Abstract
Background
Ambient air pollutant (AAP) exposure is associated with adverse pregnancy outcomes, such as preeclampsia, preterm labor, and low birth weight. Previous studies have shown methylation of immune genes associate with exposure to air pollutants in pregnant women, but the cell-mediated response in the context of typical pregnancy cell alterations has not been investigated. Pregnancy causes attenuation in cell-mediated immunity with alterations in the Th1/Th2/Th17/Treg environment, contributing to maternal susceptibility. We recruited women (n = 186) who were 20 weeks pregnant from Fresno, CA, an area with chronically elevated AAP levels. Associations of average pollution concentration estimates for 1 week, 1 month, 3 months, and 6 months prior to blood draw were associated with Th cell subset (Th1, Th2, Th17, and Treg) percentages and methylation of CpG sites (IL4, IL10, IFNγ, and FoxP3). Linear regression models were adjusted for weight, age, season, race, and asthma, using a Q value as the false-discovery-rate-adjusted p-value across all genes.
Results
Short-term and mid-term AAP exposures to fine particulate matter (PM2.5), nitrogen dioxide (NO2) carbon monoxide (CO), and polycyclic aromatic hydrocarbons (PAH456) were associated with percentages of immune cells. A decrease in Th1 cell percentage was negatively associated with PM2.5 (1 mo/3 mo: Q < 0.05), NO2 (1 mo/3 mo/6 mo: Q < 0.05), and PAH456 (1 week/1 mo/3 mo: Q < 0.05). Th2 cell percentages were negatively associated with PM2.5 (1 week/1 mo/3 mo/6 mo: Q < 0.06), and NO2 (1 week/1 mo/3 mo/6 mo: Q < 0.06). Th17 cell percentage was negatively associated with NO2 (3 mo/6 mo: Q < 0.01), CO (1 week/1 mo: Q < 0.1), PM2.5 (3 mo/6 mo: Q < 0.05), and PAH456 (1 mo/3 mo/6 mo: Q < 0.08). Methylation of the IL10 gene was positively associated with CO (1 week/1 mo/3 mo: Q < 0.01), NO2 (1 mo/3 mo/6 mo: Q < 0.08), PAH456 (1 week/1 mo/3 mo: Q < 0.01), and PM2.5 (3 mo: Q = 0.06) while IL4 gene methylation was positively associated with concentrations of CO (1 week/1 mo/3 mo/6 mo: Q < 0.09). Also, IFNγ gene methylation was positively associated with CO (1 week/1 mo/3 mo: Q < 0.05) and PAH456 (1 week/1 mo/3 mo: Q < 0.06).
Conclusion
Exposure to several AAPs was negatively associated with T-helper subsets involved in pro-inflammatory and anti-inflammatory responses during pregnancy. Methylation of IL4, IL10, and IFNγ genes with pollution exposure confirms previous research. These results offer insights into the detrimental effects of air pollution during pregnancy, the demand for more epigenetic studies, and mitigation strategies to decrease pollution exposure during pregnancy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13148-022-01254-2.
Keywords: Air pollution, DNA methylation, Pregnancy, Immunology, Th1, Th2, IL4, IL10, IFNγ, PM2.5
Background
Ambient air pollutant (AAP) exposure is associated with adverse pregnancy outcomes, such as preeclampsia, preterm labor, and low birth weight, but the immune mechanisms involved are not clearly understood [1–4]. T-helper (Th) cells modulate immune responses and can be broadly classified into Th1 cells, involved in cellular immunity, and Th2 cells, involved in humoral immunity. In a typical pregnancy, there is an attenuation in cell-mediated immunity which shifts from a Th1 to a Th2-dominant environment [5, 6], contributing to overall maternal susceptibility to intracellular pathogens [7, 8] and air pollutants [9]; this environment of immune tolerance seems to dominate in the second trimester [10].
Immune alterations in utero may occur due to environmental factors such as smoking [11, 12], exposure to secondhand smoke [13], and elevated exposure to AAPs [14, 15]. AAP exposure can also lead to cytokine dysregulation and T cell polarization associated with epigenetic modifications [16]. These environmental impacts during pregnancy highlight the need to better understand immune alterations from AAP exposure and find effective ways to mitigate, treat, and/or prevent any adverse health effects to both mother and baby.
More specifically, Th1 cells produce interferon-gamma (IFNγ) which is considered pro-inflammatory while Th2 cells, produce the anti-inflammatory interleukins (IL)4 and IL10 [17, 18]. During pregnancy, Th2 cytokines promote placental changes, differentiation, and inhibition of pro-inflammatory Th1 cytokines [19, 20]. However, the Th1/Th2 paradigm has more recently expanded into a Th1/Th2/Th17 and T-regulatory (Treg) paradigm [18, 21]. Th17 cells cause inflammation which may play an important role in preventing pathological infections during pregnancy but have also been associated with preeclampsia [21]. In contrast, Treg cells, which are identified by the expression of FoxP3, play a central role in immunoregulation and tolerance, including maternal–fetal tolerance during pregnancy [18].
Studies have linked decreases in levels of IL10 with exposure to AAPs such as nitrogen oxides (NOx) [22] and PM10 [23]. Others have found that exposure to particulate matter leads to the elevation of IL4 in animal studies [24]. Our group previously showed that exposure to polycyclic aromatic hydrocarbons with 4,5,6-rings (PAH456) is associated with decreased IL10 gene methylation [25]. Furthermore, we have found links between AAPs and DNA methylation of specific CpG sites of IL10 and FoxP3 genes in children with asthma [26] and more recently that AAP exposure was associated with altered methylation of CpG sites for IFNγ, IL4, IL10, and FoxP3 genes [27].
