Abstract
Background
Low back and neck pain are a leading cause of disease burden globally. Opioids are recommended in guidelines for acute low back and neck pain; however, there is a lack of compelling efficacy data to support this.
Methods
The OPAL trial is a prospectively registered, triple-blinded, randomised, placebo-controlled trial. Patients with acute (≤12 weeks duration) back and/or neck pain receive guideline care plus either an opioid (oxycodone + naloxone, up to 20 mg per day) or a placebo for up to 6 weeks or earlier, if pain is resolved. The primary outcome is pain measured using the Pain Severity Score of the Brief Pain Inventory with the primary time point being 6 weeks. Secondary outcomes include physical function, time to recovery, quality of life, adverse events and risk of opioid misuse. Outcomes are collected at weeks 2, 4, 6, 12, 26 and 52. Analysis will be done on an intention-to-treat principle. p values of < 0.05 will be considered significant and 95% confidence intervals will be reported. Repeated-measures linear mixed models will be used to assess the effect of the treatment group on the primary outcome and continuous secondary outcomes. Adverse events will be compared between groups using Fisher’s exact test. Cost-effectiveness analyses will be conducted if a treatment effect on pain is seen at week 6. Subgroup analyses will be performed to assess whether pain duration and pain location are treatment effect modifiers.
Discussion
The OPAL trial will provide important evidence about whether a short course of opioids is effective in the treatment of acute non-specific low back and/or neck pain. This pre-specified statistical analysis plan details the methodology for the analysis of the OPAL trial results.
Trial registration
ACTRN12615000775516. The trial has completed recruitment. Follow-up on the last patient will be completed in March 2022.
Keywords: Opioids, Low back pain, Neck pain, Placebo-controlled trial, Statistical analysis plan
Background
Low back pain and neck pain are extremely prevalent and are leading causes of disease burden globally [1]. Strong analgesics, such as opioid analgesics, are recommended by clinical guidelines for people with acute low back pain or neck pain who require more pain relief [2]. Opioid analgesics are widely and increasingly used [3], but there is a lack of compelling efficacy data supporting the use of opioid analgesics for acute low back pain or neck pain [4]. A placebo-controlled trial of opioids for acute non-specific spinal pain has not previously been conducted; therefore, we cannot be certain what benefits opioids might provide. Concerns regarding opioid use are further heightened by the risks of adverse events, some of which can be serious (e.g. dependency, misuse and overdose) [5].
The OPAL study is the world’s first randomised placebo-controlled trial which is investigating the efficacy and safety of opioid analgesics in people with acute low back and/or neck pain [6]. Three-hundred and forty-seven participants are randomised to receive either the study medication (modified release oxycodone up to 20 mg per day) or a placebo for up to 6 weeks in addition to guideline-recommended care. The primary outcome is pain severity at 6 weeks. Other outcomes include pain severity, physical functioning, time to recovery, quality of life (physical and mental subscales) and occurrence of adverse events, measured at regular time points up to 12 months.
Methods/design
Study objectives
The primary objective is to investigate differences in pain severity at 6 weeks between participants randomised to the opioid arm and those randomised to placebo. We hypothesise that participants randomised to treatment with an opioid analgesic will have lower pain severity. The null hypothesis is that there is no difference in mean pain severity score between the two arms at 6 weeks.
Secondary objectives are to investigate whether the opioid analgesic:
Reduces pain at 12 weeks
Improves disability, time to recovery, quality of life (physical and mental) and global improvement over the 6-week treatment period and at 12 weeks
Is tolerated
Is cost-effective from the health system and societal perspectives
Does not result in opioid analgesic misuse at 3, 6 and 12 months
Patient population
Participants are recruited as they present to general practitioners (GP) or a hospital emergency department with a primary complaint of low back and/or neck pain. A doctor screens the patient against the inclusion and exclusion criteria. If they are eligible, the doctor seeks informed consent prior to enrolling them into the study.
Participants in the community experiencing acute back and/or neck pain can also be identified via advertisements on social media. Potential participants respond to the social media advertisement by completing an online pre-screening questionnaire and are encouraged to contact the OPAL trial team if they meet the pre-screening criteria. They are then referred to a study GP for further screening.
Inclusion criteria
Low back pain (pain between the 12th rib and buttock crease) and/or neck pain (pain in the area below the occiput to the most distal cervical spine) with or without distal radiation to the leg (for low back pain) or arm (for neck pain)
The current episode of pain is no more than 12 weeks since onset and preceded by at least a 1-month pain-free period (to screen out those with persistent pain)
Pain severity is at least moderate (as measured by adaptations of item 7 of the SF-36, i.e. how much low back pain or neck pain have you had in the last week? None/very mild/mild/moderate/severe/very severe).
