Abstract
Background
Perioperative neurocognitive disorders (PND) are common complications of major surgery among elderly patients, remarkably decreasing patients’ life quality. Platelet count has been proved to be an essential factor in inflammation. However, as far as we know, the relationship between platelet count and PND is not clear yet in the orthopedic area. PND could be a long-term disease, which sometimes lasts for several years, and it is meaningful to find a biomarker of PND at the early stage. Thus, we designed this study to find out the association between perioperative platelet count and occurrence of PND, and determine whether preoperative platelet count could be a biomarker of the early stage of PND.
Methods
A prospective observational study was performed on the patients who would take total knee arthroplasty or total hip arthroplasty. Their peripheral platelets were counted by blood routine examination 1 day before and 3 days after the surgery. And we assessed their neurocognitive functions 1 day before and 3 days after the surgery. These data were recorded and analyzed to find out the relationship between platelet count and the occurrence of PND.
Results
Eventually, 70 patients finished the whole process, and 14 of them developed PND. The median preoperative platelet count in the PND group was significantly higher than that in the non-PND group (239 vs 168 × 10^9/L, p = 0.009). Preoperative platelet count was an independent risk factor for PND (odds ratio = 1.014, 95% confidence interval [CI] 1.000–1.027, P = 0.043) in the logistic multivariable regression, while the area under the curve of the receiver operating characteristic curve of the prediction model was 0.796 (95% CI 0.676–0.916).
Conclusions
The higher preoperative and postoperative level of platelet count in the peripheral blood were associated with the early stage of PND, and preoperative platelet count could be a potential predictor of the early stage of PND in patients undergoing major orthopedic surgeries.
Trial registration
Chinese Clinical Trial Registry: ChiCTR2000033001, registration date: 17 May 2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-02899-7.
Keywords: Perioperative neurocognitive disorders, Perioperative platelet count, Biomarker, Elderly major orthopedic surgery
Background
Perioperative neurocognitive disorders (PND), which were brought up in 2018, are common complications of major surgery among elderly patients, including cognitive impairment diagnosed before operation and postoperative acute and chronic cognitive dysfunction that could be diagnosed from 1 day to 1 year after surgery [1]. This concept combines the postoperative delirium (POD) and postoperative cognitive dysfunction (POCD). At the same time, POD is defined as an acute disorder of attention and cognition after surgery, and POCD used to be a concept but not a clinical diagnosis, representing a significant decline in neurocognitive performance in attention, orientation, memory, verbal fluency, coordination and so on [2, 3]. PND has been proved to increase the cost of care, reduce the quality of life, increase the risk of long-term cognitive decline and mortality [4–9]. Furthermore, the relationship between PND and cardiac surgery has been discussed widely. In contrast, in non-cardiac surgery, the incidence of PND ranges between 8.9 and 46.1%, depending on the study and type of surgery [10]. As the PND may have a long course and may even develop to Alzheimer‘s disease (AD) [11], which is hard to be cured by most clinical drugs [12], it is essential to diagnose and treat it at the early stage. Meanwhile, many recent studies focused on mechanisms and biomarkers at the early stage of PND, such as on the third day after surgery [13–15]. However, platelet count has not been reported as a biomarker in the early stage of PND.
Among many non-cardiac surgeries, total knee arthroplasty (TKA) and total hip arthroplasty (THA) are prevalent types of major orthopedic surgery. As the proportion of older people increases in China, the prevalence of osteoarthritis on the knee has risen to 21.85% [16], becoming a heavy burden for society. TKA is one of the most helpful solutions to knee osteoarthritis [17], while THA has a similar circumstance to TKA [18, 19]. Recent studies have shown that THA and TKA could induce an obvious inflammation reaction process via cell-immune response, genomic storm, or postoperative infection [20–22]. Moreover, several studies have proved that TKA and THA are risky for venous thromboembolism [23, 24]. Besides being the main reason for thrombosis, platelets have been an essential factor in inflammation by transferring signals in immune cell-cell interactions, activating and recruiting leukocytes to the inflammation site. Even its secreting exosomes can promote inflammation [25–27]. PND has been reported to result from a central neuroinflammatory response to surgery [28, 29]. However, as far as we know, the relationship between platelet count and occurrence of PND has never been investigated yet in the orthopedic area.
In this study, we designed this prospective observational study to determine whether perioperative platelet count would be a biomarker of the early stage of PND. Besides, a predicting model for the occurrence of PND was also constructed among elderly patients undergoing major orthopedic surgery.
Methods
Patients
The patients who took TKA or THA in West China Hospital between June 2020 and May 2021 were included in the study. The inclusion criteria were: (1) patients older than 55 years; (2) requiring a TKA or a THA surgery; The exclusion criteria were: (1) history of mental diseases; (2) patients with current Neurological disease; (3) left ventricle ejection fraction < 40%; (4) patients who refused or were unable to finish the neurocognitive evaluation; (5) preoperative Mini-Mental Stare Examination (MMSE) less than required score (illiteracy < 17; primary school < 20; middle school and higher < 24).
Clinical data collection
Common demographic factors were collected when the patients were admitted to the hospital, for example, age, gender, body mass index (BMI), and education level. Moreover, professional doctors performed a cardiac examination for the New York Heart Association classification (NYHA). The hemocyte indexes, hepatic and renal functions were assessed by blood routine examination 1 day before and 3 days after the surgery. Perioperative change of platelet was defined as postoperative platelets minus preoperative platelets.
Blood collection
A well-trained nurse collected the blood samples 1 day before and 3 days after the surgery, and the blood analysis was performed by the hematology analyzer (SYSMEXXN-10, Sysmex, Japan) in the clinical lab. Therefore, we mainly focused on the platelet count before and after the surgery.
Neurocognitive evaluation
To evaluate the patients’ neurocognitive levels, a well-trained investigator performed a questionnaire to roundly assess their neurocognitive functions 1 day before and 3 days after the surgery. The questionnaire included: (1) Mini-Mental State Examination (MMSE); (2) Word Immediate Recall Test, testing ability of short-memory; (3) Image Immediate Recall Test, testing ability of short-term visual memory; (4) Trail Making Test A, testing hand-eye coordination; (5) Digit Span Test, testing concentration as well as attention; (6) Digit Symbol Coding Test, testing psychomotor speed; (7) Word Delayed Recall Test, testing ability of long-memory; (8) Word Delayed Interference Test; (9) Image Delayed Recall Test, testing ability of delay recall; (10) Image Delayed Interference Test and (11) Verbal Fluency Test, testing fluency and executive function. At the very beginning, patients with severe cognitive dysfunction were excluded by the lower MMSE score than we required. Each qualified patient gets scores of these tests preoperatively and postoperatively. We collected all of these scores and then obtained the standard deviation (SD) of every preoperative test. We defined a worse test performance as a negative changed score, with an absolute value larger than 1.5 times SD. Patients with no less than two negative changed scores, except for MMSE, were diagnosed as PND [30, 31]. According to this standard, we divided the participants into the PND group and the non-PND group. Finally, we defined two decreased scores as mild PND and more than two decreased scores as severe PND.