Residents from Fresno, California, have consistently elevated exposure to AAPs. According to the 2020 State Of The Air report for US cities, Fresno is ranked first in short-term particle pollution, second in year-round particle pollution, and fourth in ozone (O3) exposure [28]. Here, we identified differentially methylated regions in the maternal IFNγ, IL4, IL10, and FoxP3 genes during pregnancy and associated them with exposure to AAPs. Our primary hypothesis is that exposures to elevated levels of elemental carbon (EC), CO, O3, NO2, NOx, PAH456, PM2.5, and coarse particulate matter (PM10) are associated with increased methylation of the IL4, IL10, IFNγ, and FoxP3 genes. Our secondary hypothesis is that Th cell percentages are associated with exposure to high levels of AAPs. In addition, based on our previous findings of exposure to AAPs and gene methylation in children [26, 27], we aimed to explore associations between altered methylation of IFNγ, IL4, IL10, and FoxP3 genes from the mothers and their children’s cord blood, as well as blood samples drawn in these offspring at 12 and 24 months old.
Results
Subject characteristics
The demographics of the 186 women recruited at aproximately 20 weeks of pregnancy are summarized in Table 1. The median age (quartile 1; quartile 3) was 27 years (23; 33). The median weight at the start of the pregnancy (quartile 1; quartile 3) was 72.73 kg (59.09; 85.00). Most enrolled subjects were Hispanic (73%).
Table 1.
Demographic characteristics of subjects
| Overall | |
|---|---|
| N = 186 | |
| Age of mother at baseline (median [IQR]) years | 27 (23, 33) |
| Weight at start of pregnancy (kgs) (median [IQR]) | 72.7 [59.1, 85.0] |
| Race n (%) | |
| Hispanic | 135 (72.6) |
| African American | 20 (10.8) |
| White | 17 (9.1) |
| Asian or Pacific Islander | 13 (7.0) |
| Household income last year before taxes (%) | |
| ≤ $15 K | 59 (31.7) |
| > $15–$30 K | 78 (41.9) |
| > $30–$50 K | 31 (16.7) |
| > $50 K | 6 (3.2) |
| Number of weeks pregnant (median [IQR]) | 22 (20, 23) |
| Season of baseline visit (%) | |
| 1: Oct–Jan (winter) | 54 (29.0) |
| 2: Feb–May (spring) | 64 (34.4) |
| 3: Jun–Sep (summer) | 68 (36.6) |
| Ever diagnosed Asthma (%) | 44 (23.7) |
The mean AAP exposure averages were calculated at 1 week 1, 3, and 6 months before each subject’s blood draw (Additional file 1: Fig. S1).
Methylation is associated with ambient air pollution
We explored associations between AAP exposures (CO, elemental carbon (EC), O3, NO2, NOx, PAH456, PM10, PM2.5) and methylation of CpG sites in the IFNγ, IL4, IL10, and FoxP3 genes. We constructed a linear regression model on each individual gene methylation as a function of each AAP exposure, adjusting for weight, age, season, race, and asthma. The p-values for the association with exposure across all genes were adjusted for multiple testing using the Benjamini–Hochberg procedures that control the false-discovery rate (FDR). We used a Q value which denotes the adjusted p-value across all genes based on the regression model, and Q < 0.1 was considered as statistically significant. Coefficients and corresponding 95% confidence intervals for each AAP exposure were summarized in Additional file 19: Table S3.
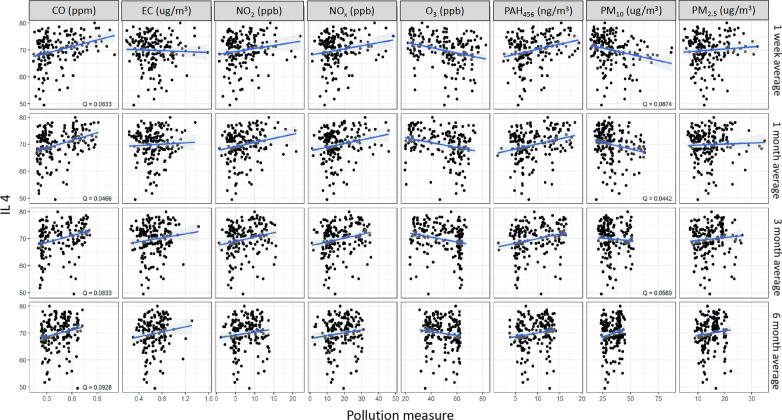
Methylation of IL4 was positively associated with CO (1 w: Q = 0.08, 1 m: Q = 0.05, 3 m: Q = 0.08 and 6 m: Q = 0.09) and negatively associated with PM10 (1 w: Q = 0.08, 1 m: Q = 0 0.04, 3 m: Q = 0.06) (Fig. 1). Methylation of IL10 was positively associated with CO (1 w: Q = 0.01, 1 m: Q < 0.01, 3 m: Q < 0.01), EC (3 m: Q = 0.05 and 6 m: Q = 0.04), NO2 (1 m: Q = 0.05, 3 m: Q < 0.01 and 6 m: Q = 0.08), NOx (1 m: Q = 0.04, 3 m: Q < 0.01 and 6 m: Q = 0.06), PAH456 (1 w: Q < 0.01, 1 m: Q < 0.01 and 3 m: Q < 0.01), PM2.5 (3 m: Q = 0.06) and negatively associated with O3 (1 w: Q < 0.01, 1 m: Q < 0.01 and 3 m: Q < 0.01) and PM10 (1 m: Q = 0.05) (Fig. 2). In addition, IFNγ methylation was positively associated with CO (1 w: Q = 0.05, 1 m: Q = 0.01, and 3 m: Q = 0.04) and PAH456 (1 w: Q = 0.02, 1 m: Q < 0.01, 3 m: Q = 0.06) (Fig. 3) while FoxP3 methylation was only positively associated with PM10 (6 m: Q = 0.05) (Additional file 2: Fig. S2).
Fig. 1.
Associations between IL4 and ambient air pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. IL4 refers to average DNA methylation across 6 CpG sites in the gene. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter
Fig. 2.
Associations between IL10 and ambient air pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. IL10 refers to average DNA methylation across 4 CpG sites in the gene. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter
Fig. 3.
