Abstract
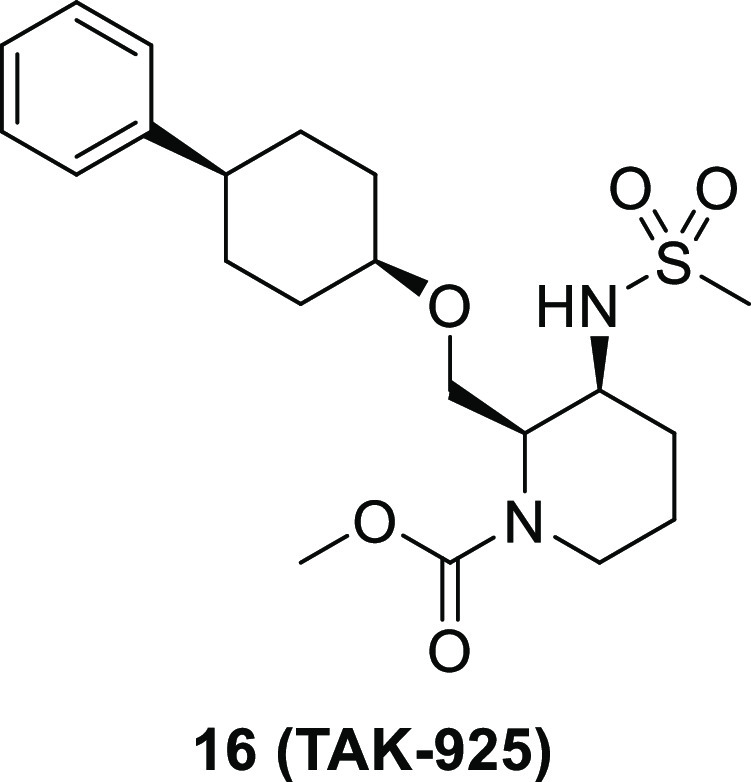
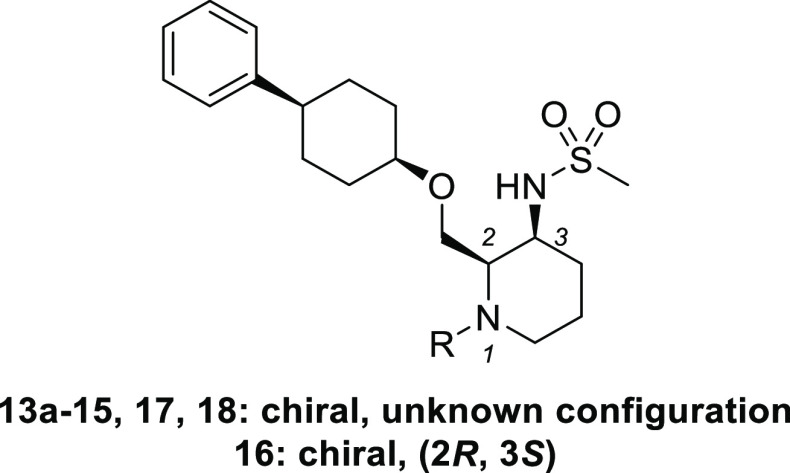
TAK-925, a potent, selective, and brain-penetrant orexin 2 receptor (OX2R) agonist, [methyl (2R,3S)-3-((methylsulfonyl)amino)-2-(((cis-4-phenylcyclohexyl)oxy)methyl)piperidine-1-carboxylate, 16], was identified through the optimization of compound 2, which was discovered by a high throughput screening (HTS) campaign. Subcutaneous administration of compound 16 produced wake-promoting effects in mice during the sleep phase. Compound 16 (TAK-925) is being developed for the treatment of narcolepsy and other related disorders.
Keywords: Orexin 2 receptor agonist, OX2R, TAK-925
Orexin A (OX-A) and orexin B (OX-B) are the hypothalamic neuropeptides which are known as important regulators of sleep/wakefulness states.1,2 Loss of orexinergic neurons in the brain is associated with the cause of narcolepsy type 1 (NT1) characterized by excessive daytime sleepiness, cataplexy, hypnagogic/hypnopompic hallucinations, sleep paralysis, and disturbed nighttime sleep.3−6
Orexin neuropeptides exert their effects through the activation of the G protein-coupled receptors identified as orexin receptor type 1 (OX1R) and type 2 (OX2R). OX2R knockout (KO) mice exhibit apparent narcolepsy-like phenotypes including fragmentation of sleep/wakefulness and cataplexy-like episodes, while OX1R KO mice do not show significant behavioral abnormalities.7,8 Thus, OX2R activation is anticipated to be a promising therapeutic option for NT1.
