Abstract
Objectives:
To evaluate differences in physical impairment, muscle strength, muscle mass and muscle density between patients with hypermobile Ehlers Danlos Syndrome (hEDS), hypermobile spectrum disorder (HSD), and healthy controls.
Methods:
Female adults with hEDS (n=20) and HSD (n=23), diagnosed to the most recent criteria, and age-matched healthy controls (n=28) completed the Arthritis Impact Measurement Scale (physical functioning) and performed maximal muscle strength and strength endurance tests of lower and upper limbs (hand grip, posture maintenance, 30 seconds chair rise and isokinetic tests). Muscle mass and density were evaluated by dual-energy X-ray absorptiometry and peripheral quantitative computed tomography.
Results:
No differences in physical functioning and muscle strength were found between adults with hEDS and HSD. Furthermore, no differences in muscle mass and density were observed between the three groups. Nevertheless, when both patient groups were compared to controls, physical functioning, maximal muscle strength and muscle strength endurance were significantly lower (all p<0.001), except for the hand flexors.
Conclusion:
Physical functioning, muscle strength, density and mass did not significantly differ between individuals with hEDS and HSD. Compared to controls, physical functioning and muscle strength (maximal and endurance) were significantly lower. Consequently, (functional) strength training in individuals with hEDS and HSD is necessary.
Keywords: Ehlers-Danlos Syndrome, Hypermobility, Muscle Mass, Muscle Strength, Physical Functioning
Introduction
Hypermobile Ehlers-Danlos syndrome (hEDS) is a hereditary connective tissue disorder characterized by generalized joint hypermobility (GJH), generalized soft tissue fragility (e.g. organ prolapse, unusually soft or velvety skin, mitral valve prolapse etc.) and secondary musculoskeletal symptoms such as (sub)luxations and pain. Diagnosis is solely established on a clinical basis as the causative gene(s) is (are) unknown and unmapped[1]. Similar to hEDS, patients with hypermobility spectrum disorder (HSD) also show GJH which results in secondary musculoskeletal symptoms, though they have less signs of generalized tissue fragility[2].
Despite the clear distinction in generalized soft tissue fragility between hEDS and HSD, previous research indicated that there are no differences concerning symptom severity and physical activity between these patient populations[3,4]. Both diseases are marked by a broad spectrum of clinical symptoms, among which decreased muscle strength is of primary importance[5]. Insufficient muscle strength may further compromise joint stability in these patients and consequently contribute to overload injuries and recurrent joint dislocations, which further impacts their quality of life[6].
Rombaut et al.[7] already demonstrated decreased muscle strength and physical impairment in patients with hEDS. However, these patients were diagnosed according to older diagnostic criteria (Villefranche, 1997), which are less specific as they do not clearly define the required exclusion and inclusion criteria to establish the diagnosis of hEDS[8-10]. Consequently, it is unclear whether muscle strength and physical functioning is affected to the same extent in individuals with hEDS and HSD, diagnosed according to the most recent criteria[1]. This knowledge is of primary importance as this might assist health practitioners to tailor therapy to the needs of patients with hEDS and HSD, and in the decision whether or not they should treat patients with hEDS and HSD differently. Therefore, this study aims to evaluate muscle strength (maximal muscle strength and muscle strength endurance), muscle mass, muscle density, and physical functioning in patients with hEDS in comparison with HSD. Furthermore, both patient groups will be compared with a healthy control population and the association between physical functioning and muscle strength will be evaluated. We hypothesize that there are no significant differences regarding muscle strength and physical functioning between hEDS and HSD patients, though that these parameters are decreased in comparison with healthy controls and are associated with impaired physical functioning.
Materials and methods
Participants
This study protocol was reviewed and approved by the Ethical Committee of Ghent University Hospital (EC number 2018/1089), and written informed consent was obtained from all participants. As there is a female predominance in hEDS and females tend to be more hypermobile, only females were recruited[8,11]. Twenty adults with hEDS and 23 with HSD were recruited in the Center for Medical Genetics at Ghent University Hospital based on the diagnostic criteria of 2017[1,2]. Furthermore, 28 healthy age-matched (±3 years) female control participants were recruited through social media and flyers. Participants were excluded if they: (1) were pregnant or less than one year after giving birth, (2) had heart arrhythmias or heart failure, or (3) had neurologic disorders. Additionally, healthy control participants were excluded if they had GJH (Beighton score of ≥5/9 in adults <50 years old and ≥4/9 in adults ≥50 years old), rheumatic diseases or musculoskeletal injuries or surgery of the lower or upper limbs in the last two years[1,8].
Procedures
Participants were invited by e-mail or phone to participate in this cross-sectional study conducted in Ghent University Hospital. Subject characteristics including age, height (stadiometer), weight (digital scale), body mass index (BMI, kg/m2), GJH (Beighton score), pain (general visual analogue scale score or VAS; ranging from 0 to 10) and sports (self-designed questionnaire) were collected. Furthermore, total body dual x-ray absorptiometry (DXA) was performed with a Hologic QDR-Discovery device (software version 2.3.1; Hologic, Bedford, MA, USA) to evaluate subtotal lean mass (whole body lean mass minus the head (SLM); kg), lean mass of the dominant leg (LMDL; kg), lean mass of both arms (dominant arm: LMDA, non-dominant arm: LMnDA; kg) and subtotal fat percentage (whole body fat percentage minus the head; %). Furthermore, muscle density, which is related to the lipid content of the muscle (the lower the muscle density, the higher the lipid content of the muscle), of the dominant leg (tibial shaft; mg/cm3) was assessed by peripheral quantitative computed tomography (pQCT) with a XCT-2000 device (Stratec, Medizintechnik, Pforzheim, Germany)[12]. Finally, muscle strength was evaluated as described below.
