Abstract
Objectives:
Whole-body vibration (WBV) is applied to the sole of the foot, whereas local mechanical vibration (LMV) is applied directly to the muscle or tendon. The time required for the mechanical stimulus to reach the muscle belly is longer for WBV. Therefore, the WBV-induced muscular reflex (WBV-IMR) latency may be longer than the tonic vibration reflex (TVR) latency. The aim of this study was to determine whether the difference between WBV-IMR and TVR latencies is due to the distance between the vibration application point and the target muscle.
Methods:
Eight volunteers participated in this study. The soleus reflex response was recorded during WBV, LMVs, and tendon tap. LMVs were applied to the Achilles tendon and sole of the foot. The latencies were calculated using the cumulative averaging technique.
Results:
The latency (33.4±2.8 ms) of the soleus reflex induced by the local foot vibration was similar to the soleus TVR latency (30.9±3.2 ms) and T-reflex (32.0±2.4 ms) but significantly shorter than the latency of the soleus WBV-IMR (42.3±3.4 ms) (F(3,21)=27.46, p=0.0001, partial η2=0.797).
Conclusions:
The present study points out that the neuronal circuitries of TVR and WBV-IMR are different.
Keywords: Exercise, Muscle Spindle, Muscle Vibration, Stretch Reflex, Tendon Vibration
Introduction
Whole-body vibration (WBV), as a rehabilitation and exercise training method, is becoming increasingly popular in physical therapy, rehabilitation, and professional sports due to its claimed beneficial effects on the neuromuscular system[1,2]. These benefits include improved muscle strength and power[3-9]. Although little is known about the physiological mechanisms underlying the effects of WBV on muscular performance, the presence of reflex muscle activity during WBV has been shown[3,11-14]. This reflex, whose receptor origin has not been clearly defined, is called the WBV-induced muscle reflex[12-15]. Although there is no conclusive evidence that tonic vibration reflex (TVR) genuinely occurs during WBV, it is the most cited mechanism to explain the effects of WBV on muscular performance[3,10,11,16,17]. A previous study on the comparison of reflex latencies observed an eight milliseconds difference between the reflex times of WBV and local mechanical vibration (LMV) on the soleus muscle[15]. This result disputes the view that the beneficial neuromuscular effects of WBV can be explained by TVR. This discrepancy may be explained by the different vibration application techniques[18].
There are two main differences between WBV and LMV in terms of application techniques[18]. First, the vibration frequencies are different. LMV frequency is above 100 Hz, whereas WBV frequency is between 5-50 Hz[18,19]. However, the dependence of sound speed on frequency is usually insignificant in frequencies lower than ten thousand hertz[20]. Therefore, the effect of frequency on latency can be negligible. The second difference is that LMV is applied directly to the muscle or tendon. However, for WBV training, vibration is applied to the sole of the foot and transmitted to the targeted muscles through the feet[18]. The distance between the vibrated body site and the target muscle belly is longer for WBV than LMV. Since the longer the distance the vibration wave travels, the longer the time it takes to propagate, the time taken for the vibration stimulus to reach the muscle’s belly will be longer for the WBV than for the tendon vibration. In the current study to overcome this problem, vibration stimuli with the same frequency and amplitude were applied to the foot sole and the Achilles tendon.
Taking this basic knowledge of physics into account, we have developed the following hypotheses that can harmoniously explain the views put forward in previous research on WBV: Firstly, the latency of the soleus reflex induced by the LMV (soleus TVR) is shorter than the latency of the soleus reflex induced by the sole vibration. Secondly, the latency of soleus reflex induced by the sole vibration and the latency of soleus reflex induced by the WBV are similar. The aim of this study was to determine whether the difference between WBV-IMR and TVR latencies is due to the distance between the vibration application point and the target muscle.
Materials and Methods
This cross-sectional study was performed at a tertiary referral unit specialized in Physical Medicine and Rehabilitation. The right soleus reflex response was recorded during WBV, LMVs, and Achilles tendon tap. The LMVs were applied to the right Achilles tendon and sole of the right foot. WBV-induced muscular reflex (WBV-IMR) latency, TVR latency, foot vibrations latency, T-reflex latency were then calculated.
Participants
Eight healthy (five females, three males) individuals aged 20 to 40 years volunteered to participate in this study. All participants gave written informed consent to the experimental procedures following the Declaration of Helsinki and were approved by the local ethics committee (Approval Number, 2020-398) and registered with the Protocol Registration ClinicalTrials.gov (NCT04516798). This study was completed with eight healthy volunteers (five women, three men). Their mean ± standard deviation of the age was 27.7±2.7 years; body height 170.7±8.4 cm.
