Abstract
Background
Inflammatory mammary cancer (IMC), the counterpart of human inflammatory breast cancer (IBC), is the deadliest form of canine mammary tumors. IMC patients lack specific therapy and have poor outcomes. This proof-of-principle preclinical study evaluated the efficacy, safety, and effect on survival of neoadjuvant intratumoral (in situ) empty cowpea mosaic virus (eCPMV) immunotherapy in companion dogs diagnosed with IMC.
Methods
Ten IMC-bearing dogs were enrolled in the study. Five dogs received medical therapy, and five received weekly neoadjuvant in situ eCPMV immunotherapy (0.2–0.4 mg per injection) and medical therapy after the second eCPMV injection. Efficacy was evaluated by reduction of tumor growth; safety by hematological and biochemistry changes in blood and plasma; and patient outcome by survival analysis. eCPMV-induced immune changes in blood cells were analyzed by flow cytometry; changes in the tumor microenvironment were evaluated by CD3 (T lymphocytes), CD20 (B lymphocytes), FoxP3 (Treg lymphocytes), myeloperoxidase (MPO; neutrophils), Ki-67 (proliferation index, PI; tumor cell proliferation), and Cleaved Caspase-3 (CC-3; apoptosis) immunohistochemistry.
Results
Two neoadjuvant in situ eCPMV injections resulted in tumor shrinkage in all patients by day 14 without systemic adverse events. Although surgery for IMC is generally not an option, reduction in tumor size allowed surgery in two IMC patients. In peripheral blood, in situ eCPMV immunotherapy was associated with a significant decrease of Treg+/CD8+ ratio and changes in CD8+Granzyme B+ T cells, which behave as a lagging predictive biomarker. In the TME, higher neutrophilic infiltration and MPO expression, lower tumor Ki-67 PI, increase in CD3+ lymphocytes, decrease in FoxP3+/CD3+ ratio (p<0.04 for all comparisons), and no changes in CC-3+ immunostainings were observed in post-treatment tumor tissues when compared with pretreatment tumor samples. eCPMV-treated IMC patients had a statistically significant (p=0.033) improved overall survival than patients treated with medical therapy.
Conclusions
Neoadjuvant in situ eCPMV immunotherapy demonstrated anti-tumor efficacy and improved survival in IMC patients without systemic adverse effects. eCPMV-induced changes in immune cells point to neutrophils as a driver of immune response. Neoadjuvant in situ eCPMV immunotherapy could be a groundbreaking immunotherapy for canine IMC and a potential future immunotherapy for human IBC patients.
Keywords: vaccination; neutrophil Infiltration; immunogenicity, vaccine; breast neoplasms; drug evaluation, preclinical
Introduction
Inflammatory breast cancer (IBC) is a rare, aggressive, and highly metastatic form of human breast cancer (BC). At time of diagnosis, all IBC patients have lymph node involvement and ~40% have distant identifiable metastases.1 2 IBC accounts for roughly 2.5% of all newly diagnosed BC in the US, but is responsible for ~10% of BC-related deaths,3 4 making it the deadliest form of BC. Anthracycline-taxane-based chemotherapy, with targeted therapy when appropriate, remains the backbone of neoadjuvant therapy for IBC5 6; and its efficacy is minimally effective as shown by a 15-year survival rate of ~20%–30%.6 The inability to identify effective therapy has multiple factors, but two major contributing factors are the absence of optimal models to identify mechanisms involved in the aggressive behavior of IBC, and the associated lack of knowledge of in vivo IBC biology. The limited knowledge we do have has been obtained from in vitro studies using cell lines and patient-derived xenograft models using immunodeficient mice.7 While cell lines are useful for drug sensitivity studies, and published data reveal a remarkable correlation between drug responses in patient-derived xenograft models and clinical patient outcomes,8 9 a direct extrapolation of useful data obtained from these models into human clinical trials is not always possible. This is particularly true for immunotherapy studies since human xenografts can only be studied in vivo using immunodeficient mice.
Rodent cancer immunotherapy studies usually use syngeneic cell lines in inbred mice, but there is no rodent cell line model of IBC or canine inflammatory mammary cancer (IMC). Canine cancer patients, however, can bridge rodent lab studies and human clinical trials.10–12 Cancers in dogs occur spontaneously, have clinical and pathophysiological presentation equivalent to human cancers, and share genomic and immune features. Canine patients are outbred, have intact immune systems and a tumor that, like spontaneous human tumors, is predominantly ‘self’ immunologically, making them a uniquely valuable model for immunotherapy studies. The progression of disease in dogs recapitulates disease course in humans to a much greater extent than rodent cancer models.10–12 Canine mammary tumors are the most frequent neoplasia in sexually intact female dogs.13 14 The annual incidence of mammary tumors within all canine tumors is ~17%,14 and the frequency of malignant tumors varies from 48% to 70% of the overall mammary tumor patients.13 14 Strikingly, dogs are also affected by IMC which, as in human IBC, is the most aggressive type of mammary tumors, with a reported incidence of 18% of all canine malignant mammary tumors.15 IMC dogs live an average of 1 month post diagnosis in absence of specific treatment.15 As we have recently reviewed, canine IMC represents a unique and excellent therapeutic model to evaluate the clinical efficacy of new anticancer agents, approaches or combinations, including immunotherapy.16
In our previous studies, we extensively documented the ability of in situ vaccination using cowpea mosaic virus (CPMV) nanoparticles and empty CPMV (eCPMV) virus-like nanoparticles to stimulate antitumor immune responses and improve outcomes in various syngeneic murine tumor models, including BC,17–19 and canine oral melanoma.20 In this proof-of-principle study, we evaluated the clinical efficacy of the highly immunogenic eCPMV nanoparticles against canine IMC. CPMV and eCPMV are identical in their protein content, but eCPMV lacks RNA and, therefore, is non-infectious toward plants, offering safety from an agricultural perspective. Our results demonstrated robust clinical efficacy of neoadjuvant in situ eCPMV immunotherapy (eCPMV immunotherapy from here on) leading to tumor reduction in all treated IMC dogs and improved survival of dogs treated with this agent. eCPMV immunotherapy induced a strong neutrophilic tumor infiltration associated with necrosis and supported neutrophils as a driver of tumor cell death. This study is the first step towards human clinical trials using eCPMV to treat IBC which therapeutically remains an orphan disease.
Materials and methods
Canine patient recruitment and selection criteria
Inclusion criteria at diagnosis, histopathological and histological grade classification are described in detail in online supplemental file 1. Diagnosis of IMC was based on the presence of characteristic clinical signs including erythema, edema, warmth, pain, and histopathological confirmation of neoplastic invasion of superficial dermal lymphatic vessels.15 21 The characteristics of the 10 IMC patients included in this study are summarized in table 1. All patients’ owners signed an informed consent. For this study, we followed the ARRIVE guidelines 2.0.22
Table 1.