Anesthesia and surgery
All patients got the same preoperative preparation, including ECG monitoring, blood oxygen saturation monitoring, arterial blood pressure, and central venous pressure monitoring. As for the anesthesia method, we took combined anesthesia of inhalation and intravenous. The main anesthetic included sevoflurane, desflurane, propofol, cisatracurium besylate, and lidocaine for topical anesthesia. During the surgery, the patients were given mechanical ventilation at 12 times/min, with a tidal volume of 6–8 ml/kg. After the surgery, patients were treated with a drainage tube, which was placed locally to reduce the hematoma and relieve pain. And antibiotics were administered on the day before and on the day of surgery to prevent infection, which could be a catastrophic complication of TKA and THA. The second day after surgery, low molecular weight heparin was injected to avoid a deep venous thrombosis. Early rehabilitation training was encouraged.
Statistics analysis
All of the continuous variables were shown as median with interquartile range (IQR). And all of the categorical variables were shown as a number with a percentage. We used the Mann-Whitney U test for comparisons of continuous variables between different groups and the Fisher exact test for comparisons of categorical variables. The receiver operating characteristic curve (ROC curve) was used to determine the most appropriate threshold of platelet count decided by the Youden Index, and the area under the curve (AUC), 95% confident interval (95%CI), as well as specificity (spe) and sensitivity (sen) were displayed. Furthermore, multivariate logistic regression analysis, which included potential risk factors, was performed to determine the independent risk factors of the early stage of PND. Results were shown as odds ratio (OR) and 95%CI. As a result, we also built a prediction model for the occurrence of PND and performed the ROC curve to evaluate the prediction effect of the model. We took education, gender, duration of surgery, and preoperative platelet into consideration. Among them, the preoperative platelet and duration of surgery were analyzed as continuous variables. On the contrary, education was divided into two groups by the middle school; gender was divided into two groups as well. All statistical analyses were performed by the IBM SPSS Statistical 26. We regarded a two-sided P < 0.05 as statistically significant.
Results
Patients
Totally 87 patients were enrolled in the study, and 70 of them finished the whole process (Fig. 1). According to the neurocognitive evaluation, 14 were classified into the PND group, while the others were assigned to the non-PND group. All the baseline information and laboratory examinations of the PND and non-PND groups were shown in Tables 1 and 2. We observed that women developed significantly more PND than men (Table 1). In addition, no significant cognitive difference existed between these two groups before the surgery, suggesting that all of the patients were in a similar cognitive condition at the beginning (Table 3). However, after surgery, the PND group performed significantly worse than the non-PND group in Word Immediate Recall Test (P = 0.009), word delayed recall test (P = 0.001), word delayed interference test (P = 0.001), and Image Delayed Recall Test (P = 0.028) (Table 4). Meanwhile, we did not observe a significant difference between the mild PND group and severe PND group in their baseline information and clinical examinations (Supplementary Files 1 and 2).
Fig. 1.
Flow diagram of participants. The figure showed the whole process of patients’ inclusion and exclusion
Table 1.
Preoperative variables of the total cohort, PND and non-PND group
| Preoperative variables | Total cohort N = 70 | PND group N = 14 | Non-PND group N = 56 | P |
|---|---|---|---|---|
| Age (y) | 67.0 (62.3–73.0) | 69.5 (64.3–73.5) | 67.0 (62.8–73.0) | 0.670 |
| BMI (kg/m2) | 24.9 (22.0–27.3) | 24.9 (21.7–28.1) | 25.0 (22.2–27.2) | 0.831 |
| Gender | 0.026* | |||
| Male | 23 (32.9%) | 1 (4.3%) | 22 (95.7%) | |
| Female | 47 (67.1%) | 13 (27.7%) | 34 (72.3%) | |
| Education | 0.760 | |||
| No lower than middle school | 45 (64.3%) | 8 (17.8%) | 37 (82.2%) | |
| Lower than middle school | 25 (35.7%) | 6 (24.0%) | 19 (76.0%) | |
| Operation type | 0.148 | |||
| TKA | 37 (52.9%) | 5 (13.5%) | 32 (86.5%) | |
| THA | 33 (47.1%) | 9 (27.3%) | 23 (72.7%) | |
| NYHA classification | 1.000 | |||
| ≥ ΙΙ | 23 (32.9%) | 4 (17.4%) | 19 (82.6%) | |
| < ΙΙ | 47 (67.1%) | 10 (21.3%) | 37 (78.7%) | |
| Hypertension | 0.744 | |||
| Yes | 19 (27.1%) | 3 (15.8%) | 16 (84.2%) | |
| No | 51 (72.9%) | 11 (21.6%) | 40 (78.4%) | |
| If atherosclerosis in lower limbs | 1.000 | |||
| Yes | 30 (42.9%) | 6 (20.0%) | 24 (80.0%) | |
| No | 40 (57.1%) | 8 (20.0%) | 32 (80.0%) | |
| Hemoglobin (g/L) | 136 (127–143) | 135 (125–145) | 136 (127–143) | 0.848 |
| Hematokrit | 0.42 (0.39–0.44) | 0.42(0.38–0.45) | 0.42(0.39–0.44) | 0.918 |
| Platelet (10^9/L) | 184 (151–232) | 239(191–255) | 168(151–215) | 0.009** |
| Leukocyte (10^9/L) | 5.78 (4.85–6.64) | 5.85(4.72–6.81) | 5.76(4.85–6.71) | 0.901 |
| Neutrophil percentage (%) | 62.5 (55.4–69.3) | 60.9(54.7–67.4) | 63.0(55.5–70.1) | 0.528 |
| Neutrophil count (10^9/L) | 3.57 (3.04–4.25) | 3.72(3.09–4.25) | 3.53(2.98–4.32) | 0.947 |
| Lymphocyte (10^9/L) | 1.52 (1.19–1.96) | 1.70(1.10–2.21) | 1.52(1.22–1.96) | 0.676 |
| PLR | 118.3 (83.6–170.0) | 136.5(91.3–210.1) | 117.0(83.6–161.3) | 0.311 |
| Monocyte (10^9/L) | 0.39 (0.33–0.48) | 0.37(0.35–0.49) | 0.39(0.32–0.48) | 0.581 |
| ALT (U/L) | 17.0 (12.8–23.3) | 15.5(13.5–31.5) | 18(12.3–22.8) | 0.982 |
| AST (U/L) | 21.5 (19.9–26.0) | 20.5(18.0–30.0) | 22(19–26) | 0.895 |
| ALB (g/L) | 43.9 (41.6–46.2) | 44.2(41.3–46.3) | 43.9(42.0–46.2) | 0.769 |
| TBil (μmol/L) | 10.2 (8.0–13.4) | 10.6(8.8–14.1) | 10(8–13.5) | 0.725 |
| HDL (mmol/L) | 1.4 (1.1–1.7) | 1.5(1.2–1.7) | 1.4(1.1–1.6) | 0.29 |
| LDL (mmol/L) | 3.2 (2.3–4.1) | 3.4(2.6–4.1) | 3.2(2.3–4.1) | 0.803 |
| Serum creatinine (μmol/L) | 66.0 (56.8–78.0) | 63.5(55.5–71.8) | 66.0 (57.0–79.8) | 0.287 |
| Blood glucose (mmol/L) | 5.5 (5.1–6.2) | 5.6(4.8–6.0) | 5.5(5.2–6.3) | 0.681 |
| PT (s) | 10.5 (10.1–10.8) | 10.5(9.9–10.7) | 10.6(10.1–10.8) | 0.423 |
| APTT (s) | 26.2 (25.0–27.1) | 25.7(24.7–26.7) | 26.2(25.0–27.1) | 0.366 |
| INR | 0.97 (0.92–1.00) | 0.97(0.91–0.98) | 0.96(0.93–1.00) | 0.591 |
| Duration of surgery (min) | 64.5 (54.5–85.0) | 61.5(54.3–95.8) | 65.0 (53.5–83.8) | 0.994 |
| Duration of anesthesia (min) | 113.0 (100.0–144.5) | 109.0 (99.8–153.5) | 114.5(100.3–142.3) | 0.78 |
Data are presented as median with IQR for continuous variables and as number for categorical variables. The P-value is calculated by the Mann-Whitney U test for continuous variables and by Fisher’s exact test for categorical variables
PND Perioperative neurocognitive disorders, BMI Body mass index, TKA Total knee arthroplasty, THA Total hip arthroplasty, NYHA New York Heart Association, PLR Platelet-to-lymphocyte ratio, ALT Alanine aminotransferase, AST Aspartate aminotransferase, ALB Albumin, TBil Total bilirubin, HDL High density lipoprotein, LDL Low density lipoprotein, PT Prothrombin time, APTT Activated partial thromboplastin time, INR International normalized ratio
*P means P-value < 0.05
**P means P-value < 0.01. We diagnose the systolic pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg preoperative as hypertension
Table 2.