Procedures
Whole-Body Vibration
WBV was performed using a Power Plate Pro5 device (POWER PLATE® International, Amsterdam, The Netherlands). During WBV, subjects were asked to stand upright on both feet on a vibration platform with their knees locked. Subjects were barefooted, and no sponge or foam was placed between the vibration platform and their feet. Participants were asked to hold the handle of the WBV device to maintain a static balance. The whole plate oscillated with a linear movement upward and downward with an amplitude of two millimeters. Different vibration frequencies (30, 35, and 40 Hz), each lasting for 30 s with 5-s rest intervals, were delivered in random order to negate any order/time effect.
Local (Isolated) Vibration
The subjects were asked to lie on the examination table in a prone position. The right ankle joint was fixed in a neutral position (Figure 1a). The LMV was applied using a custom-made vibrator consisting of a DC motor (Bosch™, with frequency between 5000-20000), vibration head (oscillating with a peak-to-peak amplitude of 2 mm), and control unit. Before the experiments, the frequency of the vibrator was calibrated and monitored online during the experiment. The vibration was applied to the tendon or sole with a constant force by one of the researchers[15].
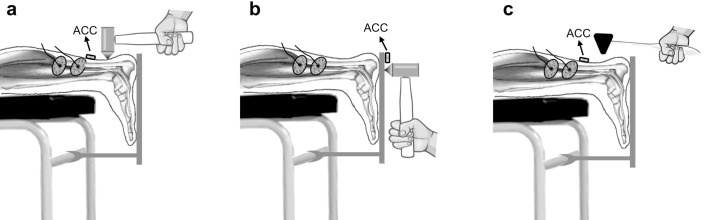
Figure 1.
Illustration of the experimental setup. Surface EMG recordings were taken from soleus muscle a. To elicit soleus TVR, vibrations were applied to the Achilles tendon (Achilles tendon vibration) using a local vibrator. b. To elicit soleus LFV-IMR, foot vibrations were applied to the platform underlying the foot (Foot vibration) using a local vibrator. c. To elicit soleus T-reflex, taps were applied to the Achilles tendon (Achilles tendon tap) using a reflex hammer. Acc: Accelerometer.
To elicit soleus TVR, three different vibration frequencies (100, 135, and 150 Hz) with an amplitude of two millimeters, each lasting for 30 s with 5-s rest intervals, were delivered to the right Achilles tendon in random order to negate any order/time effect. To elicit the local foot vibration-induced muscular reflex (LFV-IMR), the LMV was then applied to the right foot’s sole in the same position (Figure 1b). Three different vibration frequencies (100, 135, and 150 Hz), each lasting for 30 s with 5-s rest intervals, were delivered to the right foot of the subject in random order to negate any order/time effect.
Soleus T-Reflex
Twenty consecutive taps with 3-5 second intervals were applied to the right Achilles tendon using a reflex hammer while subjects were lying in the same prone position with the right ankle fixed at a neutral angle (Figure 1c).
Data Acquisition
The surface EMG (SEMG) from the right soleus and acceleration data were recorded simultaneously using the data acquisition and analysis system (POWERLAB® ADInstruments Co, Oxford, UK). SEMG was recorded using a bipolar recording technique. Disposable self-adhesive Ag/AgCl (Kendall® Arbo) SEMG electrodes were placed on the right soleus belly four centimeters apart[21]. The skin overlying the muscle was shaved; light abrasion was applied; the skin was cleaned with alcohol to reduce skin resistance[22]. The reference electrode was placed on the medial malleolus. To prevent the sway of electrode cables, they were fixed to the body. Three accelerometers (LIS344ALH; Ecopack, Mansfield, TX, USA) were used to determine WBV-IMR latency, LFV-IMR latency, T-reflex latency, and TVR latency (Figure 1). The sampling rate was 40 kHz for SEMG and accelerometer signals.