Associations between IFNγ and ambient air pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. IFNγ refers to average DNA methylation across 3 CpG sites in the gene. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter
Frequency of cell populations is associated with ambient air pollution
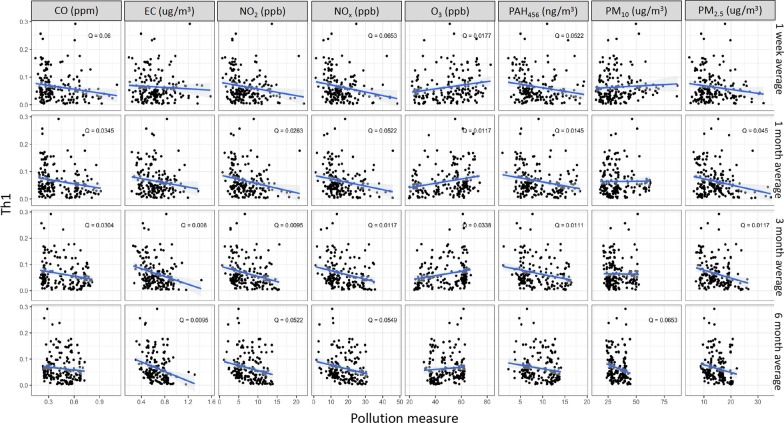
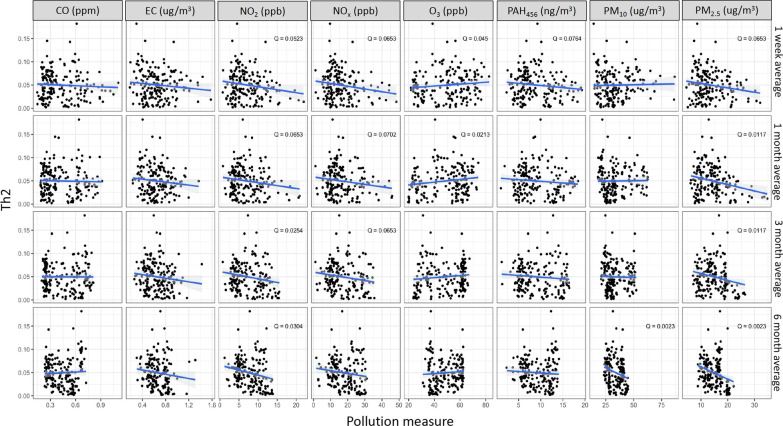
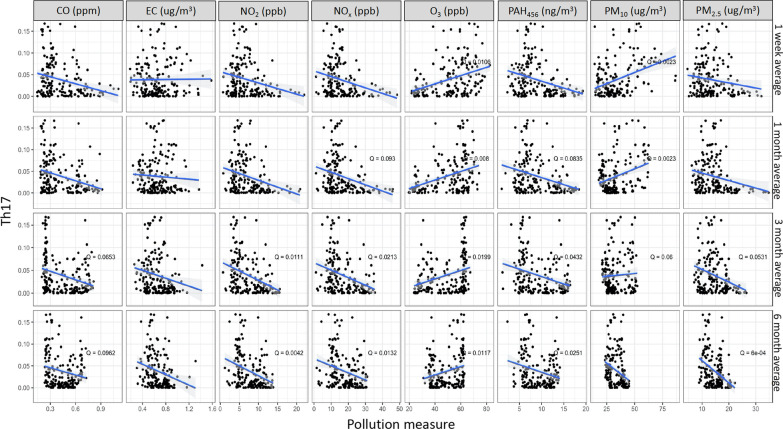
Figure 4 illustrates the percentage of Th1 cells negatively associated with exposures to PM2.5 (1 m: Q = 0.05 and 3 m: Q = 0.01), NO2 (1 m: Q = 0.03, 3 m: Q = 0.01, and 6 m: Q = 0.05), NOx (1 w: Q = 0.07, 1 m: Q = 0.05, 3 m: Q = 0.01, 6 m: Q = 0.05), and PAH456 (1 w: Q = 0.05, 1 m: Q = 0.01, and 3 m: Q = 0.01). We present in Fig. 5 the percentages of Th2 cells which were negatively associated with exposures to NO2 (1 w: Q = 0.05, 1 m: Q = 0.06, 3 m: Q = 0.02 and 6 m: Q = 0.03), NOx (1 w: Q = 0.07, 1 m: Q = 0.07, 3 m: Q = 0.07), PAH456 (1 w: Q = 0.08) and PM2.5 (1 w: Q = 0.06, 1 m: Q = 0.01, 3 m: Q = 0.01 and 6 m: Q < 0.01) and positively associated with exposure to O3 (1 w: Q = 0.04, 1 m: Q = 0.02). Lastly, Fig. 6 shows that Th17 was negatively associated with exposures to CO (3 w: Q = 0.07, 1 m: Q < 0.1), NO2 (3 w: Q = 0.01, 6 m: Q = 0.004), NOx (1 m: Q < 0.1, 3 m: Q = 0.02, 6 m: Q = 0.01), PAH456 (1 m: Q = 0.8, 3 m: Q = 0.04, 6 m: Q = 0.03) and PM2.5 (3 m: Q = 0.05 and 6 m: Q < 0.01) and positively associated with exposures to O3 (1 m: Q < 0.01,1 3 m: Q = 0.04, 6 m: Q = 0.02) and PM10.(1 w: Q < 0.01, 1 m: Q < 0.01. 3 m: Q = 0.06). No associations were found between Treg cell percentages and AAP (Additional file 3: Fig.S3).
Fig. 4.
Associations between Th1 percentage and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter
Fig. 5.
Associations between Th2 percentage and ambient air pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter
Fig. 6.