Epidemiological and clinicopathological characteristics of eCPMV-treated and control canine IMC patients
| Patient | Age, y. | Weight, kg | TS,* cm | TS,† cm3 | Breed | Type | Histo. grade | Histo. type | sdLVI | LNI | Therapy | OS, days |
| eCPMV-treated IMC patients | ||||||||||||
| P1 | 11.0 | 10.3 | 15.1 | 74.8 | Mixed | Primary | III | Special type | Yes | Yes | +FCT | 174 |
| P2 | 13.5 | 25.6 | 15.7 | 45.7 | Mixed | Secondary | III | Simple | Yes | Yes | +FCT | 156 |
| P3 | 10.7 | 8.2 | 19.6 | 104.2 | Poodle | Secondary | III | Simple | Yes | Yes | +FCT | 109 |
| P4 | 10.7 | 17.0 | 17.3 | 52.7 | Kerry Blue Terrier | Secondary | III | Simple | Yes | Yes | +FCT | 165 |
| P5 | 11.9 | 2.7 | 3.9 | 4.3 | Bichon Frise | Secondary | III | Simple | Yes | Yes | +FCT | 67 |
| Control IMC patients | ||||||||||||
| P6 | 13.0 | 26.2 | 14.0 | 252.0 | Mixed | Secondary | III | DA | Yes | Yes | FCT | 27 |
| P7 | 14.2 | 7.6 | 7.0 | 14.0 | Maltese | Secondary | III | Simple | Yes | Yes | FCT | 40 |
| P8 | 9.7 | 10.3 | 4.0 | 32.0 | Mixed | Secondary | III | Simple | Yes | Yes | FCT | 132 |
| P9 | 13.0 | 9.3 | 5.0 | 30.6 | Miniature Schnauzer | Secondary | III | Simple | Yes | Yes | FCT | 63 |
| P10 | 8.9 | 7.7 | 3.0 | 9.4 | Poodle | Secondary | III | Simple | Yes | Yes | FCT | 73 |
Age at diagnosis in years.
+FCT indicates that eCPMV-treated dogs received FCT therapy starting after second eCPMV injection until surgery or death, and P1 and P5 continued on FCT as adjuvant therapy until death.
*TS refers to the largest diameter of the target tumor in cm.
†TS refers to tumor volume in cm3; type, refers to primary and secondary IMC.
DA, ductal associated; eCPMV, empty cowpea mosaic virus; FCT, firocoxib+cyclophosphamide+toceranib; Histo, histologic; IMC, inflammatory mammary cancer; LNI, regional lymph node involvement; OS, overall survival time; sdLVI, superficial dermal lymphovascular invasion; TS, tumor size.
jitc-2021-004044supp001.pdf (2.7MB, pdf)
eCPMV immunotherapy protocol and follow-up
A pretreatment incisional biopsy of the target tumor was taken from all patients followed by intratumoral eCPMV injection immediately after obtaining the biopsy sample. Collection of the tumor biopsy is described in online supplemental file 1. Incisional biopsies from two patients with confirmed IMC status referred from a different institution were not available. eCPMV production, dosage, number of the tumor injections, and the tumor injection procedure are described in online supplemental file and figure S1. In situ eCPMV vaccination was administered once a week (day 0 (D0) and day 7 (D7)). Reduction in tumor volume (Tv) was observed by D14 and a few owners of IMC patients agreed to additional eCPMV immunotherapy (table 2). The IMC patients treated in this study presented one (patients P3, P4 and P5) or two individual tumor masses (patients P1 and P2). For P1, only the larger tumor was injected during the first two treatments, and both tumors were injected in the subsequent treatments. For P2, both tumors were always injected.
Table 2.
Tv changes in eCPMV-treated and control dogs
| Patient | Day | eCPMV dose, mg | Treatments | Tv, cm3 | %TGI | P value |
| P1 (T1) | D0 | 0.200 | 8 | 67.4 | 0.0 | |
| D7 | 0.200 | 49.5 | −26.5 | |||
| D19 | 0.200 | 36.9 | −45.3 | 0.063 | ||
| DFU | 13.2 | −80.4 | 0.007 | |||
| P1 (T2) | D0 | 6 | 7.4 | 0.0 | ||
| D7 | 10.0 | 35.1 | ||||
| D19 | 0.200 | 17.8 | 140.7 | 0.179 | ||
| DFU | 5.2 | −29.7 | 0.977 | |||
| P2 (T1) | D0 | 0.350 | 7 | 39.6 | 0.0 | |
| D7 | 0.350 | 28.6 | −27.8 | |||
| D14 | 0.350 | 23.6 | −40.4 | 0.137 | ||
| DFU | 0.350 | 43.1 | 8.9 | 0.920 | ||
| P2 (T2) | D0 | 0.050 | 7 | 6.1 | 0.0 | |
| D7 | 0.050 | 2.1 | −64.9 | |||
| D14 | 0.050 | 0.7 | −89.3 | 0.163 | ||
| DFU | 0.050 | 2.9 | −52.3 | 0.379 | ||
| P3 | D0 | 0.400 | 2 | 104.2 | 0.0 | |
| D7 | 0.400 | 25.2 | −75.8 | |||
| D14 | 24.7 | −76.3 | 0.330 | |||
| P4 | D0 | 0.400 | 3 | 52.7 | 0.0 | |
| D7 | 0.400 | 61.4 | 16.6 | |||
| D15 | 0.400 | 51.0 | −3.1 | 0.907 | ||
| P5 | D0 | 0.200 | 2 | 4.3 | 0.0 | |
| D9 | 0.200 | 4.5 | 3.5 | |||
| D17 | 4.2 | −3.5 | 0.667 | |||
| Control canine IMC patients | ||||||
| P6 | D0 | 252.0 | ||||
| D25 | 459.0 | 82.2 | ||||
| P7 | D0 | 14.0 | ||||
| NA | NA | |||||
| P8 | D0 | 32.0 | ||||
| D73 | 75.3 | 124.6 | ||||
| P9 | D0 | 30.6 | ||||
| D35 | 94.6 | 209.0 | ||||
| P10 | D0 | 9.4 | ||||
| D39 | 30.8 | 228.6 | ||||
D0, D7, D14, DFU, day 0, 7, 14 and at last follow-up (D92 in P1 and D79 in P2), respectively; T1, target tumor; T2, second tumor treated; NA, not available; Treatments refer to the total number of eCPMV injections; %TGI, percentage of tumor growth inhibition. P values obtained by regression analysis as described in Methods. The first P value shows regression analysis from D0 to D14; the second, from D0 to last follow-up.
DFU, date of follow-up; eCPMV, empty cowpea mosaic virus; NA, not available; TGI, tumor growth inhibition; Tv, tumor volume.
IMC patients are not eligible for a surgical procedure.23 The medical therapy24 (described in online supplemental file) was added to eCPMV immunotherapy after the second eCPMV injection. Patient P1 had severe adverse events to toceranib and was taken off medical therapy after 23 days of administration. After diagnosis, all IMC control dogs not treated with eCPMV received the medical therapy until death (table 1).
When eCPMV-induced tumor reduction allowed surgery, the tumor was resected with collection of a surgical biopsy, and the medical therapy was maintained as adjuvant treatment. Surgical procedures were performed per institutional standard of care protocol (described in online supplemental file). After surgery, follow-up was performed once a month until death or euthanasia. Thoracic radiographs and abdominal ultrasound were performed every 2 months to search for distant metastases.
Quality of life and tumor response evaluation
Each canine patient was closely observed by the attending veterinarian in the clinic for 4 hours after each injection and subsequently by the owner on a daily basis to evaluate the potential adverse events induced by eCPMV vaccination. The quality of life (QOL) of each patient was evaluated at first eCPMV dose and at D7 and D14 using a preestablished survey.25
The tumor response to the eCPMV vaccination was evaluated once a week during the treatment period by measuring the Tv with calipers using the formula Tv=0.5 × long axis × short axis2. The percentage of tumor growth inhibition (%TGI) was estimated by the formula %TGI=100 x(final Tv-initial Tv)/initial Tv. All measurements are in cubic centimeters (cm3). Although the Response Evaluation Criteria in Solid Tumors in cancer immunotherapy trials (iRECIST) is not designed to address in situ vaccination in canine patients (27), the iRECIST criteria are also provided in online supplemental table S1.