Exclusion criteria
Known or suspected serious spinal pathology (e.g. infection, cauda equina syndrome, spinal fracture)
Contraindications to opioid analgesics (including previous intolerance to opioids or adverse reaction, opioid addiction history, allergy to any ingredient in the opioid or placebo tablets, prior or current abuse of psychoactive drugs, prior or current alcoholism) or scoring ‘high risk’ (a score of 9 or above) on the Opioid Risk Tool
Have taken a prescription opioid analgesic for the current episode of low back pain and/or neck pain at a dose > 15 mg of oral morphine equivalent per day for 5 or more consecutive days
Spinal surgery in the preceding 6 months
Scheduled or being considered for surgery or interventional procedures for low back pain and/or neck pain during the 6-week treatment period
Less than 18 years of age
Not having sufficient English language skills to understand trial procedures or complete assessments, or suitable translation is not available
For female participants: planning conception or are pregnant or breastfeeding
There were changes to the exclusion criteria during the trial to facilitate recruitment and in response to the up-scheduling of codeine from over-the-counter to a prescription-only medicine in Australia in February 2018. The original protocol (version 1.0) states that participants must have had back and/or neck pain for a minimum of 2 weeks and excluded those who have taken any prescription opioid for the current episode of back and/or neck pain.
Outcomes
| Outcome | Measurement tool | Measurement occasions |
| Primary outcome | ||
| Pain severity | Pain Severity Score of the Brief Pain Inventory | Baseline, weeks 2, 4, 6 and 12 |
| Secondary outcomes | ||
| Physical functioning (generic) | Pain Interference Score of the Brief Pain Inventory. | Baseline, weeks 2, 4, 6 and 12 |
| Physical functioning (condition-specific) | Roland-Morris Disability Questionnaire (24 items, for participants reporting low back pain only) and Neck Disability Questionnaire (for participants reporting neck pain only) | Baseline and 6 weeks |
| Time to recovery (average daily pain of 0 or 1 of 10 for the past seven consecutive days) | Pain diary | Daily until recovery or up to 12 weeks |
| Quality of life (physical) | SF-12 | Baseline, weeks 2, 4, 6 and 12 |
| Quality of life (mental) | SF-12 | Baseline, weeks 2, 4, 6 and 12 |
| Participants’ rating of global improvement | Global Perceived Effect scale | Baseline, weeks 2, 4, 6 and 12 |
| Other outcomes | ||
| Adverse events | Self-report and doctor report |
Self-report at weeks 2, 4, 6 and 12 Doctor report after each follow-up visit |
| Work absenteeism | Self-report | Baseline, weeks 2, 4, 6 and 12 |
| Use of treatment or health care services | Self-report | Baseline, weeks 2, 4, 6 and 12 |
| Compliance to study medication | Self-reported adherence recorded in a medication diary compared against doctor prescription data, supported by returned medicine count |
Medicine diary is recorded daily over 6 weeks Doctor prescription data at each visit Returned medicine count at end of treatment period (≤6 weeks) |
| Success of blinding | Participants are asked to estimate their allocation group as active opioid, inactive placebo or do not know | Week 6 |
| Long-term outcomes | ||
| Pain severity | Pain Severity Score of the Brief Pain Inventory | Weeks 26 and 52 |
| Use of treatment or health care services | Self-report | Weeks 26 and 52 if still experiencing low back pain and/or neck pain (> 1/10) |
| Risk of misuse | Current Opioid Misuse Measure | Weeks 12, 26 and 52 |
Intervention
Participants randomised to the treatment group receive modified release oxycodone plus naloxone up to 20 mg per day for up to 6 weeks. Participants randomised to the placebo group receive identical placebo tablets. Both groups also receive guideline care (reassurance of a positive prognosis, advice to stay active and avoid bed rest and, if required, other guideline-recommended treatments) [2].
The medication regimen starts at a dose of 5 mg oxycodone/naloxone, 2 times a day. This is gradually titrated up to the maximum dose of 10 mg, 2 times a day based on individual participant progress, tolerability and sedation score, before down titration to cessation. Treatment will continue until ‘adequate improvement’ (0 to 1 out of 10 pain for 3 consecutive days) or for a maximum of 6 weeks. For participants screened by a trial GP, their treatment is provided and monitored by the GP. For participants screened at the emergency department, their treatment is provided and monitored by a hospital rheumatologist.
Randomisation and blinding
An a priori allocation sequence was prepared by an independent statistician. Trial medication packs were prepared according to the sequence and were prepared and dispatched to trial pharmacies by an independent supplier. Upon recruitment, the participant is allocated the next sequentially numbered medication pack, randomising the participant on a 1:1 ratio. Blinding of the trial doctors, pharmacist, participant, assessor and data analyst is ensured by randomisation and concealed allocation. Blinding will be maintained until all data have been collected and analysis and interpretation have been completed and agreed upon. The success of participant blinding will be tested at 6 weeks.