Associations between Th17 percentage and ambient air pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter
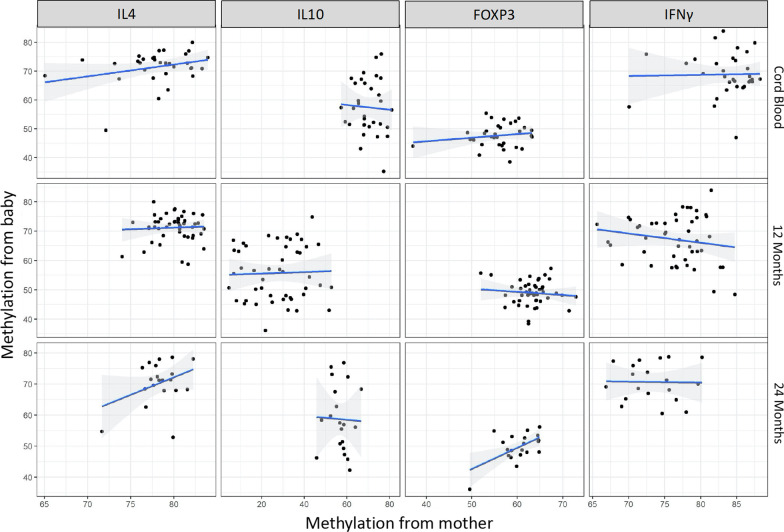
To determine whether the methylation level is driven by sub-cell type frequency we did further data analysis on the association between DNA methylation and sub-cell type frequency (Additional file 14: Fig. S14).There was an association between Th1 cell frequency and methylation level of the IFNγ gene regions, but the Th1 cell frequency was also associated with IL4 methylation. There was no significant association between methylation level of IL4 gene region and the Th2 cell frequency. Also, the mother and baby’s IL4 and IL10 methylation levels were not associated, after univariate analysis and FDR adjustment at 0.1 significance level (Fig. 7). Pearson’s correlation coefficients and corresponding 95% confidence intervals between mother’s and baby’s methylation levels were summarized in Additional file 19: Table S4.
Fig. 7.
Associations between each pair of mother’s methylation and baby’s cord blood, at 12 months and 24 months. Bivariate analysis using Pearson’s correlation. False discovery rate adjustment at 0.1 significance level was applied
Discussion
In this cohort of pregnant women from Fresno, California, a metropolitan area known for elevated AAP levels, we found levels of AAPs to be associated with alterations in both pro and anti-inflammatory cytokines as well as a decrease in some Th cell population subsets. In general, these associations were sustained over time for short-term and mid-term AAP exposure.
Methylation of CpG sites in the IL4 and IL10 genes is related to anti-inflammatory responses, while methylation of the IFNγ gene would alter proinflammatory responses. IL4 aids in the polarization of antigen-stimulated naïve Th cells into Th2 effector cells and propagates Th2 responses, while IL10 downregulates Th1 cells [19, 29]. Our results indicate that, across multiple time exposure periods, higher levels of CO and PM2.5 are positively associated with methylation of the IL4 gene, while higher levels of CO, NO2, and PAH456 are positively associated with methylation of the IL10 gene. While the literature on methylation of IL4 and IL10 genes during pregnancy is scarce, prior studies have demonstrated an association between exposure to AAPs with IL4 in mice [24] and with IL10 in humans during late pregnancy [23]. Also, associations per pollutant and time average considered were not consistent between IL4 and IL10 gene methylation. For example, IL10 methylation was significantly associated with PAH456 but not with IL4 methylation. This is similar to another study where high levels of PAH456 were associated with decreases in protein expression of the IL10 gene from 24 h to 1-year exposure, but not the IL4 gene [30]. However, we also found that exposures to CO and PAH456 were associated with the methylation of IFNγ, which is pro-inflammatory. During the second trimester, these relationships could potentially be regulated by a gradual reversal of immune tolerance during mid to late pregnancy [10]. Furthermore, methylation of the FoxP3 gene was only associated with exposure to PM10.
Exposures to PM2.5, NO2, and NOX were inversely associated with Th2 cell percentages from 1 week to 6 months before the blood draw, while Th1 cells were inversely associated with CO, NOx, and PAH456 exposure from 1 week to 3 months. Th17 cells were also inversely associated with exposure to CO, NO2, NOx, PAH456, and PM2.5. Despite the relevance of these findings, there is scarce research on the direct effect of AAPs directly on T cell subsets during pregnancy. In murine models, PM and NO2 exposures exacerbate airway inflammation creating a Th1/Th2 imbalance [31]. Sasaki et al. reported that air pollutants can affect signaling pathways and expression of T cells associated with reduced expression of the IFNγ and IL10 perturbing the Th1/Th2 balance [32]. Finally, PAH456 molecules within air pollution may play a role in the maintenance and function of Th17 responses [33].
Epigenetic marks may serve as biomarkers that can be used to support the prevention of adverse pregnancy events. The novelty of this study is the identification of multiple sites within a gene as well as gene regions sensitive to AAP exposures. Additional relationships with specific gene regions are available (Additional file 5: Fig. S5–Additional file 17: Fig. S17). Some of our findings were consistent with our a priori hypothesis, however, for O3, we found associations contrary to our hypothesis. This may be due to the chemical equilibrium between ambient concentrations of O3 and NO2 such that local increases in NO2 are invariably accompanied by local decreases in O3 [34].
We did not find any association between the methylation of the mother’s blood and the baby’s cord blood or peripheral blood from 12 or 24 months. This is consistent with the findings by Friedman and colleagues who did not find consistent associations between infant outcomes and inflammatory biomarkers in the mother [35]. It is possible that individual genotypes at specific loci may result in different patterns of DNA methylation, which could influence extended genomic regions [36]. These methylation quantitative trait loci (meQTLs) may be responsible for direct gene-environment interactions which can vary over time under different conditions, such as exposure to AAPs.
To our knowledge, this is the first study to evaluate the association between T cell subsets and AAP exposure along with IL4, IL10, IFNγ, and FoxP3 gene methylation in a sample of women at mid-pregnancy (20–23 weeks). Previous studies have not specified a critical time of exposure duration for effects on gene methylation or immune phenotype changes. However, our findings suggest some of the associations are sustained during pregnancy across the 6-month period before the blood draw.
One limitation is that the blood draws occurred across different seasons. To address this, we accounted for season, the distribution of subjects, and the spatial variation in pollutants in our statistical analysis plan. The 6-month exposure could potentially serve as a reference that could account for seasonal variations and could explain some of the unexpected trends for PM10. The estimated individual exposures to AAPs were assessed using modeling methods, rather than personal monitoring, so some exposure misclassification is likely. Also, to determine whether the methylation level could be driven by sub-cell type frequency, we performed further data analyses on the possible association between DNA methylation and sub-cell type frequency (Additional file 18: Fig. S18). There was no significant association between methylation levels of tested IL4 gene regions and the Th2 cell frequency. There was an association between Th1 cell frequency and methylation level of the IFNγ gene region. Therefore, we cannot rule out the possibility that the sub-cell proportions may be a potential confounder.