Since the endogenous orexin peptides cannot efficiently penetrate the blood-brain barrier (BBB),9 brain-penetrant and small-molecule orexin agonists would be attractive for the treatment of the sleep-related disorders including NT1.10
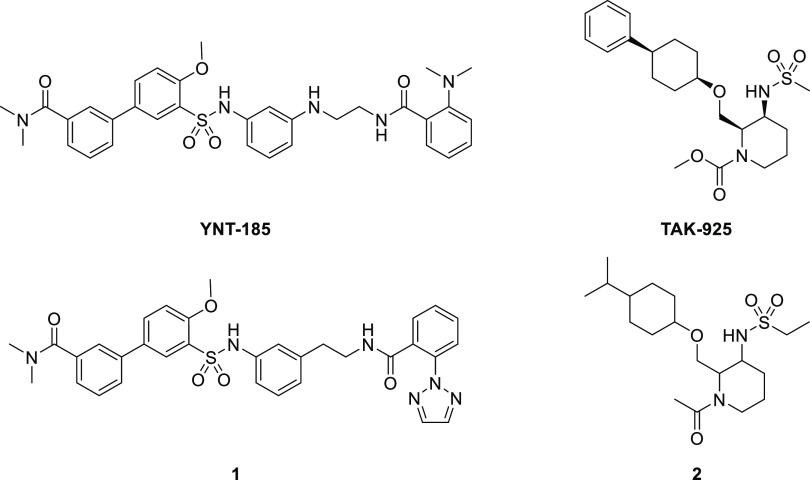
YNT-185 (Figure 1) was reported as the first nonpeptide OX2R agonist.11 The analogous compound 1(12) was recently published with its cocomplex structure with active-state OX2R obtained by cryogenic electron microscopy (cryo-EM). The analogous compounds with OX1R/OX2R agonistic activities have been also disclosed.13 However, structurally different OX2R agonists with smaller molecular weights compared with those in this series (YNT-185: 616, compound 1: 624) should be explored to develop brain-penetrant therapeutic OX2R agonist.14
Figure 1.
Chemical structures of reported OX2R agonists and our hit compound 2.
Recently, we reported that TAK-925 (Figure 1), developed as a potent and selective agonist for OX2R, shows a therapeutic potential for diseases associated with hypersomnia in mice.15 TAK-925 has been investigated as a drug for the treatment of hypersomnia including NT1 (Clincaltrials.gov Registry Identifier: NCT03332784). In this paper, we report the design, synthesis, and discovery of brain-penetrant small molecule OX2R agonist TAK-925 starting from a high throughput screening (HTS) campaign, followed by the optimization of hit compound.
An HTS campaign to discover OX2R agonists was performed by measuring calcium flux as a functional determinant of OX2R agonism using a fluorometric imaging plate reader (FLIPR) assay system. As a result, the hit compound 2 (diastereomeric mixtures, EC50 = 570 nM, maximum response [Emax] = 94%) was identified with moderate EC50 but full OX2R agonistic activity comparable to OX-A (Figure 1). Compound 2 exhibited good selectivity against OX1R agonism (EC50 > 100 000 nM), as well as the good characteristics for central nervous system (CNS) drugs such as smaller molecular weight (389) and favorable topological polar surface area (TPSA, TPSA = 76), indicating that compound 2 is a promising starting point for the development of selective and brain-penetrant OX2R agonist drug candidates.
Our hit compound 2 was a diastereomeric mixture, thus our first effort was an evaluation of all possible diastereomers (3–6; Table 1). Among four cis and trans isomers for each ring A and B, cis-cis derivative 3 showed the most potent OX2R agonistic activity (EC50 = 270 nM). In addition, compound 3 maintained a good selectivity against OX1R agonism (EC50 > 100 000 nM). These results imply that the OX2R receptor strongly recognizes the compound stereochemistry.
Table 1. In Vitro Activities of Compounds 3–6.
