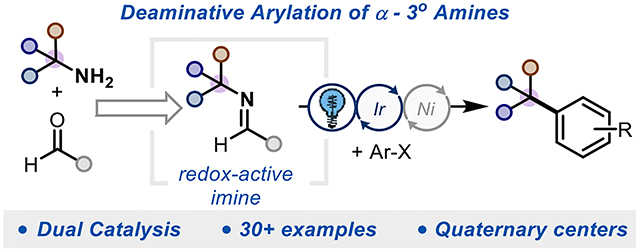
Abstract
We report a method to activate α−3° amines for deaminative arylation via condensation with an electron-rich aldehyde and merge this reactivity with nickel metallaphotoredox to generate benzylic quaternary centers, a common motif in pharmaceuticals and natural products. The reaction is accelerated by added ammonium salts. Evidence is provided in support of two roles for the additive - inhibition of nickel black formation and acceleration of the overall reaction rate. We demonstrate a robust scope of amine and haloarene coupling partners and show an expedited synthesis of ALK2 inhibitors.
Graphical Abstract
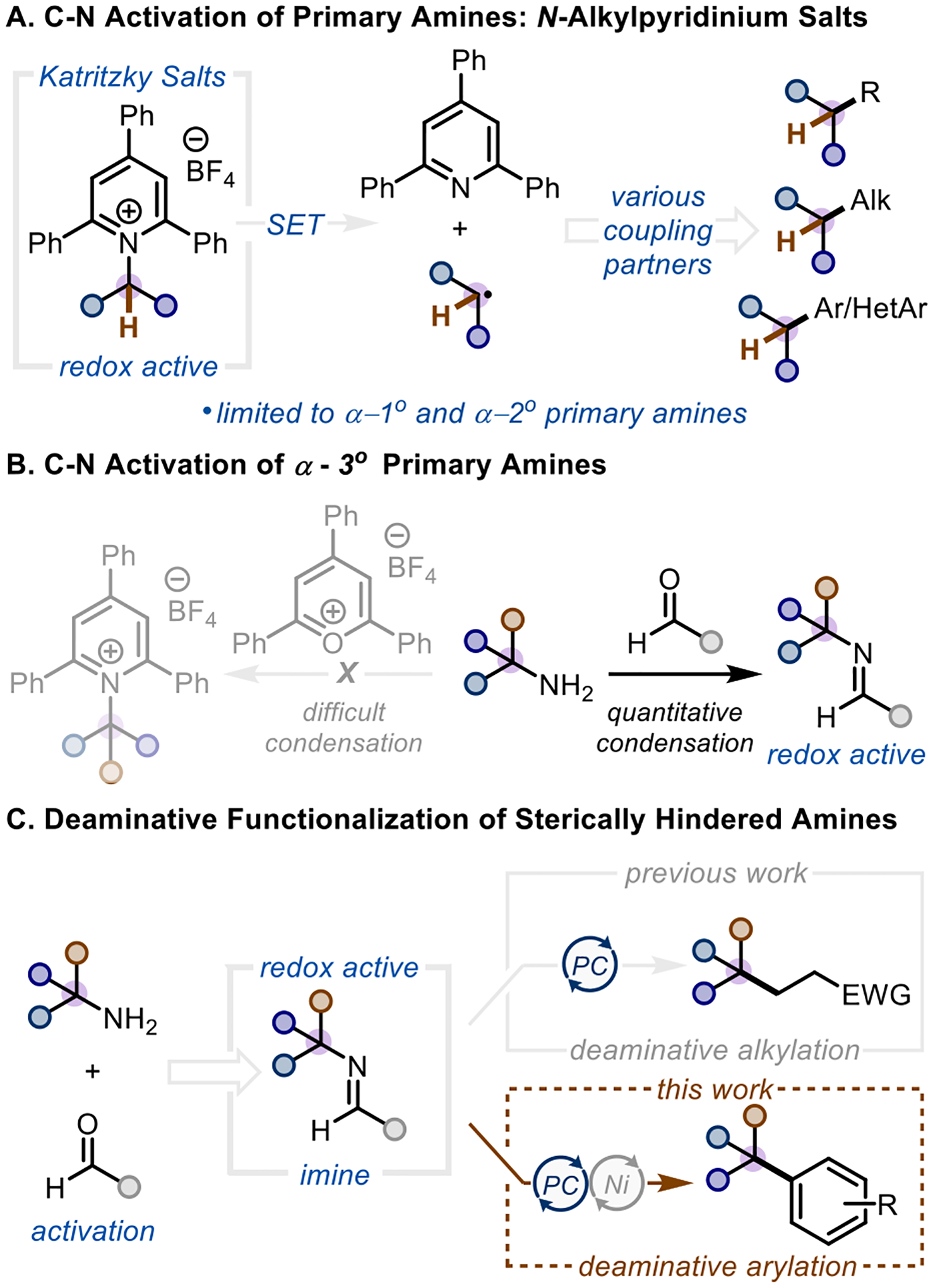
Primary amines are present in a variety of building blocks, easily prepared and purified, and are amenable to late-stage functionalization. The ubiquity of primary alkyl amines as advanced intermediates and drugs1,2 makes them attractive reagents for alkyl precursors. Thus, discovery chemists can take advantage of the plethora of primary amines that they obtain in their inventories to utilize them as coupling partners. In 2017, the Watson group published a nickel-catalyzed Suzuki-Miyaura coupling demonstrating the use of unactivated primary amines as alkyl precursors via formation of Katritzky pyridinium salts (Scheme 1A).3 Since this seminal publication, the use of Katritzky salts as a functional handle has been widely popularized due to their ease of synthesis and accessible reduction potential (~ −0.9 V vs SCE).4 The corresponding pyridinium salts are amenable to many transition metal-catalyzed5–12 and photoredox-catalyzed13–19 deaminative transformations,20,21 but are limited to α-1° and α-2° amines, as sterically encumbered α-3° amines are unable to condense onto triphenyl-pyrylium salts (Scheme 1B).10,21 In an effort to address this shortcoming, we developed a complementary method to activate the C-N bond of α-3° amines via condensation onto an electron rich aldehyde activating group (Scheme 1C, top).22 This allows for generation of a nucleophilic carbon radical via single-electron oxidation that can be coupled to electron-deficient olefins to generate Giese products. This deaminative method complements the Katritzky salts in two ways: (1) tertiary alkyl amines are used as viable alkyl precursors, and (2) the radical reactivity is revealed through an oxidative rather than reductive pathway.
