Abstract
Background
Secondary ischaemia is a frequent cause of poor outcome in patients with aneurysmal subarachnoid haemorrhage (SAH). Besides vasospasm, platelet aggregation seems to play a role in the pathogenesis of secondary ischaemia. Experimental studies have suggested that antiplatelet agents can prevent secondary ischaemia.
Objectives
To determine whether antiplatelet agents change outcome in patients with aneurysmal SAH.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched August 2006), MEDLINE (1966 to August 2006) and EMBASE databases (1980 to August 2006). We also searched reference lists of identified trials.
Selection criteria
All randomised controlled trials (RCTs) comparing any antiplatelet agent with control in patients with aneurysmal SAH.
Data collection and analysis
Two review authors independently extracted the data and assessed trial quality. Relative risks (RR) were calculated with regard to poor outcome, case fatality, secondary ischaemia, haemorrhagic intracranial complications and aneurysmal rebleeding according to the intention‐to‐treat principle. In case of a statistically significant primary analysis, a worst case analysis was performed.
Main results
Seven RCTs were included in the review, totalling 1385 patients. Four of these trials met the criteria for good quality studies. For any antiplatelet agent there were reductions of a poor outcome (RR 0.79, 95% confidence interval (CI) 0.62 to 1.01) and secondary brain ischaemia (RR 0.79, 95% CI 0.56 to 1.22) and more intracranial haemorrhagic complications (RR 1.36, 95% CI 0.59 to 3.12), but none of these differences were statistically significant. There was no effect on case fatality (RR 1.01, 95% CI 0.74 to 1.37) or aneurysmal rebleeding (RR 0.98, 95% CI 0.78 to 1.38). For individual antiplatelet agents, only ticlopidine was associated with statistically significant fewer occurrences of a poor outcome (RR 0.37, 95% CI 95% CI 0.14 to 0.98) but this estimate was based on only one small RCT.
Authors' conclusions
This review shows a trend towards better outcome in patients treated with antiplatelet agents, possibly due to a reduction in secondary ischaemia. However, results were not statistically significant, thus no definite conclusions can be drawn. Also, antiplatelet agents could increase the risk of haemorrhagic complications. On the basis of the current evidence treatment with antiplatelet agents in order to prevent secondary ischaemia or poor outcome cannot be recommended.
Plain language summary
Antiplatelet therapy for aneurysmal subarachnoid haemorrhage
A subarachnoid haemorrhage (SAH) is a type of stroke due to bleeding in the subarachnoid space, which is the small space between the brain and the skull, and which contains blood vessels that supply the brain. The cause of the bleeding is usually a rupture of a bulge in one of these vessels, which is called an aneurysm. The outcome of patients after SAH is generally poor: 50% of patients die within one month after the haemorrhage, and of those who survive the initial month, 50% remain dependent on someone else for help with activities of daily living (eg, walking, dressing, bathing). One of the causes of poor outcome is a complication of SAH called secondary ischaemia (ischaemia means lack of blood). This complication occurs four to 10 days after the haemorrhage (hence secondary). The cause is not exactly known, but besides contraction of the blood vessels in the brain, there is evidence that clotting of blood platelets plays a role as well. Therefore, trials have been performed with agents that prevent clotting of blood platelets (antiplatelet agents). In this review of seven trials, including 1385 patients, that studied the effects of antiplatelet agents on the outcome after SAH, we found that patients who were treated with antiplatelet agents had a poor outcome less often, and secondary ischaemia less often than patients that received no antiplatelet agent, but the results were not statistically significant and so no definite conclusion can be drawn. Moreover, patients who are treated with antiplatelet agents might have a slightly higher risk of bleeding. Based on these results we conclude that antiplatelet agents after SAH cannot be recommended at the present time.
Background
Aneurysmal subarachnoid haemorrhage (SAH) is a subset of stroke with an incidence of nine per 100,000 person years (de Rooij 2007). Half the patients are younger than 55 years of age (ACROSS 2000), and around 70% of patients die or remain dependent on help for activities of daily living as result of the haemorrhage (Hop 1997). Patients who survive the initial hours after the SAH are at risk of deterioration from aneurysmal rebleeding, hydrocephalus, secondary ischaemia and medical complications.
Secondary ischaemia occurs in up to one third of patients (Brilstra 2000) and is still an important cause of death and dependency on help for activities of daily living after SAH (Roos 2000), even in patients treated with nimodipine, which results in a risk reduction of secondary ischaemia of 13% (Rinkel 2005). Secondary ischaemia develops most often between the fourth and tenth day after the SAH (Brilstra 2000). The pathogenesis has not been elucidated yet, but is often attributed to vasospasm of the intracranial arteries (Dorsch 1995; Rabinstein 2004). However, vasospasm cannot be the only initiator of secondary ischaemia, as severe vasospasm does not always result in secondary ischaemia and not all patients with secondary ischaemia have severe vasospasm (Rabinstein 2004). Platelet aggregation may also play a role in the development of secondary ischaemia. In animal research both rupture of an artery and the presence of blood at the outside of an intact artery have been shown to activate platelet aggregation (Honma 1989), and to reduce antiplatelet activity of the endothelium (Okhuma 1993). In patients with SAH, activation of platelet aggregation and the associated release of thromboxane B2, a potent vasoconstrictor, are increased from the third day after the onset of the SAH (Juvela 1991; Okhuma 1991; Vinge 1988). The increase of thromboxane B2 is larger in patients who developed secondary ischaemia than in patients without secondary ischaemia (Vinge 1988). Furthermore, platelet count is decreased after SAH, and this decrease is larger in patients developing secondary ischaemia, probably due to increased platelet consumption (Hirashima 2005).
Acetylsalicylic acid (aspirin) is a well known inhibitor of platelet aggregation (Patrono 1994; Vane 2003). Two observational studies showed that patients who used acetylsalicylic acid before the onset of the SAH had a reduced risk for developing secondary ischaemia (Juvela 1995; Toussaint 2004). These results suggest that the administration of platelet inhibitors may reduce the risk of secondary ischaemia, and thereby the risk of poor outcome. However, platelet inhibitors could also lead to more haemorrhagic complications and thereby to a negative effect on outcome. We performed a systematic literature review to evaluate the results of all randomised controlled trials (RCTs) of antiplatelet therapy in aneurysmal SAH.
Objectives
To determine whether antiplatelet therapy changes the outcome in patients with aneurysmal SAH.
Methods
Criteria for considering studies for this review
Types of studies
We tried to identify all unconfounded RCTs comparing antiplatelet therapy with control in patients with aneurysmal SAH. We excluded uncontrolled studies, as well as quasi‐randomised controlled trials where allocation to treatment or control group was not concealed (eg, allocation by alternation, open random number list, date of birth, day of the week, or hospital number).
Types of participants
Patients of any age and either gender with SAH documented by either computerised tomography (CT) scan or cerebrospinal fluid examination were included in the analysis. A ruptured cerebral aneurysm should preferably be proven by angiography, or at least be highly likely, judged by the pattern of haemorrhage on CT.
Types of interventions
Treatment with any antiplatelet agent versus control during the clinical course of patients with SAH. Study medication had to be started within seven days after the SAH and had to be continued for at least one week.
Types of outcome measures
To provide an intention‐to‐treat analysis, we aimed to extract from each RCT the outcome assessments, at the end of follow up (at least one month after the haemorrhage), for all patients who were originally allocated to each treatment group.
Primary outcome measure
Poor outcome (death, or dependence on help for activities of daily living)
Secondary outcome measures
(1) Case fatality (2) Secondary ischaemia (clinical signs of cerebral ischaemia, as well as CT or magnetic resonance (MR) documented cerebral infarction alone). There is no consensus on a definition of secondary ischaemia, and previous research has shown that trials use different definitions for secondary ischemia or do not give a well‐defined definition at all (van der Schaaf 2002). For this reason, we decided beforehand to use the number of patients with secondary ischaemia given by the authors of the RCT included in the review, without adjusting these numbers to a predefined definition of secondary ischaemia. (3) Intracranial haemorrhagic complications (4) Aneurysmal rebleeding
Search methods for identification of studies
See: 'Specialized register' section in Cochrane Stroke Group
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Review Group Co‐ordinator in August 2006. In addition, we searched MEDLINE (1966 to August 2006) and EMBASE (1980 to August 2006) (Appendix 1).