Postoperative variables of the total cohort, PND and non-PND group
| Postoperative variables | Total cohort N = 70 | PND group N = 14 | Non-PND group N = 56 | P |
|---|---|---|---|---|
| Hemoglobin | 118 (110–126) | 118(110–128) | 118(110–125.5) | 0.769 |
| Hematokrit | 0.36 (0.34–0.38) | 0.36(0.33–0.41) | 0.37(0.34–0.38) | 0.947 |
| Platelet (10^9/L) | 164 (139–208) | 208(188–221) | 156(137–194) | 0.003** |
| Perioperative Change of platelet | −17.5 (−37.0–0.5) | −25.5 (−38.3- -8.5) | −14.5 (−28.8–1.5) | 0.290 |
| Leukocyte (10^9/L) | 10.54 (8.91–13.62) | 11.66(9.33–13.67) | 10.41(8.86–13.80) | 0.849 |
| Neutrophil percentage (%) | 85.1 (81.1–87.9) | 84.1(82.9–87.3) | 85.2(80.7–88.0) | 0.763 |
| Neutrophil count (10^9/L) | 9.09 (7.26–11.78) | 9.81(8.05–11.47) | 8.86(7.21–11.80) | 0.775 |
| Lymphocyte (10^9/L) | 1.04 (0.77–1.33) | 1.2(0.77–1.45) | 1.00(0.76–1.29) | 0.374 |
| PLR | 170.9 (113.5–230.2) | 184.6(128.6–251.3) | 165.7(111.0–225.3) | 0.284 |
| Change of PLR | 39.3 (2.0–83.7) | 51.6(−1.1–89.2) | 38.2(3.2–73.6) | 0.725 |
| Monocyte (10^9/L) | 0.70 (0.45–0.88) | 0.73(0.32–0.79) | 0.68(0.45–0.89) | 0.638 |
| ALT (U/L) | 16.0 (13.0–20.3) | 17.5(14–29.5) | 15.5(12.3–19) | 0.245 |
| AST (U/L) | 21.5 (18.8–27.3) | 24.5(19.8–34.3) | 21(18–26) | 0.112 |
| ALB (g/L) | 37.9 (35.8–39.7) | 37.2(36.1–40.9) | 38.1(35.7–39.6) | 0.953 |
| TBil (μmol/L) | 11.4 (9.5–15.6) | 11.1(8.9–14.1) | 11.6(9.9–15.7) | 0.454 |
| HDL (mmol/L) | 1.3 (1.1–1.6) | 1.5(1.3–1.7) | 1.3(1.1–1.6) | 0.207 |
| LDL (mmol/L) | 2.7 (2.2–3.6) | 2.7(2.1–3.5) | 2.7(2.2–3.6) | 0.843 |
| Serum creatinine (μmol/L) | 67.0 (58.8–80.0) | 63(58–71.8) | 67(58.3–80.8) | 0.287 |
| Blood glucose (mmol/L) | 7.1 (6.2–8.1) | 7.1(6.0–7.3) | 7.2(6.2–8.3) | 0.287 |
Data are presented as median with IQR for continuous variables and as the number for categorical variables. The P-value is calculated by the Mann-Whitney U test for continuous variables and by Fisher’s exact test for categorical variables
PND Perioperative neurocognitive disorders, PLR Platelet-to-lymphocyte ratio, ALT Alanine aminotransferase, AST Aspartate aminotransferase, ALB Albumin, TBil Total bilirubin, HDL High density lipoprotein, LDL Low density lipoprotein
*P means P-value < 0.05
**P means P-value < 0.01
Table 3.
Difference of preoperative cognitive function
| Tests | Total cohort | PND group | Non-PND group | P |
|---|---|---|---|---|
| MMSE | 26(25–28) | 26(25–29) | 27(25–28) | 0.853 |
| Word Immediate Recall Test | 13(11–16) | 13.5(10–17) | 13(11–16) | 0.848 |
| Image Immediate Recall Test | 9(7–11) | 9.5(9–11) | 9(7–11) | 0.404 |
| Trail Making Test A | 100.5(71–144.5) | 108.5(69–159) | 100.5(69–136) | 0.809 |
| Digit Span Test | 18(16–21) | 18.5(16–21) | 18(16–21.5) | 0.735 |
| Digit Symbol Coding Test | 25(19–32) | 25(18.5–34) | 25(19–32) | 0.808 |
| Word Delayed Recall Test | 4(2–6) | 4(2.5–6) | 4(2–5.5) | 0.377 |
| Word Delayed Interference Test | 22(20–23) | 21.5(19.5–23) | 22(21–23) | 0.666 |
| Image Delayed Recall Test | 3(2–4) | 3(2–4) | 3(2–4) | 0.493 |
| Verbal Fluency Test | 33(28–38) | 29(26–35) | 35(29–38.5) | 0.126 |
Data are presented as median with IQR. The P-value is calculated by the Mann-Whitney U test
PND Perioperative neurocognitive disorders
*P means P-value < 0.05
**P means P-value < 0.01
Table 4.