Other limitations include lack of adjustment for dietary exposure and exposure to other toxicants from ambient/indoor air, water, and other sources which could contribute to DNA methylation and potentially confound results. The population considered was a convenience sample predominantly of Hispanic descent. Asthma status was self-reported by the participants rather than measured, but studies have shown good agreement between patient self-report and physician clinical diagnosis [37, 38].
In summary, we demonstrate that methylation levels at specific CpG sites in the IL4, IL10, and IFNγ genes are associated with exposure to the pollutants CO, NO2, PM2.5, and PAHs sustained up to 6 months before the blood draw. Also, the percent of Th1, Th2, and Th17 cells was negatively associated with several AAPs (CO, NO2, NOx, PAH456, and PM2.5). The findings from this study increase our understanding of the epigenetic effects of exposure to AAPs and may impact the development of therapies to alter the methylation of these sites. With the development of new therapies targeting specific gene sites and improved efforts to reduce exposure to ambient air pollutants, we aim to mitigate the impacts associated with AAP exposure during pregnancy, thereby reducing adverse health outcomes for both mother and baby.
Conclusion
These results offer insights into the detrimental effects of air pollution during pregnancy, elucidating the role of epigenetic biomarkers and their possible uses for preventing or predicting epigenetic damage resulting from air pollution exposure.
Methods
Subject recruitment and study center visit
From November 2014 to August 2016, we recruited a convenience sample of 186 pregnant women from Fresno, California (median age = 27 years). Recruitment was done in cooperation with UCSF-Fresno-affiliated and Clinica Sierra Vista obstetric clinics, and the pregnant women were approached to participate in the clinic waiting areas by our field office staff.
Interested women were screened for eligibility (between 18 and 25 weeks gestation, residence in Fresno or Clovis for at least the past 3 months, residence within 20 km of the central air quality monitoring site, no plans to move from the Fresno/Clovis area in the next 2 years, English- or Spanish-speaking, smoked < 50 cigarettes during their pregnancy, and no cancer, HIV, or autoimmune disease). If interested and eligible, the pregnant women were invited to visit the study center at UCSF-Fresno. All study protocols were approved by the Institutional Review Boards at the University of California, Berkeley; the University of California, San Francisco-Fresno (UCSF-Fresno); and Stanford University. Written, informed permission was obtained from each subject.
During each visit, we collected information on height and weight, a health and general history questionnaire, and blood samples. We ensured that subjects that took oral immunosuppressants within 5 days of the blood draw, had a history of allergen immunotherapy within 1 year of the visit, had a chronic disease other than allergies or asthma, or had an acute infection were not considered in the analysis.
Cord blood was collected at birth from a convenience sample (n = 35) of enrolled mothers who were invited to enroll their babies and come in for office visits when the babies were approximately 12- (n = 58) and 24-months old (n = 20). After the mothers gave written permission for their babies to participate in the study, we collected information for the babies on length and weight, conducted a health and general history questionnaire, and also took blood samples from a convenience sample of the babies to explore methylation associations between mother-baby pairs at each of three time points: birth (cord blood), 12 months and 24 months. We did not include samples from twin births.
Collection and processing of blood specimens
We extracted human peripheral blood mononuclear cells (PBMCs) and plasma from the collected blood sample using a Ficoll procedure which allows rapid and efficient isolation of mononuclear cells and stored them in liquid nitrogen and at − 80 °C as detailed in previous research [39].
Methylation sequencing and analysis
We extracted DNA from frozen PBMCs using the QIAamp DNA blood Mini Kit (Qiagen, Valencia, CA) which was subjected to a bisulfite modification through the EZ DNA Methylation Kit (Zymo Research, Orange, CA) based on manufacturer recommendations as described in published works [25]. We conducted a methylation analysis on IL4, IL10, FoxP3, and IFNγ genes. Based on previous findings, we selected key sites of important immunoregulatory genes that modulate immune tolerance [40–42]. We selected 6 CpG sites in the intron 2 region of the IL4 gene (Chr5:132673938, Chr5:132673907, Chr5:132675095, Chr5:132675115, Chr5:132675133, Chr5:132675242) and 4 CpG sites in the intron 4 region of the IL10 gene (Chr1:206769266, Chr1:206769234, Chr1:206769230, Chr1:206769214), respectively. We also selected 2 CpG sites in the promoter region of the FoxP3 gene (ChrX:49264916, ChrX:49264956) and 3 CpG sites in the promoter region of the IFNγ gene (Chr12:68159798, Chr12:68159930, Chr12:68160040). We included internal controls for bisulfite conversion efficiency in each pyrosequencing assay and followed standardized operating procedures (SOPs) and quality control from the Sean Parker Center for Allergy and Asthma Research’s laboratory as done in previous work [43]. We explored associations between AAP exposure (CO, O3, NO2, NOx, PAH456, PM2.5, and PM10) and methylation levels at the aforementioned CpG sites.
Flow cytometry
We also considered the frequency of different T helper (Th1, Th2, Th17, and Treg) subset populations using flow cytometry. We resuspended the cells in PBS in flow-cytometry staining tubes. We used live/dead staining (Molecular Probes/Invitrogen) in all our samples and identified live cells by the intracellular conversion of a calcein ester to free calcein, which is fluorescent in the green spectrum; we identified dead cells by the red staining of internal nucleic acids using ethidium homodimer. We further stained the fraction with CD4-Pacific Blue (clone RPA-T4, BD Biosciences), CD25-phycoerythrin (PE)-Cy7 (clone BC96, BioLegend), and CD127-FITC (BD Biosciences). We gated CD127 low-expressing CD4 + CD25hi cells to ensure separation of conventional activated T cells from stable Treg populations, which further allowed us to identify highly-enriched FoxP3 + cells [25].
