Abstract
Background
We previously reported a trial using a DNA vaccine encoding prostatic acid phosphatase (MVI-816, pTVG-HP), given over 12 weeks concurrently or sequentially with pembrolizumab, in patients with mCRPC. We report the final analysis of this trial following two additional treatment arms in which patients with mCRPC continued concurrent treatment until progression.
Materials and methods
Patients with mCRPC were treated with MVI-816 and pembrolizumab every 3 weeks (arm 3, n=20) or MVI-816 every 2 weeks and pembrolizumab every 4 weeks (arm 4, n=20). The primary objectives were safety, 6-month progression-free survival (PFS), median time to radiographic progression, and objective response rates. Secondary objectives included immunological evaluations.
Results
In 25 patients with measurable disease, there were no complete response and one confirmed partial response in a patient who subsequently found to have an MSIhi tumor. 4/40 patients (10%) had a prostate-specific antigen decline >50%. The estimated overall radiographic PFS rate at 6 months was 47.2% (44.4% arm 3, 61.5% arm 4). Accounting for all off-study events, overall median time on treatment was 5.6 months (95% CI: 5.4 to 10.8 months), 5.6 months for arm 3 and 8.1 months for arm 4 (p=0.64). Thirty-two per cent of patients remained on trial beyond 6 months without progression. Median overall survival was 22.9 (95% CI: 16.2 to 25.6) months. One grade 4 event (hyperglycemia) was observed. Immune-related adverse events (irAEs) >grade 1 were observed in 42% of patients overall. Interferon-γ and/or granzyme B immune response to prostatic acid phosphatase was detected in 2/20 patients in arm 3 and 6/20 patients in arm 4. Plasma cytokines associated with immune activation and CD8+ T-cell recruitment were augmented at weeks 6 and 12. The development of irAE was significantly associated with a prolonged time on treatment (HR=0.42, p=0.003). Baseline DNA homologous recombination repair mutations were not associated with longer time to progression.
Conclusions
Findings here demonstrate that combining programmed cell death 1 blockade with MVI-816 is safe, can augment tumor-specific T cells, and can result in a favorable 6-month disease control rate. Correlative studies suggest T-cell activation by vaccination is critical to the mechanism of action of this combination. Future randomized clinical trials are needed to validate these findings.
Trial registration number
Keywords: immunogenicity, vaccine; prostatic neoplasms; clinical trials, phase II as topic; costimulatory and inhibitory T-cell receptors
Introduction
Metastatic castration-resistant prostate cancer (mCRPC) is the lethal form of prostate cancer, accounting for nearly 30,000 deaths per year in the USA.1 Over the last 15 years, several therapies have been approved by Food and Drug Administration based on their ability to prolong overall survival in patients with mCRPC.2–8 Among these approved agents is sipuleucel-T, an autologous vaccine that targets the prostate tumor antigen prostatic acid phosphatase (PAP).5 The approval of sipuleucel-T demonstrates that immune-based therapies can have a positive role in the treatment of mCRPC. Given this, sipuleucel-T has been investigated in combination with other agents, including ipilimumab or abiraterone.9 10 Unfortunately, these trials have not demonstrated increased clinical activity.
Other immune-based treatments have demonstrated significant activity against many other solid tumor types, notably T-cell checkpoint inhibitors targeting cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) or programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1). However, these have demonstrated little benefit when used as monotherapies for patients with prostate cancer.11–14 The KEYNOTE-199 phase 2 trial treated 258 patients with mCRPC with pembrolizumab monotherapy in one of three cohorts depending on PD-L1 expression and whether patients had measurable disease. The objective response rate and 6-month disease control rate (DCR) were low, such that further pursuit of pembrolizumab as a monotherapy has not been pursued.15 Similarly, a phase 1a trial of 35 patients with mCRPC treated with atezolizumab monotherapy showed a similar rate of prostate-specific antigen (PSA) and objective responses, and median overall survival was 14.7 months. Two large randomized phase 3 trials using CTLA-4 blockade with ipilimumab as monotherapy in patients with mCRPC, either before or after chemotherapy, were negative for overall benefit in terms of overall survival.13 16 Trials conducted with nivolumab and ipilimumab in advanced prostate cancer have demonstrated slightly greater efficacy in terms of objective response rates and PSA declines, and perhaps in particular in patients with baseline homologous recombination repair (HRR) mutations, but this treatment elicited more adverse effects.17 18 Taken together, these prior trials have suggested that combination approaches with checkpoint blockade may be necessary, but combinations other than PD-1 blockade with CTLA-4 blockade may be preferable. Pembrolizumab and atezolizumab have been combined with other agents for advanced prostate cancer, including androgen-targeted agents (enzalutamide), chemotherapy (docetaxel), and poly (ADP-ribose) polymerase inhibition, and several of these trials are underway.19
We have previously reported that a DNA vaccine encoding PAP (pTVG-HP, aka MVI-816) could be safely administered to patients with early PSA-recurrent prostate cancer and elicit/augment PAP-specific type 1 T helper-biased T cells.20 21 In preclinical studies, we demonstrated that vaccination activates CD8+ T cells that then express PD-1.22 Blockade of PD-1 or PD-L1 at the time of vaccination led to greater antitumor activity, likely due to preventing ligation of PD-1 expressed on activated CD8+ T cells by PD-L1 in the tumor microenvironment.22 23 We tested this approach in a pilot clinical trial in 26 patients with advanced prostate cancer. Patients with mCRPC received MVI-816 delivered over 12 weeks, and received pembrolizumab either concurrently with vaccination over these 12 weeks, or over the subsequent 12 weeks. We have previously reported the safety and outcomes of that trial, wherein we observed that concurrent treatment was associated with PSA declines and decreases in tumor volume.24 That trial was then expanded to explore continued concurrent treatment until disease progression, beyond 12 weeks, and with two different schedules of treatment. We report here the final analysis of this trial, with emphasis on the 40-patient extended treatment arms.
Materials and methods
Study agent and regulatory information
MVI-816 (Madison Vaccines, Madison, Wisconsin, USA) is a plasmid DNA encoding the full-length human PAP cDNA.25 The study protocol was reviewed and approved by all local and federal regulatory entities. All patients gave written informed institutional review board (IRB)-approved consent for participation.