Reflex Latency Measurement
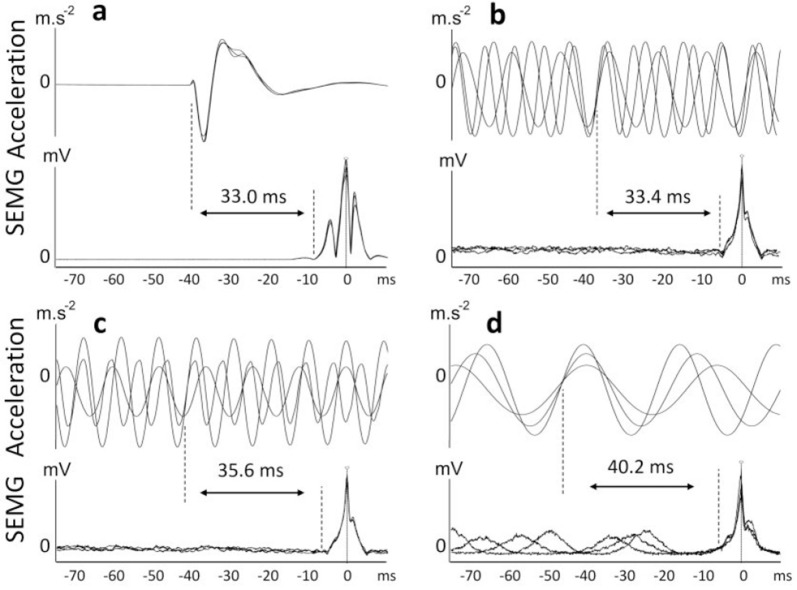
Reflex latencies were determined using the cumulative average method[12]. Firstly, to minimize the movement artifacts from the EMG signals, they were filtered using a high-pass digital filter set at 60 Hz for determining WBV-IMR: 160 Hz high-pass digital filter for determining TVR latency. Filtered signals were then full-wave rectified to bring out genuine peaks to be used as triggers in a spike-triggered averaging algorithm. Accelerometer signals were also filtered with a high-pass digital filter set at 5 Hz. The peaks of the artifact-free rectified EMG signals were used as the triggers and acceleration record as the source in a spike-triggered averaging algorithm to determine the latency of reflexes. This procedure was performed for all three vibration frequencies that were tested. The averaged data belonging to different vibration frequencies were then superimposed to generate a cumulative average. To precisely determine the intersection point of the accelerometer trace of three different vibration frequencies, the standard error (SE) was calculated for each data point throughout the trace of the cumulated average of the accelerometer. The point in time in an accelerometer cycle where the SE was the lowest was considered the effective stimulus time point (Figure 2). This point effectively represents the threshold for activation of the receptor responsible for the reflex response[12]. Using the same technique, the onset of the EMG (onset of reflex response) was determined. The peaks of the artifact-free rectified EMG signals were used as the triggers and EMG record as the source in a spike-triggered averaging algorithm to determine the latency of reflexes. This averaging process was then separately conducted for the three vibration frequencies. Averaged EMG data belonging to three different vibration frequencies were then combined to generate the cumulated average of the EMG for each participant. The point in time in an EMG trace where the SE was the lowest was considered the onset of reflex response. Figure 2 clearly illustrates that the spike-triggered averaging approach combined with the cumulated average technique works very well to indicate the EMG reflex response’s effective start point.
Figure 2.
Reflex latency measurement using the cumulative average method for soleus muscle: (a) T-reflex latency, (b) tonic vibration reflex latency, (c) local foot vibration-induced muscular reflex latency, and (d) whole-body vibration-induced muscular reflex latency The open circle represents the positive peak of the rectified EMG that was used as the trigger to average the accelerometer data and the rectified EMG data.
Reflex latency negatively correlates with body height[23]. Therefore, all latencies were adjusted to the body height of each participant. Latency was expressed as milliseconds (ms). SEMG and accelerometer data were processed and analyzed offline using LABCHART 7® Software Version 7.3.3 (PowerLab®system ADInstruments, Oxford, UK).
Statistical Analysis
The minimum number of participants required was determined by an a priori power analysis (G*Power version 3.1.9.4, Franz Faul). Based on a previously published study in which the effect size was 1.932 for the difference between WBV-IMR and TVR latency[15]. With an alpha=0.05 and power=0.99, the projected total sample size needed with this effect size was estimated to be eight. All data were checked for normal distribution using the Shapiro-Wilk test. The arithmetic mean and standard deviation (SD) were calculated for each variable. The comparison of mean reflex latencies was performed by an ANOVA appropriate for multiple dependent variables with repeated measures paired t-test. The Bonferroni test was applied for pair-wise comparisons. Differences in the mean values and effect sizes with a 95% confidence interval were calculated, and a p-value<0.05 was considered to indicate statistical significance. The software package used for data management was SPSS ver18 software.