Ambient air pollution exposure assessment
Average air pollutant exposures were calculated for each mother over several different time periods: 1 week, and 1, 3 and 6 months before the blood sample was collected. To calculate air pollution exposures, we modeled outdoor residential air pollution exposure to AAPs, specifically CO, O3, NO2, NOx, EC, PAH456, PM2.5, and PM10 (PAH456 is defined as polycyclic aromatic hydrocarbons with 4, 5 and 6 rings (fluoranthene, benz[a]anthracene, chrysene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[k]flouoranthene, benzo[ghi]perylene, indeno[1,2,3-cd]pyrene, and dibenz[a,h]anthracene). Two methods were used for individual-level exposure assessment depending on the specific pollutant of interest: interpolation of data extracted from the US Environmental Protection Agency’s (EPA) Air Quality System using inverse distance-squared weighting for CO, NO2, NOx, O3, PM2.5, and PM10, and regression modeling incorporating monitoring data and field sampling data for EC, and PAH456.
EPA’s Air Quality System offered hourly, quality-assured, ambient pollutant concentrations and meteorological data from Fresno’s site monitoring stations. Elemental carbon (BC) was determined from Aethalometer™ (model AE42; Magee Scientific, Berkeley, CA) measurements of the optical absorption of PM2.5 ambient aerosol at 880 nm. Particle-bound PAHs were monitored with the PAS 2000 (EcoChem Analytics, League City, TX) which uses a photoelectric aerosol sensor to measure ambient PAH with three or more rings. Data from these real-time continuous monitors are available from 2002 through 2017 covering the time window of our study. The air pollution data were subject to rigorous quality assurance which included the comparison of values at nearby sites and consistency with historical patterns for each pollutant. These methods have been described in detail elsewhere [44–46]. Lastly, a residential address history was obtained from each participating mother, and exposure was matched to participants’ residential street addresses.
Statistical analysis
The DNA methylation was calculated as the average of the selected CpG sites for each gene. To evaluate the associations between gene methylation and air pollution, we constructed a linear regression model on each methylation as a function of each AAP exposure, adjusting for weight, age, season, race, and asthma. The p-values for the association with exposure across all genes were adjusted for multiple testing using the Benjamini–Hochberg procedures that control the false-discovery rate (FDR). The selection of covariates is based on our previous epigenetic work [26, 27]. Seasons were defined as described in previous work [47]. For race, the respondents were allowed to select up to 4 race/ethnicity responses for themselves, which were upcoded to Hispanic, Black/African American, Non-Hispanic White, Asian/Pacific Islander, and American Indian/Alaska Native. Any subject coded as Hispanic in any reported category was defined as Hispanic, and among those remaining, the first other listed race was used. We further constructed the same model for each CpG site as a supplementary analysis. We adjusted the p-values of exposures across all CpG sites for multiple testing using the same method. The correlations between mother’s and cord blood, and with baby’s methylation levels at 12 months and 24 months were assessed using Pearson’s correlation coefficients; p-values across all genes were adjusted for multiple testing using the same method. All tests were two-sided. We used a Q value to denote the adjusted p-value, and Q < 0.1 was considered statistically significant. The statistical analysis was performed using the R statistical computing software (R 3.4.1).
Supplementary Information
Additional file 1: Figure S1. Ambient air pollutant (AAP) concentration levels and counts per participant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 2: Figure S2. Associations between Foxp3 and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. FoxP3 refers to average DNA methylation across 3 CpG sites in the gene. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 3: Figure S3. Associations between Treg percentage and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 4: Figure S4. Associations between each T cell sublet percentage and each CpG site methylation. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant.
Additional file 5: Figure S5. Associations between IL4_CpG4 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 6: Figure S6. Associations between IL4_CpG3 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 7: Figure S7. Associations between IL4_CpG24 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 8: Figure S8. Associations between IL4_CpG22 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 9: Figure S9. Associations between IL4_CpG21 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 10: Figure S10. Associations between IL4_lossCpG23 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 11: Figure S11. Associations between IL10_CpG38 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 12: Figure S12. Associations between IL10_CpG39 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all cell types, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 are shown. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 13: Figure S13. Associations between IL10_CpG40 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 14: Figure S14. Associations between IL10_CpG41 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 15: Figure S15. Associations between IFNγ_CpG5 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all cell types, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 are shown. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 16: Figure S16. Associations between IFNγ_CpG4 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 17: Figure S17. Associations between IFNγ_CpG3 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 18: Figure S18. Associations between each cell type percentage vs each gene. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant.
Additional file 19: Supplementary file with tables of (1) flow cytometry panel analysis, (2) summary of CpG site of the 4 genes Foxp3, Il-4, Il-10 and IFN-γ, (3) estimates and coefficients of gene and pollutant data by time estimate, and (4) estimates and correlation coefficients of mother and baby data.
Acknowledgements
The authors would like to thank the UCSF Fresno research team (Griselda Aguilar, Christian Bonilla, Karina Corona, Cynthia Cortez, Alexa Lopez, Carolina Orozco, and Janna Blaauw) for their hard work in conducting the clinical visits; Natalie Myren, Kimberly Meyer, Megan Flaviano, Anmol Sidhu and Beth MacDonald for general assistance with study management; and all of the participating children and families for their patience and dedication to the study.
Authors' contributions
JA, XH, SC, MP, KN contributed to the conception, design, data collection, data analysis, and/or manuscript writing. JA drafted the manuscript. LL, JB and TT contributed to the cohort design, data collection, and data preparation. KH, EN, FL contributed with the air pollution exposure data. SC, XH, MP were responsible for statistical analyses and interpretation. KN, MP supervised the study. VS, MP, KN helped with manuscript writing and editing. TB, PU, PK, NA, HM added additional content and expertise to revisions and peer comments. All authors read and approved the final manuscript.