Hematological, flow cytometry, biochemical and cytokine analyses
A blood sample (~10 mL) was collected from each patient at D0, D7, and D14 to evaluate changes induced by eCPMV immunotherapy. Hematological analyses were done using a standard hematology analyzer (ADVIA 120, Siemens Healthcare, Madrid, Spain). Samples collected at D0, D14, and at various time points after surgery were used to isolate peripheral blood mononuclear cells (PBMCs) for flow cytometry using the Lymphocyte Isolation Reagent kit per manufacturer’s instructions (Ficoll 1.077 g/mL solution, Rafer, Zaragoza, Spain). Isolated cells were transferred to a freezing medium (70% RPMI, 20% DMSO and 10% fetal bovine serum), frozen at −80°C overnight, and then transferred into liquid nitrogen until sample processing. Flow cytometry analysis was performed using a 14-color panel (online supplemental table S2) using reagents and procedures as previously described.26 Stained samples were acquired using LSR Fortessa II (BD Biosciences, San Jose, California, USA) equipped with 4 lasers and 16 detectors. Flow cytometric analysis was performed using FlowJo software (V.10.7.1; BD Bioscience).
The biochemical panel (glucose, creatinine, urea and alanine aminotransferase (ALT)) of each patient was performed by reflection spectrophotometry (Refrovet Plus, Scil animal care company, Viernhheim, Germany) and for total proteins by Biuret’s colorimetric test (Bradford Diagnostics, Sigma-Aldrich, St. Louis, MO, USA). Cytokine measurement in plasma samples was performed using the MILLIPLEX Canine Cytokine/Chemokine Magnetic Bead Panel (Merck Millipore, Burlington, MA, USA). The list of cytokines is provided in online supplemental file 1. The evaluation of hematological, biochemical and other adverse events related to eCPMV immunotherapy was performed according to the Veterinary Cooperative Oncology Group criteria in the V.2.27
Histopathology and immunohistochemistry assays
Single 3 µm tumor tissue sections were used for histopathology and immunohistochemistry (IHC). The IHC assays for Ki-67, CC-3, myeloperoxidase (MPO), IL-8, CD3, CD20, and FoxP3, and the scoring of all markers are described in online supplemental file 1 and are reported following REMARK guidelines.28
Statistical analyses
Primary outcomes were efficacy, measured by reduction in Tv; biosafety, measured by evaluation of hematological and biochemistry changes in blood and plasma; and, survival of treated patients, measured by tracking clinical status of patients. The Kaplan-Meier method with the log-rank test was used to estimate survival. Unpaired Student’s t-test, Mann-Whitney U test, Fisher’s exact test, and the χ2 test were used as appropriate. Two-tailed p values less than 0.05 were considered statistically significant. Statistical analyses were carried out using IBM SPSS Statistics program (V.25) and GraphPad Prism (V.7.02; GraphPad San Diego, California, USA) software. Full details of statistical analysis are provided in online supplemental file 1.
Results
eCPMV immunotherapy vaccination induces tumor shrinkage in IMC patients
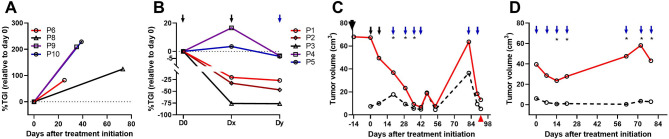
Of the 10 enrolled IMC patients, 5 did not receive the eCPMV immunotherapy because the owners declined treatment. These patients were used as control cases (figure 1A; table 2). The remaining five IMC patients were treated with eCPMV immunotherapy: Patients P3 and P5 received two injections, P4, three injections (figure 1B), P1, eight injections (figure 1C), and P2, seven injections (figure 1D). Owners of patients P3 and P4 refused additional treatment for personal reasons. P3 died at D109 of unknown cause and P4 was euthanized at D165 due to disease progression (table 1). P1 and P2 are described in detail below. Treatment response evaluated by %TGI demonstrated shrinkage of Tv at D7 in three patients (P1, P2, and P3, figure 1B and table 2) and tumor growth (pseudoprogression) in patients P4 and P5 with tumor shrinkage by D14 (figure 1B and table 2). The eCPMV immunotherapy resulted in tumor reduction sufficient to enable surgery in P5 and P1 after 2 and 8 injections, respectively (figure 1B, C). Regression analyses indicate that all patients had a reduction in tumor burden after two eCPMV injections (table 2).
Figure 1.
Neoadjuvant in situ eCPMV immunotherapy induced tumor regression in canine IMC patients. IMC patients were treated and followed up as described in the Methods. (A, B) Tumor growth inhibition (TGI) as percentage of growth relative to D0. (A) %TGI in control canine IMC patients treated with medical therapy from D0, but no eCPMV therapy. Tumor volume changes in P7 during follow-up were not available. (B) %TGI in eCPMV-treated IMC patients. Dx refers to measurements done at D7 (P1, P2, P3, P4) and D9 (P5). Dy refers to measurements at D14 (P2, P3); D15 (P4), D17 (P5) and D19 (P1). Black arrows indicate eCPMV immunotherapy for all dogs; blue, for P1, P2 and P4. (C, D) Tumor growth kinetics in P1 (C) until surgery at D92 indicated by a red arrowhead, and P2 (D) until last day of follow-up at D79. Large black arrow in C indicates start of anti-COX2 therapy (15 days before eCPMV immunotherapy). Black arrows in C indicate eCPMV immunotherapy given to T1 and *, medical therapy as described in the Methods section. P1 and P2 patients have a second tumor mass (black broken line) (C, D). Blue arrows indicates treatment provided to the largest (T1; solid red line) and smallest (T2; interrupted black line) tumors in P1 (C) and both tumors in P2 (D). eCPMV, empty cowpea mosaic virus; IMC, inflammatory mammary cancer.
Patients P1 and P2 received more treatments and were followed up in more detail. P1 presented two concurrent large masses classified as special type carcinoma located in different mammary chains (table 2). Before eCPMV treatment, this patient was on anti-COX-2 (firocoxib) therapy for 15 days without responding to that therapy. As shown in figure 1C, the tumor growth kinetics demonstrate that eCPMV immunotherapy resulted in an immediate response that was sustained following each weekly eCPMV injection. At D20, medical therapy was added for 3 weeks to the eCPMV immunotherapy, but due to toxicities, toceranib was removed (at D42, after 23 days of toceranib treatment), and the dog received only firocoxib and metronomic cyclophosphamide along with eCPMV immunotherapy for three additional treatments. With regards to durability of treatment response, a rapid tumor growth was observed during the time the patient was receiving firocoxib and metronomic cyclophosphamide, but not eCPMV immunotherapy because the owners and the dog were not available (D54–D82 in figure 1C). However, as soon as the eCPMV injections were resumed, a sharp decrease in tumor growth was observed to the point that the patient underwent bilateral radical mastectomy to remove the tumor masses (D92 in figure 1C). Of note, treatment of a second IMC mass was also started on D19 and the clinical response was similar to the largest mass treated at the same time (figure 1B). This patient lived ~6 months and died of noncancer-related renal failure.