Patient population
Eligible subjects were those with a histological diagnosis of prostate adenocarcinoma with metastases and evidence of castration resistance. Patients were required to have progressive disease, as per Prostate Cancer Working Group 2 (PCWG2) criteria.26 There was no exclusion for patients with visceral metastases, except for brain metastases. Prior treatment with second-generation androgen receptor (AR)-targeted agents, including abiraterone and/or enzalutamide, was allowed, and treatment with cytotoxic chemotherapy greater than 6 months before registration was permitted. There was no exclusion for patients who had received prior immunotherapy. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance score of ≤2, and normal bone marrow, liver, and renal function.
Study design and procedures
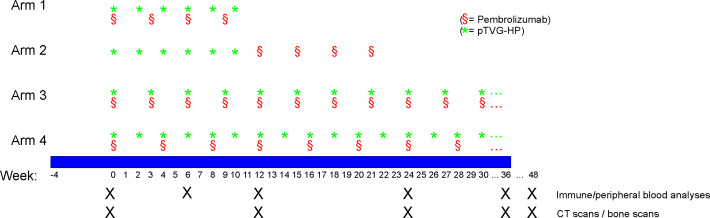
This study was originally designed as an open-label, randomized pilot trial to evaluate concurrent versus sequential treatment with MVI-816 and pembrolizumab. The original accrual goal was 32 subjects, based on the goal of detecting an anticipated 45% increase in progression-free survival (PFS) rate at 6 months in the concurrent treatment with 80% power at the one-sided 10% significance level. Results from this pilot trial were reported after accrual of 26 subjects.24 The trial was then amended to further explore concurrent treatment in 20 additional subjects. A fourth study arm of 20 additional subjects was subsequently added to explore a separate concurrent treatment schedule. The treatment schemas for each study arm are shown in figure 1. Each vaccination used 100 µg MVI-816 plasmid coadministered with 200 µg granulocyte-macrophage colony-stimulating factor (GM-CSF, Leukine, sargramostim) and was delivered intradermally on the left lateral arm in two divided injections. Patients underwent blood draws prior to treatment and at weeks 6, 12, 36, and 48 for immunological assessments. All patients received a tetanus immunization prior to study treatment as a positive control for immunological testing. Blood tests were performed every 3–4 weeks and included complete blood counts (CBC), creatinine, electrolytes, glucose, bilirubin, alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, amylase, lactate dehydrogenase, and thyroid stimulating hormone (TSH). All toxicities were graded according to the National Cancer Institute Common Terminology Criteria Grading System, version 4. Plasma from study participants (61 evaluable of 66) was evaluated for 105 oncogenic and DNA HRR mutations in cell-free DNA using the xF gene liquid biopsy panel (Tempus, Chicago, Illinois, USA).
Figure 1.
Treatment schema for the original study arms evaluating treatment sequence (arms 1 and 2), and the arms evaluating treatment until progression (arms 3 and 4). pTVG-HP, DNA vaccine encoding prostatic acid phosphatase.
Clinical response evaluation
Serum PSA values were collected every 3–6 weeks. PSA progression was defined as the first 50% increase over nadir (or pretreatment PSA if no PSA decline), confirmed by a second value 3 or more weeks later. CT of abdomen/pelvis and bone scans were obtained within 6 weeks prior to the first day of treatment, and then at 12-week intervals following day 1. Tumor response measurements were made as per PCWG2 recommendations.26 By protocol, scans at week 12 were used as the radiographic “baseline” for determining subsequent response or progression to permit possible delayed immunological treatment effects and account for possible pseudoprogression. Patients came off trial for radiographic progression, clinical progression, or at the discretion of the patient or treating physician; however, discontinuation for PSA rise alone was discouraged.
Immunological response evaluation
Measures of antigen-specific immune response were performed by interferon-γ (IFN-γ) and granzyme B fluorescent ELISpot with fresh (not cryopreserved) peripheral blood mononuclear cells (PBMCs) as previously described.27 Antigens used included tetanus toxoid protein (Calbiochem), pools of 15-mer peptides spanning the amino acid sequence of PAP or PSA and overlapping by 11 amino acids (LifeTein), or phytohemaglutinin as a positive control. Immune response resulting from immunization was defined as a PAP-specific response detectable post-treatment that was statistically significant (compared with media only control by t-test), at least threefold higher than the pretreatment value, and with a frequency >1:100 000 PBMC, as we have previously reported.21 24 28 In separate analyses, ELISpot assays were conducted using purified CD4+ or CD8+ T cells and cocultured with antigen and autologous CD14+ monocytes (Stem Cell Technologies, Vancouver, British Columbia, USA).
Sera samples obtained at baseline and various post-treatment time points were evaluated for IFN-γ concentration by ELISA, as previously reported.29 Plasma samples were also evaluated by Luminex multiplex analysis for 35 cytokine and chemokine analytes, according to the manufacturer’s instructions (Cytokine 35-plex human panel, ThermoFisher). These included epidermal growth factor (EGF), eotaxin, FGF-basic, G-CSF, GM-CSF, hepatocyte growth factor (HGF), IFN-α, IFN-γ, interleukin (IL)-1β, IL-1α, IL-1RA (IL-1 receptor antagonist), IL-2, IL-2R, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40/p70) IL-13, IL-15, IL-17A, IL-17F, IL-22, interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), monokine induced by gamma (MIG, CXCL9), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, RANTES, tumor necrosis factor α (TNF-α), and vascular endothelial growth factor (VEGF).