Sample size
A sample size of 173 per group (346 total) will be sufficient to detect a clinically meaningful between-group difference of 1 on a 10-point pain scale at 6 weeks, assuming a SD of 2.5, power of 90% and α of 5% and allowing for 5% dropout and 10% non-compliance. No studies have reported on a minimum clinically important difference for acute spinal pain, but we expect patients would need to perceive at least one point of pain reduction to consider the treatment worthwhile.
Statistical analysis
General principles
This analysis plan applies to all data collected during the OPAL trial. Analyses will be conducted using SAS Enterprise Guide version 8.3 or above (SAS Institute Inc. 2012). Analyses will be conducted by the trial team and by an independent biostatistician using randomly permuted (‘scrambled’) treatment allocations. Real treatment allocations will be used once the validation is completed and the statistical code finalised. Data interpretation will first be performed using the real but masked treatment allocation.
Confidence intervals and p values
A p value of < 0.05 will be considered statistically significant. 95% confidence intervals will be reported to aid interpretation of precision and clinical importance of the findings.
Timing of final analysis
All outcomes will be analysed collectively at the completion of participant recruitment and following up. Recruitment was completed in March 2021 (347/346 participants recruited). The expected date of the final visit is March 2022 (approximately 12 months after completion of recruitment).
Interim analysis
A data safety and monitoring committee met on 2 occasions and oversaw interim pain intensity and adverse event outcomes during the conduct of the trial. No formal interim efficacy analysis was conducted.
Multiplicity adjustment
Given the clear outcome hierarchy and limited number of related secondary outcomes, we do not plan to adjust for multiplicity.
Data sets to be analysed
The intention-to-treat (ITT) population is all participants randomised regardless of compliance to study treatment. Unless otherwise specified, all analyses will be conducted on an intention-to-treat basis.
Subject disposition
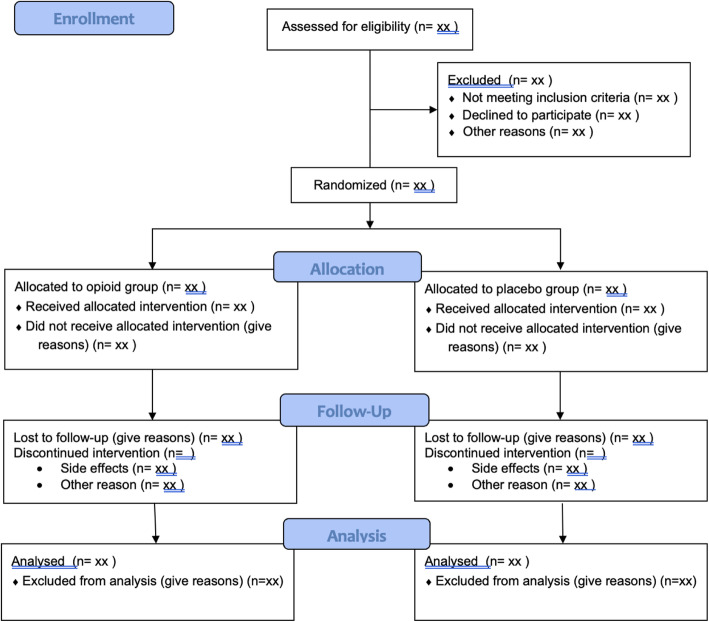
The flow of participants through the trial including the number of withdrawals and participants lost to follow-up will be presented in a CONSORT flow diagram (see Fig. 1 in the Appendix). Timing and reasons for withdrawal and lost to follow-up will also be presented in the flow diagram.
Participant characteristics and baseline comparisons
Baseline participant characteristics will be presented in a table (see Table 1 in the Appendix). Discrete variables will be summarised by frequencies and percentages. Percentages will be calculated according to the number of participants for whom data are available. Continuous variables will be summarised using mean and SD, and median and interquartile range (Q1–Q3). We do not intend to perform statistical tests comparing groups on the baseline data. Baseline characteristics collected are:
Sex
Age
Body mass index (kg/m2)
Location of pain (back/neck/both)
Days since onset of pain
Number of previous episodes of back and/or neck pain
Employment status
Employment classification
Whether pain is compensable
Household weekly income
Health insurance status
Pain severity
Disability (generic- and condition-specific)
Quality of life (physical and mental subscales)
Global perceived effect since onset of pain
Healthcare utilisation prior to enrolment, including use of opioid medications
Compliance to the study intervention
Compliance data will be displayed as per Table 2 in the Appendix. Compliance is determined primarily by looking at the participant’s medication reported in the daily clinical trial medicines diary when compared to the doctor’s prescription (and supply) of clinical trial medicines. Acceptable compliance will be defined as consuming ≥80% of their prescribed clinical trial medication. Secondarily, we will also look at the returned medicine count to confirm the participant’s self-reported medication diary data. Compliance with the randomised intervention will be summarised using the following variables:
Number of treatment discontinuations
Discontinuation reasons (where available)
Daily dose of medication taken
Cumulative dose of medication taken over the course of the study
Compliance, defined as taking at least 80% of prescribed medication
Protocol deviations are defined as any deviations from the study protocol (1), including eligibility criteria, concomitant medications or study conduct. A list of deviations will be presented in a table including the participant ID, date of deviation, details of the deviation, classification of deviation, whether the data was excluded from analysis and whether the participant needed to be replaced. See protocol deviations in Table 3 in the Appendix.