Difference of postoperative cognitive function
| Tests | Total cohort | PND group | Non-PND group | P |
|---|---|---|---|---|
| Word Immediate Recall Test | 14(11–18) | 11.5(7.5–14) | 15.5(11–19) | 0.009** |
| Image Immediate Recall Test | 9.5(6–12) | 8.5(5–12) | 10(6–12) | 0.338 |
| Trail Making Test A | 104.5(66–145) | 111(64.5–177.5) | 104.5(65–139.5) | 0.607 |
| Digit Span Test | 18(14–20) | 14.5(12.5–20) | 18(15–21) | 0.103 |
| Digit Symbol Coding Test | 24.5(18.5–31) | 21.5(16.5–28) | 25(20–31.5) | 0.338 |
| Word Delayed Recall Test | 3(1.5–6) | 2(0–2) | 4(3–6.5) | 0.001** |
| Word Delayed Interference Test | 21(18–23) | 18(15.5–19.5) | 22(20–23) | 0.001** |
| Image Delayed Recall Test | 3(2–5) | 2(0.5–3) | 3(2–5) | 0.028* |
| Verbal Fluency Test | 33(28–38) | 29(22.5–36.5) | 33.5(28–39) | 0.179 |
Data are presented as median with IQR. The P-value is calculated by the Mann-Whitney U test
PND Perioperative neurocognitive disorders
*P means P-value < 0.05
**P means P-value < 0.01
Relationship between preoperative platelet count and occurrence of PND
The preoperative platelet count level in the PND group was significantly higher than that in the non-PND group (P = 0.009, Table 1). Then, according to the ROC curve, the cut-off value (229, spe:0.839, sen:0.643) of preoperative platelet count was determined by Youden Index, and the AUC was 0.728 (P = 0.009, 95%CI 0.569–0.888, Fig. 2). To ensure whether preoperative platelet count was an independent risk factor of occurrence of PND, a multivariable logistic regression analysis was performed. The results showed that the P-value of the preoperative platelet count was 0.043 and OR was 1.014 (95%CI 1.000–1.027), meaning that it was an independent risk factor of occurrence of PND (Table 5). Finally, the prediction model of PND was shown, and the model’s predictive effect was examined by the ROC curve (Fig. 3), whose AUC was 0.796 (P = 0.001, 95%CI 0.676–0.916). The results were barely affected by the adjustment for preoperative platelet count, which was to fill in the missed values.
Fig. 2.
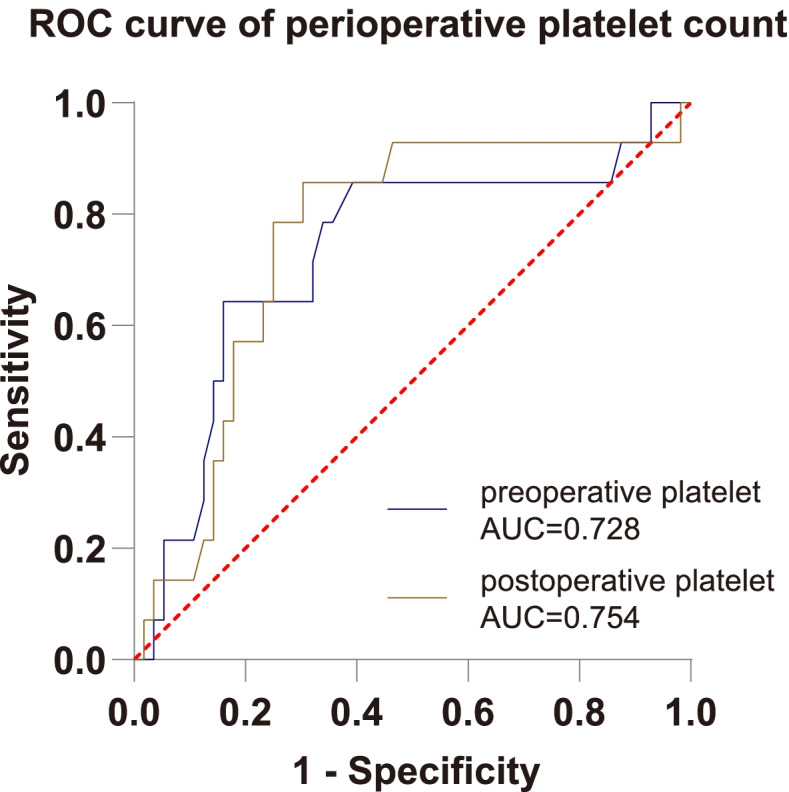
Receiver-operating characteristic (ROC) curves for preoperative platelet and postoperative platelet. The figure showed the ROC curves for preoperative platelet and postoperative platelet, whose area under the curve (AUC) were 0.728 and 0.754 respectively
Table 5.
Multivariate logistic regression analysis
| Variables | OR (95%CI) | P |
|---|---|---|
| Education (Lower than middle school) | 2.073 (0.545–7.888) | 0.285 |
| Gender (Female) | 6.367 (0.728–55.689) | 0.094 |
| Duration of surgery (min) | 1.009 (0.980–1.038) | 0.556 |
| Preoperative platelet (10^9/L) | 1.014 (1.000–1.027) | 0.043* |
OR Odds ratio, CI Confidence interval
*P means P value < 0.05
Fig. 3.
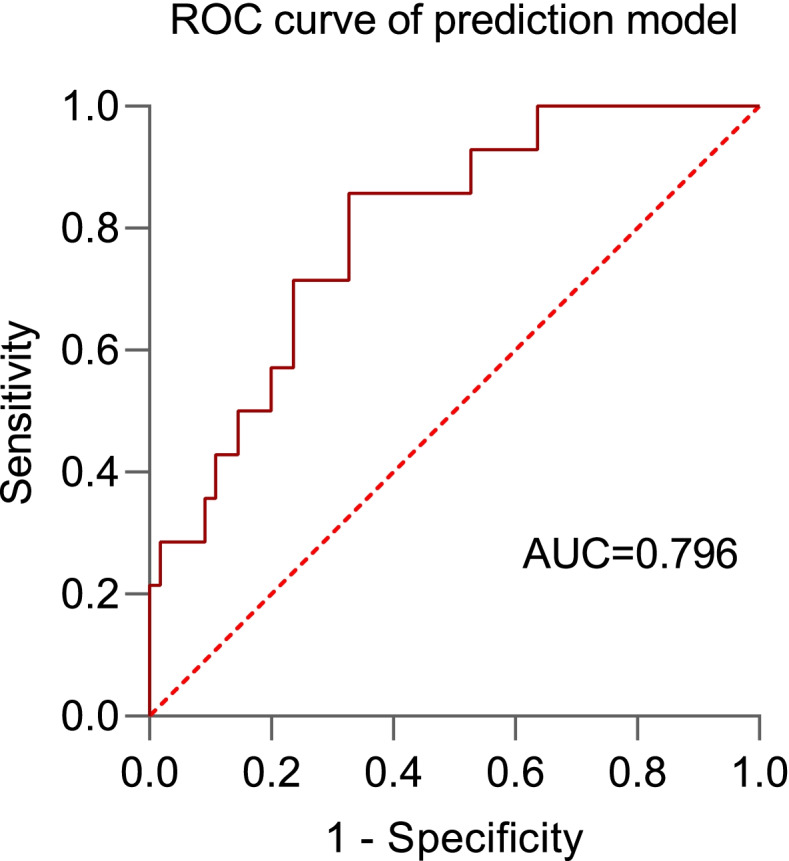
Receiver-operating characteristic (ROC) curve for prediction model of occurrence of PND. The figure showed the ROC curves for the prediction model of occurrence of PND, whose area under the curve (AUC) was 0.796
In addition, the participants were divided into higher preoperative platelet group and lower preoperative platelet group by the cut-off value of 299. The characteristics and laboratory examinations were compared and listed in Table 6. We found that the occurrence of PND, percentage of females, preoperative platelet-to-lymphocyte ratio (PLR), and the level of postoperative platelet count were all increased, while the PT and INR were decreased in the higher preoperative platelet group.