P2 with two tumor masses classified as simple carcinomas in different mammary chains was enrolled from an external clinic at D57 of her diagnosis. For simplicity, the first eCPMV injection is represented here as D0. This tumor showed fluctuation in the response to eCPMV immunotherapy with a decrease in tumor size followed by tumor growth up to D70, and again, a decrease in tumor size up to D79 (figure 1D). Shortly after that visit the patient was euthanized in a different clinic at about D99 due to dyspnea and metastatic lung disease. Similar to patient P1, tumor growth was observed during the time the patient was not on treatment because the Hospital was closed due to COVID-19 lockdown (D21 to D62 in figure 1D). Interestingly, a second eCPMV-treated mass in this patient was more responsive to the therapy and after a decrease in the Tv, it never significantly grew again and remained almost unchanged during the observation period (figure 1D; table 2). This patient lived ~5 months.
eCPMV immunotherapy is not toxic
No adverse reactions at injection site or systemic reactions were observed during the 4-hour period after each eCPMV administration. eCPMV immunotherapy did not induce significant fluctuations in hematocrit and hemoglobin levels in any dog over the 14-day period. Fluctuations remained within the normal range in all eCPMV treated dogs (online supplemental figure S2). Further, no significant changes in total proteins (albumin and globulins levels) were observed during the treatment period (online supplemental table S3 and figure S3A). Despite fluctuations in the levels of glucose, urea, creatinine, and ALT during eCPMV immunotherapy, these changes remained within the normal ranges (online supplemental figure S3B–E). Hence, these findings indicate that eCPMV immunotherapy with this dosing does not negatively affect hepatic, renal and digestive functions in the vaccinated dogs. No changes were observed in blood cell numbers, and protein levels in the control patients not treated with eCPMV (online supplemental table S4).
Per our QOL questionnaire, QOL was improved in three dogs (P1, P2, and P3) and two dogs reported no changes in QOL during the 14-day observation time (P4 and P5; data not shown).
eCPMV immunotherapy induces changes in immune blood cell populations
White blood cell analysis indicates a decrease in lymphocyte, monocyte, and mature neutrophil numbers at D7 in three dogs (P3, P4, and P5), with a subsequent increase close to pretreatment levels in lymphocytes and mature neutrophils, and a further decrease in the monocyte levels (online supplemental figure S4A–C). Immature neutrophils in these three cases remain close to or slightly higher than pretreatment levels (online supplemental figure S4D). In the other two eCPMV-treated dogs (P1 and P2), lymphocytes and monocytes remained increased at D14. An increase in mature and immature neutrophils was observed in both dogs, with P1 showing a large increase in immature neutrophils without reaching statistical significance (online supplemental figure S4D). Change in blood cell numbers during treatment in all treated dogs is shown in online supplemental table S3. These findings suggest that eCPMV immunotherapy induces an increase in peripheral blood inflammatory cells.
Immunophenotyping by flow cytometry on canine PBMCs collected at D0 and after eCPMV immunotherapy detected a decrease of the regulatory T cells (Treg)/CD8+ ratio in treated IMC patients to various degrees (figure 2A; p=0.019; gating strategy is shown in online supplemental figure S5). Individual changes in CD8+, Treg+, Treg+/CD8+ cells ratio, and CD8+ granzyme B (GZMB)+ cells numbers is presented in online supplemental table S5. Individual analysis of immune cell changes in patients P1, P2, P4, and P5 over time during eCPMV immunotherapy revealed a general trend of decreased Treg+/CD8+ ratio in the various patients with some fluctuations in P1 and P5 (figure 2B). Of note, as we observed for the %TGI, an increase in Treg+/CD8+ ratio was observed in P1 around the time when eCPMV immunotherapy was interrupted (D54 to D82 in figure 2B). However, as soon as the treatment was restarted at D82, a subsequent sharp decrease in Treg+/CD8+ ratio was observed in P1 (figure 2B). In addition, a sharp increase in Treg+/CD8+ ratio post-surgery was observed in P1 and P5 patients, the only two dogs that underwent surgery (figure 2B).
Figure 2.
Neoadjuvant in situ eCPMV immunotherapy induced changes in T cell populations in canine IMC patients. PBMCs were processed for flow analysis as described in Methods. (A) Change of Treg+/CD8+ ratio in IMC patients before (D0) and at first isolation after eCPMV treatment (Dx). Asterisk (*) indicates p<0.05 in a paired t-test. (B) Individual changes in Treg+/CD8+ ratio in IMC patients treated with eCPMV. Black arrows indicate eCPMV therapy for all dogs; blue for P1, P2, and P4; red for P1, and brown for P2. (C) Percentage change in CD8+GZMB+ T cell population during eCPMV treatment; values are represented as percentage change over D0. Red arrowheads in the x-axis indicate surgery time on D17 for P5 and D92 for P1. eCPMV, empty cowpea mosaic virus; IMC, inflammatory mammary cancer; PBMCs, peripheral blood mononuclear cells.
Changes in CD8+ T cells expressing cytotoxicity marker GZMB were also evaluated in four patients (figure 2C and online supplemental table S5). A fluctuation in CD8+GZMB+ T cells was observed in the treated IMC patients, with an initial decrease in circulating CD8+GZMB+ T cells in patients P2, P4, and P5 and a subsequent increase in patients P2 and P5. Patient P1 showed increases in CD8+GZMB+ T cells from the start of eCPMV immunotherapy up to D84 when the levels started decreasing until D92 when surgery was performed. Then, a sharp postsurgery increase in CD8+GZMB+ T cells was observed until the last follow-up on D117 (figure 2C). This increase pattern was significant over the treatment period (figure 2C; p=0.007 (data not shown)).
eCPMV immunotherapy induces changes in IL-8 plasma levels
Of the 13 cytokines analyzed, no significant eCPMV-induced changes were observed at D7 and D14 when compared with D0, except for IL-8 which increased at D7 in P1, P4, and P5. The IL-8 levels decreased by D14 in all dogs, except in P1 where the levels showed a 10-fold increase when compared with pretreatment levels (online supplemental figure S6; P = 0.039). Average changes in IL-8 plasma levels in all patients during treatment are shown in online supplemental table S3.
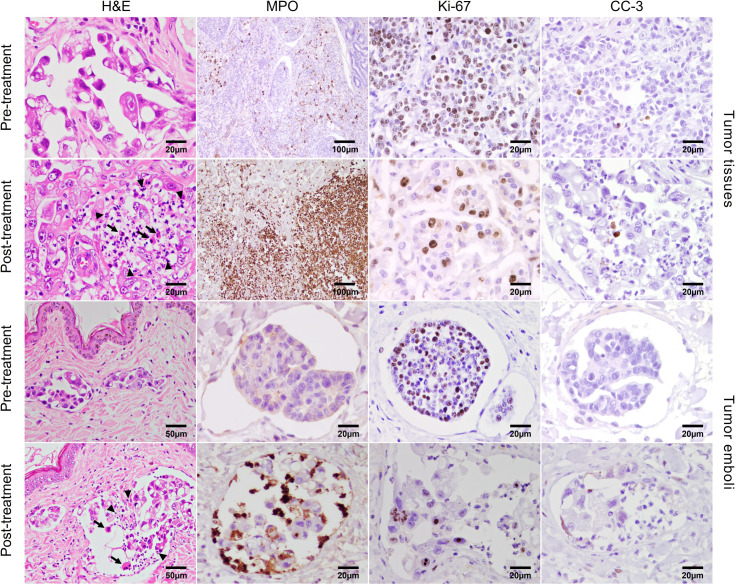
Neoadjuvant in situ eCPMV immunotherapy is associated with a strong neutrophilic infiltration and tumor cell death in tumor tissues and tumor emboli
eCPMV immunotherapy induced a large neutrophilic infiltration and associated tumor cell death (necrosis) in post-treatment as compared with pretreatment tumor samples (table 3; figure 3; H&E staining) and emboli (figure 3; H&E staining). The percentage of total necrosis area and neutrophil-associated necrosis area was significantly higher in post-treatment as compared with pretreatment tumor tissues (p=0.024, for both; online supplemental table S6; table 3). Furthermore, MPO expression, an enzyme secreted by neutrophils during inflammation, was significantly higher in post-treatment than in pretreatment tumor specimens (p=0.032; online supplemental table S6; figure 3; table 3). In addition, tumor Ki-67 proliferation index (PI) was lower in post-treatment than in pretreatment tumor samples (figure 3; table 3; online supplemental table S6; P=0.038). The percentage of tumor cells undergoing apoptosis (CC-3+) was not different between pretreatment and post-treatment tumor samples (figure 3; table 3; online supplemental table S6), indicating that apoptosis is not the mechanism of tumor cell death. Of note, although it was not possible to quantify the CC-3 expression in tumor emboli due to insuficient number of cells in emboli to apply the scoring system, a similar pattern of neutrophil influx, changes in MPO, CC-3, and Ki-67 immunostaining as in the tumor tissue were observed in tumor emboli from post-treatment samples as compared with pretreatment samples (figure 3). Further, a significant increase in CD3+ T lymphocyte density was observed in post-treatment compared with pretreatment samples (p=0.024; online supplemental table S6; table 3; online supplemental figure S7). An increase in CD20+ B lymphocytes in post-treatment was observed in two of three IMC patients (table 3; online supplemental table S6 and figure S7), and a decrease in FoxP3+ lymphocytes was observed in two of three post-treatment samples (table 3; online supplemental table S6 and figure S7). In addition, the FoxP3+/CD3+ ratio decreased significantly in post-treatment samples (p=0.012; online supplemental table S6).