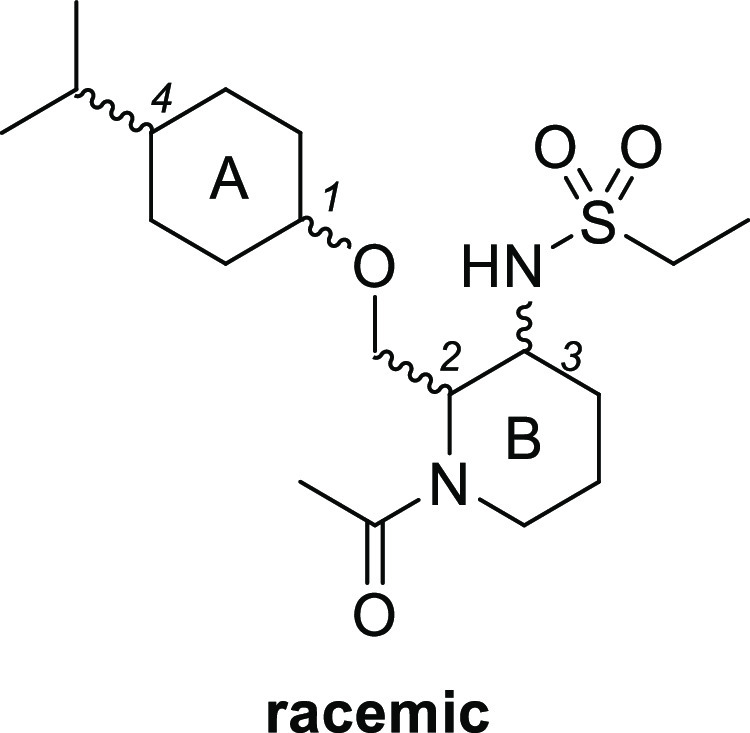
| EC50 (nM)a |
||||
|---|---|---|---|---|
| compd | ring A 1,4-position | ring B 2,3-position | OX2R agonistic activityb | OX1R agonistic activityb |
| 3 | cis | cis | 270 (250–300) | >100 000 |
| 4 | cis | trans | >30 000 | >100 000 |
| 5 | trans | cis | >30 000 | >100 000 |
| 6 | trans | trans | >30 000 | >100 000 |
| OX-A | 0.18 (0.15–0.21) | 0.068 (0.053–0.086) | ||
EC50 values and 95% confidence intervals were calculated from duplicate measurements. All values are rounded to two significant digits. n = 2.
Calcium flux assay with Chinese hamster ovary cells expressing human OX2R or human OX1R.
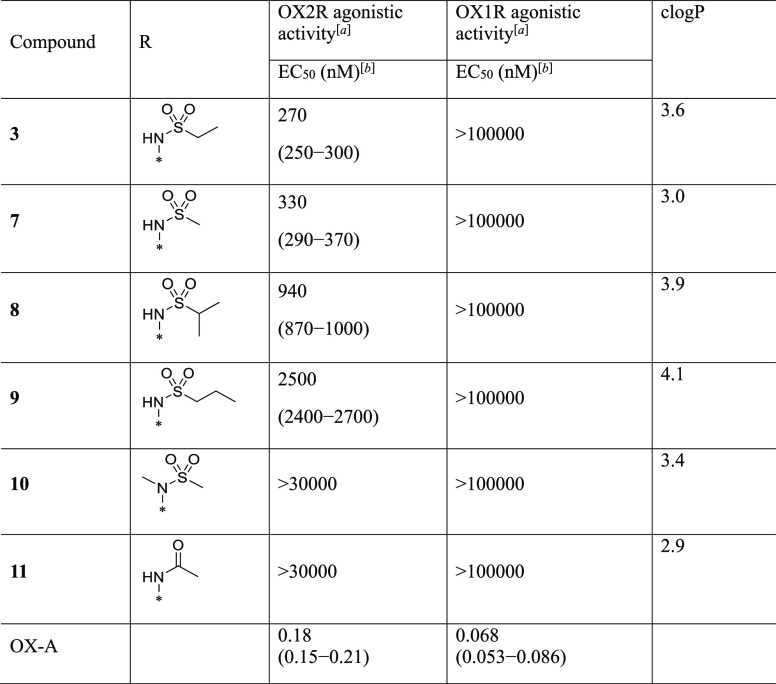
We conducted optimization of the sulfonamide, isopropyl, and carbonyl parts of compound 3 to increase the OX2R agonistic activity (Figure 2).
Figure 2.
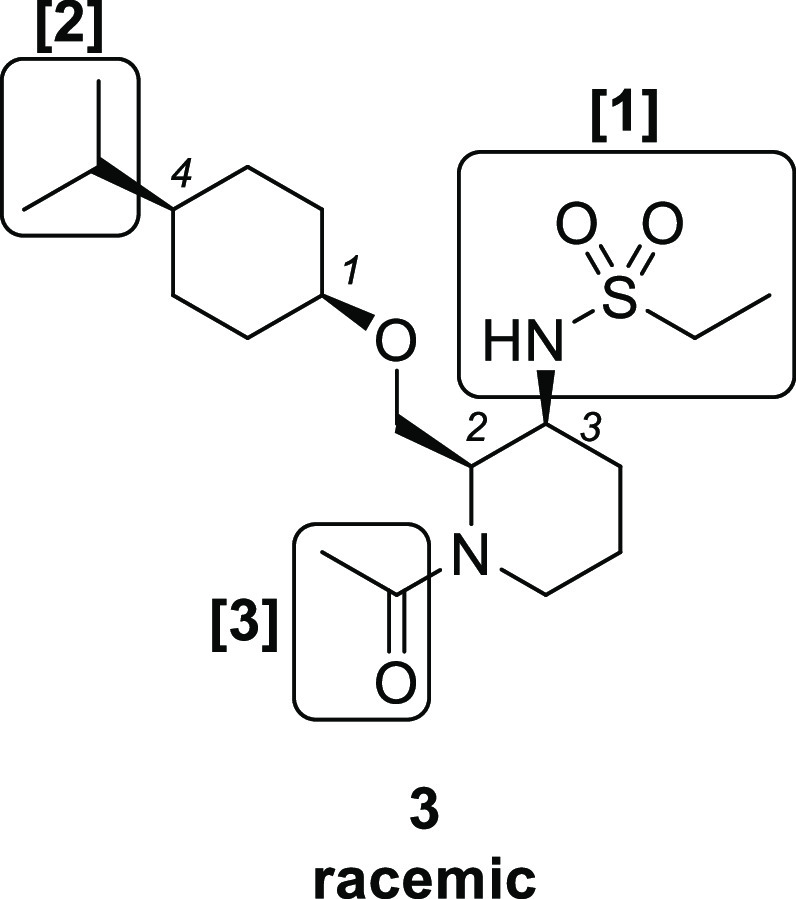
Lead optimization of compound 3.