In an effort to identify further published, ongoing and unpublished studies we searched the reference lists of all relevant trials. As there is an abundance of different antiplatelet drugs, and the most common antiplatelet drug, acetylsalicylic acid, does not have a patent and can be produced by any company or pharmacy in the world, we chose not to contact pharmaceutical companies or pharmacies as the list of companies would be endless.
Data collection and analysis
Data extraction
One author (SMDM) screened the titles of all references found by the search strategy and discarded all articles that were clearly not about subarachnoid haemorrhage or were definitely not a trial of antiplatelet agents. Next, the same author screened the abstracts of all remaining references; references were discarded if the abstract convincingly showed the article was not a trial of antiplatelet agents in subarachnoid haemorrhage. Of the remaining references, the same author read the articles and decided whether they were about a trial of antiplatelet therapy after SAH. When in doubt, the reference was discussed with a second author (WMvdB). Of the remaining trials, two authors (SMDM and WMvdB, AA or GJER) independently extracted details of randomisation methods, blinding of treatments and outcome assessments, definition of outcome measures, number of patients excluded or lost to follow up, and data on major prognostic factors such as clinical condition at admission and age. From these data, we decided whether treatment groups were comparable with regard to major prognostic risk factors for outcome, and whether intention‐to‐treat analysis was possible. All authors assessed the methodological quality of each RCT on the basis of the extracted data. In addition, we recorded study drug dose, route and timing of the drug administration, duration of follow up and adverse effects of study drugs. If patients were excluded or lost to follow up after randomisation, or if any of the above data were not available from the publications, we sought further information by contacting the trialists to allow an intention‐to‐treat analysis. Based on this data, we assessed RCTs on their quality as follows:
A ‐ Adequate, double‐blinded treatment concealment, losses to follow up or post‐randomisation exclusions together less than 10%;
B ‐ Double‐blinded but loss to follow up or post‐randomisation exclusions together more than 10%;
C ‐ Single‐blinded or no blinding of treatment concealment for outcome assessment.
Data analysis
We aimed to calculate relative risks (RR) according to the intention‐to‐treat principle. We calculated an estimate of the treatment effect across trials (relative risk (RR) with a 95% confidence interval (CI)) with the fixed‐effect model. We also calculated absolute risk reductions and numbers needed to treat, if the effect estimate was statistically significant. To quantify inconsistency across trials, the I‐squared statistic was used, which describes the percentage of the variability in effect estimates that is caused by heterogeneity. A value greater than 50% may be considered substantial heterogeneity.
Additional subgroup‐analyses were based on: (1) RCTs that started study medication before and studies that started study medication after aneurysm occlusion; (2) RCTs that started study medication within four days of the SAH; (3) effects of separate agents.
To test for a difference between subgroups, the Deeks method was used (Deeks 2001), based on the chi‐squared statistics of each subgroup.
Sensitivity analyses
(1) Exclusion of poor quality studies. Studies in categories B and C were excluded from this analysis. (2) In case of a statistically significant result in the primary analysis, secondary analyses were performed according to the worst‐case‐scenario method. A worst‐case‐scenario analysis assumes that those patients who had been excluded or lost to follow up in the treatment group have the worst possible outcome while those patients who had been excluded or lost to follow up in the control group have the best possible outcome. Only if the effects of primary and secondary meta‐analyses were of the same direction and magnitude was a definitive conclusion made about the treatment effectiveness.
Results
Description of studies
We identified 15 trials of antiplatelet drugs in aneurysmal SAH (Fujita 1990; Hirashima 2001; Hop 2000; Mendelow 1982; Mizukami 1981; Niemi 1999; Ono 1984; Shaw 1985; Suzuki 1981; Suzuki 1985; Suzuki 1989; Tani 1984; Tokiyoshi 1991; Yano 1993; van den Bergh 2006). The trials were performed between 1981 and 2006. We included seven RCTs in the review (Hop 2000; Mendelow 1982; Ono 1984; Shaw 1985; Suzuki 1989; Tokiyoshi 1991; van den Bergh 2006). We excluded the other trials for the following reasons: five trials had no randomised treatment allocation (Hirashima 2001; Suzuki 1981; Suzuki 1985; Tani 1984; Yano 1993); for one trial it was not clear from the available information whether treatment allocation was randomised (Fujita 1990); one trial was excluded because study medication was given for only three days after surgery (Niemi 1999); and another because there were incomplete data available on trial design and outcome measures (Mizukami 1981). We did not succeed in contacting the authors of the trials with incomplete data.
Size of the trials and treatment modes
The review includes seven RCTs including 1385 patients (743 in the treatment group and 642 in the control group). The number of randomised patients per RCT ranged from 24 patients (Tokiyoshi 1991) to 677 patients (Shaw 1985).
There were three RCTs of acetylsalicylic acid: two RCTs used suppositories 100 mg or placebo for 21 days after surgery (Hop 2000) or 14 days after surgery (van den Bergh 2006); one RCT administered acetylsalicylic acid or placebo 600 mg daily orally or via rectal retention enema until discharge (Mendelow 1982).
There were two RCTs of OKY‐046 (Cataclot), a selective thromboxane synthetase inhibitor. One of these RCTs randomised patients in three groups, placebo, 80 mg OKY‐046, or 400 mg OKY‐046 daily, by continuous infusion until 10 to 14 days after surgery (Suzuki 1989). The presence of two treatment groups and one control group in this trial explains the difference in included patients between the treatment and control groups. The other RCT administered Cataclot or placebo 1 ug/kg/min via continuous infusion, until 8 to 14 days after surgery (Tokiyoshi 1991).
One RCT studied dipyridamole 100 mg orally or 10 mg intravenously until three months after surgery, no placebo was used (Shaw 1985).
One RCT studied ticlopidine or placebo 300 mg per day orally for two weeks after the haemorrhage (Ono 1984).
Five RCTs started trial medication only after surgery (Hop 2000; Ono 1984; Suzuki 1989; Tokiyoshi 1991; van den Bergh 2006), two RCTs before surgery: immediately after admission (Shaw 1985) or 72 hours after admission (Mendelow 1982).
Aneurysm treatment
In six of the seven RCTs aneurysms were occluded by means of neurosurgical clipping only. In one RCT all patients were operated on within two days after the haemorrhage (Tokiyoshi 1991), in three RCTs within four days after the SAH (Hop 2000; Ono 1984; Suzuki 1989). In two RCTs study treatment was begun before aneurysm occlusion; in one RCT only 29 of the 53 patients were operated on (timing of treatment is not mentioned) (Mendelow 1982) and in the other only 348 of the 677 patients (the median day of surgery in the treatment group was 9.8 days and in the control group 9 days) (Shaw 1985).
In one RCT, 52 of the 161 included patients were treated by endovascular coiling and 108 by surgical clipping within four days after the haemorrhage (van den Bergh 2006).
Inclusion and exclusion criteria
Two RCTs included patients only when an aneurysm was confirmed on (CT‐) angiography (Hop 2000; Tokiyoshi 1991) and in five RCTs patients were eligible only if surgical occlusion was performed before the fourth day after the SAH (Hop 2000; Ono 1984; Suzuki 1989; Tokiyoshi 1991; van den Bergh 2006). Two RCTs enrolled patients with subarachnoid haemorrhage without further specification, in one RCT an aneurysm was not confirmed in 190 patients of the 667 (Shaw 1985), and in the other in 20 of the 53 patients (Mendelow 1982). These patients were included in all analyses if possible; one study did not give information about poor outcome for patients with an unconfirmed aneurysm (Shaw 1985). Three RCTs selected patients based on clinical condition at admission; one RCT excluded patients in a very good or very poor clinical condition (Hunt and Hess grade 1 and 5) (Ono 1984), the other two RCTs excluded patients in a very poor condition: Hunt and Hess grade 5 (Tokiyoshi 1991), and Japan Coma Scale less than 30 (Suzuki 1989).