Scheme 1.
Use of Primary Amines as Alkyl Precursors.
Expanding the coupling partner from electron deficient olefins to aryl halides through dual photoredox/nickel catalysis (Scheme 1C, bottom) would allow for the use of primary α-3° amines as radical precursors in C(sp3)-C(sp2) cross coupling, a bond construction recently identified as a missing link in crosscoupling technology,23 and of increasing interest to medicinal chemistry as a way to expand and diversify the chemical landscape of pharmaceuticals.23–27 While similar C(sp3)-C(sp2) cross couplings can be achieved with other alkyl precursors, namely halides,28,29 alkyl trifluoroborates,30 Grignard reagents,31,32 olefins,33,34 and redox-active esters,35 our strategy adds a valuable building block to the synthetic toolbox for generating quaternary centers from ubiquitous primary amines.
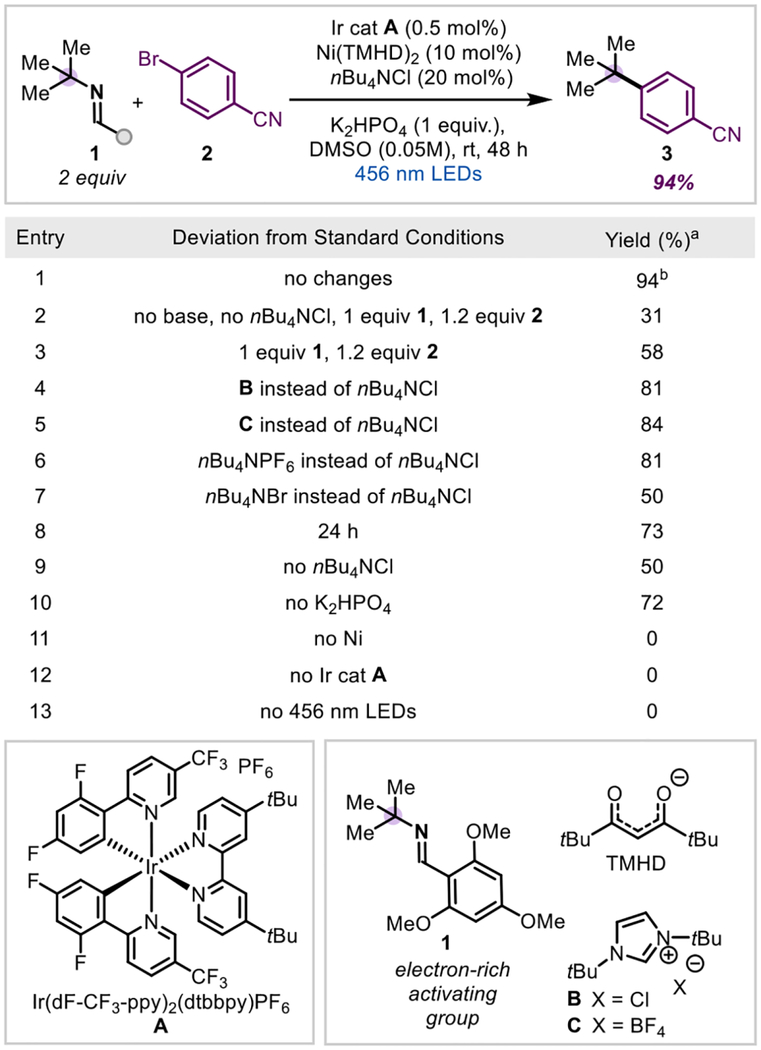
We began our initial screen by using tetramethylheptanedionato (TMHD) nickel(II),28,30 1 equivalent of imine, and 1.2 equivalents of aryl halide (Table 1, entry 2) to afford the product in 31% yield (see SI for additional ligands screened). An extensive additive screen identified tetra-alkyl ammonium salts and imidazolium salts as helpful for reactivity, with nBu4NCl giving the best results (see SI for full list of additives screened). The use of nBu4NBr did not cause the same increase in yield.36 2,4,6-trimethoxybenzonitrile, the byproduct of imine fragmentation, was consistently formed in 10–20% higher yield than 3. With heavier aliphatic amine-derived imines, we observe the products of C-N reduction and elimination (alkane and alkene). Therefore, adjusting stoichiometries of the starting material and adding an equivalent of inorganic base increased the yield to 94%. As we noticed in our previous work, the imine nitrogen is sufficiently basic to promote desired reactivity in the absence of other bases, giving a 72% yield (entry 10).
Table 1.
Optimization and Control Reactions.
|
GCMS yields with mesitylene as an internal standard.
isolated yield.