Statistical analysis
Time to radiographic progression was defined from the start of treatment to date of radiographic disease progression or last day of radiographic follow-up. Overall survival was defined from the start of treatment to date of death for any cause or last day of follow-up. Time on trial was defined as the number of months from the start of treatment date to the date the patient discontinued treatment or completed the trial. By protocol criteria, radiographic progression was defined using the month 3 scans as the “baseline,” to account for delayed response to immunotherapy. Only one patient had a confirmed progression event so that for the primary analysis, both unconfirmed and confirmed progression events were treated as events for the evaluation of time to radiographic progression. In a secondary analysis, only confirmed progression events were treated as events while unconfirmed progression events were censored. Exploratory analyses were conducted to analyze time on trial. In these analyses, the off-study dates due to any cause were treated as events. Time to radiographic progression, overall survival, and time on trial were analyzed using the Kaplan-Meier method, and comparisons between study arms were conducted using univariate Cox proportional hazard regression modeling. HRs and the corresponding 95% CIs were reported. The radiographic PFS (rPFS) rate at 6-month rates for each arm were obtained from the Kaplan-Meier analysis. The number of responses were summarized in terms of frequencies. Toxicities and adverse events (AEs) with an attribution of at least possibly related to treatment were summarized by type and severity in tabular format, stratified by arms. Best changes in PSA levels were calculated for each participant as the highest percentage decreases and reported using waterfall plots. The analyses of the primary and secondary efficacy endpoints were analyzed using the intent-to-treat population, which included all randomized participants meeting eligibility criteria. AEs were analyzed using the safety population which included all participants who received at least one dose of treatment. Changes in cytokine and chemokine serum levels from pretreatment to weeks 6 and 12 were summarized in graphical format using profile plots. The log-rank test was used to evaluate associations between changes in immunological/cytokine parameters (<median vs ≥median change from pretreatment to week 6) and time to radiographic progression and time on trial. Analogously, the log-rank test was used to evaluate the associations between immune responses, baseline HRR mutation status, and time to event clinical outcomes. The comparisons of immune response rates between groups were conducted using Fisher’s exact test. All reported p-values are two-sided, and p-value <0.05 was used to define statistical significance. Statistical analyses were conducted using SAS software (SAS Institute, Cary, North Carolina, USA), V.9.4.
Results
Patient population and adverse events
Sixty-six patients with progressive mCRPC were enrolled in this trial between August 2015 and October 2020 at the University of Wisconsin Carbone Cancer Center. Twenty-six patients were enrolled in two treatment arms to evaluate the sequence of DNA vaccine with PD-1 blockade, and the results from these treatment arms were previously reported.24 Forty patients were then accrued sequentially to two treatment arms evaluating different schedules of treatment, with vaccination and pembrolizumab delivered at the same time every 3 weeks (arm 3), or vaccination delivered every 2 weeks and pembrolizumab delivered every 4 weeks (arm 4). The schema for these treatment arms is shown in figure 1. Demographic information and prior treatments for all patients are shown in table 1. The median age of participants was 70 years (range 47–86 years). Forty-one per cent had been treated with a second-generation AR-targeted therapy, and 27% had received prior taxane chemotherapy (13/66 in the castration-sensitive setting and 6/66 in the castration-resistant setting). AEs for patients treated in arms 1 and 2 were previously reported.24 For patients treated in arms 3 and 4, a single grade 4 AE (hyperglycemia) was observed (table 2). This event occurred within 28 days after the patient had come off trial and had started abiraterone and prednisone. While this was felt to be most likely due to prednisone, and was not considered an irAE, attribution to prior study treatment could not be excluded. Grade 3 events included hyperthyroidism, increased AST, hypoxia, adrenal insufficiency, abdominal pain, colitis, diarrhea, vomiting, increased amylase, back pain, bone pain, muscle weakness, pruritus, and rash. Thirteen grade 3 or 4 events were observed in arm 4, and four grade 3 or 4 events were observed in arm 3. Online supplemental table 1 shows AE for all patients in all study arms. Over all study arms, irAEs of grade 2 or higher were observed in 28/66 (42%) of patients and included adrenal insufficiency (n=2), hepatitis (n=2), colitis (n=4), thyroid dysfunction (n=16), pancreatitis (n=1), pneumonitis (n=1), and rash (n=2). For patients in the extended treatment arms, 19/40 (48%) developed an irAE grade 2 or higher. 6/40 (15%) required high-dose steroid treatment, and 7/40 (18%) discontinued pembrolizumab due to toxicity. Of these patients, two, both in arm 4, discontinued pembrolizumab due to irAE but continued treatment with MVI-816. Both patients had subsequent PSA declines, including one patient who developed a complete PSA response (data not shown).
Table 1.
Demographics
| Overall | Arm 1 | Arm 2 | Arm 3 | Arm 4 | |
| Number | 66 | 13 | 13 | 20 | 20 |
| Age (years) | |||||
| Median | 70 | 67 | 73 | 71 | 66 |
| Range | 47–86 | 60–82 | 54–83 | 47–77 | 50–86 |
| Race/ethnicity, n (%) | |||||
| White (non-Hispanic) | 63 (95%) | 12 (92%) | 12 (92%) | 20 (100%) | 19 (95%) |
| White (unknown ethnicity) | 2 (3%) | 1 (8%) | 1 (8%) | 0 (0%) | 0 (0%) |
| Black or African-American | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) |
| Prior treatments, n (%) | |||||
| Bicalutamide | 58 (88%) | 13 (100%) | 12 (92%) | 17 (85%) | 16 (80%) |
| Any second-generation AR-targeted agent | 27 (41%) | 4 (31%) | 8 (62%) | 5 (25%) | 10 (50%) |
| Enzalutamide | 18 (27%) | 3 (23%) | 6 (46%) | 3 (15%) | 6 (30%) |
| Abiraterone | 12 (18%) | 1 (8%) | 2 (15%) | 2 (10%) | 7 (35%) |
| TAK-700/apalutamide | 4 (6%) | 2 (15%) | 1 (8%) | 1 (5%) | 0 (0%) |
| Taxane (docetaxel and/or cabazitaxel) | 18 (27%) | 2 (15%) | 3 (23%) | 4 (20%) | 9 (45%) |
| Radium 223 | 3 (5%) | 1 (8%) | 0 (0%) | 1 (5%) | 1 (5%) |
| Sipuleucel-T | 1 (2%) | 0 (0%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Metastatic disease sites, n (%) | |||||
| Bone | 55 (83%) | 13 (100%) | 13 (100%) | 13 (65%) | 16 (80%) |
| Lymph node | 25 (38%) | 4 (31%) | 4 (31%) | 9 (45%) | 8 (40%) |
| Visceral | 7 (11%) | 1 (8%) | 2 (15%) | 3 (15%) | 1 (5%) |
| Baseline PSA (ng/mL) | |||||
| Median | 37.1 | 34.4 | 31.8 | 26.3 | 25.7 |
| Range | 2.6–811 | 3.1–173 | 5.8–245 | 7.3–356 | 2.6–811 |
Table 2.