We first examined the modification of the sulfonamide part of compound 3 (Table 2). Replacement of the ethyl group of compound 3 with a methyl group maintained the OX2R agonistic activity (7, EC50 = 330 nM), whereas the isopropyl (8, EC50 = 940 nM) and propyl (9, EC50 = 2500 nM) derivatives showed moderate activity. Methylation of nitrogen atoms in the sulfonamide (10, EC50 > 30 000 nM) or replacement of sulfonamide with acetamide (11, EC50 > 30000 nM) reduced agonist activity, indicating that the secondary sulfonamide may play an important role in OX2R agonistic activity. Considering the ligand lipophilicity efficiency16,17 (LLE, LLE = (pEC50) – clogP, clogP: calculated using ChemDraw) of these compounds as a drug likeness index, compound 7 (LLE = 3.5, clogP = 3.0), which showed higher value than compound 3 (LLE = 3.0, clogP = 3.6) was selected as a lead compound for further exploration.
Table 2. In Vitro Activities of Compounds 3 and 7–11.
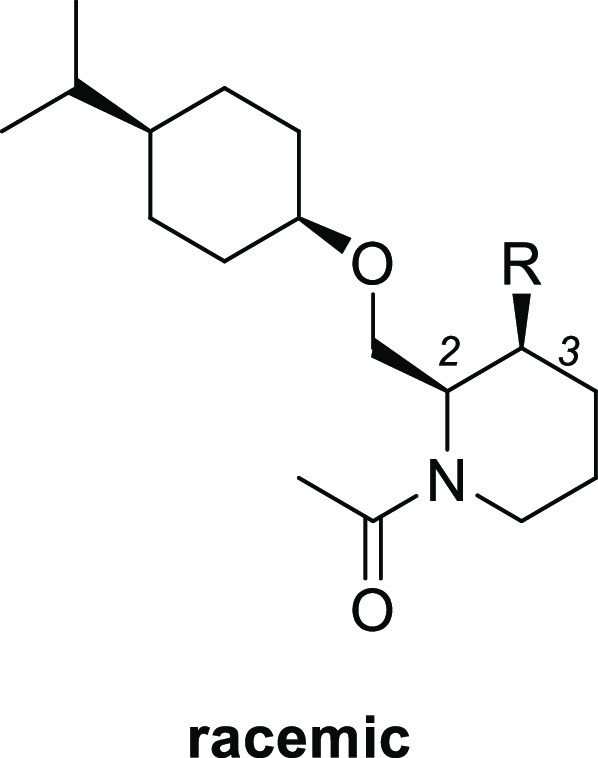
Calcium flux assay with Chinese hamster ovary cells expressing human OX2R or human OX1R.
EC50 values and 95% confidence intervals were calculated from duplicate measurements. All values are rounded to two significant digits. n = 2.