Concomitant therapies
Type and frequency of concomitant interventions will be categorised and presented as per Table 5 in the Appendix.
Analysis of the primary outcome
Descriptive analysis will consist of raw mean scores at each visit (baseline, weeks 2, 4 and 6 and months 3, 6 and 12) (see Table 4 in the Appendix). Raw data will also be reported on a longitudinal mean plot together with 95% confidence intervals.
Main analysis
Repeated-measure linear mixed models will be used to assess the effect of the treatment group on pain severity. The model will include outcome data collected at every post-baseline visit, i.e. at weeks 2, 4, 6, 12, 26 and 52. Fixed effects will include the randomised treatment allocation, the follow-up time points as a categorical variable with 6 levels, the interaction between treatment and time point as well as the baseline pain severity score.
Correlations between repeated measures (weeks 2, 4, 6, 12, 26 and 52 assessments) will be modelled using a repeated effect with a compound-symmetry structure. The primary treatment effect will be estimated as the adjusted mean difference in pain severity at the week 6 visit between the opioid and placebo arms together with its 95% CI.
The same model will be used to estimate the effect of the treatment at weeks 12 and 52.
Adjusted analysis
We will conduct sensitivity analyses after adding duration of the current pain episode and site of pain, that is, low back or neck, as covariates to the main model. This will be done both on the non-imputed and imputed (if applicable) data.
Subgroup analyses
The primary analysis described under the ‘main analysis’ heading will be repeated according to the site of pain (low back or neck). We will consider sex (male or female) as an additional subgroup. We anticipate that this will be reported in an appendix of the main report or in a separate manuscript.
This will be done by adding the subgroup variable to the main model together with its interaction with the randomised treatment, with the visit and with the treatment by visit interaction.
Heterogeneity by subgroup will be assessed by estimating the effect of the intervention at week 6 within each subgroup as well as through the corresponding interaction p value (at week 6 only). The results will be presented on a forest plot.
In addition, heterogeneity of treatment effect according to pain duration will be assessed using pain duration as a continuous variable.
Complier average causal effect
In case of substantial departure from compliance (see definition above), we will estimate the effect of the intervention in compliers using a Complier Average Causal Effect (CACE) with a propensity score and/or a joint-modelling approach. Details of the approach will be defined after reviewing the main results. The CACE analysis will therefore be considered exploratory.
Treatment of missing data
In case > 10% of the primary outcome data at week 6 are missing, multiple imputations will be used to conduct sensitivity analyses for the longitudinal linear mixed model of the primary outcome. The imputation model will include the treatment arm, all baseline variables (listed above) and all the pain severity score, the physical functioning scores, the quality of life scores and the global improvement score collected at baseline and all follow-up visits. One hundred sets will be imputed using fully conditional specification with discrete variables imputed using a discriminant function [7]. Each imputed set of data will be analysed using the same model as the one used for the main analysis, both without and with adjustment for additional covariates (see the ‘Adjusted analysis’ section). Subgroup analyses will not be conducted on the imputed data set.
Analysis of secondary outcomes
Results will be presented as per Table 4 in the Appendix.
Continuous secondary outcomes
Continuous secondary outcomes include the following scores:
Disability measured by the Pain Interference Score
Disability measured by the Roland-Morris Disability score
Disability measured by the Neck Disability Questionnaire score
SF-12 scores
Participants’ rating of global improvement
All will be described using a longitudinal mean plot and analysed using repeated-measure linear mixed models mirroring the approach used for the analysis of the primary outcome to estimate the effect of the treatment at weeks 6 and 12.
The same imputed datasets as the one created in the imputation of the primary outcome will be used to repeat the analysis of these five continuous secondary outcomes. Multiple imputations will only be performed if the primary outcome at week 6 is missing for more than 10% of subjects.
Time to recovery
Daily pain data collected from the patient diary will be described using a heatmap. The heatmap will consist of one colour-coded (by pain intensity) square per day (x-axis) per patient (y-axis) and two side-by-side panels (opioid vs placebo) up to week 6.