Measurements
Maximal muscle strength
Following the protocol described by Rombaut et al.[7], maximal muscle strength of the knee flexors (Hamstrings) and extensors (Quadriceps) of the dominant leg was evaluated by an isokinetic test (Biodex, at an angular velocity of 60°/sec)[9]. Test results with a coefficient of variation >20%, which indicates large variety between repetitions, were discarded and repeated. Absolute peak torque (PT; Nm) and relative peak torque (PT/LMDL; Nm/kg) were calculated for both muscle groups (knee flexors and extensors).
To evaluate maximal strength of the hand flexors in both hands, the JAMAR Hydraulic Hand Dynamometer was used, which has proven to be a reliable tool for measuring handgrip strength[13,14]. Participants were seated upright with the feet flat on the ground, shoulders in a neutral position, the elbow against the side and 90° flexed, and the wrist in a neutral position[13,15]. Next, participants were asked to perform a maximal isometric contraction. The highest value (Newton) of three attempts was recorded and normalized for lean mass of the evaluated arm (LMDA or LMnDA; N/kg).
Additionally, pain severity (VAS) was evaluated before and immediately after each maximal muscle strength test.
Muscle strength endurance
Following the same protocol as described above, muscle strength endurance of the knee flexors and extensors of the dominant leg was evaluated by isokinetic tests (Biodex, 30 repetitions at an angular velocity of 240°/sec). The amount of work during 30 repetitions (total work; J), the first ten repetitions (work first third; J), the last ten repetitions (work last third; J) and the ratio of difference between the first and last ten repetitions (work fatigue; %) were calculated. Additionally, total work was normalized for LMDL (relative values; J/kg).
Subsequently, functional strength of the lower limbs was evaluated with the 30 seconds chair rise test (30s CRT). Participants were asked to get as quickly as possible from a sitting to a standing position (and back) with the arms folded across the chest, starting from a sitting position with the hips and knees 90° flexed and feet placed flat on the ground. The number of stands in 30 seconds was recorded and normalized for body weight (number of stands/kg)[16].
Furthermore, muscle strength endurance of the lower limbs was measured by two posture maintenance tests (dominant hip flexors and wall sitting) in which participants had to maintain a specific position as long as possible, as described by Rombaut et al.[7]. Additionally, muscle strength endurance of the shoulder abductors was evaluated by bilateral 90° shoulder abduction hold in standing position. The length of time (sec) a participant could maintain the correct position was recorded.
Afterwards, strength endurance of the hand flexors of the dominant hand was evaluated in the same position as described above (maximal muscle strength measurements of the hand flexors) by asking the participants to squeeze as long and hard as possible until force dropped down under 50% of their maximal value. Total time (sec) was recorded.
Additionally, pain severity (VAS) and fatigue (Borg scale, ranging from 6 to 20) were evaluated before, immediately after and one minute after each muscle strength endurance test.
Physical impairment
Physical impairment was evaluated by the Arthritis Impact Measurement Scale (AIMS). Only the physical health component of the AIMS was evaluated, which is the mean of the following subscales (all ranging from 0 to 10): movement abilities, walking and bending, hand and finger function, arm function, household activities and personal care. Higher scores on the AIMS questionnaire indicate higher physical impairment[17].
Statistical analyses
Data analysis was performed using the statistical package SPSS version 26. Data normality was evaluated by the Shapiro-Wilk test and visual inspection of the Q-Q plots. Normally distributed data are shown as mean ± standard deviation, non-normally distributed data (AIMS, work fatigue, posture maintenance tests, endurance test of the hand flexors, VAS and Borg scores) as median and interquartile ranges. Normally distributed variables were compared between the three groups (hEDS, HSD and controls) using an univariate analysis of variance (ANOVA) test or Welch ANOVA if the statistical assumption of homogeneity of variance (Levene’s test) was violated. If significant differences were identified, post-hoc Tukey (homogeneous variances) or Games-Howell (variances not homogeneous) tests were performed to pairwise compare the three groups. Non-normal distributed data were analyzed with a Kruskall-Wallis test and were pairwise compared (Dunn-Bonferroni) if significant differences were observed. Due to the small sample size, important factors impacting muscle strength, such as BMI and pain, were not included as covariates, though all primary variables were normalized for (i.e. divided by) body weight or lean mass (LMDL, LMDA or LMnDA).
Furthermore, associations between physical functioning (AIMS questionnaire – component physical health) and muscle strength were determined by correlation coefficients (Spearman’s rho or rs; 0.8-0.99: very strong, 0.5-0.8: moderately strong, 0.3-0.5: fairly strong, <0.3: poor)[18,19].
Results
Characteristics
Participant characteristics are shown in Table 1. No significant differences were observed between the hEDS and HSD group. Beighton score (p<0.001), general VAS score (p<0.001) and subtotal fat percentage (hEDS – controls: p=0.012; HSD – controls: p=0.006) were significantly higher in the patient groups compared to the controls. Age, BMI, muscle density, subtotal lean mass, and lean mass of the dominant leg and both arms were similar in all groups. Fourteen patients (70%) with hEDS, twenty patients (91%) with HSD and 28 (100%) healthy controls performed sports (e.g. swimming, riding the bike, aquagym etc.).