Table 6.
Difference between high preoperative platelet and low preoperative platelet groups divided by 229
| Variables | High platelet group | Low platelet group | P |
|---|---|---|---|
| > 229, N = 18 | < 229, N = 52 | ||
| Age (y) | 66(60.8–71) | 67.5(63–75.5) | 0.117 |
| BMI (kg/m2) | 25(21.6–27.2) | 24.8(22.3–27.5) | 0.783 |
| PND | 0.001** | ||
| Yes | 9 (50%) | 5 (9.6%) | |
| No | 9 (50%) | 47 (90.4%) | |
| Gender | 0.039* | ||
| Male | 2 (11.1%) | 21 (40.4%) | |
| Female | 16 (88.9%) | 31 (59.6%) | |
| NYHA classification | 0.145 | ||
| ≥ ΙΙ | 3 (16.7%) | 20 (38.5%) | |
| < ΙΙ | 15 (83.3%) | 32 (61.5%) | |
| Preoperative platelet (10^9/L) | 252.5(245.3–276.0) | 166.0(142.3–189.3) | < 0.001 |
| Preoperative PLR | 145.9(105.7–217.5) | 113.8(80.7–153.4) | 0.014* |
| Preoperative PT (s) | 10.3(9.7–10.6) | 10.6(10.2–10.9) | 0.004** |
| Preoperative INR | 0.94(0.90–0.97) | 0.97(0.94–1.00) | 0.041* |
| Postoperative platelet (10^9/L) | 222.5(207.8–251.0) | 150.5(132.5–178.3) | < 0.001 |
Data are presented as median with IQR for continuous variables and as the number for categorical variables. The P-value is calculated by the Mann-Whitney U test for continuous variables and by Fisher’s exact test for categorical variables
PND Perioperative neurocognitive disorders, BMI Body mass index, NYHA New York Heart Association, PLR Platelet-to-lymphocyte ratio, PT Prothrombin time, INR International normalized ratio
*P means P-value < 0.05
**P means P-value < 0.01
Relationship between postoperative platelet count and occurrence of PND
The postoperative platelet in the early stage of PND was distinctly higher than that in the non-PND group (P = 0.003, Table 2). Moreover, as the ROC curve showed, the cut-off value (179.5, sen:0.857, spe:0.696) of postoperative platelet count was determined by Youden Index, and the AUC was 0.754 (P = 0.003, 95%CI 0.611–0.897, Fig. 2). The perioperative change of platelet and the change of PLR did not show a significant difference between PND and non-PND groups (Table 2).
Moreover, the participants were divided into higher postoperative platelet group and lower postoperative platelet group by the cut-off value of 179.5. The characteristics and laboratory examinations were compared and listed in Table 7. We found that the occurrence of PND, percentage of females, preoperative and postoperative platelet-to-lymphocyte ratio (PLR), and the level of preoperative platelet count were all significantly increased in the higher postoperative platelet group.
Table 7.
Difference between high postoperative platelet and low postoperative platelet groups divided by 179.5
| Variables | High platelet group | Low platelet group | P |
|---|---|---|---|
| > 179.5, N = 29 | < 179.5, N = 41 | ||
| Age (y) | 66(61.5–71) | 70(63–76) | 0.08 |
| BMI (kg/m2) | 24.8(21.7–27.5) | 25.2(22.5–27.2) | 1.000 |
| PND | < 0.001 | ||
| Yes | 12 (41.4%) | 2 (4.9%) | |
| No | 17 (58.6%) | 39 (95.1%) | |
| Gender | 0.005** | ||
| Male | 4 (13.8%) | 19 (46.3%) | |
| Female | 25 (86.2%) | 22 (53.7%) | |
| NYHA classification | 1.000 | ||
| ≥ ΙΙ | 10 (34.5%) | 13 (31.7%) | |
| < ΙΙ | 19 (63.5%) | 28 (68.3%) | |
| Preoperative platelet (10^9/L) | 235(203–258) | 159(138–180.5) | < 0.001 |
| Preoperative PLR | 144.8(120.2–217.0) | 98.6(80.0–139.0) | 0.001** |
| Postoperative platelet (10^9/L) | 212(196.5–237.0) | 143(123.5–159.5) | < 0.001 |
| Postoperative PLR | 189.2(152.7–255.6) | 149.1(105.6–193.7) | 0.005** |
| Postoperative ALB (g/L) | 38.3(37.0–40.3) | 37.1(35.1–39.5) | 0.048* |
Data are presented as median with IQR for continuous variables and as number for categorical variables. The P-value is calculated by the Mann-Whitney U test for continuous variables and by Fisher’s exact test for categorical variables
PND Perioperative neurocognitive disorders, BMI Body mass index, NYHA New York Heart Association, PLR platelet-to-lymphocyte ratio, ALB Albumin
*P means P-value < 0.05
**P means P-value < 0.01
Discussion
PND is a prevalent complication in aged people after major surgery, and it is hard to perform a scaled assessment due to patients’ poor condition and uncooperative behavior [32–34]. Thus, it is essential to find a new and easily accessible biomarker to predict PND occurrence at the early stage. This prospective observational study included 70 patients who took TKA or THA and analyzed the relationship between their perioperative platelet count and occurrence of PND on the third day after surgery. We found a significant increase in the perioperative platelet counts in the PND group than in the non-PND group. Besides, preoperative platelet count showed a significant difference in logistic analysis. It might be an independent risk factor and a potential predictor of PND. To our knowledge, this is the first study to estimate an association between perioperative platelet count in peripheral blood and the early stage of PND in patients undergoing major orthopedic surgery.