Results
The reflex responses were obtained in all cases. Adjusted reflex latency for WBV-IMR, soleus TVR, local foot vibration and T-reflex were 42.3±3.4 ms, 30.9±3.2 ms, 33.4±2.8 ms and, 32.0±2.4 ms, respectively (F(3,21)=27.46, p=0.0001, partial ƞ2=0.797). The PostHoc analysis showed that the WBV-IMR latency was significantly longer than the latency of soleus TVR, the latency of LFV-IMR, and the latency of T-reflex (p=0.004, p=0.004, p=0.003, respectively, with Bonferroni correction). There was no significant difference between the latency of soleus TVR, the latency of local foot vibration-induced reflex, and T-reflex latency (Figure 3).
Figure 3.
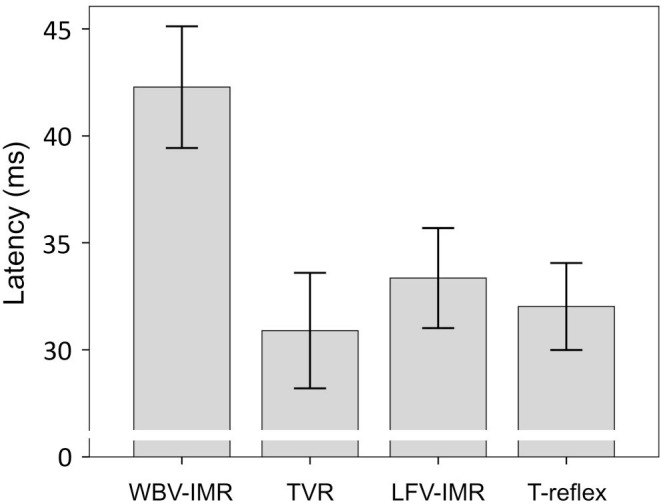
Mean of the latency of whole-body vibration-induced muscular reflex (WBV-IMR), tonic vibration reflex (TVR), local foot vibration-induced muscular reflex (LFV-IMR), and T-reflex (with 95% confidence interval).
Discussion
This study’s main finding is that the latency of the soleus reflex induced by the local foot vibration is similar to the soleus TVR latency but shorter than the soleus WBV-IMR. This finding does not support the view that TVR can explain the beneficial neuromuscular effects of WBV-IMR.
It is well known that mechanical vibration applied to the muscle belly or tendon can elicit a reflex muscle contraction. It is defined as TVR, a spinal polysynaptic spindle-based reflex[24-26]. Indeed, the primary endings of the muscle spindle can respond in 1:1 synchrony up to about 100-150 Hz vibrations[27]. In this study, 100, 135, and 150 Hz frequencies were used for LMV. As in a previous study[15], the current report found that the soleus TVR latency was similar to soleus T-reflex latency but shorter than soleus WBV-IMR latency. This study’s original finding is that it reveals that the foot sole’s further distance is not the reason for the long latency of WBV-IMR compared to local foot vibration and Achilles tendon LMV. It suggests that their neuronal circuitries are different.
The mechanical oscillations are defined as vibrations, which are closely linked to the concept of waves. Vibration propagates as an acoustic wave in a medium (air, water, metals, or biological tissue, etc.). The acoustic wave is a longitudinal wave. The acoustic wave leads to the compressions and rarefactions of the particles while it propagates through the medium. In other words, the particles of the medium periodically move back and forth in a line parallel to the line in which the wave moves[28]. It is well known that acoustic waves travel at speeds greater than 1000 m/s through soft tissue[29]. Considering that the soleus muscle length is less than 50 cm, vibration stimulus applied to the Achilles tendon, or sole takes less than 0.5 ms to reach the soleus muscle. The rapid propagation of acoustic waves in biological tissues may explain the similarity of soleus reflex latencies induced by vibration applied to the Achilles tendon or the foot’s sole. However, it does not explain the time difference between TVR and WBV-IMR.
Strengths and limitations
WBV is performed in a standing position[18,19]. However, in this study, high-frequency (100-150 Hz) vibration was applied to the sole of the foot in the prone position, not while standing. This may be a limitation of the present study. But in our opinion, when high-frequency WBV is applied to the sole of the foot while standing, the latency of the soleus reflex will not change. Because the soleus is a postural muscle and its activity increases when standing upright. Ogiso et al. showed that soleus stretch reflex latency did not change at various isometric voluntary contraction levels[30]. However, future studies can be considered to clarify this issue.