Outcome measures and follow up
All RCTs reported on clinical outcome and case fatality. Two RCTs used the modified Rankin scale (Hop 2000; van den Bergh 2006), for the current review Rankin greater than 3 was defined as a poor outcome. One RCT used the Glasgow Outcome Scale (GOS) (Shaw 1985), GOS less than 4 was defined as poor outcome. The other four RCTs used a self‐designed grading scale. One RCT used a four‐point scale base on the GOS (Tokiyoshi 1991): (1) excellent, (2) good (combined to good outcome), (3) poor, (4) dead (combined to poor outcome). A second RCT used a three‐point scale (Mendelow 1982): A ‐ no neurological deficit (good outcome), B ‐ neurological disability, C ‐ death (combined to poor outcome). A third RCT used a five‐point scale (Ono 1984): (1) excellent, (2) good (combined to good outcome), (3) poor, (4) vegetative state, (5) death (combined to poor outcome). A fourth RCT used a nine‐point scale (Suzuki 1989): 0 to 4 good outcome, 5 to 8 poor outcome. Five RCTs reported on secondary ischaemia, either on clinical signs (Hop 2000; Ono 1984; Tokiyoshi 1991; van den Bergh 2006) or infarction on CT scan (Hop 2000; Suzuki 1989; van den Bergh 2006). Four RCTs reported on intracranial haemorrhagic complications (Hop 2000; Shaw 1985; Suzuki 1989; van den Bergh 2006) and only two RCTs on rebleeding (Shaw 1985; van den Bergh 2006). Follow up was one month in one RCT (Tokiyoshi 1991), three months in four RCTs (Ono 1984; Shaw 1985; Suzuki 1989; van den Bergh 2006), four months in one RCT (Hop 2000) and six months in one RCT (Mendelow 1982).
Risk of bias in included studies
Five RCTs used a double‐blinded (patients and treating physicians both blinded for treatment), placebo‐controlled design (Hop 2000; Mendelow 1982; Ono 1984; Suzuki 1989; van den Bergh 2006), one RCT did not use a placebo, but outcome assessment was performed blinded for treatment allocation (Shaw 1985), one RCT did not specify whether treatment allocation and outcome assessment were blinded, but did use a placebo (Tokiyoshi 1991). Four RCTs randomised patients with consecutive series of identical study medication boxes, tablets or vials (Hop 2000; Ono 1984; Suzuki 1989; van den Bergh 2006), one RCT used a randomisation code stratified according to Hunt and Hess grade (Mendelow 1982) and two RCTs did not specify the exact method of randomisation (Shaw 1985; Tokiyoshi 1991). Four RCTs did not have any losses to follow up and intention‐to‐treat analysis was possible for all outcome measures (Hop 2000; Mendelow 1982; Tokiyoshi 1991; van den Bergh 2006). In one RCT only one patient per group was lost to follow up (1.5%) (Ono 1984). In one RCT 166 patients (49%) in the treatment group and 163 patients in the control group (49%) were excluded after randomisation because they did not come to surgery, and another 14 patients per group (8%) were withdrawn for unknown reasons, for poor outcome analysis only (Shaw 1985). For the other outcome measures (case fatality, rebleeding, intracranial haemorrhagic complications) intention‐to‐treat analysis was possible. In another RCT 27 patients (9.5%) were excluded after randomisation equally distributed between treatment and control groups, before opening of the key code because of failures to comply with protocol stipulations and another two patients (0.8%) in the treatment group were excluded because of discontinuation of study therapy due to side effects (Suzuki 1989). Based on this information, three of the seven trials were classified as poor quality trials (category B or C): Suzuki 1989 (category B), Shaw 1985 (category C) and Tokiyoshi 1991 (category C).
Effects of interventions
Comparisons 01.01 and 01.02: Poor outcome
Data on poor outcome were available for all seven RCTs, totalling 997 patients. The pooled RR was 0.79 (95% CI 0.62 to 1.01) (Comparison 01.01). Since the result was not statistically significant, no worst‐case analysis was performed. For each antiplatelet agent separately, none of the risk estimates was statistically significant except one: the RR for ticlopidine was 0.37 (95% CI 0.14 to 0.98), but this analysis included only one small RCT (Ono 1984). The RR for acetylsalicylic acid was 0.81 (95% CI 0.51 to 1.28), for Cataclot/OKY‐046 0.88 (95% CI 0.54 to 1.44) and for dipyridamole 0.86 (95% CI 0.57 to 1.29).
For RCTs that started study medication before aneurysm occlusion (Mendelow 1982; Shaw 1985), the RR was 0.88 (95% CI 0.62 to 1.25), and for RCTs that started study medication only after aneurysm occlusion (Hop 2000; Ono 1984; Suzuki 1989; Tokiyoshi 1991; van den Bergh 2006) the RR was 0.72 (95% CI 0.51 to 1.02) (Comparison 01.02). These were the same RCTs in which study medication was started before day four in all cases. There were no statistical differences between the subgroups of different antiplatelet agents (P = 0.44) or between the subgroups according to start of study medication (P = 0.48) as determined by the Deeks method (Deeks 2001).
In a sensitivity analysis after exclusion of poor quality studies (Shaw 1985; Suzuki 1989; Tokiyoshi 1991), the RR was essentially unchanged: 0.67 (95% CI 0.45 to 1.02).
Comparisons 02.01 and 02.02: Case fatality
Data on case fatality were available for all RCTs, totalling 1354 patients. This number is greater than in the poor outcome analysis because data on case fatality were available for the 357 patients excluded from the poor outcome analysis by Shaw (Shaw 1985). The pooled RR was 1.01 (95% CI 0.74 to 1.37) (Comparison 02.01). Also, for individual agents none of the risk estimates were statistically significant and ranged from 0.35 (95% CI 0.10 to 1.23) for ticlopidine to 1.17 (95% CI 0.57 to 2.41) for Cataclot/OKY‐046.
There were minute differences between RCTs that started study medication before aneurysm occlusion (RR 1.10, 95% CI 0.74 to 1.64) (Mendelow 1982; Shaw 1985) and RCTs that started study medication after aneurysm occlusion (RR 0.88, 95% CI 0.54 to 1.45) (Hop 2000; Ono 1984; Suzuki 1989; Tokiyoshi 1991; van den Bergh 2006) (Comparison 02.02). These are the same RCTs that started study medication within four days. There were no statistical differences between the subgroups of different antiplatelet agents (P = 0.37) or between the subgroups according to start of study medication (P = 0.56). In a sensitivity analysis after exclusion of poor quality studies (Shaw 1985; Suzuki 1989; Tokiyoshi 1991), the RR was 0.74 (95% 0.41 to 1.33). As there was no statistically significant risk estimate in the primary analysis, no worst‐case‐scenario analysis was performed.
Comparisons 03.01 and 03.02: Secondary ischaemia
Four RCTs, totalling 368 patients, reported on clinical signs of secondary ischaemia (Hop 2000; Ono 1984; Tokiyoshi 1991; van den Bergh 2006). In all these RCTs study medication was started after aneurysm occlusion and within four days after the SAH. The pooled RR was 0.79 (95% CI 0.53 to 1.16) (Comparison 03.01). For acetylsalicylic acid only, the RR was 1.34 (95% CI 0.76 to 2.34), for Cataclot/OKY‐046 0.53 (95% CI 0.24 to 1.15) and for ticlopidine 0.39 (95% CI 0.17 to 0.86). The difference between these subgroups was statistically significant (P = 0.03). After exclusion of one poor quality study (Tokiyoshi 1991) the RR was 0.85 (95% CI 0.55 to 1.32).