Time to recovery is defined as an average daily pain of 0 or 1 (out of 10) for seven consecutive days). The day of ‘event’ will be the first day of the recovery, i.e. day 1 of the seven consecutive days. In the absence of event, data will be censored at 12 weeks (day 84) or at the point when no more daily data is collected, whichever is sooner.
Time to recovery will be described using a Kaplan-Meier plot and summarised as the median number of days to recovery together with quartiles (if available). Differences in survival curves will be assessed using the log-rank test (see Table 4 in the Appendix).
Additionally, we will compare the proportion of participants who have recovered between groups (up to week 12) as a dichotomous measure to support the recovery outcome.
Healthcare utilisation
We will describe the type and frequency of services and medications used and assess between-group differences using Fisher’s exact test. We will also report what proportion of participants in each group (opioid and placebo) are still experiencing low back pain or neck pain at 26 and 52 weeks and the use of healthcare services, including opioid analgesics, in these participants. Differences in proportions will be tested using Fisher’s exact test (see Table 5 in the Appendix).
Risk of misuse
We will report the proportion in each group deemed to be at risk of misuse as per the COMM risk of misuse screening tool (scoring ≥9 indicates the risk of misuse) at weeks 12, 26 and 52. Differences in proportions will be tested using Fisher’s exact test (see Table 4 in the Appendix).
Cost-effectiveness
Two analyses will be conducted if between-group differences are found in the primary analysis: (1) a cost-effectiveness analysis using pain severity as a measure of effectiveness and (2) a cost-utility analysis where health state utilities (quality-adjusted life years or QALY) will be based on measures obtained from the SF-12 and transformed into utilities via the SF-6D algorithm. These analyses will not be included in the main paper and will be refined further following the main results.
The primary analysis will be conducted from the health sector’s perspective to assess the incremental cost per 1-point pain reduction or per QALY gained between the two treatment groups over 12 weeks.
A secondary analysis will entail a societal perspective in which costs associated with the use of community services (e.g. exercise classes) and work absenteeism related to low back pain or neck pain will be included. Costs of community services will be based on the self-reported costs. Costs of absenteeism from paid employment will be estimated by the number of days absent from work multiplied by the average wage rate. Sensitivity analyses will test uncertainty in key parameters such as the selection of cost weights and statistical variation in quality-of-life scores.
To obtain costs, public health services will be valued at published standard rates (e.g. the Medical Benefits Scheme standard fees, the Pharmaceutical Benefits Scheme costs). Private non-medical health services (e.g. physiotherapy) will be valued at published standard rates, if available, or as reported by participants.
Analysis of safety outcomes
Between-group analysis of safety outcomes will be presented as per Table 5 in the Appendix.
Adverse events
All adverse events are reported per ICD-10 code to level 3. Serious adverse events (see definition below) are also categorised for relatedness (yes/no) and expectedness (yes/no). The proportion of participants with adverse events overall and within each category (serious and nonserious) will be compared between the two groups using Fisher’s exact test. Self-reported data will be used as the primary source of data and supported by data reported by the doctor. Where participants and doctors have reported the same AE on the same dates, we will exclude the doctor data to avoid duplication. Please see Table 5 in the Appendix for aggregate data and Table 6 in the Appendix for detailed adverse events and serious adverse events.
Serious adverse events are any untoward medical occurrence that [8]
Results in death
Is life-threatening
Requires hospitalisation or prolongation of existing hospitalisation
Results in persistent or significant disability or incapacity
Is a congenital anomaly or birth defect or
Is a medically significant or important event or reaction
Conclusion
The OPAL trial will be the world’s first placebo-controlled trial of opioids for acute non-specific spinal pain and aims to provide high-quality evidence on the efficacy of a short course of opioid analgesics for the treatment of acute, non-specific low back and/or neck pain. The results of the OPAL trial will provide much needed evidence to clinicians and is likely to influence international clinical guidelines. The findings of the OPAL trial will impact the healthcare system and society by helping to ensure that opioids are only being used where the benefits outweigh the harms. The planned statistical analysis is detailed here to provide transparency.
Acknowledgements
We deeply appreciate the support from 157 doctors who screened and recruited participants to the trial and the 94 pharmacies who held and dispensed study medication between 2016 and 2021.
Abbreviations
- OPAL
OPioids for acute SpinAL pain
- GP
General practitioner
- CONSORT
Consolidated Standards of Reporting Trials
- SF-12
12-item Short Form survey (quality of life measurement tool)
- ITT
Intention-to-treat
- SD
Standard deviation
- ID
Identification
- CI
Confidence interval
- QALY
Quality-adjusted life years
- SF-6D
Short Form Six Dimension
- ICD-10
International Classification of Disease version 10
- COMM
Current Opioid Misuse Measure
- BPI
Brief Pain Inventory
- RMDQ
Roland-Morris Disability Questionnaire
- NDI
Neck Disability Index
- SF12v2
Short form 12, version 2
Appendix: Planned tables and figures
Fig. 1.