Adverse events
| Arm 3 | Arm 4 | |||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Endocrine | ||||||||
| Adrenal insufficiency | 1 | |||||||
| Hyperthyroidism/decreased TSH | 2 | 3 | 1 | 3 | 2 | |||
| Hypothyroidism/increased TSH | 5 | 4 | ||||||
| Gastrointestinal | ||||||||
| Abdominal pain | 1 | 1 | ||||||
| Colitis | 1 | 2 | ||||||
| Diarrhea | 1 | 1 | 3 | 2 | ||||
| Nausea | 1 | 3 | 1 | |||||
| Vomiting | 2 | 1 | ||||||
| General | ||||||||
| Chills | 3 | 5 | ||||||
| Fatigue | 3 | 3 | 10 | 1 | ||||
| Injection site reaction | 5 | 5 | ||||||
| Laboratory investigations | ||||||||
| Increased AST | 1 | 1 | ||||||
| Increased amylase | 1 | 1 | ||||||
| Metabolism and nutrition | ||||||||
| Hyperglycemia | 1 | 1 | ||||||
| Musculoskeletal and connective tissue | ||||||||
| Arthralgias/arthritis | 1 | 3 | ||||||
| Back pain | 1 | 1 | ||||||
| Bone pain | 1 | 1 | 1 | |||||
| Muscle weakness | 1 | 1 | ||||||
| Myalgia | 2 | 2 | ||||||
| Nervous system | ||||||||
| Dizziness | 2 | 1 | 1 | |||||
| Headache | 2 | 2 | ||||||
| Respiratory, thoracic | ||||||||
| Hypoxia | 1 | |||||||
| Skin and subcutaneous | ||||||||
| Pruritus | 1 | 1 | 1 | |||||
| Rash maculopapular | 1 | 1 | 2 | 1 | ||||
All adverse events with a frequency >5%, and any adverse events with grade >grade 2, that were believed to be at least possibly related to treatment are shown for patients treated in arms 3 and 4. The numbers represent the number of patients experiencing a particular event at any point during the treatment period, with the highest grade reported for any single individual.
TSH, thyroid-stimulating hormone.
jitc-2021-004198supp001.pdf (629.2KB, pdf)
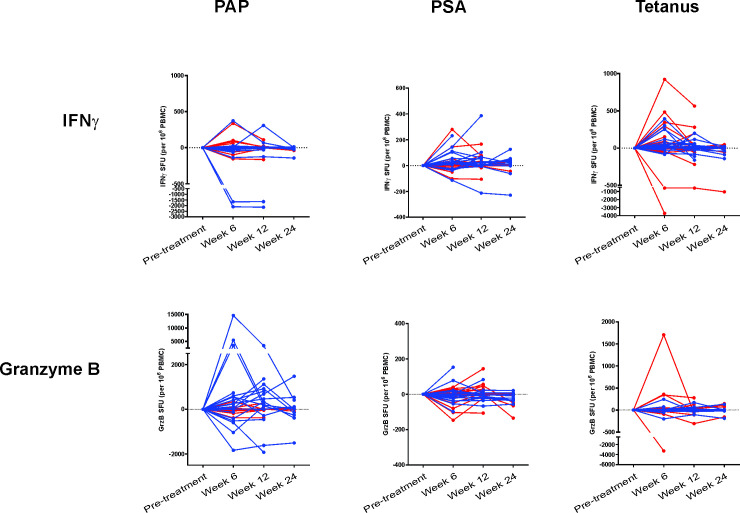
Immunological response
Similar to what was previously described for the first two study arms, patients in arms 3 and 4 were evaluated prior to treatment, and at weeks 6, 12 and 24 for evidence of T-cell immunity to the PAP target antigen by antigen-specific secretion of IFN-γ and/or granzyme B, as detected by fluorescent ELISpot. As shown in figure 2, IFN-γ- and granzyme B-secreting T cells in response to PAP restimulation were observed, most notably with antigen-specific granzyme B secretion. The magnitude of these responses was greater in patients treated in arm 4. IFN-γ-secreting response specific for PSA, an off-target antigen, was detected in some individuals, notably patients treated in arm 4. Multifunctional T cells, secreting both IFN-γ and granzyme B, were also detected, predominantly in patients treated in arm 4 (online supplemental figure 1). Using purified CD4+ and CD8+ T cells, PAP-specific IFN-γ secretion was detected in both CD4+ and CD8+ T cells, and PAP-specific granzyme B secretion was found primarily in CD8+ T cells (online supplemental figure 2). Overall, 2/20 (10%) patients in arm 3 had significant increases in PAP-specific T cells that were detected at least twice in post-treatment specimens up to 1 year and 6/20 (30%) patients in arm 4. In contrast, 7/20 (35%) patients in arm 3, and 5/20 (25%) patients in arm 4, had significant responses detectable to tetanus toxoid at least twice in post-treatment specimens. For arm 3 and arm 4 combined, there was no significant association detected between immune response to PAP and the incidence of irAE (p=0.12).
Figure 2.
Immunological response: interferon-γ (IFN-γ) and granzyme B (GrzB) fluorescent ELISpot. Peripheral blood mononuclear cells (PBMCs) were collected from subjects (n=19, arm 3; n=20, arm 4) at baseline, 6 weeks, 12 weeks, and 24 weeks and evaluated for antigen-specific IFN-γ or GrzB secretion by fluorescent ELISpot. Shown are the spot-forming units (SFUs) for IFN-γ secretion (top panels) or GrzB secretion (bottom panels) following stimulation with a prostatic acid phosphatase (PAP) peptide library, PSA peptide library (non-specific control), or tetanus (positive control) for each patient. Patients treated in arm 3 are colored red, and patients treated in arm 4 are colored blue.