In this study, the mean difference between soleus TVR and soleus WBV-IMR latencies was 11.4 ms. Although this study’s sample size was small, the effect size (partial eta squared) was considerable, and the study power (99%) was high.
In the present study, accelerometer and EMG recordings were recorded with a sample rate of 40 kHz. This high sampling rate allowed latency measurements to be made with a sensitivity of 0.025 ms.
Conclusion
This study showed that TVR was activated when a high-frequency LMV was applied to the sole or Achilles tendon and that the significant difference between soleus TVR and soleus WBV-IMR latencies was not due to the application of WBV to the sole rather than the Achilles tendon. This study’s findings support the view that the neuronal circuitry of TVR and WBV-IMR is different[15].
There is currently no standardized vibration training guideline for athlete prescription. A complete understanding of the basic mechanisms underlying the effects of vibration and their dependence on the intervention characteristics and the exercise protocol may help develop more effective vibration exercise protocols. Further studies are needed to identify the precise afferent type and/or receptor origin of the WBV-IMR pathway.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.van Heuvelen MJG, Rittweger J, Judex S, Sañudo B, Seixas A, Fuermaier ABM, et al., editors. Reporting Guidelines for Whole-Body Vibration Studies in Humans, Animals and Cell Cultures: A Consensus Statement from an International Group of Experts. Biology (Basel) 2021;10(10):965. doi: 10.3390/biology10100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteiro-Oliveira BB, Coelho-Oliveira AC, Paineiras-Domingos LL, Sonza A, Sá-Caputo DDC, Bernardo-Filho M. Use of surface electromyography to evaluate effects of whole-body vibration exercises on neuromuscular activation and muscle strength in the elderly: a systematic review. Disabil Rehabil. 2021;26:1–10. doi: 10.1080/09638288.2021.1994030. [DOI] [PubMed] [Google Scholar]
- 3.Mester J, Kleinöder H, Yue Z. Vibration training: benefits and risks. J Biomech. 2006;39(6):1056–65. doi: 10.1016/j.jbiomech.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Cidem M, Karacan I, Diraçoğlu D, Yıldız A, Küçük SH, Uludağ M, et al. A Randomized Trial on the Effect of Bone Tissue on Vibration-induced Muscle Strength Gain and Vibration-induced Reflex Muscle Activity. Balkan Med J. 2014;31(1):11–22. doi: 10.5152/balkanmedj.2013.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SY, Son WM, Kwon OS. Effects of whole body vibration training on body composition, skeletal muscle strength, and cardiovascular health. J Exerc Rehabil. 2015;11(6):289–95. doi: 10.12965/jer.150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bemben D, Stark C, Taiar R, Bernardo-Filho M. Relevance of Whole-Body Vibration Exercises on Muscle Strength/Power and Bone of Elderly Individuals. Dose Response. 2018;16(4) doi: 10.1177/1559325818813066. 1559325818813066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centner C, Ritzmann R, Schur S, Gollhofer A, König D. Blood flow restriction increases myoelectric activity and metabolic accumulation during whole-body vibration. Eur J Appl Physiol. 2019;119(6):1439–1449. doi: 10.1007/s00421-019-04134-5. [DOI] [PubMed] [Google Scholar]
- 8.Aydın T, Kesiktaş FN, Baskent A, Karan A, Karacan I, Türker KS. Cross-training effect of chronic whole-body vibration exercise: a randomized controlled study. Somatosens Mot Res. 2020;37(2):51–58. doi: 10.1080/08990220.2020.1720635. [DOI] [PubMed] [Google Scholar]
- 9.Rasti E, Rojhani-Shirazi Z, Ebrahimi N, Sobhan MR. Effects of whole body vibration with exercise therapy versus exercise therapy alone on flexibility, vertical jump height, agility and pain in athletes with patellofemoral pain: a randomized clinical trial. BMC Musculoskelet Disord. 2020;21(1):705. doi: 10.1186/s12891-020-03732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kvorning T, Bagger M, Caserotti P, Madsen K. Effects of vibration and resistance training on neuromuscular and hormonal measures. Eur J Appl Physiol. 2006;96(5):615–25. doi: 10.1007/s00421-006-0139-3. [DOI] [PubMed] [Google Scholar]