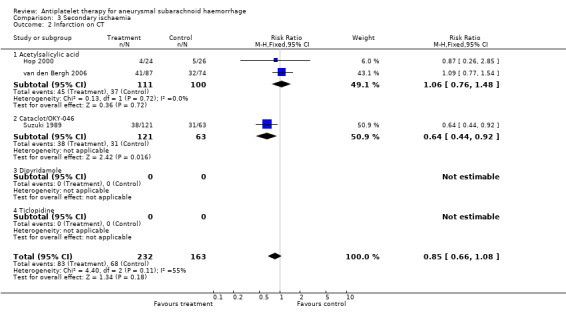
Three RCTs, totalling 395 patients, reported on cerebral infarction on CT scan (Hop 2000; Suzuki 1989; van den Bergh 2006); the RR was 0.85 (95% CI 0.66 to 1.08) (Comparison 03.02). Again, differences between individual antiplatelet agents were statistically significant (P = 0.04).
Comparisons 04.01 and 04.02: Intracranial haemorrhagic complications
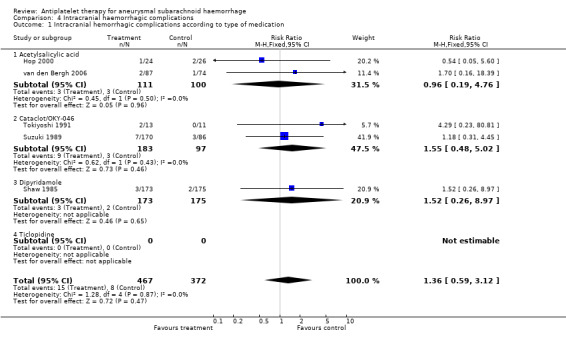
Data on intracranial haemorrhagic complications were available for five RCTs, totalling 839 patients (Hop 2000; Shaw 1985; Suzuki 1989; Tokiyoshi 1991; van den Bergh 2006). The overall risk of haemorrhagic complication was non‐significantly higher in the antiplatelet group: RR 1.36 (95% CI 0.59 to 3.12) (Comparison 04.01). For acetylsalicylic acid only, the RR was 0.96 (95% CI 0.19 to 4.76), for Cataclot/OKY‐046 1.55 (95% CI 0.48 to 5.02) and for dipyridamole 1.52 (95% CI 0.26 to 8.97).
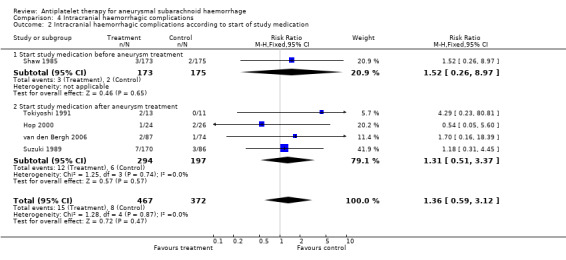
The RRs were not essentially different for the one RCT that started study medication before aneurysm occlusion (RR 1.52, 95% CI 0.26 to 8.97) (Shaw 1985) and RCTs that started study medication after aneurysm occlusion (RR 1.31, 95% CI 0.51 to 3.37) (Hop 2000; Suzuki 1989; Tokiyoshi 1991; van den Bergh 2006) (Comparison 04.02). These were the same RCTs in which study medication was started within four days in all cases. There were no statistical differences between the subgroups of different antiplatelet agents (P = 0.90) or between the subgroups according to start of study medication (P = 0.86). After exclusion of poor quality studies (Shaw 1985; Suzuki 1989; Tokiyoshi 1991), only the two RCTs of acetylsalicylic acid remained (RR 0.96, 95% CI ‐0.19 to 4.76). Since the risk estimate was not statistically significant in the primary analysis, no worst‐case‐scenario analysis was performed.
Comparison 05.01: Rebleeding
Data on rebleeding were only available for two RCTs, totalling 838 patients (Shaw 1985; van den Bergh 2006). The RR was 0.98 (95% CI 0.70 to 1.38). No further subgroup analyses or sensitivity analyses were performed.
Discussion
Effect of antiplatelet therapy on clinical outcome and secondary ischaemia
This meta‐analysis shows a trend for antiplatelet therapy to reduce the occurrence of poor outcome in patients with a SAH. In the subgroup analyses for each individual antiplatelet agent, for trials that started trial medication before or after aneurysm treatment and trials that started trial medication before or after day four, the risk estimates for poor outcome were essentially the same. However, none of the risk estimates, except for the subgroup analysis for ticlopidine that constituted of only one trial, reached statistical significance. The sample size of this ticlopidine study was small (Ono 1984). No power calculation was given, and the reason for stopping the trial after only 133 patients were included was not stated. As these data are lacking, there is a considerable risk that the statistical significance of this trial is a chance finding. Furthermore, ticlopidine has shown a high risk of haematological complications in ischemic stroke and coronary stenting, and is not commonly used for this reason (Cosmi 2001; Weinberger 2005). There were no important differences between RCTs that started study medication before or after aneurysm occlusion. Also, in the sensitivity analysis with exclusion of poor quality studies, the RR was essentially unchanged; however, in the worst case scenario no beneficial effect remained. This loss of evidence of effectiveness in the worst case scenario analysis is explained mainly by the relatively high number of patients lost to follow up in the RCTs. The risk estimates for secondary ischaemia showed a favourable trend as well, indicating that reduction of the occurrence of secondary ischaemia might be the mechanism how antiplatelet therapy might reduce the frequency of poor outcome. For separate antiplatelet agents, all agents except acetylsalicylic acid showed a trend towards a reduction of secondary ischaemia. Antiplatelet therapy did not show any effect on case fatality: all risk estimates were close to one, except for the ticlopidine trial (Ono 1984), but the result was not statistically significant.
Effect of antiplatelet therapy on haemorrhagic complication and rebleeding
For haemorrhagic complications, the risk estimate of the main analysis as well as most of the subgroup analyses indicated a slightly increased risk, but none of the estimates was statistically significant. There were no obvious differences for RCTs that started study medication before or after aneurysm occlusion. Data on rebleeding were available for only two RCTs and results were inconclusive.
Methodological issues of the present overview
We have assessed all randomised controlled trials in which any type of antiplatelet agent was compared with control in patients with SAH. The RCTs were comparable with respect to study design and selection of patients, and all had a well balanced distribution of prognostic factors for poor outcome between treatment and control groups, which adds to the validity of the results. The difference between the numbers in the treatment and control groups can be explained by the fact that one study randomised patients into three groups, two treatment groups (different medication dosage) and one control group. Three RCTs were defined as poor quality trials, in two treatment allocation and outcome were not blinded (Shaw 1985) or did not mention blinding (Tokiyoshi 1991), and in two RCTs strict intention‐to‐treat analysis was not possible because patients were excluded from the analyses (Shaw 1985; Suzuki 1989). However, after exclusion of these RCTs from the main analyses, the results were essentially unchanged. Four different antiplatelet agents were used, and they differ in the way they inactivate platelet function. However, in most analyses there were only small differences between the separate agents. Only in the analyses for secondary ischaemia did the RCTs of acetylsalicylic acid show a trend contrary to the other agents, and there was inconsistency across trials (I‐squared statistic greater than 50%). In all other analyses there was no inconsistency across trials. For some RCTs information was incomplete, but because these RCTs were performed in the 1980s, additional information could not be retrieved.
Authors' conclusions
Implications for practice.
This review shows a trend towards better outcome in patients treated with antiplatelet agents, possibly due to a reduction in secondary ischaemia. However, results were not statistically significant and no beneficial effect remained in the worst‐case‐scenario analysis. Thus no definite conclusion can be drawn. Also, antiplatelet agents could increase the risk of haemorrhagic complications. On the basis of the current evidence treatment with antiplatelet agents cannot be recommended for patients with SAH.
Implications for research.