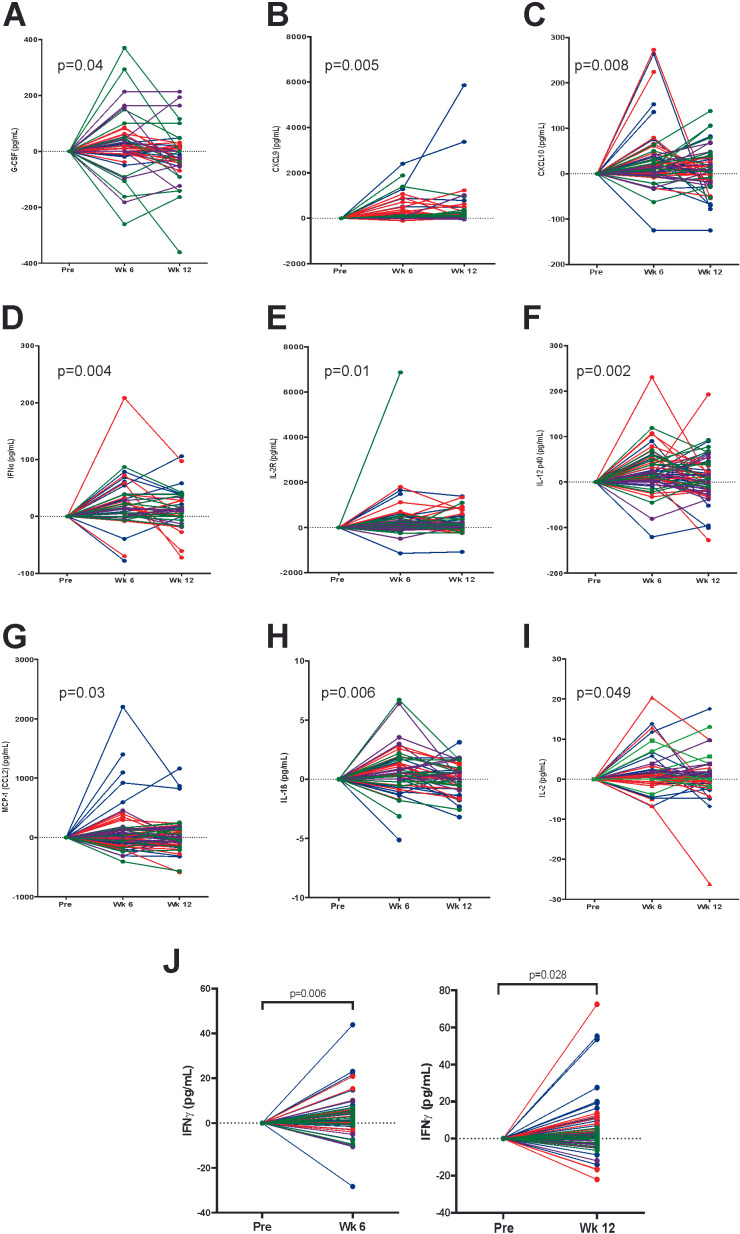
Changes in serum levels, from pretreatment to weeks 6 and 12, of 35 cytokines and chemokines, were evaluated for all patients by multiplex Luminex analysis or ELISA. Significant changes were observed for G-CSF (figure 3A), CXCL9 (figure 3B), CXCL10 (figure 3C), IFN-α (figure 3D), IL-2R (figure 3E), IL-12 (figure 3F), MCP-1 (figure 3G), IL-1β (figure 3H), IL-2 (figure 3I), and IFN-γ (figure 3J), with the greatest increases observed for most cytokines at week 6. While patients treated in arm 2 (receiving vaccine only for the first 12 weeks) showed increases in many of these cytokines, significant increases were only detected in IL-1β (p=0.049) and IL-2 (p=0.01, data not shown), suggesting that most of the changes in cytokines observed were due to the addition of pembrolizumab. For arm 3 and arm 4 combined, there was no significant association detected between increased IFN-γ in the serum at 6 weeks (above the median) and the presence of irAE (p=0.10).
Figure 3.
Treatment elicits increases in serum cytokines and chemokines. Plasma obtained from patients prior to treatment, and at weeks 6 and 12 of treatment, were evaluated for 35 cytokines and chemokines by multiplex Luminex assay. Shown are the changes in concentration from baseline for each patient, grouped by study arm (arm 1=green, arm 2=purple, arm 3=red, arm 4=blue), for those cytokines with significant change over time (by mixed-effects model): (A) G-CSF, (B) CXCL9, (C) CXCL10, (D) IFNα, (E) IL-2R, (F) IL-12, (G) MCP-1 (CCL2), (H) IL-1β, and (I) IL-2. (J) IFN-γ was below the level of detection for this multiplex assay and was separately measured from pretreatment to week 6 or pretreatment to week 12 by ELISA, with statistical comparison by paired t-test.
Clinical effects
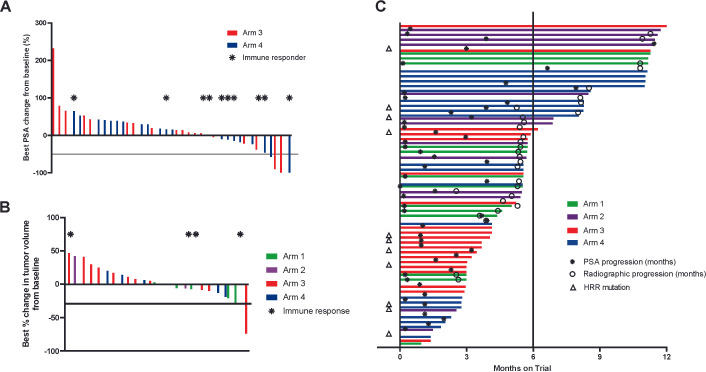
PSA values were obtained every 3–4 weeks. Shown in figure 4A is the best PSA change from baseline. Fourteen patients (35%) had any PSA decline, and four patients (10%) had a confirmed PSA decline >50%; three patients had a PSA decline >90%. Five of these 14 patients had received prior second-generation AR-targeted therapy, and three had received prior taxane chemotherapy. The detection of PSA decline was not significantly associated with T-cell immune response to PAP (43% immune responders had PSA decline vs 17% immune responders had no PSA decline, p=0.12). Only one of four patients with PSA decline >50% had a detectable immune response to PAP. The median time to PSA progression for the entire trial was 1.6 months (95% CI: 1.1–3.2 months), 2.5 months for patients in arm 3, and 3.9 months for patients in arm 4. Sixteen patients in arms 3 and 4 had measurable disease, of whom five had any evidence of tumor volume decrease. One patient in arm 3, who was subsequently found to have an MSIhi tumor, had a confirmed partial response, and there were no complete responses. By protocol criteria, radiographic progression was determined using the month 3 scans as the “baseline,” to account for delayed response to immunotherapy. Patients were to come off trial with evidence of disease progression beyond that time point, in the event of clinical progression prior to that or at the discretion of the treating physician or patient that other treatments were warranted. 21/40 patients in arms 3 and 4 had evidence of progression at 3 months compared with baseline, of which 17 came off trial, 3 had evidence of progression at 6 months, and 1 had no progression at 6 months. Overall median time to progression (TTP) across all arms was 5.6 months (95% CI: 5.4 to 10.8 months), 5.6 (95% CI: 4.6–not been reached) months for arm 3, and 8.1 (95% CI: 5.3 to 10.8) months for arm 4 (figure 4C, p=0.64). For the trial overall, 21/66 (32%) of patients remained on trial without radiographic progression after 6 months, previously defined as the “disease control rate” for this population.15 The DCR was 10% (2/20) for arm 3 and 45% (9/20) for arm 4 (p=0.03). Median overall survival for the entire trial was 22.9 months, and patients remain in follow-up for up to 2 years after treatment.