PND has been demonstrated to be associated with central neuroinflammation [13]. Surgery and anesthesia could stimulate peripheral inflammation, which could be manifested as increased IL-6, C reaction protein, and TNF-α [35–37]. Meanwhile, it was also reported that the peripheral inflammatory factors could go through the blood-brain barrier (BBB) more than usual because of the increased BBB permeability during pathological state [38]. Besides, the microglial cells in the aged brain are more sensitive to inflammatory factors and can secret more proinflammatory cytokines [39]. Once microglial cells are stimulated, central neuroinflammation occurs, which usually harms the hippocampus, resulting in the occurrence of PND [40]. In recent years, there were also increasing researches on platelet and suggested that platelet could exacerbate peripheral inflammation by self-degranulation or interacting with the immune cells through membrane surface receptors. For example, a bacterial infection could promote platelet to activate leukocytes via Toll-like receptors [41], and virus infection might lead to platelet releasing IL-1β, which is a core cytokine of the cascade of inflammation [42]. In addition, inflammatory mediators can make platelet self-degranulate and release chemokines, which can combine with receptors on the surface of immune cells [43]. And the previous study showed that higher platelet was associated with inflammation in colorectal cancer [44]. With more and more evidence, the platelet is deeply related to the severity of inflammation. Thus, in our study, a higher peripheral platelet level may be associated with more severe peripheral inflammation, which could cause more severe damage to the central nervous system and induce cognitive decline. And this might be the reason that preoperative platelet was an independent risk factor of the occurrence of PND at the early stage in our study. Compared with the stable condition before surgery, the patients undergoing the stress of surgery and anesthesia may suffer a higher risk of bacteria, virus infection, or non-infectious inflammation. Consistently, we found the patients in the PND group had higher postoperative platelet count. For the first time to explore the influence of platelet on the occurrence of PND after orthopedic surgery, more researches should be further performed to investigate the underlying pathological mechanism.
Tables 6 and 7 showed that patients with higher perioperative platelet count also had higher perioperative platelet-to-lymphocyte ratio (PLR). PLR has been proved to be a biomarker of inflammation in many diseases, which might explain why PLR is covaried with platelet [45–47]. However, the change of PLR showed no differences in the elderly patients between the two groups in our study. Therefore, more studies are required to ensure whether the change of PLR might be covariate with perioperative platelet. What’s more, lower PT and INR were observed in the higher preoperative platelet group. A past study has shown that high PT and INR and low platelet count could denote the same disease together [48]. So it was reasonable that lower PT and INR occurred in the higher preoperative platelet count group.
Eighty-four patients participated in this study, and 70 of them finished the whole process. We defined a decreased performance as the absolute value of reduced score more significant than 1.5 times SD of overall preoperative scores [31]. Then 14 of them developed PND, with a ratio of 14/70 (20%), which accorded with the reported percentage [7, 10, 49]. In terms of their baseline information, there was only a significant gender difference. Generally speaking, commonly reported risk factors are age and education [34]. As gender is often reported in cardiac surgery [50–52], there is limited evidence in orthopedic. Gender distribution in PND after major orthopedic surgery requires more studies. All of the patients were at a similar baseline preoperatively, while the patients in the PND group showed worse performance in several cognitive domains after surgery. On the one hand, surgery could induce inflammation and blood loss, which may cause cerebral hypoperfusion [53]. On the other hand, anesthesia may harm cognitive function by different drugs, methods, and depth [54–56]. In addition, the scores of PND patients were mainly decreased in word short-term recall, delayed recall, and delayed interference tests, indicating their neurocognitive damage were basically in aspects of short-term and delayed memory, almost consistent with that of the previous study [2]. Besides, we compared the baseline information and blood examinations between the mild PND group and severe PND group that no significant difference was displayed. The possible reason was that our mild and severe sample sizes were too small to gain a meaningful result. Larger samples are required to reduce the sampling bias.
As for the logistic multivariable regression and the prediction model, we could only take preoperative platelet count, gender, duration of surgery and education into consideration due to the size of the sample. Preoperative platelet count and gender were significantly different in our single variable analysis. While duration of surgery and education have been reported as comorbidities of PND in previous literature [57, 58]. With the result, we built a prediction model for PND occurrence, and its AUC of the ROC curve was 0.796. Because platelet count, gender, duration of surgery, and education were all easily accessible, our model might be meaningful to predict PND at the early stage after major orthopedic surgery. It can guide special care for the patients at high risk of PND in advance. Despite Dr. Wang and his colleges having built a prediction model for elderly orthopedic patients, the model was based on POD in 24 h [59]. Therefore, it is still meaningful to explore the role of preoperative platelet count and build a prediction model for patients in the early stage of PND after TKA and THA, while a larger scale of clinical investigation is also needed in the future.
Our study had several strengths. First, to our knowledge, we were the first to study the relationship between platelet count and occurrence of PND at the early stage after major orthopedic surgery in elderly patients, which could be helpful to patients’ care in the orthopedics department. Second, we provided a potential predictor of the early stage of PND. Preoperative platelet count can be examined much more quickly than a scale test. Third, we built a prediction model for the occurrence of PND, and it had an acceptable AUC. Forth, the data were credible because of well-trained investigators and reliable clinical examination in West China Hospital. On the contrary, our main limitation was the relatively small sample size, which could cause some bias and limit our model. Generally speaking, the multivariable logistic regression analyses should be used with a minimum of 10 events per predictor variable. So we can only take preoperative platelet count, education, gender and duration of surgery into consideration. Moreover, the patients were usually discharged from the hospital in 3 days, making it difficult for us to collect blood samples in a more extended follow-up period. Last but not least, although platelet counts in PND patients were elevated, they were still within the normal range. However, the elevated platelet could still give us clues to monitor and intervene in the elderly patients’ cognitive function at the early stage.
In summary, our prospective observational study has demonstrated that a higher perioperative platelet count in peripheral blood might be the biomarkers of PND at the early stage in aged patients after major orthopedic surgery. And preoperative platelet count could be a potential biomarker of the early stage of PND. Thus, elderly patients in the orthopedics department with preoperative platelet higher than 229 or with a high probability of PND in our model should be taken special care of after surgery. Besides, memory and cognitive function should be specially trained perioperatively to keep an excellent cognitive condition.
Conclusion
Perioperative higher levels of platelet count were associated with a higher risk of occurrence of PND at the early stage in patients after major orthopedic surgery. Therefore, patients with a higher perioperative platelet count should be treated more carefully in many aspects to decrease PND occurrence. Moreover, preoperative platelet count seems to be a clinically valuable biomarker to predict PND early after major orthopedic surgery. In contrast, the platelet count is easily accessible and always contained by the blood routine examinations. And a prediction model constructed including education, gender, duration of surgery, and preoperative platelet count would be effective in predicting the occurrence of PND. However, more extensive clinical investigations and basic experiment studies are required to ensure our findings and further clarify the potential mechanisms and communications.
Supplementary Information
Additional file 1: Supplementary Table 1. Preoperative variables of mild and severe PND groups.
Additional file 2: Supplementary Table 2. Postoperative variables of mild and severe PND groups.
Acknowledgements
Not applicable.