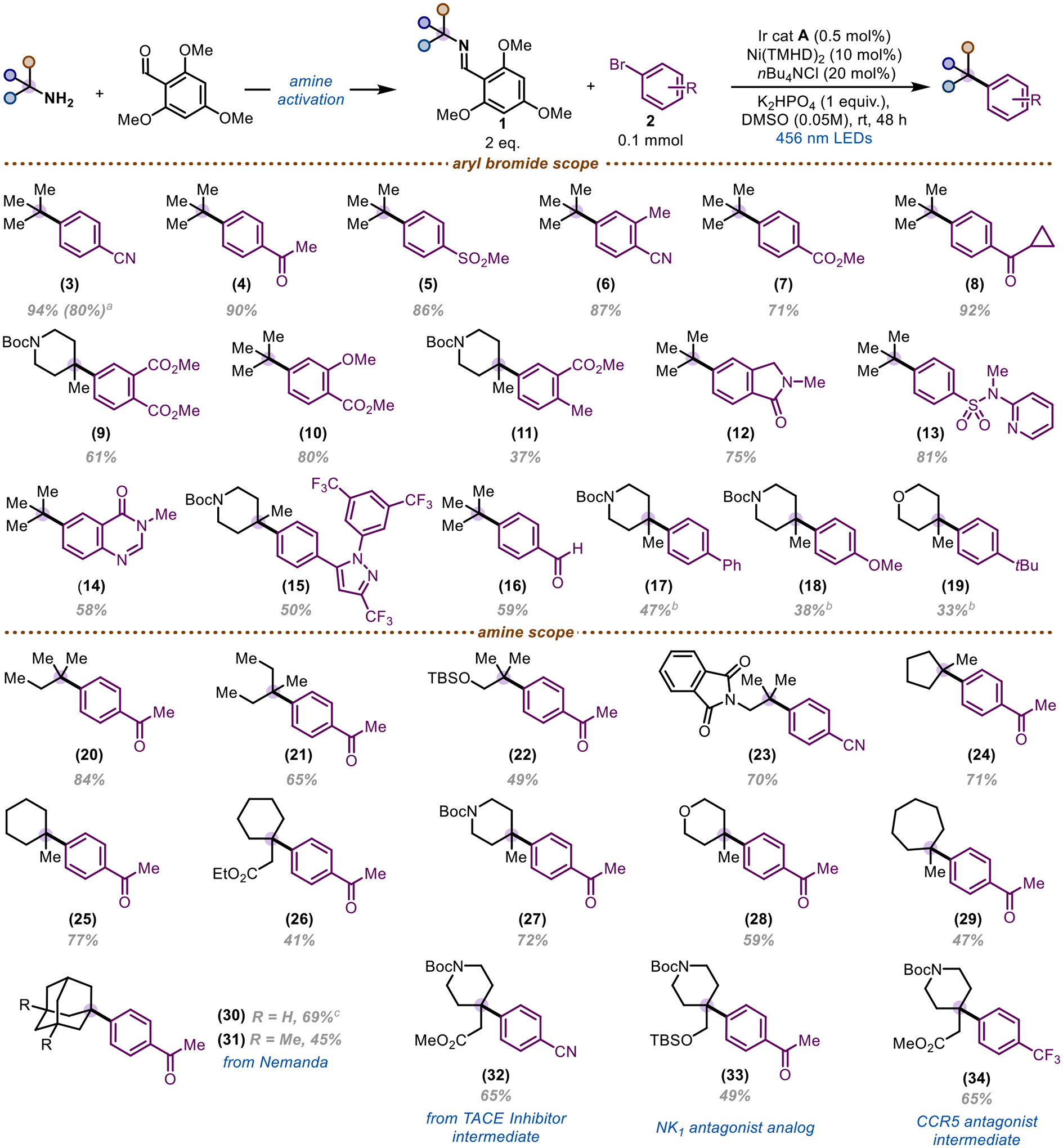
With respect to the scope of the reaction, electron-deficient aryl bromides give desired products in excellent yield (Scheme 2). Scaling the reaction up by 10x results in only a slight decrease in yield (3). Notably, electrophilic moieties that are incompatible with organometallic reagents31,32,35 are tolerated, including nitriles (3, 6), ketones (4, 8), aldehydes (16), and esters (7, 9–11). Aryl bromides containing heteroatoms proceed with high efficiency – cyclic amide 12, sulfonamide 13, quinazolinone 14, and Celecoxib derivative 15 – while N-heterocycles provide trace product.30,37 Electron-neutral aryl bromides still provide modest yields, whereas electron-rich bromides afford trace product. This is accompanied by the formation of nickel black, suggesting that electron-rich aryl bromides lead to catalyst deactivation.38 Notably, we found that replacing the electron-rich bromide with the corresponding aryl iodide and adding LiCl as an additive can restore yields to ~30–40% (18,19).29,39
Scheme 2.
Scope
a) done on a 1.0 mmol scale. b) from the aryl iodide and 1 equiv. of LiCl was added. c) NiCl2(dtbbpy)(H2O)4 was used in place of Ni(TMHD)2
Acyclic α-tertiary amines provide desired product in good yields, tolerating protected alcohols (22) as well as protected amines (23). Five- and six-membered ring systems containing functional groups such as esters, protected amines, and ethers deliver product in high yields (24–28, 32–34), tolerating additional substitution in modest yields. Notably, no isomerization is observed using our methodology. Alpha methyl cycloheptyl amine also proved to be a competent coupling partner, furnishing 29 in moderate yield. Adamantylamine 30 and Nemanda derivative 31 also provide desired product. Notably, bipyridines are also capable ligands with these substrates, due to a more sterically accessible, pseudo-tetrahedral carbon-centered radical. This allows for facile inner-sphere reductive elimination, whereas traditional tertiary planar radicals proceed via an outer-sphere mechanism.40 Amines used as intermediates en route to pharmaceuticals, such as TNF-α converting enzyme (TACE) inhibitor intermediate 32, give desired product in good yields.41 Drug derivatives, such as NK1 analog 33 and CCR5 inhibitor intermediate 34, can also be synthesized utilizing our method.42,43
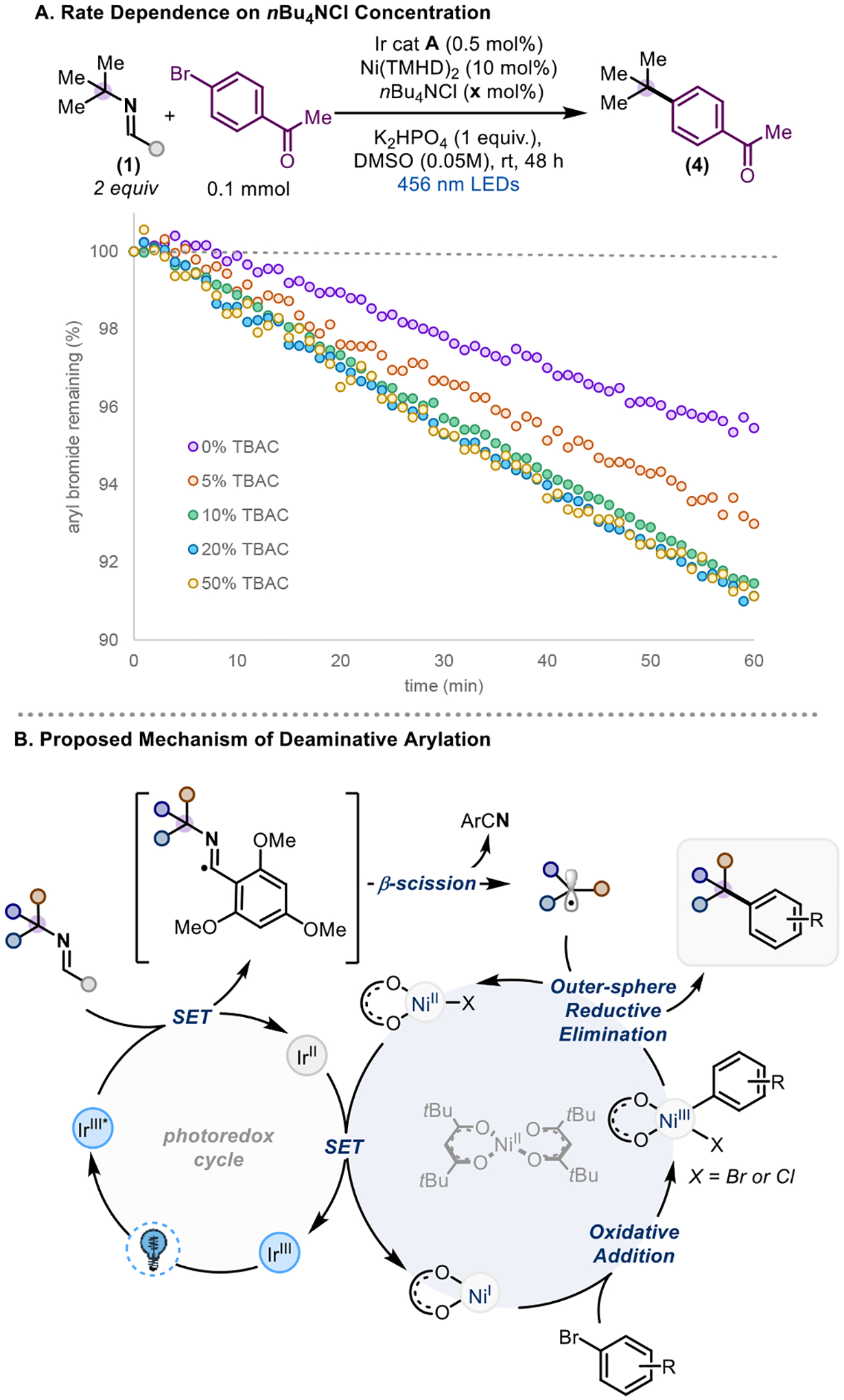