Consort 2010 flow diagram
Table 1.
Baseline characteristics
| Opioid (n = xxx) | Placebo (n = xxx) | |
|---|---|---|
| Female | n/N (%) | n/N (%) |
| Age (years) | xx.x (SD), n | xx.x (SD), n |
| BMI (kg/m2) | xx.x (SD), n | xx.x (SD), n |
| Location of pain | ||
| Back | n/N (%) | n/N (%) |
| Neck | n/N (%) | n/N (%) |
| Both | n/N (%) | n/N (%) |
| Days since onset of pain | Med (IQR) | Med (IQR) |
| Number of previous episodes of pain | Med (IQR) | Med (IQR) |
| Currently employed | n/N (%) | n/N (%) |
| Employment classification | n/N (%) | n/N (%) |
| Manager | n/N (%) | n/N (%) |
| Technician and trade worker | n/N (%) | n/N (%) |
| Clerical and administrative | n/N (%) | n/N (%) |
| Machinery operator or driver | n/N (%) | n/N (%) |
| Professional | n/N (%) | n/N (%) |
| Community or personal services worker | n/N (%) | n/N (%) |
| Sales worker | n/N (%) | n/N (%) |
| Labourer | n/N (%) | n/N (%) |
| Compensable back/neck pain | n/N (%) | n/N (%) |
| Household income/week (years) (AUD) | ||
| No income | n/N (%) | n/N (%) |
| $1–$799 ($1–$41,599) | n/N (%) | n/N (%) |
| $800–$1999 ($41,600–$103,999) | n/N (%) | n/N (%) |
| $2000–$3999 ($104,000–$207,999) | n/N (%) | n/N (%) |
| $4000 or more ($208,000 or more) | n/N (%) | n/N (%) |
| Chose not to answer | n/N (%) | n/N (%) |
| Health insurance status | ||
| None | n/N (%) | n/N (%) |
| Private hospital only | n/N (%) | n/N (%) |
| Private extras only | n/N (%) | n/N (%) |
| Private hospital and extras | n/N (%) | n/N (%) |
| DVA | n/N (%) | n/N (%) |
| Chose not to answer | n/N (%) | n/N (%) |
| Pain severity (BPI pain severity subscale average) (x/10) | Mean (SD) | Mean (SD) |
| Disability (BPI pain interference subscale average) (x/10) | Mean (SD) | Mean (SD) |
| Condition-specific disability | ||
| Back – RMDQ (0–24) | N = mean (SD) | N = mean (SD) |
| Neck – NDI (0–50) | N = mean (SD) | N = mean (SD) |
| Quality of life – Physical SF12v2 | Mean (SD) | Mean (SD) |
| Quality of life – Mental SF12v2 | Mean (SD) | Mean (SD) |
| Global perceived effect scale (−5 to 5) | Mean (SD) | Mean (SD) |
| Healthcare utilisation prior to enrolment | ||
| Took prescription opioid medication for back/neck pain | n/N (%) | n/N (%) |
| Saw a physiotherapist | n/N (%) | n/N (%) |
| Had imaging | n/N (%) | n/N (%) |
| Other healthcare | n/N (%) | n/N (%) |
| On medication for other pain or mood, sleeping, seizures or mental health problems during this episode of pain | n/N (%) | n/N (%) |
BPI Brief Pain Inventory, RMDQ Roland-Morris Disability Questionnaire, NDI Neck Disability Index, SF12v2 Short form 12, version 2
Table 2.
Compliance
| Opioid (n = xxx) | Placebo (n = xxx) | |
|---|---|---|
| GP prescription daily dose of study medication — either opioid or placebo (mg/day) | Mean (95% CI), n | Mean (95% CI), n |
| Week 1 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 2 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 3 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 4 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 5 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 6 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Cumulative dose (over entire treatment period) | xxx mgs | xxx mgs |
| Self-reported daily dose (mg/day) | Mean (95% CI), n | Mean (95% CI), n |
| Week 1 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 2 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 3 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 4 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 5 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Week 6 | xx.x (xx.x to xx.x), n | xx.x (xx.x to xx.x), n |
| Cumulative dose | xxx mgs | xxx mgs |
| Participants returning study medicines | n/N (%) | n/N (%) |
| Participants consuming ≥80% of prescribed dose | ||
| Per participant medicine dairy | n/N (%) | n/N (%) |
| Per returned medicines | n/N (%) | n/N (%) |
| Participants discontinuing study medication | ||
| n/N (%) | n/N (%) | |
| Reasons for discontinuation | ||
| Pain resolved | n/N (%) | n/N (%) |
| Felt medicine was ineffective | n/N (%) | n/N (%) |
| Others | n/N (%) | n/N (%) |
| Assessment of participant blinding (participant estimation) | ||
| Opioid | n/N (%) | n/N (%) |
| Placebo | n/N (%) | n/N (%) |
| Do not know | n/N (%) | n/N (%) |
mg/day milligrammes per day
Table 3.