Before antiplatelet drugs can be recommended in clinical practice, adequately powered RCTs are needed. Based on the effect estimate for poor outcome found in this review (RR 0.79, 95% CI: 0.62 to 1.01), 2000 to 2500 patients need to be included in such a trial (with α = 5% and a power of 80%). However, it is difficult to make recommendations as to which antiplatelet agent is the most promising. Firstly, there were no large differences between the antiplatelet drugs. Ticlopidine yielded the most promising results in this review but this was based on only one small study conducted in 1984, and ticlopidine is not commonly used anymore due to haematological complications. Acetylsalicylic acid did show a trend towards a better overall clinical outcome, but also a trend towards an increased risk for secondary ischaemia. Secondly, there was only one study that included patients whose aneurysms were treated by endovascular coiling, whereas effects of antiplatelet drugs may differ between patients treated with surgical or endovascular techniques. Thus, it could be worthwhile performing a new pilot study in patients whose aneurysms are treated by coiling, before conducting a very large trial in which most patients will be treated by coiling, based on data from the current review in which most patients were treated by surgical clipping of the aneurysm.
What's new
| Date | Event | Description |
|---|---|---|
| 22 July 2008 | Amended | Converted to new review format. |
Acknowledgements
None
Appendices
Appendix 1. MEDLINE search strategy
The following search strategy, using a combination of controlled vocabulary and free text terms, was used for MEDLINE and modified for EMBASE.
MEDLINE (Ovid)
1. Subarachnoid Hemorrhage/ 2. intracranial hemorrhages/ or cerebral hemorrhage/ 3. Intracranial Aneurysm/ 4. Rupture, Spontaneous/ 5. 3 and 4 6. Aneurysm, Ruptured/ 7. exp brain/ or exp meninges/ 8. 6 and 7 9. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 10. Vasospasm, Intracranial/ 11. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 12. sah.tw. 13. 1 or 2 or 5 or 8 or 9 or 10 or 11 or 12 14. exp Platelet aggregation inhibitors/ 15. (antiplatelet$ or anti‐platelet$ or antiaggreg$ or anti‐aggreg$ or (platelet$ adj5 inhibit$) or (thrombocyt$ adj5 inhibit$)).tw. 16. (Dispril or Albyl$ or Ticlid$ or Persantin$ or Plavix).tw. 17. exp Platelet glycoprotein gpiib‐iiia complex/ai, de 18. (((glycoprotein iib$ or gp iib$) adj5 (antagonist$ or inhibitor$)) or GR144053 or GR‐144053 or abciximab$ or tirofiban$ or eftifibatid$).tw. 19. (ReoPro or Integrilin$ or Aggrastat).tw. 20. exp Platelet activation/de 21. exp Blood platelets/de 22. (alprostadil$ or aspirin$ or dipyridamol$ or disintegrin$ or epoprostenol$ or iloprost$ or ketanserin$ or ketorolac tromethamine$ or milrinone$ or mopidamol$ or pentoxifyllin$ or procainamide$ or ticlopidine$ or thiophen$ or trapidil$).tw. 23. (acetyl salicylic acid$ or acetyl?salicylic acid or clopidogrel$ or picotamide$ or ligustrazine$ or levamisol$ or suloctidil$ or ozagrel$ or oky046 or oky‐046 or defibrotide$ or cilostazol or satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐4151 or triflusal).tw. 24. or/14‐23 25. 13 and 24 26. limit 25 to human
Data and analyses
Comparison 1. Poor outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor outcome, according to type of medication | 7 | 997 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 1.1 Acetylsalicylic acid | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.28] |
| 1.2 Cataclot/OKY‐046 | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.44] |
| 1.3 Dipyridamole | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.29] |
| 1.4 Ticlopidine | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.98] |
| 2 Poor outcome, according to start of study medication | 7 | 997 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 2.1 Start study medication before aneurysm treatment | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.25] |
| 2.2 Start study medication after aneurysm treatment | 5 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.51, 1.02] |
1.1. Analysis.
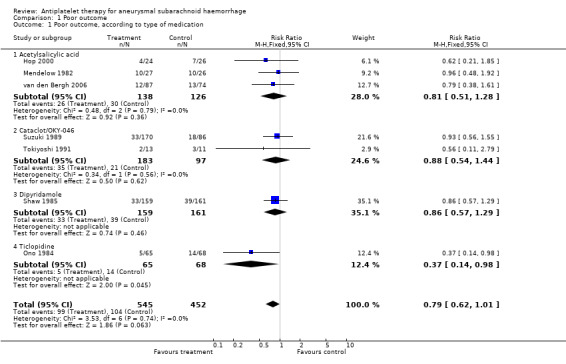
Comparison 1 Poor outcome, Outcome 1 Poor outcome, according to type of medication.
1.2. Analysis.
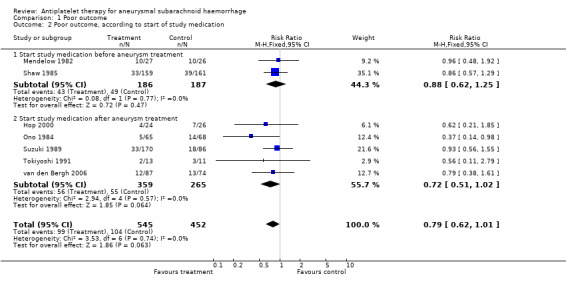
Comparison 1 Poor outcome, Outcome 2 Poor outcome, according to start of study medication.
Comparison 2. Case fatality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Case fatality, according to type of medication | 7 | 1354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.74, 1.37] |
| 1.1 Acetylsalicylic acid | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.49, 1.93] |
| 1.2 Cataclot/OKY‐046 | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.41] |
| 1.3 Dipyridamole | 1 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.74, 1.71] |
| 1.4 Ticlopidine | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.23] |
| 2 Case fatality, according to start of study medication | 7 | 1354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.74, 1.37] |
| 2.1 Start study medication before aneurysm treatment | 2 | 730 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.74, 1.64] |
| 2.2 Start study medication after aneurysm treatment | 5 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.45] |
2.1. Analysis.
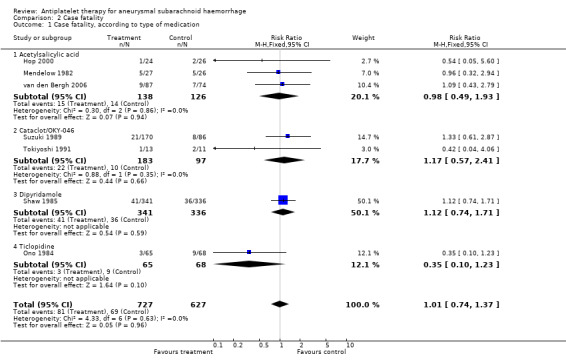
Comparison 2 Case fatality, Outcome 1 Case fatality, according to type of medication.
2.2. Analysis.
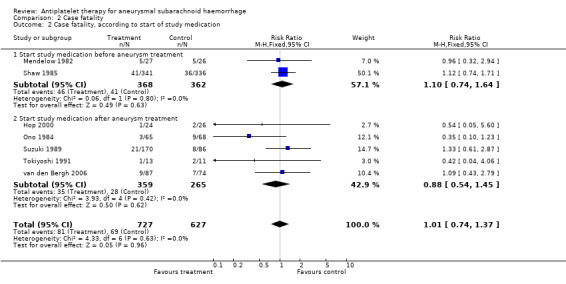
Comparison 2 Case fatality, Outcome 2 Case fatality, according to start of study medication.
Comparison 3. Secondary ischaemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical signs of secondary ischaemia | 4 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.16] |
| 1.1 Acetylsalicylic acid | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.76, 2.34] |
| 1.2 Cataclot/OKY‐046 | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.15] |
| 1.3 Dipyridamole | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Ticlopidine | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.17, 0.86] |
| 2 Infarction on CT | 3 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.08] |
| 2.1 Acetylsalicylic acid | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.48] |
| 2.2 Cataclot/OKY‐046 | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.92] |
| 2.3 Dipyridamole | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Ticlopidine | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 Secondary ischaemia, Outcome 1 Clinical signs of secondary ischaemia.