Abbreviations
- POCD
Postoperative cognitive dysfunction
- POD
Postoperative delirium
- PND
Perioperative neurocognitive disorders
- TKA
Total knee arthroplasty
- THA
Total hip arthroplasty
- MMSE
Mini Mental Stare Examination
- BMI
Body mass index
- SD
Standard deviation
- IQR
Interquartile range
- ROC
Receiver operating curve
- AUC
Area under the curve
- OR
Odds ratio
- PLR
Platelet-to-lymphocyte ratio
- BBB
Blood brain barrier
Authors’ contributions
RQ Wang and R Gao designed the study; collected the data; analyzed and interpreted the data and mainly contributed to writing the manuscript. XY Xie, Q Zhao contributed in collecting data of patients. H Chen provided study materials. XY Zhang, PL Lv, CT Zhang, LY Deng and Q Zheng analyzed and interpreted the patients’ data. T Zhu provided administrative support. C Chen provided administrative support, study material and patients and analyzed the data. All authors wrote the article and read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81870858, 82171185 and 81500937 to Chan Chen), the National Key R&D Program of China (No.2018YFC2001800 to Tao Zhu), the National Natural Science Foundation of China (No. 81671062 to Tao Zhu), the National Natural Science Foundation of China (No. 81801117 to Peilin Lv), Sichuan Province Science and Technology Support Program (No.2020YJ0051 to Chan Chen), China Postdoctoral Science Foundation (Grant No. 2020 M673234 to Rui Gao), Post-doctoral Research Project, West China Hospital, Sichuan University (Grant No.2020HXBH022 to Rui Gao) and China Postdoctoral Science Foundation (Grant No.2017 M610603 to Chen Chan).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol was approved by the West China Hospital of Sichuan University Biomedical Research Ethics Committee (Sichuan, China). The study was registered in the Chinese Clinical Trial Registry (ChiCTR-IPD-2000033001). Patients or their legal guardians have signed written informed consent before enrollment. All methods were carried out in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ruiqun Wang and Rui Gao contributed equally to this work.
References
- 1.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Q, Gao R, Liu C, Chen H, Zhang X, Guan J, Xie X, Qiu Y, Cheng X, Lv P, et al. Dynamic change of lymphocyte-to-monocyte is associated with the occurrence of POCD after cardiovascular surgery: a prospective observational study. Front Behav Neurosci. 2021;15:646528. doi: 10.3389/fnbeh.2021.646528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161–1174. doi: 10.1001/jama.2017.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price CC, Levy SA, Tanner J, Garvan C, Ward J, Akbar F, Bowers D, Rice M, Okun M. Orthopedic surgery and post-operative cognitive decline in idiopathic Parkinson's disease: considerations from a pilot study. J Parkinsons Dis. 2015;5(4):893–905. doi: 10.3233/JPD-150632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caza N, Taha R, Qi Y, Blaise G. The effects of surgery and anesthesia on memory and cognition. Prog Brain Res. 2008;169:409–422. doi: 10.1016/S0079-6123(07)00026-X. [DOI] [PubMed] [Google Scholar]
- 6.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bin Abd Razak HR, Yung WY. Postoperative delirium in patients undergoing Total joint Arthroplasty: a systematic review. J Arthroplast. 2015;30(8):1414–1417. doi: 10.1016/j.arth.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Crocker E, Beggs T, Hassan A, Denault A, Lamarche Y, Bagshaw S, Elmi-Sarabi M, Hiebert B, Macdonald K, Giles-Smith L, et al. Long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg. 2016;102(4):1391–1399. doi: 10.1016/j.athoracsur.2016.04.071. [DOI] [PubMed] [Google Scholar]
- 9.Bai J, Liang Y, Zhang P, Liang X, He J, Wang J, Wang Y. Association between postoperative delirium and mortality in elderly patients undergoing hip fractures surgery: a meta-analysis. Osteoporos Int. 2020;31(2):317–326. doi: 10.1007/s00198-019-05172-7. [DOI] [PubMed] [Google Scholar]
- 10.Androsova G, Krause R, Winterer G, Schneider R. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. 2015;7:112. doi: 10.3389/fnagi.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Z, Ou Y, Duan K, Jiang X. Inflammation: a bridge between postoperative cognitive dysfunction and Alzheimer's disease. Med Hypotheses. 2010;74(4):722–724. doi: 10.1016/j.mehy.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Zhao D, Tang Y, Suo X, Zhang C, Dou Y, Chang J. A dual-targeted multifunctional nanoformulation for potential prevention and therapy of Alzheimer's disease. Innovation (N Y) 2021;2(4):100160. doi: 10.1016/j.xinn.2021.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang X, Yu Y, Tang X, Chen M, Zheng Y, Zhu S. Transcriptome profile in Hippocampus during acute inflammatory response to surgery: toward early stage of PND. Front Immunol. 2019;10:149. doi: 10.3389/fimmu.2019.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XD, Wang LK, Wu HY, Jiao L. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiol. 2018;18(1):177. doi: 10.1186/s12871-018-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H, Li F, Ye W, Wang M, Zhou X, Feng J, Liu L, Wang X. Correlation between plasma CircRNA-089763 and postoperative cognitive dysfunction in elderly patients undergoing non-cardiac surgery. Front Behav Neurosci. 2020;14:587715. doi: 10.3389/fnbeh.2020.587715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Zhen X, Hu X, Li Y, Gu S, Gu Y, et al. Osteoarthritis in the middle-aged and elderly in China: prevalence and influencing factors. Int J Environ Res Public Health. 2019;16(23). [DOI] [PMC free article] [PubMed]
- 17.Wang D, Wang HY, Luo ZY, Meng WK, Pei FX, Li Q, Zhou ZK, Zeng WN. Blood-conserving efficacy of multiple doses of oral tranexamic acid associated with an enhanced-recovery programme in primary total knee arthroplasty: a randomized controlled trial. Bone Joint J. 2018;100-B(8):1025–1032. doi: 10.1302/0301-620X.100B8.BJJ-2017-1598.R1. [DOI] [PubMed] [Google Scholar]
- 18.Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Glazier R, Wilkins A, Badley EM. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients' preferences. Med Care. 2001;39(3):206–216. doi: 10.1097/00005650-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Zavadak KH, Gibson KR, Whitley DM, Britz P, Kwoh CK. Variability in the attainment of functional milestones during the acute care admission after total joint replacement. J Rheumatol. 1995;22(3):482–487. [PubMed] [Google Scholar]
- 20.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, et al. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med. 2014;6(255):255ra131. doi: 10.1126/scitranslmed.3009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langkilde A, Jakobsen TL, Bandholm TQ, Eugen-Olsen J, Blauenfeldt T, Petersen J, Andersen O. Inflammation and post-operative recovery in patients undergoing total knee arthroplasty-secondary analysis of a randomized controlled trial. Osteoarthr Cartil. 2017;25(8):1265–1273. doi: 10.1016/j.joca.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Hood BR, Cowen ME, Zheng HT, Hughes RE, Singal B, Hallstrom BR. Association of Aspirin with Prevention of venous thromboembolism in patients after Total knee Arthroplasty compared with other anticoagulants: a noninferiority analysis. JAMA Surg. 2019;154(1):65–72. doi: 10.1001/jamasurg.2018.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White RH, Henderson MC. Risk factors for venous thromboembolism after total hip and knee replacement surgery. Curr Opin Pulm Med. 2002;8(5):365–371. doi: 10.1097/00063198-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kuravi SJ, Yates CM, Foster M, Harrison P, Hazeldine J, Hampson P, Watson C, Belli A, Midwinter M, Nash GB. Changes in the pattern of plasma extracellular vesicles after severe trauma. PLoS One. 2017;12(8):e0183640. doi: 10.1371/journal.pone.0183640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenne CN, Kubes P. Platelets in inflammation and infection. Platelets. 2015;26(4):286–292. doi: 10.3109/09537104.2015.1010441. [DOI] [PubMed] [Google Scholar]