We next turned our attention to the role of additive on the reaction. Both nBu4NCl and tert-butyl imidazolium chloride were found to be beneficial to reactivity. Interestingly, without an additive, an appreciable amount of nickel black is observed in the reaction. Because tetra-alkyl ammonium salts and imidazolium salts are known to provide electro-steric stabilization of nanoparticles,44–48 we propose that the first role of the additive may be to prevent catalyst death by preventing aggregation that would lead to the irreversible formation of nickel black.38 By doing so, this preserves the necessary equilibrium with the active catalyst. Indeed, tracking the reaction progress over time indicates that with nBu4NCl, product formation increases steadily over time, whereas without nBu4NCl the product formation plateaus around 50% (see SI for more details). In addition, substituting chloride for other anions still provides an increase in yield (Table 1, entries 5 & 6), in line with a nanoparticle stabilization argument.49
In-situ LED-NMR kinetic analysis indicates that nBu4NCl also provides a rate acceleration within the first hour of the reaction (Scheme 3A), demonstrating that preventing catalyst death is not its only role. In this case, exchanging the chloride anion does not provide the same rate acceleration (see SI for kinetics data), indicating that chloride is necessary. The rate of starting material consumption tracks linearly between 0, 5, and 10% of nBu4NCl loading, but increased loadings (20, 50%) do not improve arene consumption. Therefore, adding more nBu4NCl than nickel catalyst loading does not affect the initial rate of reaction but improves the yield of reaction, presumably due to preventing catalyst death. This observation is consistent with saturation kinetics, where more additive relative to catalyst loading has no effect on the reactivity due to complete saturation of the catalyst. We hypothesized that we may be forming an anionic nickel complex that would undergo accelerated oxidative addition, similar to what has been seen for anionic palladium species in combination with alkyl ammonium chlorides.48,50 Thus, we attempted to form the active Ni(I) catalyst in situ via cyclic voltammetry and add in nBu4NCl in hopes of generating the anionic complex, but did not see the formation of a new species. In addition, nBu4NCl has no effect on the reduction potential from Ni(II) to Ni(I), indicating accelerated reduction to the active catalyst is not responsible for the rate acceleration.51 Lastly, UV-Vis experiments indicate no change in the catalyst upon addition of nBu4NCl, in contrast to recent work.51 Alternatively, the chloride could exchange with the Ni(III) oxidative addition complex, similar to observations in Gong’s reductive coupling,29 generating a Ni(II)-Cl species after reductive elimination, which has been shown to be more reducible than its bromide analogue.52,53
Scheme 3.