Table 3.
Histopathological and immunohistochemical changes in individual tumor samples induced by eCPMV immunotherapy
| Patient | Ki-67 (%) | CC-3 (%) | MPO (%) | IL-8 | CD3* | CD20* | FoxP3* | FoxP3/CD3 | Total necrosis (%)‡ | NAN (%) | nNAN (%) |
| eCPMV-treated canine IMC patients | |||||||||||
| P1 | |||||||||||
| Pretreated | † | † | 0.86 | 0.00 | 241.89 | 162.53 | 63.68 | 0.26 | 0.00 | 0.00 | 0.00 |
| Post-treated (day 92) | 41.42 | 12.00 | 15.45 | 2.00 | 1422.32 | 944.63 | 133.05 | 0.09 | 5.38 | 5.19 | 0.19 |
| P2 | |||||||||||
| Pretreated | 37.93 | 17.09 | 0.99 | 0.00 | 258.21 | 318.63 | 107.25 | 0.42 | 0.63 | 0.00 | 0.63 |
| Post-treated (day 156) | 29.48 | 5.18 | 2.09 | 2.00 | 343.89 | 80.42 | 77.51 | 0.23 | 0.97 | 0.47 | 0.50 |
| P5 | |||||||||||
| Pretreated | 60.75 | 1.00 | 1.14 | 0.00 | 253.79 | 130.32 | 99.73 | 0.39 | 0.59 | 0.00 | 0.59 |
| Post-treated (day 17) | 38.59 | 6.41 | 9.62 | 2.00 | 759.79 | 162.74 | 88.15 | 0.12 | 2.21 | 1.43 | 0.78 |
| Control canine IMC patients | |||||||||||
| P6 | 82.24 | 8.36 | 0.56 | 0.00 | 107.16 | 160.32 | 41.47 | 0.39 | 0.04 | 0.04 | 0.00 |
| P7 | 70.70 | 3.29 | 2.50 | 0.00 | 258.21 | 145.58 | 57.88 | 0.22 | 0.01 | 0.00 | 0.01 |
| P8 | 48.88 | 3.13 | 0.26 | 1.00 | 326.00 | 307.26 | 142.74 | 0.44 | 0.00 | 0.00 | 0.00 |
*The number of CD3-positive, CD20-positive, and FoxP3-positive cells is provided per mm2.
†Ki-67 and CC-3 percentages were not calculated for pretreatment biopsy of P1 due to the low availability of neoplastic cells in the pretreatment biopsy.
‡The presence of necrosis (total, neutrophil and non-neutrophil associated) was the only histopathological change assessed in H&E.
CC-3, cleaved caspase-3; eCPMV, empty cowpea mosaic virus; IL-8, interleukin-8; IMC, inflammatory mammary cancer; MPO, myeloperoxidase; NAN, neutrophil-associated necrosis; nNAN, non-neutrophil-associated necrosis.
Figure 3.
Neoadjuvant in situ eCPMV immunotherapy induced a high neutrophilic infiltration in tumor samples and tumor emboli. Representative histopathology and immunostaining of tumor tissues from pretreatment/control and post-treatment samples. A strong neutrophilic infiltration is seen in post-treatment tumor tissues and emboli than in pretreatment tissues and tumor emboli as indicated by H&E and myeloperoxidase (MPO) expression. Higher Ki-67 proliferation index is observed in pretreatment tumor tissues and emboli than in post-treatment tumor tissues and emboli. There is no difference in cleaved caspase 3 (CC-3) immunolabeling between pretreatment tumor tissues and emboli than in post-treatment tumor tissues and emboli. eCPMV, empty cowpea mosaic virus.
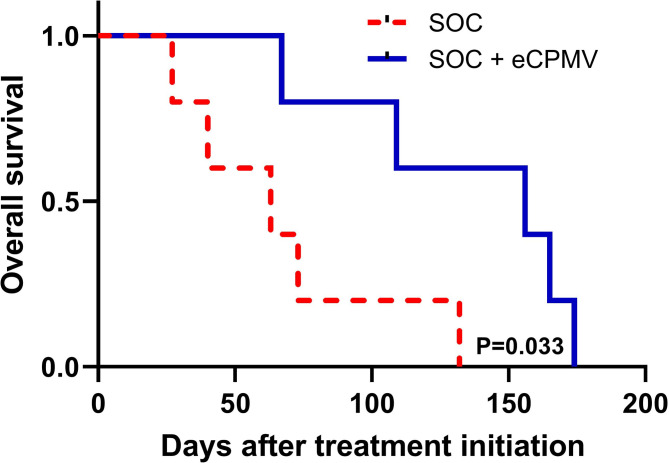
eCPMV immunotherapy is associated with improved survival in treated patients
The mean survival time of dogs treated with eCPMV was significantly higher than the mean survival time of the control group which received the medical therapy but not eCPMV immunotherapy (134 days vs 67 days (data not shown); figure 4, p=0.033). All patients, except P1 (who died of unrelated renal failure), were euthanized due to tumor progression.
Figure 4.
Neoadjuvant in situ eCPMV immunotherapy is associated with improved survival in canine IMC patients. Canine patients treated with eCPMV injections (continuous line) showed longer survival than dogs treated only with medical therapy (broken line), but not eCPMV therapy. Y-axis denotes the survival probability and x-axis the number of days after first treatment with eCPMV therapy. Kaplan-Meier analysis was performed as described in Materials and Methods. eCPMV, empty cowpea mosaic virus; IMC, inflammatory mammary cancer; SOC, standard of care.
Discussion
This study established the potential clinical utility of eCPMV nanoparticles as a novel immunotherapy against canine IMC, a highly aggressive and metastatic disease. Given the extensive local involvement, the presence of coagulopathies and distant metastatic disease, surgery is not recommended for IMC.23 To our knowledge, this is the first report of an immunotherapy-based approach to treat canine IMC patients, and the first time a treatment allowed surgical intervention in IMC patients.