Table 1.
Participant characteristics.
| hEDS (n=20) | HSD (n=23) | CTR (n=28) | P value | |
|---|---|---|---|---|
| Age (years) | 43.8 ± 14.27 | 41.1 ± 14.78 | 39.6 ± 13.90 | 0.611 |
| BMI (kg/m2) | 26.8 ± 5.54 | 28.5 ± 6.49 | 25.1 ± 4.67b | 0.090 |
| Beighton score (/9) | 5.7 ± 2.11 | 4.9 ± 1.49 | 0.2 ± 0.52 a,b | <0.001* |
| General VAS score (/10) | 5.0 ± 1.90 | 4.7 ± 2.43 | 1.0 ± 1.29 a,b | <0.001* |
| Subtotal lean mass (kg) | 39.8 ± 6.29 | 41.2 ± 7.87 | 42.7 ± 7.50 | 0.389 |
| Lean mass dominant leg (kg) | 6.97 ± 1.212 | 7.11 ± 1.657 | 7.67 ± 1.352 | 0.189 |
| Lean mass dominant arm (kg) | 2.04 ± 0.402 | 2.13 ± 0.478 | 2.23 ± 0.446 | 0.353 |
| Lean mass non-dominant arm (kg) | 1.95 ± 0.373 | 2.10 ± 0.452 | 2.13 ± 0.439 | 0.307 |
| Subtotal fat percentage (%) | 39.4 ± 5.95 | 39.6 ± 6.18 | 34.1 ± 6.11 a,b | 0.002* |
| Muscle density (mg/cm3) | 74.6 ± 3.91 | 75.7 ± 1.52 | 76.6 ± 1.71 | 0.054 |
Variables (normally distributed) are shown as mean ± standard deviation; hEDS: hypermobile Ehlers-Danlos syndrome; HSD: hypermobility spectrum disorder; CTR: healthy controls; BMI: body mass index; VAS: visual analogue scale; subtotal lean mass: whole body lean mass minus the head; kg: kilogram; mg: milligram;
: p value ANOVA test <0.05;
: significant difference between CTR and hEDS (post-hoc pairwise comparison test);
: significant difference between CTR and HSD (post-hoc pairwise comparison test).
Muscle strength measurements
Maximal muscle strength
Maximal muscle strength results are shown in Table 2. No significant differences were identified between both patient groups (hEDS and HSD).
Table 2.
Maximal muscle strength.
| hEDS (n=20) | HSD (n=23) | CTR (n=28) | P value | |
|---|---|---|---|---|
| LOWER LIMBS | ||||
| Isokinetic test at an angular velocity of 60°/sec | ||||
| PT extensors (Nm) | 86.7 ± 29.54 | 91.5 ± 32.42 | 134.3 ± 32.87 a,b | <0.001* |
| PT extensors/LMDL (Nm/kg) | 13.0 ± 4.16 | 13.2 ± 4.69 | 17.5 ± 2.89 a,b | <0.001* |
| PT flexors (Nm) | 49.1 ± 18.05 | 48.0 ± 16.77 | 72.9 ± 22.03 a,b | <0.001* |
| PT flexors/LMDL (Nm/kg) | 7.1 ± 2.29 | 6.8 ± 2.24 | 9.6 ± 2.23 a,b | <0.001* |
| UPPER LIMBS | ||||
| Hand grip strength (finger flexors) | ||||
| Dominant hand (N) | 282.5 ± 63.54 | 305.4 ± 98.20 | 331.8 ± 63.58 | 0.093 |
| Dominant hand/LMDA (N/kg) | 140.8 ± 33.1 | 146.7 ± 51.41 | 151.2 ± 26.61 | 0.515 |
| Non-dominant hand (N) | 275.6 ± 71.87 | 267.4 ± 94.20 | 311.5 ± 66.78 | 0.107 |
| Non-dominant hand/LMnDA (N/kg) | 144.5 ± 37.77 | 131.1 ± 50.28 | 149.2 ± 31.83 | 0.342 |
Variables are shown as mean ± standard deviation; hEDS: hypermobile Ehlers-Danlos syndrome; HSD: hypermobility spectrum disorder; CTR: healthy controls; PT: peak torque; LMDL: lean mass dominant leg; LMDA: lean mass dominant arm; LMnDA: lean mass non-dominant arm; N: Newton; Nm: Newton meter; Kg: kilogram;
: p value ANOVA test <0.05;
: significant difference between CTR and hEDS (post-hoc pairwise comparison test);
: significant difference between CTR and HSD (post-hoc pairwise comparison test).
In the lower limbs, absolute and relative (normalized for LMDL) values of peak torque were significantly lower in the patient groups compared to the control group (p≤0.002). In the upper limbs, maximal strength of the finger flexors (absolute and relative values) did not significantly differ between the three groups (hEDS, HSD and control group).
Additionally, VAS scores, carried out before and after the muscle strength tests, were not significantly different between the two patient groups (hEDS and HSD), whereas significantly higher scores were observed in both patient groups compared to the control group (p≤0.01).
Muscle strength endurance
Muscle strength endurance results are shown in Table 3. No significant differences were observed between the two patient groups (hEDS and HSD).