Protocol deviations
| Classification | Opioid | Placebo |
|---|---|---|
| Became apparent post-randomisation that participant did not fulfil eligibility criteria | n/N (%) | n/N (%) |
| Took opioid medicines for this episode | n/N (%) | n/N (%) |
| Pain duration > 12 weeks | n/N (%) | n/N (%) |
| Had bony metastasis | n/N (%) | n/N (%) |
| Did not receive treatment as allocated | n/N (%) | n/N (%) |
| Took concomitant opioid medication during the treatment period | n/N (%) | n/N (%) |
| Never collected medication kit | n/N (%) | n/N (%) |
| Was dispensed medicine from an incorrect kit | n/N (%) | n/N (%) |
| Discrepency in data collection procedure | n/N (%) | n/N (%) |
| Verbal consent received prior to enrolment, written consent followed | n/N (%) | n/N (%) |
| Unable to obtain baseline data | n/N (%) | n/N (%) |
| Obtained Brief Pain Inventory from a proxy | n/N (%) | n/N (%) |
| Baseline questionnaire completed > 72 h after first presentation to study doctor | n/N (%) | n/N (%) |
Table 4.
Primary and secondary outcomes
| Opioid (n = xxx) | Placebo (n = xxx) | Mean difference (95% CI), p value | |
|---|---|---|---|
| Pain Intensity (BPI-PS) | |||
| Week 2 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 4 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 6 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Week 12 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Week 26 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 52 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Physical functioning - generic (BPI-IS) | |||
| Week 2 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 4 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 6 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Week 12 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Physical functioning - back (RMDQ) | |||
| Week 6 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Physical functioning – neck (NDI) | |||
| Week 6 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Quality of life – physical score (SF-12v2) | |||
| Week 2 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 4 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 6 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Week 12 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Quality of life – mental score (SF-12v2) | |||
| Week 2 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 4 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 6 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Week 12 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Global perceived effect scale | |||
| Week 2 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 4 | xx.x (xx.x), n | xx.x (xx.x), n | |
| Week 6 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Week 12 | xx.x (xx.x), n | xx.x (xx.x), n | xx.x (xx.x to xx.x), p = 0.xxx |
| Time to recovery (days) | Median (IQR) | Median (IQR) | Log-rank p = 0.xxx |
| Recovered (yes) | |||
| Week 2 | n (%) | n (%) | |
| Week 4 | n (%) | n (%) | |
| Week 6 | n (%) | n (%) | p = 0.xxx |
| Week 12 | n (%) | n (%) | p = 0.xxx |
BPI-PS Brief Pain Inventory Pain Severity, BPI-IS Brief Pain Inventory Interference Subscale, RMDQ Roland-Morris Disability Questionnaire, NDI Neck Disability Index, SF-12 Short Form 12 Item Survey
Note: We also intend to include similar looking tables for adjusted analyses and for analyses of imputed data as supplements
Table 5.
Safety and other outcomes
| Opioid (n = xxx) | Placebo (n = xxx) | Fisher exact p value | |
|---|---|---|---|
| Safety | |||
| Serious adverse events (SAEs) | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Related SAEs | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Adverse events (AEs) | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Healthcare utilisation | |||
| Physiotherapy | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Imaging | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| General practitioner | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Specialist doctor | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| ED/hospitalisation | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Others | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Use of concomitant medications | |||
| Simple analgesia | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| NSAID | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Combination opioid | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Strong opioid | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Others | nEVT nPAT (%) | nEVT nPAT (%) | 0.xxx |
| Use of health services and concomitant medicines in patients reporting ongoing pain | |||
| Week 26 | n/N (%) | n/N (%) | 0.xxx |
| Week 52 | n/N (%) | n/N (%) | 0.xxx |
| At risk of misuse (scoring ≥9 on COMM) | |||
| Week 12 | n/N (%) | n/N (%) | 0.xxx |
| Week 26 | n/N (%) | n/N (%) | 0.xxx |
| Week 52 | n/N (%) | n/N (%) | 0.xxx |
COMM Current Opioid Misuse Measure
Table 6.