Figure 4.
Clinical effects. (A) Serum PSA values were collected from all individuals prior to treatment and over the course of treatment. Shown are the best percentage change in serum PSA from day 1. Red lines show individual patients treated in arm 3, and blue lines show individual patients treated in arm 4. The horizontal line indicates 50% decrease from baseline. Asterisks indicate those individuals with immune response to prostatic acid phosphatase (PAP). (B) Shown are the best percentage change in tumor volume for 25 individuals in all treatment arms who had measurable disease, with the horizontal line indicating 30% decline. Asterisks indicate those individuals with immune response to PAP. (C) Swimmer plot showing the time on trial for all study patients. Asterisks indicate time of PSA progression, and open circles indicate radiographic progression. Triangles indicate those individuals with baseline DNA homologous recombination repair (HRR) mutations (n=10) or MSI-high tumors (n=2). The vertical line indicates 6 months.
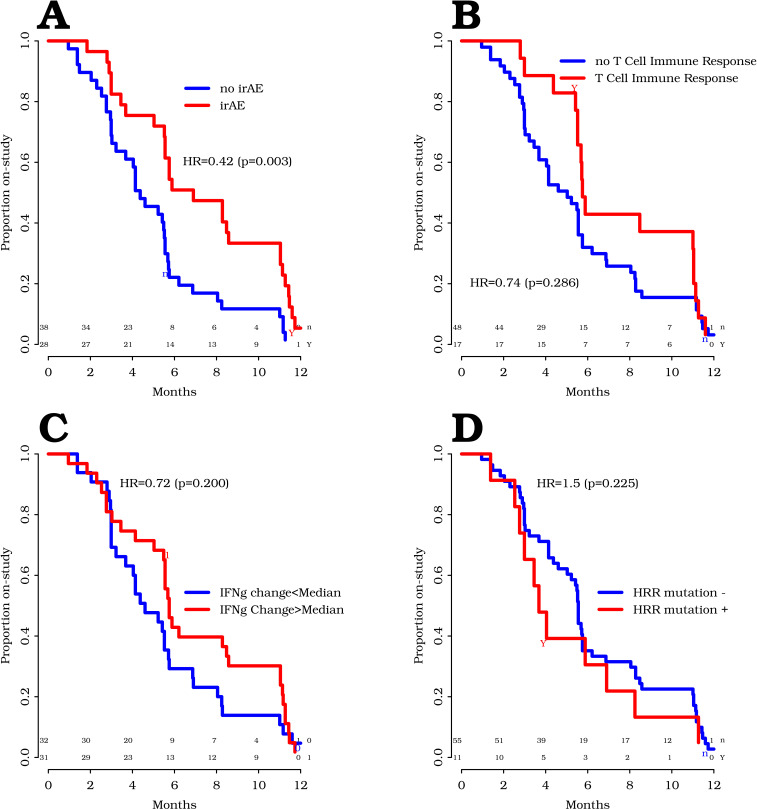
The protocol defined radiographic progression as occurring after the 3-month scan and with a confirmatory scan. However, as described above, we observed that many patients came off trial for clinical or radiographic progression prior to meeting the confirmed radiographic progression endpoint. Hence, for exploratory studies to evaluate associations with possible clinical benefit, we used time on trial rather than rPFS, as a more conservative endpoint. As shown in figure 5A, the development of irAE was significantly associated with prolonged time on trial (HR=0.42, p=0.003). Immune response to PAP was not significantly associated with longer time on trial (HR=0.74, p=0.29; figure 5B). IFN-γ increase in the serum at 6 weeks of treatment was associated with an increased time to progression (HR=0.49, p=0.056); however, no significant association was detected with time on trial (HR=0.72, p=0.20; figure 5C). Increases in CXCL-9, CXCL-10, G-CSF, IFN-α, IL-12, IL-1β, IL-2, IL-2R, and MCP-1 were not associated with increased time on trial (online supplemental figure 3). Of 61 patients for whom plasma were evaluable for baseline testing, 10 (16%) were found to have DNA HRR mutations, including MSH2, MSH3, MSH6, MLH1, MLH3, ATM, BRCA1, and FANCA mutations. Two of 61 (3%) were found to have MSI-high tumors. These specific mutations, and patient outcomes, are shown in online supplemental table 2). The presence of baseline DNA HRR mutations or MSI-high tumors was not associated with longer time on trial (HR=1.5, p=0.22; figure 5D). Similar findings were observed evaluating these associations with time to radiographic progression (online supplemental figure 4).
Figure 5.
Time on trial correlative analyses. Time on trial was assessed with respect to the development of grade 2 or higher immune-related adverse events (irAEs, A), T-cell immune response to prostatic acid phosphatase (B), increases in serum interferon-IFN-γ (C), and the presence of baseline homologous recombination repair (HRR) mutations or MSI-high tumors (D). Statistical comparisons are made using a log-rank test, with p<0.05 considered statistically significant.