The translational value of our findings relates to tumor regression in response to eCPMV immunotherapy. All IMC patients enrolled in this study who received just two injections of eCPMV immunotherapy within a ~2-week period had tumor regression. The positive response to eCMPV therapy translated into a survival of ~6 months for patient P1 who died of renal failure with no evidence of metastatic disease in the kidneys. Necropsy analysis showed that renal failure was probably caused by COX inhibitors and kidney infarcts, which could be associated with tumor-related coagulopathies.23 29 Further, overall survival benefit was statistically significant in IMC patients treated with eCMPV therapy compared with IMC control patients who lived an average of 1 month without treatment.15 30 Although an excellent survival of ~6 months was reported for IMC patients receiving more aggressive medical therapy (piroxicam, thalidomide, and toceranib) plus radiation therapy,31 to our knowledge, reports of improved survival in IMC patients receiving medical therapy and just a single agent as demonstrated in this study are very limited.24 32 Collectively, our results support the beneficial effect of eCPMV immunotherapy on survival in companion dogs diagnosed with spontaneous IMC, as previously reported in metastatic canine oral melanoma tumors.20
Although the blood analysis in eCPMV-treated IMC dogs demonstrated individual fluctuations in the levels of lymphocytes, monocytes, mature and immature neutrophils, immature neutrophils always remained above the pretreatment levels. Similar fluctuations within normal ranges were observed in blood biochemistry analysis. Hence, eCPMV immunotherapy does not induce toxic events. Of note, of the 13 cytokines analyzed, IL-8 was the only cytokine showing notable changes in the blood of eCPMV-treated IMC patients. IL-8 is secreted by blood monocytes, alveolar macrophages, fibroblasts, endothelial cells, and epithelial cells.33 The pleiotropic functions of IL-8 include varied effects such as recruitment of neutrophils, stimulation of angiogenesis and stimulation of tumor-cell proliferation.33 While high systemic IL-8 levels are associated with adverse cancer prognosis, resistance to immunotherapy in human tumors,34 35 and worse outcome in canine mammary tumors,36 changes in plasma IL-8 levels did not correlate with response in eCPMV-treated IMC dogs. These findings suggest a different role of IL-8 in the biology of IMC patients or in the response to a potent immunogenic agent such as eCPMV nanoparticles.
In previous syngeneic murine tumor models, including the breast 4T1 model, we demonstrated that eCPMV particles were rapidly taken up by and activate neutrophils in the tumor microenvironment (TME) as an important part of the antitumor immune response.17 In this canine model, we observed that eCPMV immunotherapy induced an increase in blood mature and inmature blood neutrophils, transient increases in IL-8 in the blood, and a strong neutrophilic infiltration in the tumor mass. Of note, neutrophil infiltration was predictive of treatment benefit in murine mastocytomas treated with intratumoral injection of Complete Freund’s adjuvant (CFA, containing killed mycobacteria).37 Mycobacteria triggers an immune response by activating toll-like receptors (TLR) 2 and 4.38 Furthermore, intratumoral CFA injections resulted in high neutrophil influx accompanied by extensive tumor necrosis in human renal, prostate, bladder and cervical carcinomas; and an increased B and T cell infiltration was observed in treated canine mastocytomas,37 providing evidence of a systemic immune response to the in situ treatment similar to what we observed in our eCPMV-treated dogs.
We have previously demonstrated that eCPMV induced immune response in mice by activating TLR2 and TLR4 to generate a high level inflammatory response.17 39 This leads to phagocyte activation and switching their immune suppressive status to become antigen presenting cells carrying tumor antigens to the draining lymph nodes where effector T cells are generated. These activated T cells, along with the activated myeloid cells, are responsible for the anti-tumor response and systemic effects which result in elimination of circulating and distant tumor deposits. While we cannot be sure that details of responses to eCPMV do not vary between mice and dogs, it is reasonable to assume that they are quite similar overall.
The lower Ki-67 PI of cancer cells and the absence of an increase in the percentage of tumor cells undergoing apoptosis suggest that apoptosis is not the mechanism of cell death, and that eCPMV-activated neutrophils are potentially responsible for tumor cell death. Importantly, the fact that post-treatment tumor emboli show strong neutrophilic activity and similar staining of MPO, Ki-67, and CC-3 as in post-treatment tumor samples is of clinical relevance. Tumor emboli in human IBC are associated with metastatic behavior of IBC.40 41 Induction of cell killing in tumor emboli could explain delayed metastatic formation and better survival in eCPMV-treated patients. Although significant and promising, a higher number of IMC cases treated with eCPMV are necessary to confirm these results.
Blood samples from canine cancer patients have an increased Treg+/CD8+ T cell ratio compared with healthy dogs42 and a higher ratio was associated with short survival in canine osteosarcoma.43 Our analysis demonstrated a steady decrease of the peripheral blood Treg+/CD8+ ratio in all dogs during treatment with a sharp increase observed in both surgically treated dogs (P1 and P5) after surgery. Although both metronomic cyclophosphamide and toceranib decrease Treg in dogs,44 45 we consider that eCPMV immunotherapy was the driving factor in decreasing the Treg+/CD8+ ratio because it was the only therapy given to all dogs for 2 weeks. Further, during the time the patient was not undergoing treatment, a sharp increase in the Treg+/CD8+ ratio in patient P1 was observed. At that time, the patient was on anti-COX-2 and cyclophosphamide therapy without eCPMV immunotherapy. The post-surgery sharp increase in Treg+/CD8+ ratio in patients P1 and P5 is likely surgery related. Of note, a post-treatment decrease in FoxP3+/CD3+ ratio was also observed in P1, P2, and P3 tumor samples (table 3), indirectly indicating a decrease in immunosuppressive Treg lymphocytes. Our results extend previous findings that CPMV therapy induced Treg depletion in 4T1 BC model.19 Similar to the impressive response shown in IMC dogs, in situ CPMV vaccination in mice combined with systemic low-dose cyclophosphamide chemotherapy significantly inhibited tumor growth and substantially improved survival of mice bearing 4T1 tumors.19
Changes in CD8+GZMB+ T cells in peripheral blood induced by eCPMV immunotherapy behave as a lagging biomarker of response to therapy. While the effect is clearly observed in patient P1, the most responsive patient to eCPMV immunotherapy, studies with more patients are needed to draw definitive conclusions.
Efficacious therapies combining treatments like chemotherapy with radiation therapy are associated with adverse events like cutaneous, hematologic, and gastrointestinal toxicities in dogs.31 46 Blood plasma biochemistry studies did not show abnormal changes in any of the indicators used to track potential adverse events caused by eCMPV therapy. In agreement with previous studies,20 we did not observe adverse reactions at the injection site or systemic reactions after any eCPMV administration, and QOL was even improved in three out of five patients. Hence, eCMPV therapy appears to be safe and well tolerated.
The strengths of this proof-of-principle study are: (1) This novel in situ eCPMV vaccination approach was highly effective and resulted in significant tumor reduction; it was safe, without adverse events; and it improved QOL and survival in treated IMC patients. (2) While immune changes observed in blood and tumor biopsies are still hypothesis-generating, changes in blood Treg+/CD8+ ratio and CD8+GZMB+ T cells, and tumor FoxP3+/CD3+ ratio tracked tumor reduction and could be potential predictors of response to eCPMV immunotherapy. (3) Neutrophils are potentially responsible for tumor cell death. However, a higher number of IMC cases treated with eCPMV and more analysis of neutrophil effector functions are necessary to confirm these results. (4) eCPMV immunotherapy resulted in significant tumor reduction allowing surgery in two patients. Collectively, these findings have clinical relevance and translation value for human IBC. The exceedingly high efficacy of neoadjuvant in situ eCPMV vaccination in IMC patients opens the possibility of employing in situ eCPMV vaccination as a potential novel and effective neoadjuvant immunotherapy against IBC, a therapeutically orphan disease with poor outcome.
This proof-of-principle study has limitations such as low patient numbers and lack of randomization because IMC patients are rare. A potential bias in survival analysis may result by allocating sicker dogs in the control group than in the treatment arm. However, as indicated in table 1, high-risk factors were similar in both groups and all owners of dogs in either group of the study expressed interest in experimental treatment. Dog owner refusal to participate in the trial was primarily related to their inability to return for planned eCPMV injections on the required schedule, rather than lack of interest in aggressive treatment.