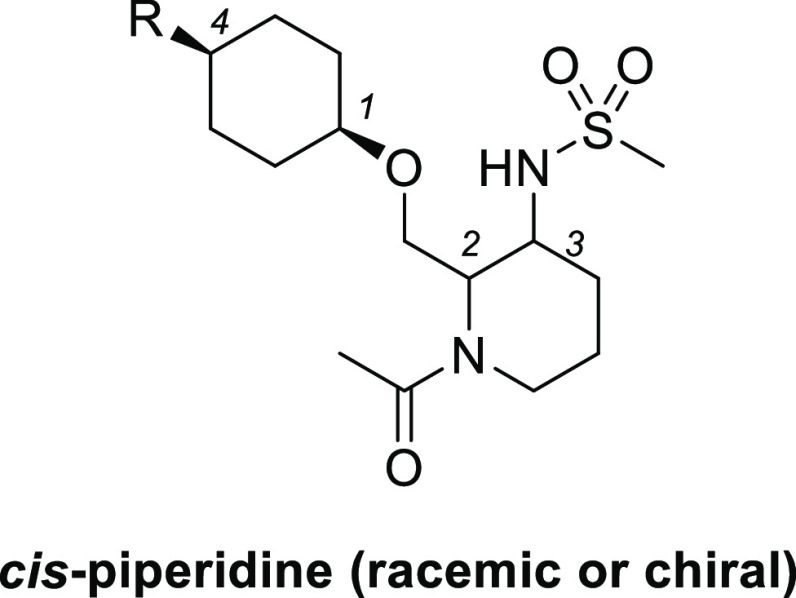
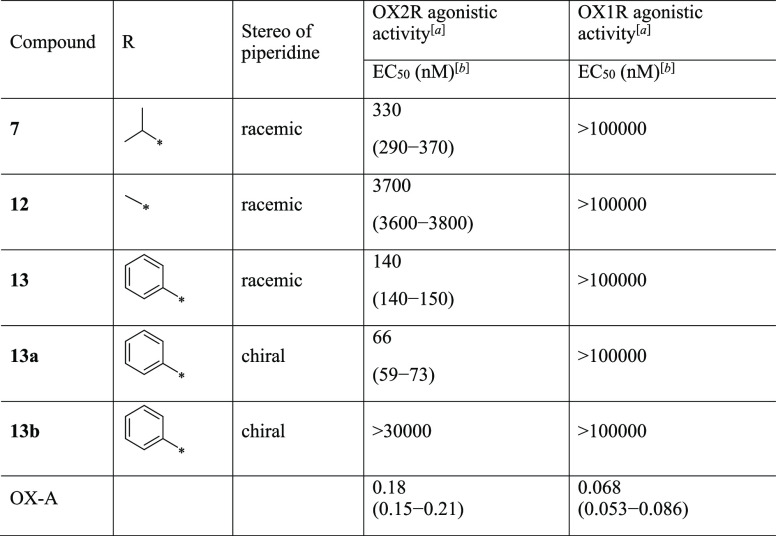
We next explored the substituent at the 4-position of the cyclohexane ring (Table 3). Compared with isopropyl compound 7, smaller methyl compound 12 showed decreased activity (EC50 = 3700 nM). On the other hand, the larger phenyl compound 13 increased the potency (EC50 = 140 nM). Chiral separation of compound 13 afforded the corresponding enantiomer pairs 13a (EC50 = 66 nM) and 13b (EC50 = > 30000 nM).
Table 3. In Vitro Activities of Compounds 7, 12, 13, 13a, and 13b.
Calcium flux assay with Chinese hamster ovary cells expressing human OX2R or human OX1R.
EC50 values and 95% confidence intervals were calculated from duplicate measurements. All values are rounded to two significant digits. n = 2.
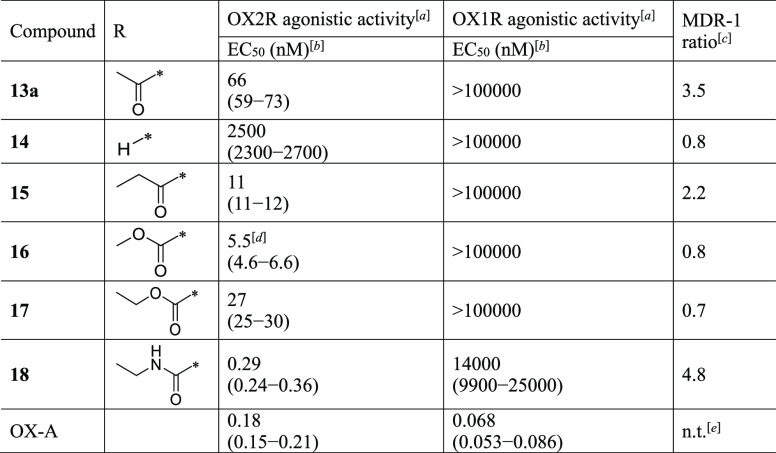
Finally, substituents at the 1-position of piperidine were explored starting from chiral compound 13a (Table 4). Removal of acetamide resulted in a large reduction in agonistic activity (14, EC50 = 2500 nM), suggesting that the carbonyl group is a key factor in potent OX2R agonistic activity. Elongation of the acetyl group to propionyl increased the activity (15, EC50 = 11 nM). Carbamate derivative 16 (EC50 = 5.5 nM) also exhibited better potency than compound 15. Further elongation of the alkyl group in compound 16 from methyl to ethyl led to the decreased potency (17, EC50 = 27 nM). On the other hand, conversion of the carbamate moiety of 18 with urea increased the activity up to subnanomolar range (18, EC50 = 0.29 nM), which is comparable to the agonist activity of endogenous OX-A peptide in our calcium flux assay system. We also measured the multidrug resistance protein 1 (MDR-1) efflux ratio of these chiral compounds, as an index for blood-brain permeability.18 Among these compounds, we selected carbamate compound 16(19) with the best balanced profile for further evaluation. Compound 16 (molecular weight: 425) exhibited good potency (OX2R EC50: 5.5 nM), selectivity (OX1R EC50: > 100 000 nM), and MDR-1 efflux ratio (0.8, A to B = 169). Compound 16 also showed good selectivity against 106 off-target enzymes and receptors.15
Table 4. In Vitro Activities of Compounds 13a, 14–18, and OX-A.