- 11.Ritzmann R, Kramer A, Gruber M, Gollhofer A, Taube W. EMG activity during whole body vibration: motion artifacts or stretch reflexes? Eur J Appl Physiol. 2010;110(1):143–51. doi: 10.1007/s00421-010-1483-x. [DOI] [PubMed] [Google Scholar]
- 12.Karacan I, Cakar HI, Sebik O, Yilmaz G, Cidem M, Kara S, et al. A new method to determine reflex latency induced by high rate stimulation of the nervous system. Front Hum Neurosci. 2014;18:536. doi: 10.3389/fnhum.2014.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cakar HI, Cidem M, Sebik O, Yilmaz G, Karamehmetoglu SS, Kara S, et al. Whole-body vibration-induced muscular reflex: Is it a stretch-induced reflex? J Phys Ther Sci. 2015;27(7):2279–84. doi: 10.1589/jpts.27.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karacan I, Cidem M, Cidem M, Türker KS. Whole-body vibration induces distinct reflex patterns in human soleus muscle. J Electromyogr Kinesiol. 2017;34:93–101. doi: 10.1016/j.jelekin.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Yildirim MA, Topkara B, Aydin T, Paker N, Soy D, Coskun E, et al. Exploring the receptor origin of vibration-induced reflexes. Spinal Cord. 2020;58(6):716–723. doi: 10.1038/s41393-020-0419-5. [DOI] [PubMed] [Google Scholar]
- 16.Pollock RD, Woledge RC, Martin FC, Newham DJ. Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol (1985) 2012;112(3):388–95. doi: 10.1152/japplphysiol.01223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang F. Rittweger J, Switzerland AG, editors. Application of Vibration Training in People with Common Neurological Disorders In: Manual of Vibration Exercise and Vibration Therapy. Springer Nature. 2020:343–354. [Google Scholar]
- 18.Souron R, Besson T, Millet GY, Lapole T. Acute and chronic neuromuscular adaptations to local vibration training. Eur J Appl Physiol. 2017;117(10):1939–1964. doi: 10.1007/s00421-017-3688-8. [DOI] [PubMed] [Google Scholar]
- 19.Rawer R. Design Principles of Available Machines. In: Rittweger J, editor. In: Manual of Vibration Exercise and Vibration Therapy. Switzerland AG: Springer Nature; 2020. pp. 39–55. [Google Scholar]
- 20.Zuckerwar AJ. New York: Academic Press; 2002. Handbook of the Speed of Sound in Real Gases. [Google Scholar]
- 21.Tucker KJ, Türker KS. A new method to estimate signal cancellation in the human maximal M-wave. J Neurosci Methods. 2005;149(1):31–41. doi: 10.1016/j.jneumeth.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Sebik O, Karacan I, Cidem M, Türker KS. Rectification of SEMG as a tool to demonstrate synchronous motor unit activity during vibration. J Electromyogr Kinesiol. 2013;23(2):275–84. doi: 10.1016/j.jelekin.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Ginanneschi F, Curti S, Marinelli F, Nft AA, Cioncoloni D, Mattioli S, et al. Reference values for distal motor conduction of the tibial nerve: Effects of demographic and anthropometric measures. Muscle Nerve. 2020;62(2):219–225. doi: 10.1002/mus.26908. [DOI] [PubMed] [Google Scholar]
- 24.De Gail P, Lance JW, Neilson PD. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry. 1966;29(1):1–11. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews PB. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966;184(2):450–72. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin BJ, Park HS. Analysis of the tonic vibration reflex: influence of vibration variables on motor unit synchronization and fatigue. Eur J Appl Physiol Occup Physiol. 1997;75(6):504–11. doi: 10.1007/s004210050196. [DOI] [PubMed] [Google Scholar]
- 27.Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rittweger J, Taiar R. The physics of vibration. In: Rittweger J, editor. In: Manual of Vibration Exercise and Vibration Therapy. Switzerland AG: Springer Nature; 2020. pp. 3–21. [Google Scholar]
- 29.Sanabria SJ, Martini K, Freystätter G, Ruby L, Goksel O, Frauenfelder T. Speed of sound ultrasound: a pilot study on a novel technique to identify sarcopenia in seniors. Eur Radiol. 2019;29(1):3–12. doi: 10.1007/s00330-018-5742-2. [DOI] [PubMed] [Google Scholar]
- 30.Ogiso K, McBride JM, Finni T, Komi PV. Short-latency stretch reflex modulation in response to varying soleus muscle activities. J Electromyogr Kinesiol. 2002;12(1):17–26. doi: 10.1016/s1050-6411(01)00030-x. [DOI] [PubMed] [Google Scholar]