Going forward, future studies will focus on expanding the therapy to a larger number of IMC patients with an in-depth analysis of tumor samples to generate insights into mechanisms of action of eCPMV nanoparticles in IMC. Further, we intend to expand our studies to evaluate combinations of eCMPV therapy with chemotherapy and/or immune checkpoint inhibitors.
Most canine tumors express PD-L1 constitutively and both innate and adaptive immune stimuli can further upregulate PD-L1 expression.47 48 We expect that combining in situ eCPMV immunotherapy with cross-functional human anti-PD-L1 inhibitors49 or canine anti-PD-1/PD-L1 inhibitors, as they become available,48 50 will result in higher efficacy than single agent used as monotherapy. We have demonstrated that in situ eCPMV synergizes with systemic checkpoint immunotherapy in various mouse models.51
Conclusion
Our findings support the implementation of neoajuvant in situ eCPMV immunotherapy as a novel and safe immunotherapy against IMC and suggest that neutrophils are drivers of tumor cell death. We envision neoadjuvant in situ eCPMV immunotherapy as a future treatment for IBC patients.
jitc-2021-004044supp002.pdf (121.4KB, pdf)
Acknowledgments
We would like to thank all canine owners who were very generous and supportive of the present study and the personnel of the Veterinary Teaching Hospital Complutense for their help and support, and Mrs. Kathleen J. Bryar for proofreading the manuscript.
Footnotes
DA-M and GV contributed equally.
LP and HA-P contributed equally.
Contributors: Conception and design: HA-P, LP, SF and NFS; Development of methodology: VB, NFS and JvB; Acquisition of data (provided animals, acquired and managed patients and provided facilities): MDP-A, DA-M, GV, AA-D, MS-R, LP, SF, NFS, JvB; Analysis and interpretation of data (eg, statistical analysis, biostatistics and computational analysis): DA-M, GV, MDP-A, LP, DG, SP, JvB, SF, HA-P; Writing, review and/or revision of the paper: HA-P, DA-M, GV, DG, SP, JvB, MDP-A, AA-D, VB, LP, SF, NFS; Administrative, technical or material support (ie, generating eCPMV, reporting or organizing data, constructing databases): HA-P, DA-M, LP, SF, NFS, JvB. Guarantor: HA-P.
Funding: This study was supported in part by the NCI (U01CA218292 and R01CA224605 to NFS and SF); Spanish Ministry of Science, Innovation and Technology (project PGC2018-094516-B-I00 to LP and MDP-A); the PhD contract at Complutense University (7026349846-Y0SC001170 to DAM); the ECVP specialization 'Residency in Veterinary Pathology' grant from Complutense University (69/2018 to AA-D); a PhD grant funded through the Mexican Council for Science and Technology (CONACYT; 515916 to GV); the Swiss Cancer Research grants (KFS-3852-02-2016 and KFS-4146-02-2017; to JvB); and the Novartis Foundation for Medical-Biological Research (#16C231; to JvB).
Competing interests: NFS and SF are co-founders of, have equity in, and have a financial interest with Mosaic ImmunoEngineering Inc. SF serves as scientific advisor and paid consultant to Mosaic; NFS serves as Director, Board Member, and Acting Chief Scientific Officer, and paid consultant to Mosaic. JvB is a cofounder and has financial interest in InCephalo AG and is a part-time employee at InCephalo. The other authors declare no potential conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This prospective study was performed at the Mammary Oncology Unit of the Veterinary Teaching Hospital at Complutense University, Madrid, Spain, from October 2018 to November 2020 (approved by the institutional animal care and use committee; Study #04/2018).
References
- 1.van Uden DJP, Bretveld R, Siesling S, et al. Inflammatory breast cancer in the Netherlands; improved survival over the last decades. Breast Cancer Res Treat 2017;162:365–74. 10.1007/s10549-017-4119-6 [DOI] [PubMed] [Google Scholar]
- 2.Fayanju OM, Ren Y, Greenup RA, et al. Extent of axillary surgery in inflammatory breast cancer: a survival analysis of 3500 patients. Breast Cancer Res Treat 2020;180:207–17. 10.1007/s10549-020-05529-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 2005;97:966–75. 10.1093/jnci/dji172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldner B, Behrendt CE, Schoellhammer HF, et al. Incidence of inflammatory breast cancer in women, 1992-2009, United States. Ann Surg Oncol 2014;21:1267–70. 10.1245/s10434-013-3439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low JA, Berman AW, Steinberg SM, et al. Long-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol 2004;22:4067–74. 10.1200/JCO.2004.04.068 [DOI] [PubMed] [Google Scholar]
- 6.Ueno NT, Buzdar AU, Singletary SE, et al. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson cancer center. Cancer Chemother Pharmacol 1997;40:321–9. 10.1007/s002800050664 [DOI] [PubMed] [Google Scholar]
- 7.Lim B, Woodward WA, Wang X, et al. Inflammatory breast cancer biology: the tumour microenvironment is key. Nat Rev Cancer 2018;18:485–99. 10.1038/s41568-018-0010-y [DOI] [PubMed] [Google Scholar]
- 8.Evans KW, Yuca E, Akcakanat A, et al. A population of heterogeneous breast cancer patient-derived xenografts demonstrate broad activity of PARP inhibitor in BRCA1/2 wild-type tumors. Clin Cancer Res 2017;23:6468–77. 10.1158/1078-0432.CCR-17-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAuliffe PF, Evans KW, Akcakanat A, et al. Ability to generate patient-derived breast cancer xenografts is enhanced in chemoresistant disease and predicts poor patient outcomes. PLoS One 2015;10:e0136851. 10.1371/journal.pone.0136851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman JD, Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Phil Trans R Soc Lond B Biol Sci 2015;370:20140231. 10.1098/rstb.2014.0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdivia G, Alonso-Diez Ángela, Pérez-Alenza D, et al. From conventional to precision therapy in canine mammary cancer: a comprehensive review. Front Vet Sci 2021;8:623800. 10.3389/fvets.2021.623800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dow S. A role for dogs in advancing cancer immunotherapy research. Front Immunol 2019;10:2935. 10.3389/fimmu.2019.02935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vascellari M, Capello K, Carminato A, et al. Incidence of mammary tumors in the canine population living in the Veneto region (northeastern Italy): risk factors and similarities to human breast cancer. Prev Vet Med 2016;126:183–9. 10.1016/j.prevetmed.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 14.Salas Y, Márquez A, Diaz D, et al. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002-2012: a growing animal health problem. PLoS One 2015;10:e0127381. 10.1371/journal.pone.0127381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez Alenza MD, Tabanera E, Peña L. Inflammatory mammary carcinoma in dogs: 33 cases (1995–1999). J Am Vet Med Assoc 2001;219:1110-4. 10.2460/javma.2001.219.1110 [DOI] [PubMed] [Google Scholar]