Table 3.
Muscle strength endurance.
| hEDS (n=20) | HSD (n=23) | CTR (n=28) | P value | |
|---|---|---|---|---|
| LOWER LIMBS | ||||
| Isokinetic test at an angular velocity of 240°/sec | ||||
| Extensors | ||||
| Total work (J) | 967.3 ± 445.17 | 1057.8 ± 409.80 | 1773.9 ± 458.33 a,b | <0.001* |
| Total work/LMDL (J/kg) | 140.9 ± 59.44 | 151.2 ± 58.22 | 231.8 ± 41.15 a,b | <0.001* |
| Work first third (J) | 395.2 ± 209.42 | 413.5 ± 183.20 | 726.3 ± 198.04 a,b | <0.001* |
| Work last third (J) | 247.9 ± 104.07 | 282.2 ± 101.28 | 451.7 ± 125.19 a,b | <0.001* |
| Work fatigue (%) | 41.4 [12.2, 47.3] | 29.7 [20.6, 37.7] | 38.9 [29.6, 44.9] | 0.273 |
| Flexors | ||||
| Total work (J) | 649.0 ± 418.27 | 633.2 ± 395.18 | 1116.5 ± 405.50 a,b | <0.001* |
| Total work/LMDL (J/kg) | 94.9 ± 57.99 | 89.3 ± 53.08 | 148.8 ± 48.95 a,b | <0.001* |
| Work first third (J) | 248.9 ± 168.39 | 250.2 ± 178.55 | 453.9 ± 168.43 a,b | <0.001* |
| Work last third (J) | 174.5 ± 116.09 | 171.0 ± 94.19 | 281.5 ± 103.28 a,b | <0.001* |
| Work fatigue (%) | 27.7 [12.9, 41.2] | 28.1 [17.7, 43.1] | 37.2 [31.8, 44.2] | 0.090 |
| Posture maintenance test | ||||
| Hip flexors (sec) | 62.2 [20.7, 89.0] | 49.2 [28.7, 76.0] | 117.8 [87.9, 172.0] a,b | <0.001* |
| Wall sit (sec) | 31.0 [15.0, 65.8] | 30.6 [18.0, 65.0] | 78.5 [58.8, 114.4] a,b | <0.001* |
| 30 seconds chair rise test | ||||
| Number of stands | 12.9 ± 6.62 | 14.9 ± 5.45 | 19.0 ± 4.28 a,b | 0.001* |
| Number of stands/BW (kg) | 0.19 ± 0.119 | 0.21 ± 0.091 | 0.28 ± 0.104 a,b | 0.009* |
| UPPER LIMBS | ||||
| Hand grip strength (finger flexors) | ||||
| Endurance test (sec) | 26.9 [18.5, 42.9] | 27.0 [16.8, 50.0] | 41.5 [26.9, 54.1] | 0.083 |
| Posture maintenance test | ||||
| Shoulder abductors (sec) | 176.5 [92.0, 245.0] | 145.0 [100.6, 240.0] | 300.0 [194.5, 300.0] a,b | 0.001* |
Normally distributed variables are shown as mean ± standard deviation, non-normally distributed variables as median [quartile 1, quartile 3]; hEDS: hypermobile Ehlers-Danlos syndrome; HSD: hypermobility spectrum disorder; CTR: healthy controls; LMDL: lean mass dominant leg; BW: body weight; J: Joule; kg: kilogram; sec: seconds;
: p value ANOVA/Kruskal-Wallis test <0.05;
: significant difference between CTR and hEDS (post-hoc pairwise comparison test);
: significant difference between CTR and HSD (post-hoc pairwise comparison test).
In the lower limbs, total work (absolute and relative values) and work performed by the knee flexors and extensors in the first and last ten repetitions were significantly lower in the patient groups compared to the control group (p≤0.003). Work fatigue did not significantly differ (for both the knee flexors and extensors). Posture maintenance tests of the hip flexors and wall sitting test showed significantly lower results in the patient groups compared to the control group (p≤0.004). Furthermore, both patient groups scored significantly lower in the 30s CRT compared to the controls (hEDS – controls: p=0,001, HSD – controls: p=0.021). However, when normalized for body weight, only significantly lower results were observed in the hEDS group compared to the controls (hEDS – controls: p=0.013, HSD – controls: p=0.052). In the upper limbs, significantly lower results were found regarding shoulder abduction maintenance in the patient groups in comparison with controls (p≤0.014). No significant differences were observed in hand grip muscle endurance between the patient groups and the control group.
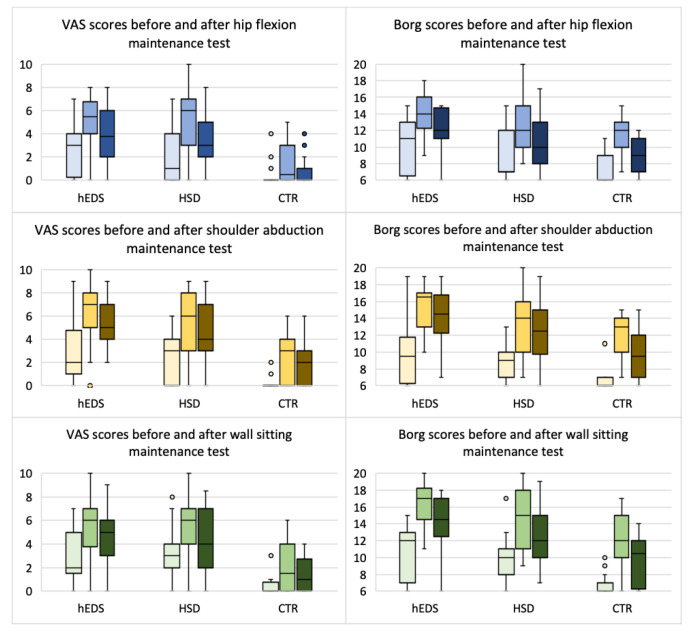
Furthermore, VAS and Borg scores, carried out before, immediately after, and one minute after the muscle strength tests, were not significantly different between the two patient groups (hEDS and HSD). However, as shown in Figure 1, VAS and Borg scores were generally significant higher in both patient groups compared to the control group (p≤0.019).