Funding
This study was supported by the NIH, Sean N Parker Center at Stanford University, Maternal and Children’s Health Program at Stanford, NIEHS R01 (Nadeau, PI). This work was also supported by the NIH/EPA-funded Children’s Environmental Health and Disease Prevention Research Centers (EPA R834596/NIEHS P01ES022849, EPA RD835435/NIEHS P20ES018173), and the NIH (NHLBI R01HL081521, NIEHS R01ES032253). Although the research has been funded in part by the US EPA, it has not been subjected to any EPA review and therefore does not necessarily reflect the views of the EPA, and no official endorsement should be inferred.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Institutional Review Boards at each participating institution approved the protocol of this study (Stanford IRB 8629, UC Berkeley IRB 2013-07-5453, UCSF-Fresno IRB 14-14785).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Mary Prunicki: Co-senior
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A. Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ Res. 2018;167:144–159. doi: 10.1016/j.envres.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Stieb DM, Chen L, Hystad P, Beckerman BS, Jerrett M, Tjepkema M, et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999–2008. Environ Res. 2016;148:513–526. doi: 10.1016/j.envres.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Šrám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113(4):375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Zhang H, Liang Q, Knibbs LD, Ren M, Li C, et al. Effects of prenatal exposure to air pollution on preeclampsia in Shenzhen, China. Environ Pollut. 2018;237:18–27. doi: 10.1016/j.envpol.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Lin H, Mosmann TR, Guilbert L, TuntiPopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151(9):4562–4573. [PubMed] [Google Scholar]
- 6.Reinhard G, Noll A, Schlebusch H, Mallmann P, Ruecker AV. Shifts in the TH1/TH2 balance during human pregnancy correlate with apoptotic changes. Biochem Biophys Res Commun. 1998;245(3):933–938. doi: 10.1006/bbrc.1998.8549. [DOI] [PubMed] [Google Scholar]
- 7.Littauer EQ, Esser ES, Antao OQ, Vassilieva EV, Compans RW, Skountzou I. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLOS Pathogens. 2017;13(11):e1006757. doi: 10.1371/journal.ppat.1006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222(6):521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Benmarhnia T, Zhang H, Knibbs LD, Sheridan P, Li C, et al. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ Int. 2018;121:317–324. doi: 10.1016/j.envint.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Shah NM, Herasimtschuk AA, Boasso A, Benlahrech A, Fuchs D, Imami N, et al. Changes in T cell and dendritic cell phenotype from mid to late pregnancy are indicative of a shift from immune tolerance to immune activation. Front Immunol. 2017;8:1138. doi: 10.3389/fimmu.2017.01138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joubert BR, Håberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120(10):1425–1431. doi: 10.1289/ehp.1205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur G, Begum R, Thota S, Batra S. A systematic review of smoking-related epigenetic alterations. Arch Toxicol. 2019;93(10):2715–2740. doi: 10.1007/s00204-019-02562-y. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen CH, Larsen A, Nielsen AL. DNA methylation alterations in response to prenatal exposure of maternal cigarette smoking: a persistent epigenetic impact on health from maternal lifestyle? Arch Toxicol. 2016;90(2):231–245. doi: 10.1007/s00204-014-1426-0. [DOI] [PubMed] [Google Scholar]
- 14.Goodrich JM, Reddy P, Naidoo RN, Asharam K, Batterman S, Dolinoy DC. Prenatal exposures and DNA methylation in newborns: a pilot study in Durban, South Africa. Environ Sci Process Impacts. 2016;18(7):908–917. doi: 10.1039/c6em00074f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21(24):3352–3357. doi: 10.1016/s0264-410x(03)00331-1. [DOI] [PubMed] [Google Scholar]
- 16.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter–induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect. 2010;118(5):640–646. doi: 10.1289/ehp.0901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–57. [PubMed] [Google Scholar]
- 18.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5:253. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzi M, Vigano A, Trabattoni D, Villa M, Salvaggio A, Clerici E, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106(1):127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu B, Tian Z, Wei H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol Immunol. 2014;11(6):564–570. doi: 10.1038/cmi.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mostafavi N, Vlaanderen J, Chadeau-Hyam M, Beelen R, Modig L, Palli D, et al. Inflammatory markers in relation to long-term air pollution. Environ Int. 2015;81:1–7. doi: 10.1016/j.envint.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, et al. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PLOS ONE. 2011;6(8):e23130. doi: 10.1371/journal.pone.0023130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Melo JO, Soto SF, Katayama IA, Wenceslau CF, Pires AG, Veras MM, et al. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett. 2015;232(2):475–480. doi: 10.1016/j.toxlet.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4):845–52.e10. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Prunicki M, Stell L, Dinakarpandian D, de Planell-Saguer M, Lucas RW, Hammond SK, et al. Exposure to NO2, CO, and PM 25 is linked to regional DNA methylation differences in asthma. Clin Epigenetics. 2018;10(1):2. doi: 10.1186/s13148-017-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prunicki M, Cauwenberghs N, Lee J, Zhou X, Movassagh H, Noth E, et al. Air pollution exposure is linked with methylation of immunoregulatory genes, altered immune cell profiles, and increased blood pressure in children. Sci Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-83577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Health_Effects_Institute. State of Global Air 2020 2020 [06/14/2021]
- 29.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17(1):701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 30.Hew K, Walker A, Kohli A, Garcia M, Syed A, McDonald-Hyman C, et al. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin Exp Allergy. 2015;45(1):238–248. doi: 10.1111/cea.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji X, Han M, Yun Y, Li G, Sang N. Acute nitrogen dioxide (NO2) exposure enhances airway inflammation via modulating Th1/Th2 differentiation and activating JAK-STAT pathway. Chemosphere. 2015;120:722–728. doi: 10.1016/j.chemosphere.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki Y, Ohtani T, Ito Y, Mizuashi M, Nakagawa S, Furukawa T, et al. Molecular events in human T cells treated with diesel exhaust particles or formaldehyde that underlie their diminished interferon-γ and interleukin-10 production. Int Arch Allergy Immunol. 2009;148(3):239–250. doi: 10.1159/000161584. [DOI] [PubMed] [Google Scholar]