3.2. Analysis.
Comparison 3 Secondary ischaemia, Outcome 2 Infarction on CT.
Comparison 4. Intracranial haemorrhagic complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intracranial hemorrhagic complications according to type of medication | 5 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.59, 3.12] |
| 1.1 Acetylsalicylic acid | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.19, 4.76] |
| 1.2 Cataclot/OKY‐046 | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.48, 5.02] |
| 1.3 Dipyridamole | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 8.97] |
| 1.4 Ticlopidine | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Intracranial haemorrhagic complications according to start of study medication | 5 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.59, 3.12] |
| 2.1 Start study medication before aneurysm treatment | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 8.97] |
| 2.2 Start study medication after aneurysm treatment | 4 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.51, 3.37] |
4.1. Analysis.
Comparison 4 Intracranial haemorrhagic complications, Outcome 1 Intracranial hemorrhagic complications according to type of medication.
4.2. Analysis.
Comparison 4 Intracranial haemorrhagic complications, Outcome 2 Intracranial haemorrhagic complications according to start of study medication.
Comparison 5. Rebleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rebleeding according to type of medication | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 1.1 Acetylsalicylic acid | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.44, 6.57] |
| 1.2 Cataclot/OKY‐046 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Dipyridamole | 1 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.67, 1.33] |
| 1.4 Ticlopidine | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.

Comparison 5 Rebleeding, Outcome 1 Rebleeding according to type of medication.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hop 2000.
| Methods | Method of randomisation: consecutive series of numbered identical boxes with either ASA or placebo Blinding: double‐blinded Analysis: intention‐to‐treat analysis Post‐randomisation exclusions: none Definition of outcome: stated | |
| Participants | Location: University Hospital Utrecht, the Netherlands Rx: 24 (16 female) Controls: 26 (21 female) Age range: mean age Rx group: 46 years (SD 13 years); mean age control group: 50 years (SD 12 years) Entry criteria: symptomatic aneurysm demonstrated by (CT‐) angiography, surgery before day 4 planned Exclusion criteria: postponement of surgery after day 5, inability to obtain informed consent, age under 18, contra‐indication for ASA, disapproval of neurosurgeon postoperatively Type/timing of aneurysm treatment: surgical clipping only, median day 2, range 0 to 4 Comparability of treatment groups: good | |
| Interventions | Rx: suppositories with 100 mg ASA for 21 days after surgery Controls: suppositories with 100 mg placebo for 21 days after surgery Start of trial medication: after surgical aneurysm treatment | |
| Outcomes | Primary outcome: (1) functional outcome after 4 months, by means of modified Rankin Scale Secondary outcomes: (1) quality of life (2) postoperative clinical deterioration (3) delayed cerebral ischaemia (4) presence of 1 or more hypodense lesions on postoperative CT (5) occurrence of haemorrhagic complications | |
| Notes | Follow up: 4 months Adverse effects: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mendelow 1982.
| Methods | Method of randomisation: randomised code stratified according to Hunt and Hess grade Blinding: double‐blinded Analysis: intention‐to‐treat analysis Post‐randomisation exclusions: none Definition of outcome: stated | |
| Participants | Location: Department of Surgical Neurology, Edinburgh, UK Rx: 27 Controls: 26 (30 of total of 53 randomised patients were female) Age range: not mentioned Entry criteria: diagnosis of SAH proven by CT or lumbar puncture Exclusion criteria: none mentioned Type/timing of aneurysm treatment: in 21 patients the aneurysm was clipped; in 8 patients the aneurysm was wrapped; timing not stated Comparability of treatment groups: no baseline table | |
| Interventions | Rx: ASA 300 mg twice daily orally or rectal retention enema, starting 72 hours after admission, before surgery Controls: placebo in same dose/route | |
| Outcomes | Primary outcome: functional outcome as defined by 3‐point scale (A: no neurological deficit; B: neurological disability; C: death) Secondary outcome: platelet function | |
| Notes | Follow up: 6 months Adverse effects: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ono 1984.
| Methods | Method of randomisation: identical tablets, code kept by study controller until end of study Blinding: double‐blinded for treatment, outcome assessment blinded Analysis: on‐treatment analyses Post‐randomisation exclusions: 1 in Rx group and 1 in placebo group Definition of outcome: stated for functional outcome, not clearly stated for neurological deficit from spasm and vasospasm on angiography | |
| Participants | Location: 15 medical centers in Japan Rx: 66 Controls: 69 (56% female patients in both groups, almost identical distribution of sex) Age range: of all patients: 13% 39 years or younger, 22% 40 to 49 years, 38% 50 to 59 years, 23% 60 to 69 years, 4% 70 years or older Entry criteria: (1) pre‐operative neurological state of Hunt and Hess grade 2‐4A; (2) ruptured intracranial aneurysm operated within 3 days after SAH; or (3) residual subarachnoid blood clot on postoperative CT within 24 hours after surgery Exclusion criteria: (1) multiple episodes of aneurysm rupture; (2) presence of intracerebral haematoma; (3) severe gastrointestinal disease; (4) severe liver disease Type/timing of aneurysm treatment: all patients were operated on within 4 days by surgery Comparability of treatment groups: good with regard to major prognostic factors | |
| Interventions | Rx: ticlopidine 100 mg 3 times a day orally for 2 weeks after the haemorrhage Control: identical tablets with lactose | |
| Outcomes | (1) Functional outcome at 3 months (2) Vasospasm on angiography 6 to 12 days after the SAH (3) Neurological deficit from spasm (4) Mortality | |
| Notes | Follow up: 3 months Adverse effects: diagnosed as side effects of study treatment: 0 in Rx group, 4 in placebo group. No significant differences in incidence of complications such as gastrointestinal bleeding, hydrocephalus, meningitis, pneumonia, etc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Shaw 1985.
| Methods | Method of randomisation: not mentioned Blinding: single‐blinded; surgical investigators not blinded for treatment allocation, however outcome assessment by blinded neurologist Analysis: intention‐to‐treat analysis for rebleeding, functional outcome only in patients who came to surgery Post‐randomisation exclusions: 166 patients in Rx group and 163 in control group were excluded for functional outcome analysis because they did not come to surgery, another 8% in each group were withdrawn from the functional outcome analysis for reasons not mentioned Definition of outcome: stated | |
| Participants | Location: Walton Hospital, Liverpool, UK and Southampton General Hospital, Southampton, UK Rx: 336, of whom 173 came to surgery Controls: 341, of whom 175 came to surgery Age range: mean age 45 years for Rx group and 45.8 years for control group Entry criteria: patients presenting with SAH Exclusion criteria: none mentioned Type/timing of aneurysm treatment: surgical treatment; median time between SAH and surgery 9.8 days in control group and 9 days in Rx group Comparability of treatment groups: good with regard to clinical condition at admission | |
| Interventions | Rx: dipyridamole 100 mg/day orally or 10 mg/day intravenously starting immediately after admission Controls: standard treatment, no placebo used | |
| Outcomes | (1) Functional outcome, by means of Glasgow Outcome Scale (2) Rebleeding | |
| Notes | Follow up: 3 months Adverse effects: diarrhoea in 2 patients in Rx group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Suzuki 1989.
