artery and Ischemic Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5321. 10.3390/ijms21155321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss A. B.; Grossfeld D.; Kasselman L. J.; Renna H. A.; Vernice N. A.; Drewes W.; Konig J.; Carsons S. E.; DeLeon J. Adenosine and the Cardiovascular System. Am. J. Cardiovasc. Drugs 2019, 19, 449–464. 10.1007/s40256-019-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Zeng X.; Li G.; Yang Q.; Xu J.; Zhang M.; Mao X.; Cao Y.; Wang L.; Xu Y.; Wang Y.; Zhang Y.; Xu Z.; Wu C.; Chen J. F.; Hoda M. N.; Liu Z.; Hong M.; Huo Y. Inactivation of endothelial adenosine A(2A) receptors protects mice from cerebral ischaemia-induced brain injury. Br. J. Pharmacol. 2019, 176, 2250–2263. 10.1111/bph.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani A.; Pantoni L.; Bordoni F.; Gianfriddo M.; Bianchi L.; Vannucchi M. G.; Bertorelli R.; Monopoli A.; Pedata F. The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003, 959, 243–250. 10.1016/S0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- Melani A.; Gianfriddo M.; Vannucchi M. G.; Cipriani S.; Baraldi P. G.; Giovannini M. G.; Pedata F. The selective A2A receptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res. 2006, 1073–1074, 470–480. 10.1016/j.brainres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Pedata F.; Gianfriddo M.; Turchi D.; Melani A. The protective effect of adenosine A2A receptor antagonism in cerebral ischemia. Neurol. Res. 2005, 27, 169–174. 10.1179/016164105X21913. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Xiong C.; Pancyr C.; Stockwell J.; Walz W.; Cayabyab F. S. Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J. Neurosci. 2014, 34, 9621–43. 10.1523/JNEUROSCI.3991-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joya A.; Ardaya M.; Montilla A.; Garbizu M.; Plaza-García S.; Gómez-Vallejo V.; Padro D.; Gutiérrez J. J.; Rios X.; Ramos-Cabrer P.; Cossío U.; Pulagam K. R.; Higuchi M.; Domercq M.; Cavaliere F.; Matute C.; Llop J.; Martín A. In vivo multimodal imaging of adenosine A(1) receptors in neuroinflammation after experimental stroke. Theranostics 2021, 11, 410–425. 10.7150/thno.51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola V. P.; Griffith M. T.; Hanson M. A.; Cherezov V.; Chien E. Y.; Lane J. R.; Ijzerman A. P.; Stevens R. C. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 2008, 322, 1211–1217. 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova A.; Thal D. M.; Nguyen A. T.; Vecchio E. A.; Jörg M.; Scammells P. J.; May L. T.; Sexton P. M.; Christopoulos A. Structure of the Adenosine A(1) Receptor Reveals the Basis for Subtype Selectivity. Cell 2017, 168, 867–877. 10.1016/j.cell.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Mihara T.; Mihara K.; Yarimizu J.; Mitani Y.; Matsuda R.; Yamamoto H.; Aoki S.; Akahane A.; Iwashita A.; Matsuoka N. Pharmacological characterization of a novel, potent adenosine A1 and A2A receptor dual antagonist, 5-[5-amino-3-(4-fluorophenyl)pyrazin-2-yl]-1-isopropylpyridone-2(1H)-one (ASP5854), in models of Parkinson’s disease and cognition. J. Pharmacol. Exp. Ther. 2007, 323, 708–719. 10.1124/jpet.107.121962. [DOI] [PubMed] [Google Scholar]
- a El Maatougui A.; Azuaje J.; González-Gómez M.; Miguez G.; Crespo A.; Carbajales C.; Escalante L.; García-Mera X.; Gutiérrez- de-Terán H.; Sotelo E. Discovery of Potent and Highly Selective A2B Adenosine Receptor Antagonist Chemotypes. J. Med. Chem. 2016, 59, 1967–1983. 10.1021/acs.jmedchem.5b01586. [DOI] [PubMed] [Google Scholar]; b Basu S.; Barawkar D. A.; Thorat S.; Shejul Y. D.; Patel M.; Naykodi M.; Jain V.; Salve Y.; Prasad V.; Chaudhary S.; Ghosh I.; Bhat G.; Quraishi A.; Patil H.; Ansari S.; Menon S.; Unadkat V.; Thakare R.; Seervi M. S.; Meru A. V.; De S.; Bhamidipati R. K.; Rouduri S. R.; Palle V. P.; Chug A.; Mookhtiar K. A. Design, Synthesis of Novel, Potent, Selective, Orally Bioavailable Adenosine A(2A) Receptor Antagonists and Their Biological Evaluation. J. Med. Chem. 2017, 60, 681–694. 10.1021/acs.jmedchem.6b01584. [DOI] [PubMed] [Google Scholar]
- Zhu T.; Fang L. Y.; Xie X. Development of a universal high-throughput calcium assay for G-protein- coupled receptors with promiscuous G-protein Galpha15/16. Acta Pharmacol Sin 2008, 29, 507–16. 10.1111/j.1745-7254.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhong W.; Su H.; Xu J.; Yang D.; Liu X.; Zhu Y. Histone methyltransferase Dot1L contributes to RIPK1 kinase-dependent apoptosis in cerebral ischemia/reperfusion. J. Am. Heart Assoc. 2021, 10, e022791 10.1161/JAHA.121.022791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulafia D. P.; de Rivero Vaccari J. P.; Lozano J. D.; Lotocki G.; Keane R. W.; Dietrich W. D. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab. 2009, 29, 534–544. 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- Mastronardi C.; Whelan F.; Yildiz O. A.; Hannestad J.; Elashoff D.; McCann S. M.; Licinio J.; Wong M. L. Caspase 1 deficiency reduces inflammation-induced brain transcription. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 7205–7210. 10.1073/pnas.0701366104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.