- 33.Glencross DA, Ho T-R, Camina N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radical Biol Med. 2020;151:56–68. doi: 10.1016/j.freeradbiomed.2020.01.179. [DOI] [PubMed] [Google Scholar]
- 34.Clapp LJ, Jenkin ME. Analysis of the relationship between ambient levels of O3, NO2 and NO as a function of NOx in the UK. Atmos Environ. 2001;35(36):6391–6405. [Google Scholar]
- 35.Friedman C, Dabelea D, Thomas DS, Peel JL, Adgate JL, Magzamen S, et al. Exposure to ambient air pollution during pregnancy and inflammatory biomarkers in maternal and umbilical cord blood: The Healthy Start study. Environ Res. 2021;197:111165. doi: 10.1016/j.envres.2021.111165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith AK, Kilaru V, Kocak M, Almli LM, Mercer KB, Ressler KJ, et al. Methylation quantitative trait loci (meQTLs) are consistently detected across ancestry, developmental stage, and tissue type. BMC Genomics. 2014;15(1):1–11. doi: 10.1186/1471-2164-15-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumeister H, Kriston L, Bengel J, Härter M. High agreement of self-report and physician-diagnosed somatic conditions yields limited bias in examining mental–physical comorbidity. J Clin Epidemiol. 2010;63(5):558–565. doi: 10.1016/j.jclinepi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Toren K, Brisman J, Järvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires: a literature review. Chest. 1993;104(2):600–608. doi: 10.1378/chest.104.2.600. [DOI] [PubMed] [Google Scholar]
- 39.Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol. 2009;85(1):7.1–7.1.8. doi: 10.1002/0471142735.im0701s85. [DOI] [PubMed] [Google Scholar]
- 40.Janson PC, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FoxP3 promoter demethylation reveals the committed Treg population in humans. PLOS ONE. 2008;3(2):e1612. doi: 10.1371/journal.pone.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunst KJ, Leung Y-K, Ryan PH, Hershey GKK, Levin L, Ji H, et al. Forkhead box protein 3 (FoxP3) hypermethylation is associated with diesel exhaust exposure and risk for childhood asthma. J Allergy Clin Immunol. 2013;131(2):592–4.e3. doi: 10.1016/j.jaci.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji-Takayama K, Suzuki M, Yamamoto M, Harashima A, Okochi A, Otani T, et al. The production of IL-10 by human regulatory T cells is enhanced by IL-2 through a STAT5-responsive intronic enhancer in the IL-10 locus. J Immunol. 2008;181(6):3897–3905. doi: 10.4049/jimmunol.181.6.3897. [DOI] [PubMed] [Google Scholar]
- 43.Kohli A, Garcia MA, Miller RL, Maher C, Humblet O, Hammond SK, et al. Secondhand smoke in combination with ambient air pollution exposure is associated with increasedx CpG methylation and decreased expression of IFN-γ in T effector cells and FoxP3 in T regulatory cells in children. Clin Epigenetics. 2012;4(1):1–16. doi: 10.1186/1868-7083-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann JK, Lutzker L, Holm SM, Margolis HG, Neophytou AM, Eisen EA, et al. Traffic-related air pollution is associated with glucose dysregulation, blood pressure, and oxidative stress in children. Environ Res. 2021;195:110870. doi: 10.1016/j.envres.2021.110870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noth EM, Hammond SK, Biging GS, Tager IB. A spatial-temporal regression model to predict daily outdoor residential PAH concentrations in an epidemiologic study in Fresno, CA. Atmos Environ. 2011;45(14):2394–2403. [Google Scholar]
- 46.Noth EM, Lurmann F, Perrino C, Vaughn D, Minor HA, Hammond SK. Decrease in ambient polycyclic aromatic hydrocarbon concentrations in California's San Joaquin Valley 2000–2019. Atmos Environ. 2020;242:117818. doi: 10.1016/j.atmosenv.2020.117818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mann JK, Balmes JR, Bruckner TA, Mortimer KM, Margolis HG, Pratt B, et al. Short-term effects of air pollution on wheeze in asthmatic children in Fresno, California. Environ Health Perspect. 2010;118(10):1497–1502. doi: 10.1289/ehp.0901292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Ambient air pollutant (AAP) concentration levels and counts per participant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 2: Figure S2. Associations between Foxp3 and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. FoxP3 refers to average DNA methylation across 3 CpG sites in the gene. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 3: Figure S3. Associations between Treg percentage and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 4: Figure S4. Associations between each T cell sublet percentage and each CpG site methylation. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant.
Additional file 5: Figure S5. Associations between IL4_CpG4 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 6: Figure S6. Associations between IL4_CpG3 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 7: Figure S7. Associations between IL4_CpG24 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 8: Figure S8. Associations between IL4_CpG22 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 9: Figure S9. Associations between IL4_CpG21 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 10: Figure S10. Associations between IL4_lossCpG23 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 11: Figure S11. Associations between IL10_CpG38 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 12: Figure S12. Associations between IL10_CpG39 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all cell types, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 are shown. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 13: Figure S13. Associations between IL10_CpG40 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 14: Figure S14. Associations between IL10_CpG41 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 15: Figure S15. Associations between IFNγ_CpG5 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all cell types, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 are shown. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 16: Figure S16. Associations between IFNγ_CpG4 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 17: Figure S17. Associations between IFNγ_CpG3 site and Ambient Air Pollutant levels. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant. CO: Carbon monoxide, EC: Elemental carbon, NO2: Nitric dioxide, NOx: Nitric oxides, O3: Ozone, PAH: Polycyclic aromatic hydrocarbons, PM: particulate matter.
Additional file 18: Figure S18. Associations between each cell type percentage vs each gene. Q value is the false-discovery-rate-adjusted p-value across all genes, based on linear regression model adjusting for weight, age, season, race, and asthma diagnosis. Q < 0.1 is considered statistically significant.
Additional file 19: Supplementary file with tables of (1) flow cytometry panel analysis, (2) summary of CpG site of the 4 genes Foxp3, Il-4, Il-10 and IFN-γ, (3) estimates and coefficients of gene and pollutant data by time estimate, and (4) estimates and correlation coefficients of mother and baby data.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.