Figure 1.
VAS and Borg scores before and after posture maintenance tests. First bar: before the test; second bar: immediately after the test; third bar: 1 minute after the test; hEDS: hypermobile Ehlers-Danlos syndrome; HSD: hypermobility spectrum disorder; CTR: healthy controls; VAS: visual analogue scale (ranging from 0-10); Borg: fatigue scale (ranging from 6-20); °: statistical outlier.
Physical impairment
Results from the AIMS questionnaire are reported in Table 4. Physical impairment (AIMS) did not significantly differ between patients with HEDS and HSD. Significantly higher results (i.e. higher physical impairment) were observed in the patient groups compared to the control group (all p<0.001). Furthermore, muscle strength was generally negatively associated with physical impairment (moderate correlation) in patients with hEDS and HSD (Supplementary Table 1). In detail, physical impairment was moderately negatively correlated to maximal strength tests in the lower limbs (both hEDS and HSD; rs ranging from -0.626 to -0.483, p<0.05) and in the upper limbs (only HSD; rs ranging from -0.774 to -0.639, p≤0.001). Except for the finger flexors, all muscle strength endurance tests were moderately negatively correlated with physical impairment in individuals with hEDS (rs ranging from -0.733 to -0.477, p≤0.05). In individuals with HSD, only moderately strong negative correlations were observed with the 30s CRT, posture maintenance tests of the lower limbs and total work of the extensors (rs ranging from -0.563 to -0.458, p ≤0.05).
Table 4.
Patient-reported outcomes.
| hEDS (n=20) | HSD (n=23) | CTR (n=28) | P value | |
|---|---|---|---|---|
| AIMS | ||||
| Movement abilities | 2.8 [1.3, 4.0] | 3.0 [1.0, 4.5] | 0.0 [0.0, 0.0] a,b | <0.001* |
| Walking and bending | 5.8 [5.0, 8.0] | 3.0 [1.0, 4.5] | 0.0 [0.0, 0.0] a,b | <0.001* |
| Hand and finger function | 3.6 [2.0, 4.5] | 2.5 [1.0, 4.0] | 0.0 [0.0, 0.0] a,b | <0.001* |
| Arm function | 1.0 [0.5, 2.3] | 1.5 [0.5, 2.0] | 0.0 [0.0, 0.0] a,b | <0.001* |
| Household activities | 3.1 [1.9, 4.4] | 3.8 [1.3, 6.3] | 0.0 [0.0, 0.0] a,b | <0.001* |
| Personal care | 0.6 [0.0, 1.3] | 0.0 [0.0, 2.5] | 0.0 [0.0, 0.0] a,b | <0.001* |
| Component physical health | 2.7 [2.1, 4.2] | 3.1 [1.2, 3.9] | 0.1 [0.0, 0.2] a,b | <0.001* |
Variables (non-normally distributed) are shown as median [quartile 1, quartile 3]; hEDS: hypermobile Ehlers-Danlos syndrome; HSD: hypermobility spectrum disorder; CTR: healthy controls; AIMS: arthritis impact measurement scale;
: p value Kruskal-Wallis test <0.05;
: significant difference between CTR and hEDS (post-hoc pairwise comparison test);
: significant difference between CTR and HSD (post-hoc pairwise comparison test). Higher scores on the AIMS indicate higher physical impairment.
Discussion
To our knowledge, this is the first study evaluating muscle strength (maximal and endurance), muscle density, muscle mass, and physical functioning in patients with hEDS and HSD, who were diagnosed according to the most recent criteria[1]. Moreover, the results of this study allowed for comparison between individuals with hEDS and HSD for the first time. As hypothesized, this study showed that muscle strength, muscle mass, muscle density, and physical impairment were similar in individuals with hEDS and HSD. When these patient groups were compared to healthy controls, muscle strength was lower in the lower limbs (maximal and endurance) and shoulder abductors (endurance). Muscle strength results in the finger flexors suggested that maximal muscle strength and muscle strength endurance did not significantly differ between the patient groups compared to controls. Finally, significantly higher fat percentages, non-significant differences (p=0.054) in muscle density, and significantly higher physical impairment were observed in both patient groups compared to the control group, whereas no significant differences were found regarding muscle mass between the three groups.