Detailed adverse events
| Opioid (n = x) | Placebo (n = x) | |
|---|---|---|
| ICD-10 code A | nEVT nPAT (%) | nEVT nPAT (%) |
| ICD-10 code B | nEVT nPAT (%) | nEVT nPAT (%) |
| (etc.) | ||
| Adverse event | ||
| ICD-10 code A | nEVT nPAT (%) | nEVT nPAT (%) |
| ICD-10 code B | nEVT nPAT (%) | nEVT nPAT (%) |
| (etc.) |
Authors’ contributions
CWCL, CM, JL, BK, LB, RD and AM conceived the trial, procured funding and contributed to the design of the trial. CJ joined as a PhD student and contributed to the running of the trial. CJ, CWCL and LB drafted the manuscript. LB provided statistical expertise and oversight. CWCL provided supervision to CJ. All authors contributed to the manuscript and approved the final version for publication.
Funding
This work is supported by the National Health and Medical Research Council (NHMRC) of Australia, grant number APP1082480. Supplementary funding was provided by ReturnToWorkSA and the Sydney Medical School Foundation. C-WCL is funded by a Career Development Fellowship and an Emerging Leadership Fellowship from the NHMRC (APP1193939). CGM is funded by a Leadership Fellowship from the NHMRC (APP1194283). The funding source has no role in the trial design and will have no role in the trial conduct, data analysis and interpretation or writing or reporting.
Availability of data and materials
All investigators have access to the study data. Raw data (de-identified) can be provided upon request.
Declarations
Ethics approval and consent to participate
Ethics approval has been granted from the Human Research Ethics Committee, The University of Sydney (Project No. 2015-004).
Consent for publication
No identifiable participant data is contained in this publication. We are willing to provide a model consent form for participation in the OPAL trial upon request.
Competing interests
AJM has received supplementary funding and/or trial medicines from GlaxoSmithKline and Pfizer (Australia) for investigator-initiated NHMRC-funded trials evaluating drug treatment of low back pain. He is also the Program Director of the NHMRC Centre for Research Excellence in Medicines and Ageing. JL has received supplementary funding and/or trial medicines from GlaxoSmithKline and Pfizer (Australia) for investigator-initiated NHMRC-funded trials evaluating drug treatment of back pain. She has received funding from Baxter Biosciences for research evaluating musculoskeletal outcomes in children with haemophilia. ROD has received supplementary funding and/or trial medicines from GlaxoSmithKline and Pfizer (Australia) for investigator-initiated NHMRC-funded trials evaluating drug treatment of back pain. ROD has been a member of an advisory board about paracetamol for GlaxoSmithKline and ibuprofen for Reckitt Benckiser (Australia). Payments went to an audited hospital account for teaching and research purposes. CGM has received supplementary funding and/or trial medicines from GlaxoSmithKline and Pfizer (Australia) for investigator-initiated NHMRC-funded trials evaluating drug treatment of low back pain.
Footnotes
Please see related article http://doi.org/10.1136/bmjopen-2016-011278
The original online version of this article was revised:"Following publication of the original article, we have been informed that the name of a supplementary funder was incorrectly reported. “WorkSafe SA” should actually be “ReturnToWorkSA”.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/27/2023
A Correction to this paper has been published: 10.1186/s13063-023-07525-4
Contributor Information
Caitlin MP Jones, Email: caitlin.jones@sydney.edu.au.
Chung-Wei Christine Lin, Email: Christine.lin@sydney.edu.au.
Richard O. Day, Email: r.day@unsw.edu.au
Bart W. Koes, Email: b.koes@erasmusmc.nl
Jane Latimer, Email: jane.latimer@sydney.edu.au.
Chris G. Maher, Email: chris.maher@sydney.edu.au
Andrew McLachlan, Email: Andrew.mclachlan@sydney.edu.au.
Laurent Billot, Email: lbillot@georgeinstitute.org.
References
- 1.Vos T, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CWC, Chenot JF, van Tulder M, Koes BW. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–2803. doi: 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- 3.Mathieson S, Wertheimer G, Maher CG, Lin C-WC, McLachlan AJ, Buchbinder R, Pearson S-A, Underwood M. What proportion of patients with chronic noncancer pain are prescribed an opioid medicine? Systematic review and meta-regression of observational studies (review) J Intern Med. 2020;287(5):458–474. doi: 10.1111/joim.13026. [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350(jan05 10):g6380. doi: 10.1136/bmj.g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BD, Bruneau J, Altice FL, Henderson G, Rahimi-Movaghar A, Larney S. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560–1579. doi: 10.1016/S0140-6736(19)32229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CC, McLachlan AJ, Latimer J, et al. OPAL: a randomised, placebo-controlled trial of opioid analgesia for the reduction of pain severity in people with acute spinal pain. Trial protocol. BMJ Open. 2016;6(8):e011278. doi: 10.1136/bmjopen-2016-011278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 8.National Health and Medical Research Council, Australian Research Council, Universities Australia . Safety monitoring and reporting in clinical trials involving therapeutic goods. Canberra: Australian Government; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All investigators have access to the study data. Raw data (de-identified) can be provided upon request.