- 27.Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34(1):5–30. doi: 10.1007/s00281-011-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxena S, Lai IK, Li R, Maze M. Neuroinflammation is a putative target for the prevention and treatment of perioperative neurocognitive disorders. Br Med Bull. 2019;130(1):125–135. doi: 10.1093/bmb/ldz010. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Xiao F, Zhang J, Wang X, Ying J, Wei G, Chen S, Huang X, Yu W, Liu X, et al. Dexmedetomidine mitigated NLRP3-mediated Neuroinflammation via the ubiquitin-autophagy pathway to improve perioperative neurocognitive disorder in mice. Front Pharmacol. 2021;12:646265. doi: 10.3389/fphar.2021.646265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X, Long G, Quan C, Zhang B, Chen J, Ouyang W. Insulin resistance predicts postoperative cognitive dysfunction in elderly gastrointestinal patients. Front Aging Neurosci. 2019;11:197. doi: 10.3389/fnagi.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. Dysfunction IgTISoPC: the assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45(3):275–289. doi: 10.1034/j.1399-6576.2001.045003275.x. [DOI] [PubMed] [Google Scholar]
- 32.Miao M, Xu Y, Sun M, Chang E, Cong X, Zhang J. BIS index monitoring and perioperative neurocognitive disorders in older adults: a systematic review and meta-analysis. Aging Clin Exp Res. 2020;32(12):2449–2458. doi: 10.1007/s40520-019-01433-x. [DOI] [PubMed] [Google Scholar]
- 33.Li YL, Huang HF, Le Y. Risk factors and predictive value of perioperative neurocognitive disorders in elderly patients with gastrointestinal tumors. BMC Anesthesiol. 2021;21(1):193. doi: 10.1186/s12871-021-01405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction - current preventive strategies. Clin Interv Aging. 2018;13:2267–2273. doi: 10.2147/CIA.S133896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wichmann MW, Huttl TP, Winter H, Spelsberg F, Angele MK, Heiss MM, Jauch KW. Immunological effects of laparoscopic vs open colorectal surgery: a prospective clinical study. Arch Surg. 2005;140(7):692–697. doi: 10.1001/archsurg.140.7.692. [DOI] [PubMed] [Google Scholar]
- 36.Jansson K, Redler B, Truedsson L, Magnuson A, Matthiessen P, Andersson M, Norgren L. Intraperitoneal cytokine response after major surgery: higher postoperative intraperitoneal versus systemic cytokine levels suggest the gastrointestinal tract as the major source of the postoperative inflammatory reaction. Am J Surg. 2004;187(3):372–377. doi: 10.1016/j.amjsurg.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Kawahito K, Adachi H, Ino T. Influence of surgical procedures on interleukin-6 and monocyte chemotactic and activating factor responses: CABG vs. valvular surgery. J Interf Cytokine Res. 2000;20(1):1–6. doi: 10.1089/107999000312676. [DOI] [PubMed] [Google Scholar]
- 38.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84(4):932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2(7):e91229. doi: 10.1172/jci.insight.91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 42.Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza FA, et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013;122(20):3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown AJ, Sepuru KM, Sawant KV, Rajarathnam K. Platelet-derived chemokine CXCL7 dimer preferentially exists in the glycosaminoglycan-bound form: implications for neutrophil-platelet crosstalk. Front Immunol. 2017;8:1248. doi: 10.3389/fimmu.2017.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vayrynen JP, Vayrynen SA, Sirnio P, Minkkinen I, Klintrup K, Karhu T, Makela J, Herzig KH, Karttunen TJ, Tuomisto A, et al. Platelet count, aspirin use, and characteristics of host inflammatory responses in colorectal cancer. J Transl Med. 2019;17(1):199. doi: 10.1186/s12967-019-1950-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang D, Bai N, Hu X, OuYang XW, Yao L, Tao Y, Wang Z. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ. 2019;7:e7132. doi: 10.7717/peerj.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39(4):345–357. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI) Transl Lung Cancer Res. 2019;8(6):886–894. doi: 10.21037/tlcr.2019.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walborn A, Williams M, Fareed J, Hoppensteadt D. International normalized ratio relevance to the observed coagulation abnormalities in warfarin treatment and disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2018;24(7):1033–1041. doi: 10.1177/1076029618772353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351(9106):857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 50.Schenning KJ, Murchison CF, Mattek NC, Kaye JA, Quinn JF. Sex and genetic differences in postoperative cognitive dysfunction: a longitudinal cohort analysis. Biol Sex Differ. 2019;10(1):14. doi: 10.1186/s13293-019-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hogue CW, Lillie R, Hershey T, Birge S, Nassief AM, Thomas B, Freedland KE. Gender influence on cognitive function after cardiac operation. Ann Thorac Surg. 2003;76(4):1119–1125. doi: 10.1016/s0003-4975(03)00817-8. [DOI] [PubMed] [Google Scholar]
- 52.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA, Neurological Outcome Research G et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 53.Salazar D, Sears BW, Andre J, Tonino P, Marra G. Cerebral desaturation during shoulder arthroscopy: a prospective observational study. Clin Orthop Relat Res. 2013;471(12):4027–4034. doi: 10.1007/s11999-013-2987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deiner S, Luo X, Silverstein JH, Sano M. Can intraoperative processed EEG predict postoperative cognitive dysfunction in the elderly? Clin Ther. 2015;37(12):2700–2705. doi: 10.1016/j.clinthera.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller D, Lewis SR, Pritchard MW, Schofield-Robinson OJ, Shelton CL, Alderson P, Smith AF. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018;8:CD012317. doi: 10.1002/14651858.CD012317.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li WX, Luo RY, Chen C, Li X, Ao JS, Liu Y, Yin YQ. Effects of propofol, dexmedetomidine, and midazolam on postoperative cognitive dysfunction in elderly patients: a randomized controlled preliminary trial. Chin Med J. 2019;132(4):437–445. doi: 10.1097/CM9.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C, Wang R, Li X, Chen J. Preoperative serum MicroRNA-155 expression independently predicts postoperative cognitive dysfunction after laparoscopic surgery for Colon Cancer. Med Sci Monit. 2016;22:4503–4508. doi: 10.12659/MSM.898397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vacas S, Degos V, Feng X, Maze M. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. 2013;106:161–178. doi: 10.1093/bmb/ldt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang G, Zhang L, Qi Y, Chen G, Zhou J, Zhu H, Hao Y. Development and validation of a postoperative delirium prediction model for elderly orthopedic patients in the intensive care unit. J Healthc Eng. 2021;2021:9959077. doi: 10.1155/2021/9959077. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Preoperative variables of mild and severe PND groups.
Additional file 2: Supplementary Table 2. Postoperative variables of mild and severe PND groups.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.