Mechanistic Investigation
Our proposed mechanism begins with oxidation of the redox-active imine by the excited state of the Ir photocatalyst (Scheme 3B). In our previous work, we provided evidence that the tertiary alkyl radical is generated via an imidoyl radical intermediate.22 Meanwhile, the nickel catalyst can undergo oxidative addition to generate a Ni(III) species. Because anionic diketone-based ligands were necessary to achieve productive reactivity, we propose that outer-sphere reductive elimination, as described by the Molander and Gutierrez groups,40 generates the desired product and NiII-X. Single electron transfer between the reduced photocatalyst and Ni(II) turns over both catalytic cycles.
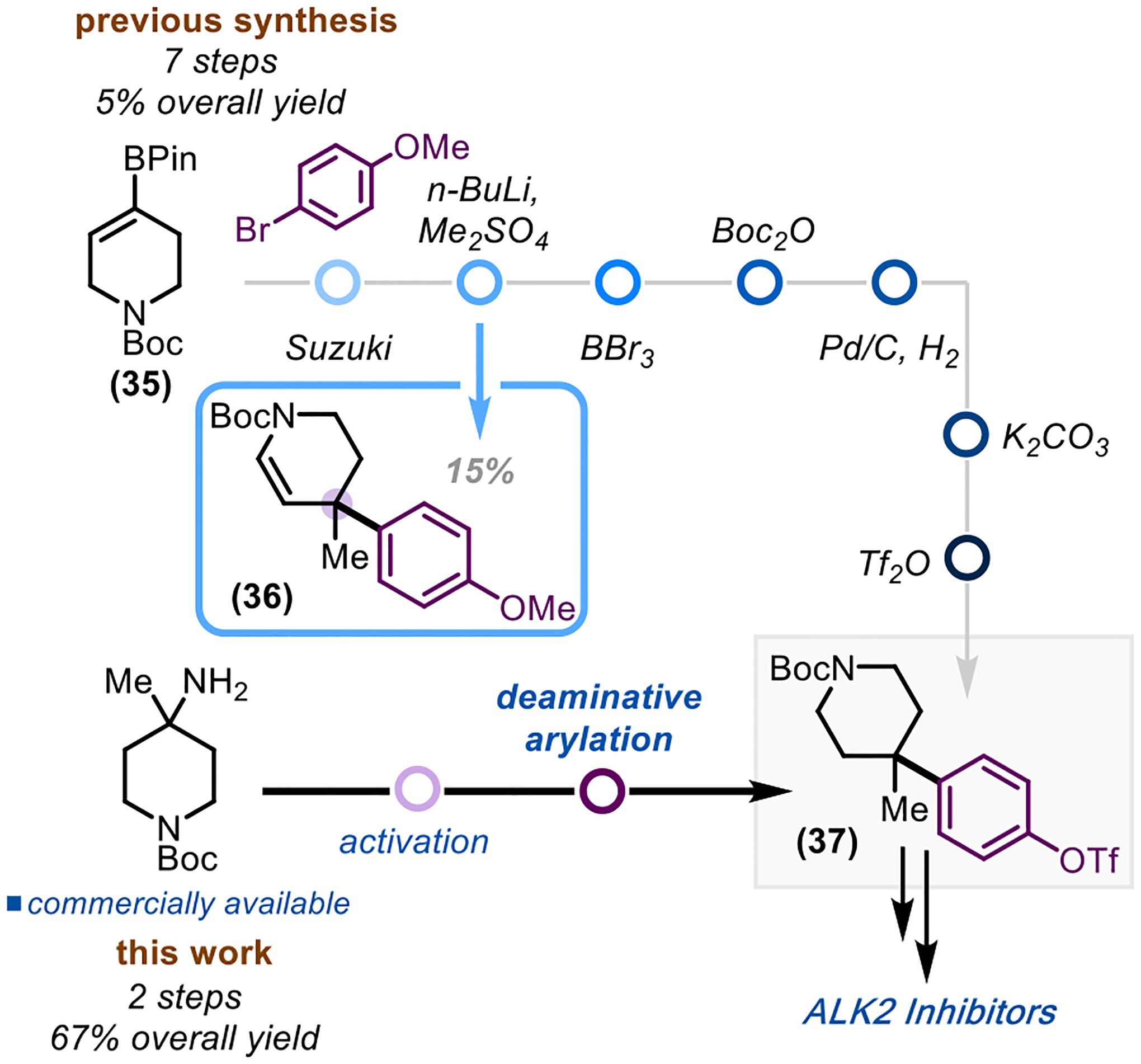
Our reaction can also be applied to medicinally relevant scaffolds, highlighting the ability to install quaternary carbons for pharmaceutical applications (Scheme 4). The reported convergent synthesis of Activin receptor-like kinase-2 inhibitors relies on N-Boc piperidine compound 37 as a key intermediate.54 This compound is synthesized in a seven-step linear sequence starting with pinacol borane 35 with a 5% overall yield. The formation of the quaternary center (36) is the bottleneck of the current route, providing product in only a 15% yield. Utilizing our method and starting from the commercially available amine allows for the expedited synthesis of compound 37, with only two steps and a 67% overall yield.
Scheme 4.
Streamlined Synthesis of ALK2 Inhibitors
In summary, we have developed a protocol to activate the C-N bond of sterically encumbered α-3° amines to utilize them as alkyl precursors for a dual photoredox/nickel-catalyzed C(sp3)-C(sp2) cross-coupling. Upon condensing primary amines onto an electron-rich activating group, we generate tertiary alkyl radicals that couple with a NiIII aryl-halide complex to generate benzylic quaternary centers. Using nBu4NCl as an additive increased the rate of the reaction while also resuscitating the nickel catalyst. Given increasing use of deaminative functionalizations with α-1° and α-2° amines via Katritzky salts, our complementary deamination addresses a synthetic need by unveiling α-3° as alkyl precursors for arylation. By leveraging amines as tertiary radical precursors, we have expanded the synthetic toolbox which will assist in diversifying the chemical space in pharmaceutical chemistry.