| Methods | Method of randomisation: identical numbered vials, key code in custody of controllers Blinding: double‐blinded for treatment, blinding for outcome assessment Analysis: on‐treatment analysis Post‐randomisation exclusions: 27 patients excluded before opening key code because of failure to meet protocol stipulation, evenly distributed among groups; 2 patients excluded in treatment groups ‐ discontinued study medication because of side effects Definition of outcome: stated | |
| Participants | Location: 48 neurosurgical services in Japan Rx: 95 in low‐dose group, 95 in high‐dose group Controls: 95 Age range: median 52.3 years Entry criteria: aneurysm surgery between day 0 and 3, aged between 16 to 70 years, suffering from initial aneurysmal rupture, consciousness better than 30 on Japan Coma Scale (30 = opens eyes barely if addressed repeatedly during pain stimulation), subarachnoid blood on CT, consent obtained Exclusion criteria: intracerebral or intraventricular haematoma on initial CT, cerebral vasospasm suspected before start of trial, severe neurological deficits before start of trial, severe complications (peptic ulcer, renal or hepatic disease), other contra‐indication considered by doctor in charge Type/timing of aneurysm treatment: surgical, between day 0 and day 4 Comparability of treatment groups: good with regard to prognostic factors | |
| Interventions | Rx: 1st group 80 mg OKY‐046 per day, 2nd group 400 mg OKY‐046 per day, by continuous infusion until 10 to 14 days, starting immediately after surgery Controls: placebo infusion in same dose/route | |
| Outcomes | (1) Location and degree of vasospasm on cerebral angiography on day 5 and 20 (2) Abnormal low and high densities on CT at day 21 (3) Functional ADL on a 9 point scale at 1 and 3 months (4) Laboratory examination (5) Overall efficiacy, safety and usefulness of drug as evaluated by doctor in charge | |
| Notes | Follow up: 3 months Adverse effects: 16% in placebo group, 19% in low‐dose group and 20% in high‐dose group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Tokiyoshi 1991.
| Methods | Method of randomisation: not mentioned Blinding: not mentioned, but placebo was used Analysis: intention‐to‐treat analysis Post‐randomisation exclusions: none Definition of outcome: stated | |
| Participants | Location: Oska University Medical School, Japan Rx: 13 (female % not mentioned) Controls: 11 (female % not mentioned) Age range: Mean age 54.2 years Entry criteria: admission within 24 hours between November 1986 and December 1989, Hunt and Hess score 1 to 4, aneurysm verified on angiography Exclusion criteria: severe cardiac, hepatic or renal insufficiency Type/timing of aneurysm treatment: surgical clipping within 48 hours after onset SAH Comparability of treatment groups: good with regard to prognostic factors | |
| Interventions | Rx: Cataclot 1ug/kg/min via continuous infusion, starting after surgery continued for 8 to 14 days Controls: placebo in same dose/route | |
| Outcomes | (1)Symptomatic vasospasm (2) Functional outcome | |
| Notes | Follow up: 1 month Adverse effects: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
van den Bergh 2006.
| Methods | Method of randomisation: consecutive series of numbered boxes, containing either ASA or placebo Blinding: double‐blinded for treatment allocation, blinded for outcome assessment Analysis: both on‐treatment analysis and intention‐to‐treat analysis Post‐randomisation exclusions: none Definition of outcome: stated | |
| Participants | Location: 3 University hospitals, the Netherlands Rx: 87 (68 female) Controls: 74 (59 female) Age range: mean age 53 years Entry criteria: admission and aneurysm treatment within 4 days after SAH, aneurysmal SAH based on typical pattern on CT, or when CT was negative, on xantochromia of cerebral spinal fluid in combination with an aneurysm confirmed on angiography Exclusion criteria: hypersensitivity to ASA, recent ASA use, age under 18 years Type/timing of aneurysm treatment: endovascular treatment (38%) or surgical treatment (61%) within 4 days after SAH Comparability of treatment groups: good | |
| Interventions | Rx: suppositories with 100 mg ASA once daily, starting after surgery for 14 days Controls: placebo in same dose/route | |
| Outcomes | Primary outcome: (1) delayed ischaemic neurological deficit defined as a new hypodense lesion on CT in combination with gradually developed focal deficits, decreased level of consciousness, or both Secondary outcomes: (1) occurrence of any new hypodensity on CT, regardless of the cause (2) post‐operative haemorrhage (3) poor outcome as defined by modified Rankin score of 4 or worse (4) non‐excellent outcome, defined by Rankin score of 1 or worse | |
| Notes | Follow up: 3 months Adverse effects: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ADL: activities of daily living ASA: acetylsalicylic acid (aspirin) CT: computerised tomography Rx: treatment SAH: subarachnoid haemorrhage SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fujita 1990 | Study of ticlopidine and albumin after SAH in 149 patients. Excluded because it was not clear whether patients were allocated to treatment groups by randomisation; duration of follow up not clear. |
| Hirashima 2001 | Study of effect of a platelet‐activating factor receptor antagonist, E5880, including 71 patients. Not randomised, historical control group used. |
| Mizukami 1981 | Study of effect of ticlopidine in 71 SAH patients. Excluded because of incomplete data. Only an abstract available; randomisation methods, blinding, inclusion or exclusion criteria, base line characteristics, start and duration of study medication and duration of follow up were not clear from the abstract. |
| Niemi 1999 | Study of ketoprofen in 18 SAH patients. Excluded because trial medication was given for only 3 days after aneurysm surgery. |
| Suzuki 1981 | Study of effect of trapidil, an antagonist and selective synthesis inhibitor of thromboxane A2. Not randomised, no control group used. |
| Suzuki 1985 | Study of effect of OKY‐046, a thromboxane synthetase inhibitor in 20 SAH patients. Not randomised, no control group used. |
| Tani 1984 | Study of effect of a selective thromboxane A2 synthetase inhibitor in 49 SAH patients. Not randomised. |
| Yano 1993 | Study of effect of thromboxane A2 synthetase inhibitor in 28 SAH patients. Not randomised. |
SAH: subarachnoid haemorrhage
Contributions of authors
SM Dorhout Mees: participated in developing the protocol, writing the first draft and final version of review. GJE Rinkel: participated in developing the protocol, commented on the first and final drafts of the review, and is the guarantor for this review. A Algra: participated in developing the protocol and commented on the first and final drafts of the review. WM van den Bergh: participated in developing the protocol and commented on the first and final drafts of the review.
Sources of support
Internal sources
University Medical Center, Utrecht, Netherlands.
External sources
The Netherlands Heart Foundation, Netherlands.
Declarations of interest
The authors were involved in the organisation and writing of two of the included RCTs (Hop 2000; van den Bergh 2006).