Calcium flux assay with Chinese hamster ovary cells expressing human OX2R or human OX1R.
EC50 values and 95% confidence intervals were calculated from duplicate measurements. All values are rounded to two significant digits. n = 2.
MDR-1 directional transport ratio (B to A/A to B).
n = 4.
n.t. = not tested.
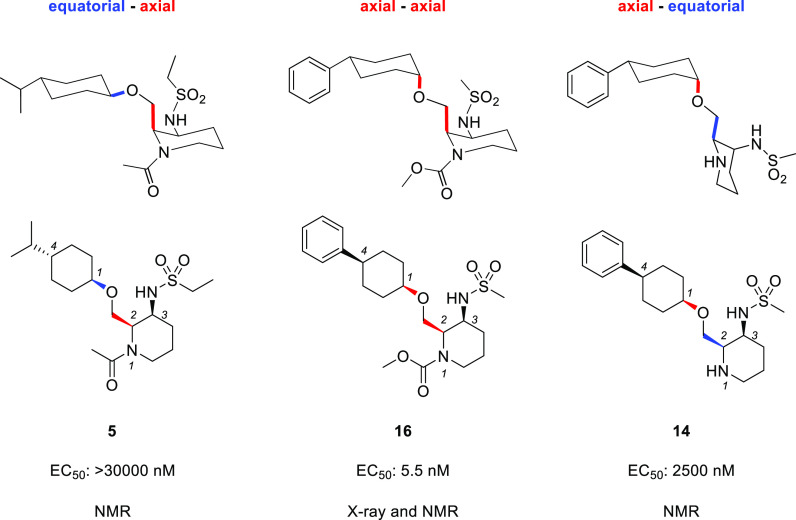
X-ray and nuclear magnetic resonance (NMR) conformational analysis of newly discovered OX2R agonists were performed to reveal the optimal conformation to show OX2R agonism (Figures 3 and 4). X-ray crystal data of highly active compound 16 (EC50 = 5.5 nM) revealed that both the methylene linker at the 2-position of the (2R,3S)-piperidine and the ether linker at the 1-position of the cyclohexane formed axial orientations (Figure 3). This conformation was also supported by the NMR nuclear Overhauser effect spectroscopy (NOESY) spectra of compound 16. Conformational analysis of compound 5 (EC50 > 30 000 nM) and compound 14 (EC50 = 2500 nM), which showed lower activity than compound 16, were also conducted by NMR NOESY studies. Compound 5 formed a chair conformation with equatorial substituent at the 1-position of the cyclohexane, while it maintained axial orientation at the 2-position of the piperidine (Figure 4). This result indicates that the axial methylene linker at the 1-position of the cyclohexane plays a crucial role in the OX2R agonistic activity. In addition, compound 14 has the equatorial substituent at the 2-position of the piperidine, keeping axial orientation at the 1-position of the cyclohexane, indicating that the axial state on the piperidine is also important for the OX2R agonistic activity. Thus, it was suggested that the unique but stable axial–axial conformation between piperidine and the cyclohexane ring of compound 16 was favorable for the OX2R agonistic activity.
Figure 3.
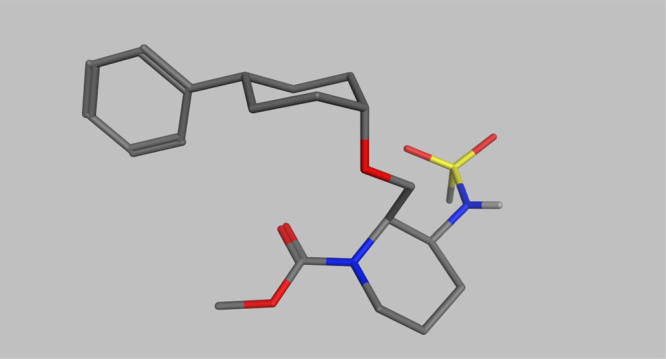
X-ray crystal structure of compound 16.
Figure 4.
Conformational analysis of compounds 5, 16, and 14.
Brain and plasma concentration of compound 16 was measured after intraperitoneal administration at 10 mg/kg in mice (Table 5). Although compound 16 showed short half-life in mice, 16 showed acceptable brain-to-plasma concentration ratio (0.2) at 0.5 and 1 h after administration respectively, indicating that compound 16 is brain-penetrant.
Table 5. Brain Concentration of Compound 16 in Mice at 10 mg/kg, ipa.
| time (h) | brain concn (ng/g) | plasma concn (ng/mL) | brain-to-plasma concn ratio |
|---|---|---|---|
| 0.5 | 183 | 880 | 0.2 |
| 1 | 63 | 288 | 0.2 |
C57BL/6J mice, intraperitoneal administration, n = 3.