Supplementary Material
ACKNOWLEDGMENT
We thank NIGMS (GM125206) for support. JRD thanks the NSF for a Graduate Research Fellowship. MAA thanks BMS for a graduate fellowship.
Funding Sources
GM125206.
Footnotes
Supporting Information. This material is available free of charge via the ACS publications website at DOI: https://doi.org/10.1021/jacs.1c10150
Experimental procedures, spectral characterization, and additional data
The authors declare no competing financial interest.
REFERENCES
- (1).Cushnie TPT; Cushnie B; Lamb AJ Alkaloids: An Overview of Their Antibacterial, Antibiotic-Enhancing and Antivirulence Activities. Int. J. Antimicrob. Agents 2014, 44 (5), 377–386. 10.1016/j.ijantimicag.2014.06.001. [DOI] [PubMed] [Google Scholar]
- (2).McGrath NA; Brichacek M; Njardarson JT A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ 2010, 87 (12), 1348–1349. 10.1021/ed1003806. [DOI] [Google Scholar]
- (3).Basch CH; Liao J; Xu J; Piane JJ; Watson MP Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C–N Bond Activation. J. Am. Chem. Soc 2017, 139 (15), 5313–5316. 10.1021/jacs.7b02389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).J.; Moore S; TrochaGrimshaw J Electrochemical Reactions. Part 26. Radicals Derived by Reduction of N-Alkylpyridinium Salts and Homologous N,N’- Polymethylenebispyridinium Salts. Cleavage of the Carbon-Nitrogen Bond. Acta Chem. Scand., Ser. B 1983, 37, 485–489. [Google Scholar]
- (5).Yu C-G; Matsuo Y Nickel-Catalyzed Deaminative Acylation of Activated Aliphatic Amines with Aromatic Amides via C–N Bond Activation. Org. Lett 2020, 22 (3), 950–955. 10.1021/acs.orglett.9b04497. [DOI] [PubMed] [Google Scholar]
- (6).Baker KM; Lucas Baca D; Plunkett S; Daneker ME; Watson MP Engaging Alkenes and Alkynes in Deaminative Alkyl–Alkyl and Alkyl–Vinyl Cross-Couplings of Alkylpyridinium Salts. Org. Lett 2019, 21 (23), 9738–9741. 10.1021/acs.or-glett.9b03899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zhu Z-F; Tu J-L; Liu F Ni-Catalyzed Deaminative Hydroalkylation of Internal Alkynes. Chem. Commun 2019, 55 (76), 11478–11481. 10.1039/C9CC05385A. [DOI] [PubMed] [Google Scholar]
- (8).Jin Y; Wu J; Lin Z; Lan Y; Wang C Merger of C–F and C–N Bond Cleavage in Cross-Electrophile Coupling for the Synthesis of Gem-Difluoroalkenes. Org. Lett 2020, 22 (14), 5347–5352. 10.1021/acs.orglett.0c01592. [DOI] [PubMed] [Google Scholar]
- (9).Wang J; Hoerrner ME; Watson MP; Weix DJ Nickel-Catalyzed Synthesis of Dialkyl Ketones from the Coupling of N-Alkyl Pyridinium Salts with Activated Carboxylic Acids. Angew. Chem. Int. Ed 2020, 59 (32), 13484–13489. 10.1002/anie.202002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).For a single example of an α-3° Katritzky salt, see: Liao J; Basch CH; Hoerrner ME; Talley MR; Boscoe BP; Tucker JW; Garnsey MR; Watson MP Deaminative Reductive Cross-Electrophile Couplings of Alkylpyridinium Salts and Aryl Bromides. Org. Lett 2019, 21 (8), 2941–2946. 10.1021/acs.orglett.9b01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Plunkett S; Basch CH; Santana SO; Watson MP Harnessing Alkylpyridinium Salts as Electrophiles in Deaminative Alkyl–Alkyl Cross-Couplings. J. Am. Chem. Soc 2019, 141 (6), 2257–2262. 10.1021/jacs.9b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cobb KM; Rabb-Lynch JM; Hoerrner ME; Manders A; Zhou Q; Watson MP Stereospecific, Nickel-Catalyzed Suzuki–Miyaura Cross-Coupling of Allylic Pivalates To Deliver Quaternary Stereocenters. Org. Lett 2017, 19 (16), 4355–4358. 10.1021/acs.orglett.7b02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zhang M-M; Liu F Visible-Light-Mediated Allylation of Alkyl Radicals with Allylic Sulfones via a Deaminative Strategy. Org. Chem. Front 2018, 5 (23), 3443–3446. 10.1039/C8QO01046C. [DOI] [Google Scholar]
- (14).Yang Z-K; Xu N-X; Wang C; Uchiyama M Photoinduced C(Sp3)−N Bond Cleavage Leading to the Stereoselective Syntheses of Alkenes. Chem. – Eur. J 2019, 25 (21), 5433–5439. 10.1002/chem.201900886. [DOI] [PubMed] [Google Scholar]
- (15).Zhu Z-F; Zhang M-M; Liu F Radical Alkylation of Isocyanides with Amino Acid-/Peptide-Derived Katritzky Salts via Photoredox Catalysis. Org. Biomol. Chem 2019, 17 (6), 1531–1534. 10.1039/C8OB02786B. [DOI] [PubMed] [Google Scholar]
- (16).Wang X; Kuang Y; Ye S; Wu J Photoredox-Catalyzed Synthesis of Sulfones through Deaminative Insertion of Sulfur Dioxide. Chem. Commun 2019, 55 (99), 14962–14964. 10.1039/C9CC08333B. [DOI] [PubMed] [Google Scholar]
- (17).Xu Y; Xu Z-J; Liu Z-P; Lou H Visible-Light-Mediated de-Aminative Alkylation of N-Arylamines with Alkyl Katritzky Salts. Org. Chem. Front 2019, 6 (23), 3902–3905. 10.1039/C9QO01175G. [DOI] [Google Scholar]