Edited (no change to conclusions)
References
References to studies included in this review
Hop 2000 {published data only}
- Hop JW, Rinkel GJE, Algra A, Berkelbach van der Sprenkel JW, Gijn J. Randomized pilot trial of postoperative aspirin in subarachnoid hemorrhage. Neurology 2000;54:872‐8. [DOI] [PubMed] [Google Scholar]
Mendelow 1982 {published data only}
- Mendelow AD, Stockdill G, Steers AJW, Hayes J, Gillingham FJ. Double‐blind trial of aspirin in patients receiving tranexamic acid for subarachnoid haemorrhage. Acta Neurochirurgica 1982;62:195‐202. [DOI] [PubMed] [Google Scholar]
Ono 1984 {published data only}
- Ono H, Mizukami M, Kitamura K, Kikuchi H. Subarachnoid hemorrhage. Agents and Actions Supplements 1984;15:259‐72. [PubMed] [Google Scholar]
Shaw 1985 {published data only}
- Shaw MDM, Foy PM, Conway M, Pickard JD, Maloney P, Spillane JA, et al. Dipyridamole and postoperative ischemic deficits in aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery 1985;63:699‐703. [DOI] [PubMed] [Google Scholar]
Suzuki 1989 {published data only}
- Suzuki S, Sano K, Handa H, Asano T, Tamura A, Yonekawa Y, et al. Clinical study of OKY‐046, a thromboxane synthetase inhibitor, in prevention of cerebral vasospasms and delayed cerebral ischaemic symptoms after subarachnoid haemorrhage due to aneurysmal rupture: a randomized double‐blind study. Neurological Research 1989;11:79‐88. [DOI] [PubMed] [Google Scholar]
Tokiyoshi 1991 {published data only}
- Tokiyoshi K, Ohnishi T, Nii Y. Efficacy and toxicity of thromboxane synthetase inhibitor for cerebral vasospasm after subarachnoid hemorrhage. Surgical Neurology 1991;36:112‐8. [DOI] [PubMed] [Google Scholar]
van den Bergh 2006 {published data only}
- Bergh WM, Algra A, Dorhout Mees SM, Kooten F, Dirven CM, Gijn J, et al. Randomized controlled trial of acetylsalicylic acid in aneurysmal subarachnoid hemorrhage: the MASH Study. Stroke 2006;37:2326‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fujita 1990 {published data only}
- Fujita K, Shirakuni T, Ehara K, Tamaki N, Matsumoto S. Prevention of the symptomatic vasospasm (SV) by antiplatelet agent (ticlopidine) and prophylactic hypervolemic hemodilution therapy ‐ evaluated by transcranial doppler sonography (TCD). Stroke 1990;21 Suppl I:1‐68. [Google Scholar]
Hirashima 2001 {published data only}
- Hirashima Y, Endo S, Nukui H, Kobayashi N, Takaku A. Effect of a platelet‐activating factor receptor antagonist, E5880, on cerebral vasospasm after aneurysmal subarachnoid hemorrhage ‐ open clinical trial to investigate efficacy and safety. Neurologia medico‐chirurgica 2001;41(4):165‐76. [DOI] [PubMed] [Google Scholar]
Mizukami 1981 {published data only}
- Mizukami M, Kikuchi H, Ono H, Taneda M. Randomized study of ticlopidine on cerebral vasospasm following ruptured aneurysm. Thrombosis and Haemostasis 1981;46:307. [Google Scholar]
Niemi 1999 {published data only}
- Niemi T, Tanskanen P, Taxell C, Juvela S, Randell T, Rosenberg P. Effects of nonsteroidal anti‐inflammatory drugs on hemostasis in patients with aneurysmal subarachnoid hemorrhage. Journal of Neurosurgical Anesthesiology 1999;11(3):188‐94. [DOI] [PubMed] [Google Scholar]
Suzuki 1981 {published data only}
- Suzuki S, Sobata E, Iwabuchi T. Prevention of cerebral ischemic symptoms in cerebral vasospasm with trapidil, an antagonist and selective synthesis inhibitor of thromboxane A2. Neurosurgery 1981;9:679‐85. [DOI] [PubMed] [Google Scholar]
Suzuki 1985 {published data only}
- Suzuki S, Iwabuchi T, Tanaka T, Kanayama S, Ottomo M, Hatanaka M, et al. Prevention of cerebral vasospasm with OKY‐046, an imidazole derivative and a thromboxane synthetase inhibitor: a preliminary co‐operative clinical study. Acta Neurochirurgica 1985;77:133‐41. [DOI] [PubMed] [Google Scholar]
Tani 1984 {published data only}
- Tani E, Maeda Y, Fukumori T, Nakano M, Kochi N, Morimura T, et al. Effect of selective inhibitor of thromboxane A2 synthetase on cerebral vasospasm after early surgery. Journal of Neurosurgery 1984;61:24‐9. [DOI] [PubMed] [Google Scholar]
Yano 1993 {published data only}
- Yano K, Kuroda T, Tanabe Y, Yamada H. Preventive therapy against delayed cerebral ischaemia after aneurysmal subarachnoid haemorrhage: trials of thromboxane A2 synthetase inhibitor and hyperdynamic therapy. Acta Neurochirurgica 1993;125:15‐9. [DOI] [PubMed] [Google Scholar]
Additional references
ACROSS 2000
- [No authors listed]. Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke 2000;31:1843‐50. [DOI] [PubMed] [Google Scholar]
Brilstra 2000
- Brilstra EH, Rinkel GJE, Algra A, Gijn J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology 2000;55:1656‐60. [DOI] [PubMed] [Google Scholar]
Cosmi 2001
- Cosmi B, Rubboli A, Castelvetri C, Milandri M. Ticlopidine versus oral anticoagulation for coronary stenting. Cochrane Database of Systematic Reviews 2001, Issue 4. [Art. No.: CD002133. DOI: 10.1002/14651858.CD002133] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Rooij 2007
- Rooij NK, linn FHH, Plas JAP, Algra A, Rinkel GJE. Incidence of subarachnoid hemorrhage: a ten year update of a systematic review with emphasis of role of region, gender, age and time trend. In preparation.
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
Dorsch 1995
- Dorsch NW. Cerebral arterial spasm: a clinical review. British Journal of Neurosurgery 1995;9:403‐12. [DOI] [PubMed] [Google Scholar]
Hirashima 2005
- Hirashima Y, Hamada H, Kurimoto M, Origasa H, Endo S. Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery 2005;102:882‐7. [DOI] [PubMed] [Google Scholar]
Honma 1989
- Honma Y, Clower BR, Haining JL, Smith RR. Comparison of intimal platelet accumulation in cerebral arteries in two experimental models of subarachnoid hemorrhage. Neurosurgery 1989;24:487‐90. [DOI] [PubMed] [Google Scholar]
Hop 1997
- Hop JW, Rinkel GJE, Algra A, Gijn J. Case‐fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28:660‐4. [DOI] [PubMed] [Google Scholar]
Juvela 1991
- Juvela S, Hillbom M, Kaste M. Platelet thromboxane release and delayed cerebral ischemia in patients with subarachnoid hemorrhage. Journal of Neurosurgery 1991;74:386‐92. [DOI] [PubMed] [Google Scholar]
Juvela 1995
- Juvela S. Aspirin and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery 1995;82:945‐52. [DOI] [PubMed] [Google Scholar]
Okhuma 1991
- Okhuma H, Suzuki S, Kimura M, Sobata E. Role of platelet function in symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1991;22:854‐9. [DOI] [PubMed] [Google Scholar]
Okhuma 1993
- Okhuma H, Ogane K, Fujita S, Manabe H, Suzuki S. Impairment of anti‐platelet‐aggregating activity of endothelial cells after experimental subarachnoid hemorrhage. Stroke 1993;24:1541‐6. [DOI] [PubMed] [Google Scholar]
Patrono 1994
- Patrono C. Aspirin as an antiplatelet drug. New England Journal of Medicine 1994;330:1287‐94. [DOI] [PubMed] [Google Scholar]
Rabinstein 2004
- Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, et al. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 2004;35:1862‐6. [DOI] [PubMed] [Google Scholar]
Rinkel 2005
- Rinkel GJ, Feigin VL, Algra A, Bergh WM, Vermeulen M, Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database of Systematic Reviews 2005, Issue 1. [Art. No.: CD000277. DOI: 10.1002/14651858.CD000277.pub3] [Google Scholar]
Roos 2000
- Roos YB, Haan RJ, Beenen LF, Groen RJ, Albrecht KW, Vermeulen M. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: a prospective hospital based cohort study in the Netherlands. Journal of Neurology, Neurosurgery and Psychiatry 2000;68:337‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toussaint 2004
- Toussaint LG, Friedman JA, Wijdicks EFM, Piepgras DG, Pichelmann MA, McIver JI, et al. Influence of aspirin on outcome following aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery 2004;101:921‐5. [DOI] [PubMed] [Google Scholar]
van der Schaaf 2002
- Schaaf IC, Ruigrok YM, Rinkel GJE, Algra A, Gijn J. Study design and outcome measures in studies on aneurysmal subarachnoid hemorrhage. Stroke 2002;33:2043‐6. [DOI] [PubMed] [Google Scholar]
Vane 2003
- Vane JR, Botting RM. The mechanism of action of aspirin. Thrombosis Research 2003;110:255‐8. [DOI] [PubMed] [Google Scholar]
Vinge 1988
- Vinge E, Brandt L, Ljunggren B, Andersson KE. Thromboxane B2 levels in serum during continuous administration of nimodipine to patients with aneurysmal subarachnoid hemorrhage. Stroke 1988;19:644‐7. [DOI] [PubMed] [Google Scholar]
Weinberger 2005
- Weinberger J. Adverse effects and drug interactions of antithrombotic agents used in prevention of ischaemic stroke. Drugs 2005;65:461‐71. [DOI] [PubMed] [Google Scholar]