- 16.Raposo TP, Arias-Pulido H, Chaher N, et al. Comparative aspects of canine and human inflammatory breast cancer. Semin Oncol 2017;44:288–300. 10.1053/j.seminoncol.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 17.Lizotte PH, Wen AM, Sheen MR, et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol 2016;11:295–303. 10.1038/nnano.2015.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerstetter-Fogle A, Shukla S, Wang C, et al. Plant virus-like particle in situ vaccine for intracranial glioma immunotherapy. Cancers 2019;11:4. 10.3390/cancers11040515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai H, Wang C, Shukla S, et al. Cowpea mosaic virus immunotherapy combined with cyclophosphamide reduces breast cancer tumor burden and inhibits lung metastasis. Adv Sci 2019;6:1802281. 10.1002/advs.201802281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoopes PJ, Wagner RJ, Duval K, et al. Treatment of canine oral melanoma with Nanotechnology-Based immunotherapy and radiation. Mol Pharm 2018;15:3717–22. 10.1021/acs.molpharmaceut.8b00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peña L, Perez-Alenza MD, Rodriguez-Bertos A, et al. Canine inflammatory mammary carcinoma: histopathology, immunohistochemistry and clinical implications of 21 cases. Breast Cancer Res Treat 2003;78:141–8. 10.1023/A:1022991802116 [DOI] [PubMed] [Google Scholar]
- 22.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 2020;18:e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marconato L, Romanelli G, Stefanello D, et al. Prognostic factors for dogs with mammary inflammatory carcinoma: 43 cases (2003-2008). J Am Vet Med Assoc 2009;235:967–72. 10.2460/javma.235.8.967 [DOI] [PubMed] [Google Scholar]
- 24.Alonso‐Miguel D, Valdivia G, García‐San José P, et al. Clinical outcome of dogs diagnosed with canine inflammatory mammary cancer treated with metronomic cyclophosphamide, a cyclooxygenase‐2 inhibitor and toceranib phosphate. Vet Comp Oncol 2021;9. 10.1111/vco.12760 [DOI] [PubMed] [Google Scholar]
- 25.Lynch S, Savary-Bataille K, Leeuw B, et al. Development of a questionnaire assessing health-related quality-of-life in dogs and cats with cancer. Vet Comp Oncol 2011;9:172–82. 10.1111/j.1476-5829.2010.00244.x [DOI] [PubMed] [Google Scholar]
- 26.Pantelyushin S, Ranninger E, Bettschart-Wolfensberger R, et al. OMIP-065: dog immunophenotyping and T-cell activity evaluation with a 14-Color panel. Cytometry A 2020;97:1024–7. 10.1002/cyto.a.24168 [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc AK, Atherton M, Bentley RT, et al. Veterinary cooperative oncology group-common terminology criteria for adverse events (VCOG-CTCAE V2) following investigational therapy in dogs and cats. Vet Comp Oncol 2021;19:311–52. 10.1111/vco.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauerbrei W, Taube SE, McShane LM, et al. Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst 2018;110:803–11. 10.1093/jnci/djy088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monteiro-Steagall BP, Steagall PVM, Lascelles BDX. Systematic review of nonsteroidal anti-inflammatory drug-induced adverse effects in dogs. J Vet Intern Med 2013;27:1011–9. 10.1111/jvim.12127 [DOI] [PubMed] [Google Scholar]
- 30.Clemente M, De Andrés PJ, Peña L, et al. Survival time of dogs with inflammatory mammary cancer treated with palliative therapy alone or palliative therapy plus chemotherapy. Vet Rec 2009;165:78–81. 10.1136/vetrec.165.3.78 [DOI] [PubMed] [Google Scholar]
- 31.Rossi F, Sabattini S, Vascellari M, et al. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radiation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet Comp Oncol 2018;16:497–504. 10.1111/vco.12407 [DOI] [PubMed] [Google Scholar]
- 32.de M Souza CH, Toledo-Piza E, Amorin R, et al. Inflammatory mammary carcinoma in 12 dogs: clinical features, cyclooxygenase-2 expression, and response to piroxicam treatment. Can Vet J 2009;50:506–10. [PMC free article] [PubMed] [Google Scholar]
- 33.Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev 2017;60:24–31. 10.1016/j.ctrv.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 34.Schalper KA, Carleton M, Zhou M, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med 2020;26:688–92. 10.1038/s41591-020-0856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuen KC, Liu L-F, Gupta V, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med 2020;26:693–8. 10.1038/s41591-020-0860-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelaleti GB, Jardim BV, Leonel C, et al. Interleukin-8 as a prognostic serum marker in canine mammary gland neoplasias. Vet Immunol Immunopathol 2012;146:106–12. 10.1016/j.vetimm.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 37.Carroll CSE, Andrew ER, Malik L, et al. Simple and effective bacterial-based intratumoral cancer immunotherapy. J Immunother Cancer 2021;9:e002688. 10.1136/jitc-2021-002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quesniaux V, Fremond C, Jacobs M, et al. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect 2004;6:946–59. 10.1016/j.micinf.2004.04.016 [DOI] [PubMed] [Google Scholar]
- 39.Mao C, Beiss V, Fields J, et al. Cowpea mosaic virus stimulates antitumor immunity through recognition by multiple MYD88-dependent toll-like receptors. Biomaterials 2021;275:120914. 10.1016/j.biomaterials.2021.120914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alpaugh ML, Tomlinson JS, Kasraeian S, et al. Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene 2002;21:3631–43. 10.1038/sj.onc.1205389 [DOI] [PubMed] [Google Scholar]
- 41.Lehman HL, Dashner EJ, Lucey M, et al. Modeling and characterization of inflammatory breast cancer emboli grown in vitro. Int J Cancer 2013;132:2283–94. 10.1002/ijc.27928 [DOI] [PubMed] [Google Scholar]
- 42.O'Neill K, Guth A, Biller B, et al. Changes in regulatory T cells in dogs with cancer and associations with tumor type. J Vet Intern Med 2009;23:875–81. 10.1111/j.1939-1676.2009.0333.x [DOI] [PubMed] [Google Scholar]
- 43.Biller BJ, Guth A, Burton JH, et al. Decreased ratio of CD8+ T cells to regulatory T cells associated with decreased survival in dogs with osteosarcoma. J Vet Intern Med 2010;24:1118–23. 10.1111/j.1939-1676.2010.0557.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton JH, Mitchell L, Thamm DH, et al. Low-dose cyclophosphamide selectively decreases regulatory T cells and inhibits angiogenesis in dogs with soft tissue sarcoma. J Vet Intern Med 2011;25:920–6. 10.1111/j.1939-1676.2011.0753.x [DOI] [PubMed] [Google Scholar]
- 45.Mitchell L, Thamm DH, Biller BJ. Clinical and immunomodulatory effects of toceranib combined with low-dose cyclophosphamide in dogs with cancer. J Vet Intern Med 2012;26:355–62. 10.1111/j.1939-1676.2011.00883.x [DOI] [PubMed] [Google Scholar]
- 46.Gaspar TB, Henriques J, Marconato L, et al. The use of low-dose metronomic chemotherapy in dogs-insight into a modern cancer field. Vet Comp Oncol 2018;16:2–11. 10.1111/vco.12309 [DOI] [PubMed] [Google Scholar]
- 47.Hartley G, Faulhaber E, Caldwell A, et al. Immune regulation of canine tumour and macrophage PD-L1 expression. Vet Comp Oncol 2017;15:534–49. 10.1111/vco.12197 [DOI] [PubMed] [Google Scholar]
- 48.Maekawa N, Konnai S, Nishimura M, et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. NPJ Precis Oncol 2021;5:10. 10.1038/s41698-021-00147-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pantelyushin S, Ranninger E, Guerrera D, et al. Cross-reactivity and functionality of Approved human immune checkpoint blockers in dogs. Cancers 2021;13:785. 10.3390/cancers13040785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Igase M, Nemoto Y, Itamoto K, et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci Rep 2020;10:18311. 10.1038/s41598-020-75533-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Steinmetz NF. A combination of cowpea mosaic virus and immune checkpoint therapy synergistically improves therapeutic efficacy in three tumor models. Adv Funct Mater 2020;30. 10.1002/adfm.202002299. [Epub ahead of print: 04 05 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-004044supp001.pdf (2.7MB, pdf)
jitc-2021-004044supp002.pdf (121.4KB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study.