- (18).Schönbauer D; Sambiagio C; Noël T; Schnürch M Photocatalytic Deaminative Benzylation and Alkylation of Tetrahydroisoquinolines with N-Alkylpyrydinium Salts. Beilstein J. Org. Chem 2020, 16 (1), 809–817. 10.3762/bjoc.16.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Yi J; Badir SO; Kammer LM; Ribagorda M; Molander GA Deaminative Reductive Arylation Enabled by Nickel/Photoredox Dual Catalysis. Org. Lett 2019, 21 (9), 3346–3351. 10.1021/acs.orglett.9b01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Correia JTM; Fernandes VA; Matsuo BT; Delgado JAC; Souza WC de; Paixão, M. W. Photoinduced Deaminative Strategies: Katritzky Salts as Alkyl Radical Precursors. Chem. Commun 2020, 56 (4), 503–514. 10.1039/C9CC08348K. [DOI] [PubMed] [Google Scholar]
- (21).Tcyrulnikov S; Cai Q; Twitty JC; Xu J; Atifi A; Bercher OP; Yap GPA; Rosenthal J; Watson MP; Kozlowski MC Dissection of Alkylpyridinium Structures to Understand Deamination Reactions. ACS Catal 2021, 8456–8466. 10.1021/acscatal.1c01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ashley MA; Rovis T Photoredox-Catalyzed Deaminative Alkylation via C–N Bond Activation of Primary Amines. J. Am. Chem. Soc 2020, 142 (43), 18310–18316. 10.1021/jacs.0c08595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Dombrowski AW; Gesmundo NJ; Aguirre AL; Sarris KA; Young JM; Bogdan AR; Martin MC; Gedeon S; Wang Y Expanding the Medicinal Chemist Toolbox: Comparing Seven C(Sp2)–C(Sp3) Cross-Coupling Methods by Library Synthesis. ACS Med. Chem. Lett 2020, 11 (4), 597–604. 10.1021/acsmedchemlett.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59 (10), 4443–4458. 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- (25).Talele TT Opportunities for Tapping into Three-Dimensional Chemical Space through a Quaternary Carbon. J. Med. Chem 2020, 63 (22), 13291–13315. 10.1021/acs.jmedchem.0c00829. [DOI] [PubMed] [Google Scholar]
- (26).Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52 (21), 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- (27).Cox B; Zdorichenko V; Cox PB; Booker-Milburn KI; Paumier R; Elliott LD; Robertson-Ralph M; Bloomfield G Escaping from Flatland: Substituted Bridged Pyrrolidine Fragments with Inherent Three-Dimensional Character. ACS Med. Chem. Lett 2020, 11 (6), 1185–1190. 10.1021/acsmedchemlett.0c00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Wang X; Wang S; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc 2015, 137 (36), 11562–11565. 10.1021/jacs.5b06255. [DOI] [PubMed] [Google Scholar]
- (29).Wang X; Ma G; Peng Y; Pitsch CE; Moll BJ; Ly TD; Wang X; Gong H Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides. J. Am. Chem. Soc 2018, 140 (43), 14490–14497. 10.1021/jacs.8b09473. [DOI] [PubMed] [Google Scholar]
- (30).Primer DN; Molander GA Enabling the Cross-Coupling of Tertiary Organoboron Nucleophiles through Radical-Mediated Alkyl Transfer. J. Am. Chem. Soc 2017, 139 (29), 9847–9850. 10.1021/jacs.7b06288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Joshi-Pangu A; Wang C-Y; Biscoe MR Nickel-Catalyzed Kumada Cross-Coupling Reactions of Tertiary Alkylmagnesium Halides and Aryl Bromides/Triflates. J. Am. Chem. Soc 2011, 133 (22), 8478–8481. 10.1021/ja202769t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lohre C; Dröge T; Wang C; Glorius F Nickel-Catalyzed Cross-Coupling of Aryl Bromides with Tertiary Grignard Reagents Utilizing Donor-Functionalized N-Heterocyclic Carbenes (NHCs). Chem.– Eur. J 2011, 17 (22), 6052–6055. 10.1002/chem.201100909. [DOI] [PubMed] [Google Scholar]
- (33).Green SA; Vásquez-Céspedes S; Shenvi RA Iron–Nickel Dual-Catalysis: A New Engine for Olefin Functionalization and the Formation of Quaternary Centers. J. Am. Chem. Soc 2018, 140 (36), 11317–11324. 10.1021/jacs.8b05868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Liu C-F; Luo X; Wang H; Koh MJ Catalytic Regioselective Olefin Hydroarylation(Alkenylation) by Sequential Carbonickelation-Hydride Transfer. J. Am. Chem. Soc 2021, 143 (25), 9498–9506. jacs.1c03228. 10.1021/jacs.1c03228. [DOI] [PubMed] [Google Scholar]
- (35).Chen T-G; Zhang H; Mykhailiuk PK; Merchant RR; Smith CA; Qin T; Baran PS Quaternary Centers by Nickel-Catalyzed Cross-Coupling of Tertiary Carboxylic Acids and (Hetero)Aryl Zinc Reagents. Angew. Chem. Int. Ed 2019, 58 (8), 2454–2458. 10.1002/anie.201814524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).We suspect the lower yield arises from competitive oxidation by bromide anion. For oxidation potentials, see: Zhang P; Le C “Chip”; MacMillan DWC Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc 2016, 138 (26), 8084–8087. 10.1021/jacs.6b04818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37). We attempted pyridine, pyrazine, and indole-based heteroaryl halides, but saw no conversion to the desired product. This is in accord with previous systems using Ni(TMHD)2, due to substrate coordination to the nickel center which halts catalysis (see Ref 30).