It has been reported that activation of the orexin system by intracerebroventricular injection of OX-A increases wakefulness in rodents.20,21 In this study, we assessed the effect of compound 16 on wakefulness time in ICR mice during the sleep phase based on the measurements of electroencephalogram and electromyogram. Subcutaneous administration of compound 16 at 3 mg/kg significantly increased total wakefulness time during 3 h after administration in ICR mice (Figure 5). These results demonstrate that compound 16 is brain-penetrant and shows arousal effect in mice.
Figure 5.
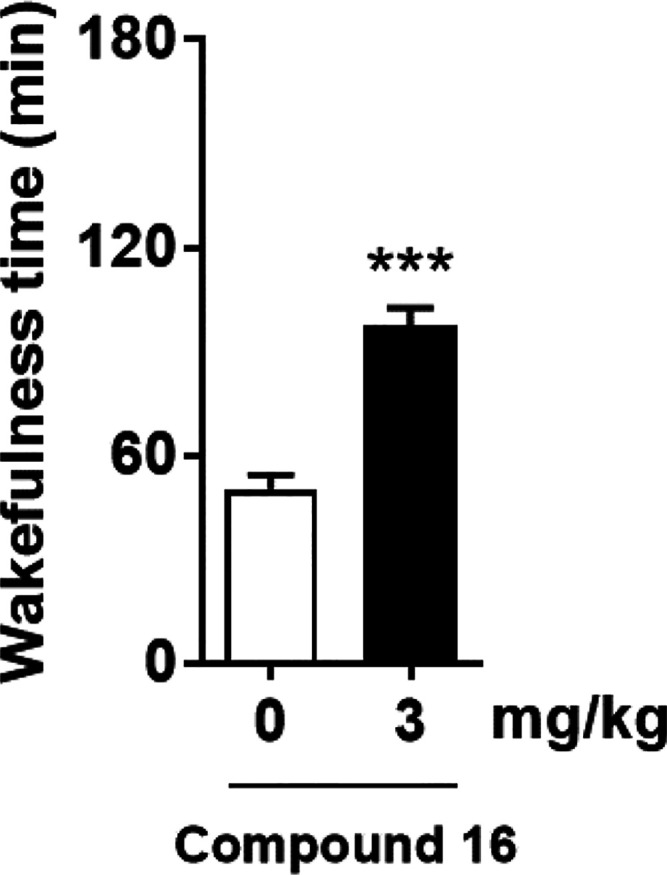
Effect of compound 16 on wakefulness time in ICR mice during the sleep phase. Compound 16 at 3 mg/kg or vehicle was administered subcutaneously to ICR mice at zeitgeber time 5, and electroencephalograms and electromyograms were recorded. Analysis was performed with data collected during 3 h after drug administration. Data were presented as the mean + standard error of the mean (n = 8). ***P < 0.001, compared with the vehicle-treated mice (two-tailed paired t test).
We have developed a potent, selective, and brain-penetrant OX2R agonist, compound 16, starting from a hit compound 2. Conformational analysis of compound 16 revealed the unique axial–axial conformation, which might contribute to the potent OX2R agonistic activity. Subcutaneous administration of compound 16 significantly increased total wakefulness time in mice during the sleep phase. Compound 16 (TAK-925) is a promising therapeutic agent as an OX2R agonist for the treatment of narcolepsy and other related disorders.
Acknowledgments
The authors thank Dr. Takeshi Sakurai for valuable discussions. We acknowledge Miki Hara and Natsumi Fujii for their work on chiral separation and analysis, Mitsuyoshi Nishitani for obtaining the single crystal X-ray structure of compound 16, and Dr. Shinobu Sasaki and Mika Inoue for the compound’s synthesis. We also thank Drs. Tohru Miyazaki, Yasuhisa Kohara, Makoto Kamata, Yoshinori Kawamura, Kenji Izawa, Michiyo Mochizuki, and Tatsuki Koike for their valuable discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00626.