Symptom severity in individuals with hEDS versus HSD
As hypothesized, no differences in maximal muscle strength, muscle strength endurance, muscle mass and density, and physical impairment were found between individuals with hEDS and HSD. This is in accordance with previous studies showing no differences in physical activity and sleep (both objectively measured by accelerometry), severity of symptoms, and comorbid features between individuals with hEDS and HSD[3,4,20]. As already suggested by our research group and Copetti et al.[4], it is as such very likely that patients with hEDS and HSD show a similar clinical profile despite the difference in generalized soft tissue fragility[3]. Therefore, we suggest that further research should, on the one hand, evaluate whether similar physiotherapy approaches are recommended in these patients and whether they may yield similar benefits, and on the other hand, elucidate the added value of introducing new elements to the current classification of patients with hEDS and HSD.
There can be suggested that patients with hEDS and HSD may also be classified – besides the diagnostic classification – based on physical impairment and severity of frequently occurring symptoms such as reduced maximal muscle strength and muscle strength endurance, fatigue and pain[21,22]. The social, psychological and societal impact for patients not meeting the 2017 diagnostic criteria for hEDS could be reduced by this supplementary classification[20]. In addition, we suggest to include multiple objective measurements (e.g. muscle strength as well as objective functional assessments) in research and clinics, in order to more adequately classify patients according to their symptom severity and level of physical impairment. Nevertheless, given the broad spectrum of symptoms patients with hEDS and HSD experience, individually tailored treatment should still be highly recommended.
Muscle strength, mass and density in individuals with hEDS and HSD
In accordance with previous studies in patients diagnosed according to the older (and less strict) criteria (Villefranche, 1997), this study showed that maximal muscle strength and muscle strength endurance were significantly lower in patients with hEDS and HSD in comparison with healthy controls[7,9]. We can hypothesize that the observed increased fat percentage and trend to decreased muscle density in patients with hEDS and HSD could negatively impact muscle strength in these patients, as a higher fat content may result in an attenuation of the relationship between muscle mass and muscle performance[23-25]. This can partially explain in turn the overall physical impairment in patients with hEDS and HSD[23,26-28]. Furthermore, this study showed higher pain levels in patients with hEDS and HSD, which can possibly further explain decreased muscle strength in these patients[3,7]. Additionally, previous research has suggested that decreased muscle strength could also result from poor proprioception, lower physical activity levels and alterations in structural integrity of the connective tissue in these patients[5,7,9]. Nevertheless, further research is required to confirm these hypotheses and elucidate the causality of decreased muscle strength and the impact of higher fat percentages in patients with hEDS and HSD.
In contrast with the abovementioned results, this study suggested that maximal hand grip strength and hand grip strength endurance are not significantly different in individuals with hEDS and HSD in comparison with healthy controls. However, patients with hEDS and HSD reported significantly lower hand, finger and arm function (subscale of the physical component score of the AIMS) compared to controls. Based on these results, we could speculate that impaired hand function is related to other typical features such as pain, fatigue and (sub)luxations, rather than due to decreased muscle strength. However, this hypothesis requires further research to understand the variability in hand grip test results and ensure it is not resulting from type II errors.
Finally, this study showed decreased muscle strength in patients with hEDS and HSD, which may be associated with impaired functioning and can presumably be related to higher fat percentages and higher levels of pain. Based on these results, we suggest that both maximal muscle strength and muscle strength endurance in lower as well as upper limbs should be carefully measured and implemented in physiotherapeutic treatment (e.g. muscle strength exercises) in patients with hEDS and HSD. Functional tests like the 30s CRT and static endurance tests of several muscle groups can be recommended to evaluate muscle strength endurance in these patients. Furthermore, in accordance with previous research of our research group (2020), we suggest that treatment should also include pain relief in order to reduce disability and optimize the effect of muscle strengthening exercises[5,9,29]. However, further research is needed to evaluate which muscle strengthening exercises are appropriate for these patients, without aggravating pain, fatigue and (sub)luxations.
Limitations and strengths
This was the first study in which muscle strength, muscle density, lean mass, and physical functioning were compared between patients with hEDS and HSD, diagnosed according to the most recent criteria of 2017, in order to identify possible differences in clinical profile. Furthermore, these patients were compared with healthy controls, matched for age and sex. Additionally, this study went beyond previous studies performed in patients with symptomatic GJH, as hand grip strength was also explored and all primary outcomes were normalized for lean mass or body weight. However, the results of this study should be viewed within the limitations of the study. Due to a high coefficient of variation (>20%), which implies high variability of results during the isokinetic test (Biodex device), maximal muscle strength test (60°/sec) results of three individuals with hEDS and two with HSD were excluded. Consequently, the power of the study (isokinetic tests) was lower, though results were more reliable given the lower variability. Furthermore, as both healthy controls and patients (hEDS and HSD) were recruited based on self-will, recruitment bias could occur. However, to minimize this bias, recruitment was done by eight people in different settings and flyers were used to reach a broad public. Finally, as limitations in daily living were not measured in this study, no conclusions can be drawn regarding the association between muscle strength, physical function and these limitations.
Conclusion
In conclusion, this study showed no differences in muscle strength (maximal and endurance), muscle density, muscle mass, and physical functioning between patients with hEDS and HSD. However, both patient groups show significantly lower muscle strength in both upper and lower limbs in comparison with healthy controls, except for hand grip strength. Therefore, physiotherapeutic treatment in patients with hEDS and HSD should address both maximal muscle strength and muscle strength endurance, in lower and upper limbs, though individually tailored to their needs given the broad clinical spectrum. On the other hand, future research is needed to address whether similar physiotherapeutic approaches in these patient groups could yield similar benefits.