Discussion
We report here the final results of a trial evaluating a DNA vaccine encoding PAP (MVI-816) in combination with pembrolizumab, evaluating sequence and dosing schedules. We have previously reported the first two arms of this trial, in which combined treatment, rather than sequential treatment, demonstrated greater efficacy as measured by objective tumor decreases and PSA declines. In this report, we evaluated continued concurrent treatment until radiographic progression and evaluated treatment with different schedules of vaccine and PD-1 blockade. We identified that treatment was safe, with adverse effects being those typically observed with PD-1 blockade alone. Patients treated in arm 4, who notably had more advanced disease, experienced higher immune response rates and prolonged time on trial, suggesting this is a preferred treatment schedule for future studies. The frequency of PSA declines, tumor volume decreases, as well as 6-month DCR, also compared favorably with what have been observed in trials with PD-1 or PD-L1 blockade alone in patients with mCRPC,14 15 supporting the use of T-cell activating therapies in combination with PD-1 blockade. The associations of time on treatment with vaccine schedule, and with measures of immune response, further support the requirement for vaccine-mediated T-cell activation in this combination.
In the current trial, we enrolled all patients with mCRPC, not restricting it to patients who had received prior chemotherapy or second-generation AR-targeted therapy, and not restricting the number of prior therapies. Hence, direct historical comparisons with PD-1 or PD-L1 blockade monotherapy trials cannot be made, as these were mostly restricted to patients who had received specific prior therapies.14 15 In the current trial, 10 patients (7 in arm 4) had received prior taxane chemotherapy and prior second-generation AR-targeted therapy and did not have MSIhi tumors. In that small subset, 2/10 (20%) had PSA declines from baseline, 4/10 (40%) remained on treatment beyond 6 months, rPFS was 5.5 months, and the median overall survival was 21.2 months. While this is a small subset, these outcomes compare favorably with trials using single agent immune checkpoint blockade.14 15 While there have been no studies evaluating MVI-816 alone in patients with mCRPC, previous experience with Prostvac and sipuleucel-T vaccines in this population suggest that treatment with vaccines alone do not lead to substantial PSA declines or prolongation of rPFS.5 30 The median rPFS was 3.7 months in patients treated with sipuleucel-T alone,5 and 3.8 months in patients treated with Prostvac alone.30 Hence, given this prior experience, we believe the rPFS and 6-month DCR observed in the current trial are likely due to the combination of vaccine and PD-1 blockade. However, randomized trials will be needed to directly evaluate this.
Several other reports have demonstrated that the development of irAEs is associated with a better outcome and longer survival for patients with other types of cancer treated with immune checkpoint blockade. This finding may be more relevant for PD-1/PD-L1 blockade than CTLA-4 blockade, as notably in the phase 3 trials with ipilimumab in prostate cancer there were frequent irAE, and no difference in overall survival. In the KEYNOTE-199 trial, irAEs were observed in 16% of patients, significantly fewer than the 42% observed in the current trial.15 Similarly, in the phase 1 trial of atezolizumab, few irAEs were observed.14 In particular, we observed thyroid-associated events occurred in 25% of patients, higher than what has been previously observed in trials with pembrolizumab alone. These findings are curious, as irAEs of any grade were observed in 23.0% of 1567 patients with melanoma treated with pembrolizumab, a disease for which treatment-related irAEs have been associated with prolonged overall survival.31 These findings suggest that either our population of patients was sufficiently different and at greater risk for irAE and response to pembrolizumab or that T-cell activation with tumor-targeted vaccination may lead to greater numbers of off-target immune toxicities. This latter possibility seems most likely given that irAEs were associated, although not significantly, with the development of T-cell response to the PAP target antigen. Notwithstanding, these events were easily manageable and did not necessarily lead to treatment discontinuation. This is the first report of irAE being associated with prolonged time to progression in patients with prostate cancer and suggests that this may serve as an independent biomarker for favorable outcome in future trials.
We identified that treatment with this combination led to an increase in several cytokines and chemokines that were detectable in the serum of patients at 6–12 weeks after beginning treatment. Notably, these included cytokines and chemokines associated with T-cell activation (eg, IFN-γ, IL-12, IL-1β, IL-2, and IFN-α) and cytolytic T-cell recruitment (eg, CXCL9 and CXCL10). CXCL9 and CXCL10 have previously been reported to be increased in plasma following treatment with PD-1 blockade in patients with non-small-cell lung cancer, and this was associated with longer PFS.32 In the current trial, we found that, while CXCL9 and CXCL10 were increased after treatment, these were not associated with longer treatment time or rPFS. In contrast, increases in IFN-γ were, suggesting that T-cell activation, presumably by vaccination, which may be important to improving the efficacy of PD-1 blockade. We previously identified that CD8+ T cells were increased in prostate tumors following vaccination and PD-1 blockade, and future studies will evaluate whether this was specifically associated with increases in IFN-γ and other cytokines and chemokines.24
In the current trial, we observed a lower immune response rate in patients treated with vaccine every 3 weeks (arm 3), compared with patients treated every 2 weeks (arm 4). The immune response rate in arm 4 (30%, 6/20) is similar to what we have reported before using vaccine alone in other stages of disease,21 27 was similar to the response rate from tetanus immunization performed in this trial, and was similar to the immune response rate to PAP detected following sipuleucel-T treatment in a previous trial.5 While these rates likely under-represent patients with immune response following treatment, given our high bar in defining an immune response and given that a functional tumor-specific T-cell response following immunization may not be detectable in the peripheral blood, it is noteworthy that this rate was lower in the treatment arm in which the frequency of immunization was reduced (arm 3). In addition, this arm had a statistically lower 6-month DCR, with the majority of patients having evidence of disease progression at 3 months. Given these findings, we believe the schedule of arm 4, with vaccine delivered every 2 weeks, is a preferable schedule for further evaluation in subsequent trials.