- (38).Gisbertz S; Reischauer S; Pieber B Overcoming Limitations in Dual Photoredox/Nickel-Catalysed C–N Cross-Couplings Due to Catalyst Deactivation. Nat. Catal 2020, 3 (8), 611–620. 10.1038/s41929-020-0473-6. [DOI] [Google Scholar]
- (39).Low yields of electron-rich aryl iodides are also consistent with outer-sphere reductive elimination because of a polarity mismatch between the electron-rich alkyl radical and electron-rich arene. As such, we have observed protodehalogenation and chlorinated arene in these cases.
- (40).Yuan M; Song Z; Badir SO; Molander GA; Gutierrez O On the Nature of C(Sp3)–C(Sp2) Bond Formation in Nickel-Catalyzed Tertiary Radical Cross-Couplings: A Case Study of Ni/Photoredox Catalytic Cross-Coupling of Alkyl Radicals and Aryl Halides. J. Am. Chem. Soc 2020, 142 (15), 7225–7234. 10.1021/jacs.0c02355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Gilmore JL; King BW; Harris C; Maduskuie T; Mercer SE; Liu R-Q; Covington MB; Qian M; Ribadeneria MD; Vaddi K; Trzaskos JM; Newton RC; Decicco CP; Duan JJW Synthesis and Structure–Activity Relationship of a Novel, Achiral Series of TNF-α Converting Enzyme Inhibitors. Bioorg. Med. Chem. Lett 2006, 16 (10), 2699–2704. 10.1016/j.bmcl.2006.02.015. [DOI] [PubMed] [Google Scholar]
- (42).Stevenson GI; Huscroft I; MacLeod AM; Swain CJ; Cascieri MA; Chicchi GG; Graham MI; Harrison T; Kelleher FJ; Kurtz M; Ladduwahetty T; Merchant KJ; Metzger JM; MacIntyre DE; Sadowski S; Sohal B; Owens AP 4,4-Disubstituted Piperidine High-Affinity NK1 Antagonists: Structure−Activity Relationships and in Vivo Activity. J. Med. Chem 1998, 41 (23), 4623–4635. 10.1021/jm980376b. [DOI] [PubMed] [Google Scholar]
- (43).Kazmierski WM; Anderson DL; Aquino C; Chauder BA; Duan M; Ferris R; Kenakin T; Koble CS; Lang DG; Mcintyre MS; Peckham J; Watson C; Wheelan P; Spaltenstein A; Wire MB; Svolto A; Youngman M Novel 4,4-Disubstituted Piperidine-Based C–C Chemokine Receptor-5 Inhibitors with High Potency against Human Immunodeficiency Virus-1 and an Improved Human Ether-a-Go-Go Related Gene (HERG) Profile. J. Med. Chem 2011, 54 (11), 3756–3767. 10.1021/jm200279v. [DOI] [PubMed] [Google Scholar]
- (44).Astruc D; Lu F; Aranzaes JR Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed 2005, 44 (48), 7852–7872. 10.1002/anie.200500766. [DOI] [PubMed] [Google Scholar]
- (45).Manojkumar K; Sivaramakrishna A; Vijayakrishna K A Short Review on Stable Metal Nanoparticles Using Ionic Liquids, Supported Ionic Liquids, and Poly(Ionic Liquids). J. Nanoparticle Res 2016, 18 (4), 103. 10.1007/s11051-016-3409-y. [DOI] [Google Scholar]
- (46).Deraedt C; Astruc D “Homeopathic” Palladium Nanoparticle Catalysis of Cross Carbon–Carbon Coupling Reactions. Acc. Chem. Res 2014, 47 (2), 494–503. 10.1021/ar400168s. [DOI] [PubMed] [Google Scholar]
- (47).Modrow H; Bucher S; Hormes J; Brinkmann R; Bönnemann H Model for Chainlength-Dependent Core−Surfactant Interaction in N(Alkyl)4Cl-Stabilized Colloidal Metal Particles Obtained from X-Ray Absorption Spectroscopy. J. Phys. Chem. B 2003, 107 (16), 3684–3689. 10.1021/jp0217740. [DOI] [Google Scholar]
- (48).Schroeter F; Strassner T Understanding Anionic “Ligand-less” Palladium Species in the Mizoroki–Heck Reaction. Inorg. Chem 2018, 57 (9), 5159–5173. 10.1021/acs.inorg-chem.8b00175. [DOI] [PubMed] [Google Scholar]
- (49).Migowski P; Dupont J Catalytic Applications of Metal Nanoparticles in Imidazolium Ionic Liquids. Chem. – Eur. J 2007, 13 (1), 32–39. 10.1002/chem.200601438. [DOI] [PubMed] [Google Scholar]
- (50).Schroeter F; Soellner J; Strassner T Cross-Coupling Catalysis by an Anionic Palladium Complex. ACS Catal 2017, 7 (4), 3004–3009. 10.1021/acscatal.6b03655. [DOI] [Google Scholar]
- (51).Wang D; XU T A Pivotal Role of Chloride Ion on Nickel-Catalyzed Enantioselective Reductive Cross-Coupling to Perfluoroalkylated Boronate Esters. ACS Catal 2021, 12469–12475. 10.1021/acscatal.1c03265. [DOI] [Google Scholar]
- (52).We have attempted to synthesize the Ni(TMHD)ArBr oxidative addition complex. However, current methods to make nickel oxidative addition complexes are reported with bipyridine ligands. As such, these methods were not suitable for diketone-based ligands. Current efforts are geared towards characterizing these complexes.
- (53).Lin Q; Dawson G; Diao T Experimental Electrochemical Potentials of Nickel Complexes. Synlett 2021, 32 (16), 1606–1620. 10.1055/s-0040-1719829. [DOI] [Google Scholar]
- (54).Blueprint Medicines Corporation; Brooijmans N; Brubaker JD; Fleming PE; Hodous BL; Kim JL; Waetzig J; Williams B; Wilson D; Wilson KJ Inhibitors of Activin Receptor-like Kinase. Patent WO2017/181117 A1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