Acknowledgements
We would like to thank the patients with hEDS and HSD and the healthy controls to participate in this study. We would also like to express our gratitude to the Department of Endocrinology for their cooperation in this study.
Appendix
Supplementary Table 1.
Association between physical impairment and muscle strength tests.
| hEDS (n=20) | P value | HSD (n=23) | P value | |
|---|---|---|---|---|
| Maximal strength tests | ||||
| Isokinetic test at an angular velocity of 60°/sec | ||||
| PT extensors/LMDL (Nm/kg) | -0.626 [-0.859; -0.179] | 0.005* | -0.483 [-0.762; -0;052] | 0.023* |
| PT flexors/LMDL (Nm/kg) | -0.615 [-0.849; -0.181] | 0.005* | -0.600 [-0.828; -0.202] | 0.003* |
| Hand grip strength (finger flexors) | ||||
| Dominant hand/LMDA (N/kg) | -0.264 [-0.639; 0.210] | 0.261 | -0.774 [-0.910; -0.486] | <0.001* |
| Non-dominant hand/LMnDA (N/kg) | -0.102 [-0.522; 0.358] | 0.670 | -0.639 [-0.845; -0.269] | 0.001* |
| Muscle strength endurance tests | ||||
| Isokinetic test at an angular velocity of 240°/sec | ||||
| Total work extensors/LMDL (J/kg) | -0.684 [-0.877; -0.299] | 0.001* | -0.458 [-0.753; -0.009] | 0.037* |
| Work first third extensors (J) | -0.498 [-0.782; -0.043] | 0.025* | -0.414 [-0.727; 0.041] | 0.062 |
| Work last third extensors (J) | -0.600 [-0.837; -0.175] | 0.005* | -0.373 [-0.701; 0.086] | 0.096 |
| Total work flexors/LMDL (J/kg) | -0.576 [-0.824; -0.142] | 0.008* | -0.414 [-0.727; 0.041] | 0.062 |
| Work first third flexors (J) | -0.477 [-0.770; -0.017] | 0.034* | -0.369 [-0.699; 0.090] | 0.100 |
| Work last third flexors (J) | -0.526 [-0.798; -0.077] | 0.017* | -0.397 [-0.716; 0.060] | 0.074 |
| 30 seconds chair rise test | ||||
| Number of stands/BW (kg) | -0.733 [-0.900; -0.380] | <0.001* | -0.542 [-0.792; -0.137] | 0.007* |
| Posture maintenance test | ||||
| Hip flexors (sec) | -0.659 [-0.866; -0.260] | 0.002* | -0.563 [-0.804; -0.164] | 0.005* |
| Wall sit (sec) | -0.486 [-0.781; -0.013] | 0.035* | -0.524 [-0.782; -0.114] | 0.010* |
| Shoulder abductors (sec) | -0.488 [-0.776; -0.031] | 0.029* | -0.362 [-0.681; 0.073] | 0.090 |
| Hand grip strength (finger flexors) | ||||
| Endurance test (sec) | -0.332 [-0.682; 0.142] | 0.153 | -0.188 [-0.568; 0.257] | 0.402 |
Values shown are Spearman’s rho (0.8-0.99: very strong, 0.5-0.8: moderately strong, 0.3-0.5: fairly strong, <0.3: poor) and 95% confidence intervals.
: p value <0.05; hEDS: hypermobile Ehlers-Danlos syndrome; HSD: hypermobility spectrum disorder; PT: peak torque; LMDL: lean mass dominant leg; LMDA: lean mass dominant arm; LMnDA: lean mass non-dominant arm; BW: body weight; J: Joule; N: Newton; Nm: Newton meter; kg: kilogram; sec: seconds.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. American journal of medical genetics Part C, Seminars in medical genetics. 2017;175(1):8–26. doi: 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- 2.Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A. A framework for the classification of joint hypermobility and related conditions. American journal of medical genetics Part C, Seminars in medical genetics. 2017;175(1):148–57. doi: 10.1002/ajmg.c.31539. [DOI] [PubMed] [Google Scholar]
- 3.Coussens DWI M, Pacey V, Malfait F, De Craemer M, Demeyer H, Rombaut L, Calders P. Physical activity and sleep in patients with hypermobile Ehlers–Danlos syndrome and patients with generalized hypermobility spectrum disorder. Edorium J Disabil Rehabil. 2020;6 100049D05MC2020. [Google Scholar]
- 4.Copetti M, Morlino S, Colombi M, Grammatico P, Fontana A, Castori M. Severity classes in adults with hypermobile Ehlers-Danlos syndrome/hypermobility spectrum disorders: a pilot study of 105 Italian patients. Rheumatology (Oxford, England) 2019;58(10):1722–30. doi: 10.1093/rheumatology/kez029. [DOI] [PubMed] [Google Scholar]
- 5.Scheper M, Rombaut L, de Vries J, De Wandele I. van der Esch M, et al., editors. The association between muscle strength and activity limitations in patients with the hypermobility type of Ehlers-Danlos syndrome: the impact of proprioception. Disability and Rehabilitation. 2017;39(14):1391–7. doi: 10.1080/09638288.2016.1196396. [DOI] [PubMed] [Google Scholar]
- 6.Tinkle BT, Levy HP. Symptomatic Joint Hypermobility: The Hypermobile Type of Ehlers-Danlos Syndrome and the Hypermobility Spectrum Disorders. The Medical clinics of North America. 2019;103(6):1021–33. doi: 10.1016/j.mcna.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Rombaut L, Malfait F, De Wandele I, Taes Y, Thijs Y, De Paepe A, et al. Muscle mass, muscle strength, functional performance, and physical impairment in women with the hypermobility type of Ehlers-Danlos syndrome. Arthritis care &research. 2012;64(10):1584–92. doi: 10.1002/acr.21726. [DOI] [PubMed] [Google Scholar]