Findings in the current report confirm our previous observation that treatment with vaccine and PD-1 blockade can lead to favorable changes in tumor growth24 and suggests that this combination, using a schedule as identified in arm 4, should be further evaluated in randomized clinical trials as a rational means to improve the efficacy of immune checkpoint blockade therapy for prostate cancer. In addition, findings from this trial suggest additional approaches that might further improve this potential efficacy. In particular, the finding that immune response to the vaccine is associated with PSA decline and increased time on treatment suggests that vaccination methods to increase the T-cell response to multiple antigens may be preferred. A trial testing this hypothesis, using one versus two vaccines targeting different tumor antigens, is currently underway (NCT04090528). Moreover, the finding that other T-cell checkpoint molecules are upregulated with T-cell activation following vaccination, notably lymphocyte activating 3 (LAG-3), suggests that future studies should evaluate vaccines in combination with multiple T-cell checkpoint blockade, as we have demonstrated in preclinical models.33
Acknowledgments
We thank referring physicians and participating patients.
Footnotes
Twitter: @HamidEmamekhoo
Contributors: DGM oversaw the experimental design and immunological analysis, wrote and edited the manuscript, and is responsible for the overall content as the guarantor. JCE was the study biostatistician, designed the trial, analyzed all results, and edited the manuscript. EW and LEJ conducted the laboratory analyses. MJB managed the clinical trial protocol. CEK, HE and JML were involved with subject recruitment and treatment. GL oversaw the conduct of the clinical trial and all safety and clinical response analyses. All authors read and approved the final manuscript.
Funding: Grant support was provided by National Institutes of Health (P30 CA014520 and R01 CA129154), a 2014 Movember-PCF Challenge Award from the Prostate Cancer Foundation, and with funding support by Madison Vaccines.
Competing interests: DGM has ownership interest, has received research support, and serves as consultant to Madison Vaccines, which has licensed material described in this manuscript and supported this trial.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Additional data, not available within the article or supplementary information, are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by University of Wisconsin Health Sciences Human Subjects Review Board (IRB) Protocol 2015-0453. Participants gave informed consent to participate in the study before taking part.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513–20. 10.1056/NEJMoa041318 [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–12. 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147–54. 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 8.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–23. 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 9.Small EJ, Lance RS, Gardner TA, et al. A randomized phase II trial of Sipuleucel-T with concurrent versus sequential abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer. Clin Cancer Res 2015;21:3862–9. 10.1158/1078-0432.CCR-15-0079 [DOI] [PubMed] [Google Scholar]
- 10.Sinha M, Zhang L, Subudhi S, et al. Pre-existing immune status associated with response to combination of sipuleucel-T and ipilimumab in patients with metastatic castration-resistant prostate cancer. J Immunother Cancer 2021;9:e002254. 10.1136/jitc-2020-002254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016;7:52810–7. 10.18632/oncotarget.10547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013;24:1813–21. 10.1093/annonc/mdt107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic Chemotherapy-Naive castration-resistant prostate cancer. J Clin Oncol 2017;35:40–7. 10.1200/JCO.2016.69.1584 [DOI] [PubMed] [Google Scholar]
- 14.Petrylak DP, Loriot Y, Shaffer DR, et al. Safety and clinical activity of Atezolizumab in patients with metastatic castration-resistant prostate cancer: a phase I study. Clin Cancer Res 2021;27:3360–9. 10.1158/1078-0432.CCR-20-1981 [DOI] [PubMed] [Google Scholar]
- 15.Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol 2019:JCO1901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700–12. 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Pachynski RK, Narayan V, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell 2020;38:489–99. 10.1016/j.ccell.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 18.Boudadi K, Suzman DL, Anagnostou V, et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 2018;9:28561–71. 10.18632/oncotarget.25564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graff JN, Beer TM, Alumkal JJ, et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J Immunother Cancer 2020;8:e000642. 10.1136/jitc-2020-000642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeel DG, Dunphy EJ, Davies JG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol 2009;27:4047–54. 10.1200/JCO.2008.19.9968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeel DG, Becker JT, Eickhoff JC, et al. Real-Time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin Cancer Res 2014;20:3692–704. 10.1158/1078-0432.CCR-14-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahm CD, Colluru VT, McNeel DG. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8+ T cells. Cancer Immunol Res 2017;5:630–41. 10.1158/2326-6066.CIR-16-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rekoske BT, Smith HA, Olson BM, et al. Pd-1 or PD-L1 blockade restores antitumor efficacy following SSX2 Epitope-Modified DNA vaccine immunization. Cancer Immunol Res 2015;3:946–55. 10.1158/2326-6066.CIR-14-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeel DG, Eickhoff JC, Wargowski E, et al. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2018;9:25586–96. 10.18632/oncotarget.25387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson LE, Frye TP, Arnot AR, et al. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP). Vaccine 2006;24:293–303. 10.1016/j.vaccine.2005.07.074 [DOI] [PubMed] [Google Scholar]
- 26.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials Working group. J Clin Oncol 2008;26:1148–59. 10.1200/JCO.2007.12.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeel DG, Eickhoff JC, Johnson LE, et al. Phase II Trial of a DNA Vaccine Encoding Prostatic Acid Phosphatase (pTVG-HP [MVI-816]) in Patients With Progressive, Nonmetastatic, Castration-Sensitive Prostate Cancer. J Clin Oncol 2019;37:3507–17. 10.1200/JCO.19.01701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyriakopoulos CE, Eickhoff JC, Ferrari AC, et al. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin Cancer Res 2020;26:5162–71. 10.1158/1078-0432.CCR-20-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahm CD, Colluru VT, McIlwain SJ, et al. TLR Stimulation during T-cell Activation Lowers PD-1 Expression on CD8 +T Cells. Cancer Immunol Res 2018;6:1364–74. 10.1158/2326-6066.CIR-18-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010;28:1099–105. 10.1200/JCO.2009.25.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert C, Hwu W-J, Hamid O, et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: a landmark analysis in patients with advanced melanoma. Eur J Cancer 2021;144:182–91. 10.1016/j.ejca.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eltahir M, Isaksson J, Mattsson JSM, et al. Plasma proteomic analysis in non-small cell lung cancer patients treated with PD-1/PD-L1 blockade. Cancers 2021;13:3116. 10.3390/cancers13133116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahm CD, Moseman JE, Delmastro LE, et al. PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy. Oncoimmunology 2021;10:e1912892. 10.1080/2162402X.2021.1912892 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-004198supp001.pdf (629.2KB, pdf)
Data Availability Statement
Data are available on reasonable request. Additional data, not available within the article or supplementary information, are available on reasonable request.