Analytical HPLC traces (compound purity in HPLC); chemical synthesis procedures; calcium flux assay (in vitro agonistic activity of OX2R and OX1R); transcellular transport study using a transporter-expression system; measurement of plasma and brain concentration of compound 16 in mice; evaluation of wakefulness time measurement of compound 16 in mice; 1H NMR and 13C NMR chart of compound 16; single-crystal X-ray structure analysis of compound 16 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sakurai T.; Amemiya A.; Ishii M.; Matsuzaki I.; Chemelli R. M.; Tanaka H.; Williams S. C.; Richardson J. A.; Kozlowski G. P.; Wilson S.; Arch J. R.; Buckingham R. E.; Haynes A. C.; Carr S. A.; Annan R. S.; McNulty D. E.; Liu W. S.; Terrett J. A.; Elshourbagy N. A.; Bergsma D. J.; Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Ohno K.; Sakurai T. Orexin neuronal circuitry: Role in the regulation of sleep and wakefulness. Front. Neuroendocrinol. 2008, 29, 70–87. 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Thorpy M. J.; Dauvilliers Y. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med. 2015, 16, 9–18. 10.1016/j.sleep.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Abad V. C.; Guilleminault C. New developments in the management of narcolepsy. Nat. Sci. Sleep 2017, 9, 39–57. 10.2147/NSS.S103467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y.; Arnulf I.; Mignot E. Narcolepsy with cataplexy. Lancet 2007, 369, 499–511. 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- Baumann C. R.; Bassetti C. L. Hypocretins (orexins) and sleep-wake disorders. Lancet Neurol. 2005, 4, 673–682. 10.1016/S1474-4422(05)70196-4. [DOI] [PubMed] [Google Scholar]
- Willie J. T.; Chemelli R. M.; Sinton C. M.; Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu. Rev. Neurosci. 2001, 24, 429–458. 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007, 8, 171–181. 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Fujiki N.; Yoshida Y.; Ripley B.; Mignot E.; Nishino S. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep 2003, 26, 953–959. 10.1093/sleep/26.8.953. [DOI] [PubMed] [Google Scholar]
- Mieda M.; Sakurai T. Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders. Rationale for development and current status. CNS Drugs 2013, 27, 83–90. 10.1007/s40263-012-0036-8. [DOI] [PubMed] [Google Scholar]
- Nagahara T.; Saitoh T.; Kutsumura N.; Irukayama-Tomobe Y.; Ogawa Y.; Kuroda D.; Gouda H.; Kumagai H.; Fujii H.; Yanagisawa M.; Nagase H. Design and synthesis of non-peptide, selective orexin receptor 2 agonists. J. Med. Chem. 2015, 58, 7931–7937. 10.1021/acs.jmedchem.5b00988. [DOI] [PubMed] [Google Scholar]
- Hong C.; Byrne N. J.; Zamlynny B.; Tummala S.; Xiao L.; Shipman J. M.; Partridge A. T.; Minnick C.; Breslin M. J.; Rudd M. T.; Stachel S. J.; Rada V. L.; Kern J. C.; Armacost K. A.; Hollingsworth S. A.; O’Brien J. A.; Hall D. L.; McDonald T. P.; Strickland C.; Brooun A.; Soisson S. M.; Hollenstein K. Structures of active-state orexin receptor 2 rationalize peptide and small-molecule agonist recognition and receptor activation. Nat. Commun. 2021, 12, 815. 10.1038/s41467-021-21087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Perrey D. A.; Decker A. M.; Langston T. L.; Mavanji V.; Harris D. L.; Kotz C. M.; Zhang Y. Discovery of arylsulfonamides as dual orexin receptor agonists. J. Med. Chem. 2021, 64, 8806–8825. 10.1021/acs.jmedchem.1c00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajouhesh H.; Lenz G. R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukitake H.; Fujimoto T.; Ishikawa T.; Suzuki A.; Shimizu Y.; Rikimaru K.; Ito M.; Suzuki M.; Kimura H. TAK-925, an orexin 2 receptor-selective agonist, shows robust wake-promoting effects in mice. Pharmacol., Biochem. Behav. 2019, 187, 172794. 10.1016/j.pbb.2019.172794. [DOI] [PubMed] [Google Scholar]
- Rankovic Z. CNS drug design: balancing physicochemical properties for optimal brain exposure. J. Med. Chem. 2015, 58, 2584–2608. 10.1021/jm501535r. [DOI] [PubMed] [Google Scholar]
- Di L.; Rong H.; Feng B. Demystifying brain penetration in central nervous system drug discovery. J. Med. Chem. 2013, 56, 2–12. 10.1021/jm301297f. [DOI] [PubMed] [Google Scholar]
- Takeuchi T.; Yoshitomi S.; Higuchi T.; Ikemoto K.; Niwa S.; Ebihara T.; Katoh M.; Yokoi T.; Asahi S. Establishment and characterization of the transformants stably-expressing MDR1 derived from various animal species in LLC-PK1. Pharm. Res. 2006, 23, 1460–1472. 10.1007/s11095-006-0285-7. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Brindisi M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015, 58, 2895–2940. 10.1021/jm501371s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan J. J.; Leslie R. A.; Patel S.; Evans M. L.; Wattam T. A.; Holmes S.; Benham C. D.; Taylor S. G.; Routledge C.; Hemmati P.; Munton R. P.; Ashmeade T. E.; Shah A. S.; Hatcher J. P.; Hatcher P. D.; Jones D. N. C.; Smith M. I.; Piper D. C.; Hunter A. J.; Porter R. A.; Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 10911–10916. 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M.; Willie J. T.; Hara J.; Sinton C. M.; Sakurai T.; Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 4649–4654. 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.