- 8.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) American journal of medical genetics. 1998;77(1):31–7. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Coussens M, Calders P, Lapauw B, Celie B, Banica T, De Wandele I, et al. Does muscle strength change over time in patients with hypermobile Ehlers-Danlos syndrome/ Hypermobility Spectrum Disorder?An 8-year follow-up study. Arthritis care &research. 2020 doi: 10.1002/acr.24220. [DOI] [PubMed] [Google Scholar]
- 10.Forghani I. Updates in Clinical and Genetics Aspects of Hypermobile Ehlers Danlos Syndrome. Balkan medical journal. 2019;36(1):12–6. doi: 10.4274/balkanmedj.2018.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gensemer C, Burks R, Kautz S, Judge DP, Lavallee M, Norris RA. Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes. Developmental dynamics: an official publication of the American Association of Anatomists. 2021;250(3):318–44. doi: 10.1002/dvdy.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. Journal of applied physiology (Bethesda, Md : 1985) 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 13.Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand Grip Strength: age and gender stratified normative data in a population-based study. BMC Res Notes. 2011;4:127. doi: 10.1186/1756-0500-4-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peolsson A, Hedlund R, Oberg B. Intra- and inter-tester reliability and reference values for hand strength. Journal of rehabilitation medicine. 2001;33(1):36–41. doi: 10.1080/165019701300006524. [DOI] [PubMed] [Google Scholar]
- 15.ASHT. Clinical assessment recommendations. (3rd edition) 2015 The Society. [Google Scholar]
- 16.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–9. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 17.Riemsma RP, Taal E, Rasker JJ, Houtman PM, Van Paassen HC, Wiegman O. Evaluation of a Dutch version of the AIMS2 for patients with rheumatoid arthritis. British journal of rheumatology. 1996;35(8):755–60. doi: 10.1093/rheumatology/35.8.755. [DOI] [PubMed] [Google Scholar]
- 18.Chan YH. Biostatistics 104: correlational analysis. Singapore medical journal. 2003;44(12):614–9. [PubMed] [Google Scholar]
- 19.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi medical journal : the journal of Medical Association of Malawi. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 20.McGillis L, Mittal N, Santa Mina D, So J, Soowamber M, Weinrib A, et al. Utilization of the 2017 diagnostic criteria for hEDS by the Toronto GoodHope Ehlers-Danlos syndrome clinic: A retrospective review. American journal of medical genetics Part A. 2020;182(3):484–92. doi: 10.1002/ajmg.a.61459. [DOI] [PubMed] [Google Scholar]
- 21.Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, et al. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. American journal of medical genetics Part C, Seminars in medical genetics. 2017;175(1):48–69. doi: 10.1002/ajmg.c.31538. [DOI] [PubMed] [Google Scholar]
- 22.Engelbert RH, Juul-Kristensen B, Pacey V, de Wandele I, Smeenk S, Woinarosky N, et al. The evidence-based rationale for physical therapy treatment of children, adolescents, and adults diagnosed with joint hypermobility syndrome/hypermobile Ehlers Danlos syndrome. American journal of medical genetics Part C, Seminars in medical genetics. 2017;175(1):158–67. doi: 10.1002/ajmg.c.31545. [DOI] [PubMed] [Google Scholar]
- 23.Moore BA, Bemben DA, Lein DH, et al. Fat Mass is Negatively associated with Muscle Strength and Jump Test Performance. Journal of Frailty &Aging. 2020;9:214–8. doi: 10.14283/jfa.2020.11. [DOI] [PubMed] [Google Scholar]
- 24.Rahemi H, Nigam N, Wakeling JM. The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. Journal of the Royal Society, Interface. 2015;12(109) doi: 10.1098/rsif.2015.0365. 20150365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallis J, James RS, Seebacher F. The effects of obesity on skeletal muscle contractile function. The Journal of experimental biology. 2018;221(Pt 13) doi: 10.1242/jeb.163840. [DOI] [PubMed] [Google Scholar]
- 26.Nonaka K, Murata S, Shiraiwa K, Abiko T, Nakano H, Iwase H, et al. Effect of Skeletal Muscle and Fat Mass on Muscle Strength in the Elderly. Healthcare (Basel, Switzerland) 2018;6(3) doi: 10.3390/healthcare6030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebrun C, van der Schouw Y. Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause. 2006;13(3):474–81. doi: 10.1097/01.gme.0000222331.23478.ec. [DOI] [PubMed] [Google Scholar]
- 28.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiologic reviews. 2013;35:51–65. doi: 10.1093/epirev/mxs006. [DOI] [PubMed] [Google Scholar]
- 29.Voermans NC, Knoop H, Bleijenberg G, van Engelen BG. Pain in ehlers-danlos syndrome is common, severe, and associated with functional impairment. Journal of pain and symptom management. 2010;40(3):370–8. doi: 10.1016/j.jpainsymman.2009.12.026. [DOI] [PubMed] [Google Scholar]