Abstract
Objectives:
Mesenchymal stem cells (MSCs) have become seed cells and basic elements for bone regeneration and bone tissue engineering. The aim of the present study was to investigate the roles and mechanisms of bone morphogenetic protein 2 (BMP-2) on osteogenic differentiation of MSCs.
Methods:
Primary MSCs were isolated from the femur and tibia bone of rats and then transfected with BMP-2 and PGC-1α adenovirus vectors. Alkaline phosphatase (ALP) activity and alizarin red staining were used to measure osteogenic differentiation of MSCs. Real-time PCR and western blot assays were performed to assess osteogenic differentiation-related proteins levels. The activities of mitochondrial respiratory chain complexes I and II and mitochondrial fluorescence intensity were used to explore mitochondria status during osteogenic differentiation of MSCs.
Results:
We found that the ability of BMP-2 overexpressed (OE) group osteogenic differentiation was significantly improved, compared with the negative control (NC) group. The results also indicated that BMP-2 can promote the activity of mitochondria. We further used the gain- and loss-of-function approaches to demonstrate that BMP-2 promotes mitochondrial activity by up-regulating PGC-1α to promote osteogenic differentiation of MSCs.
Conclusions:
These results explored the important role of BMP-2 in the osteoblast differentiation of MSCs from a new perspective, providing a theoretical and experimental basis for bone defect and repair.
Keywords: BMP-2, Mitochondrial Activity, MSCs, Osteoblast Differentiation, PGC-1α
Introduction
Although bone tissue has strong self-remodeling and repair regeneration potential, clinical problems of bone defects, osteonecrosis, and bone non-healing still exist. Bone tissue engineering is currently the most promising method for treating osteonecrosis and bone defect repair, and with seed cells as one of the three elements of tissue engineering, their selection and cultivation is the most basic link in bone tissue engineering. Mesenchymal stem cells (MSCs) have been reported to be capable of multidirectional differentiation (such as adipogenic differentiation or osteogenic differentiation) and are considered to be the most potent seed cells[1]. MSCs are capable of self-proliferation, have multidirectional differentiation potential, and can differentiate into the desired cells for research under the action of cytokines[2,3].
Bone reconstruction is hot research in the field of regenerative medicine. Tissue engineering materials and methods that promote bone growth have been widely applied to regenerate bone tissue and repair bone defects. In bone regeneration engineering, the MSCs as seed cells have broad application prospects, and the repair of fractures and bone defects has become a research hotspot[4,5]. Based on the characteristics of MSCs cultured in vitro and their ability to differentiate into osteoblasts in vivo or in vitro, researchers have begun to use in vitro expanded MSCs to implant into defects in bone tissue, which has opened up a new way for bone regeneration[6]. However, multiple cells signaling pathways in MSCs affect cell renewal and generational alternation. The mechanism of directional osteogenic differentiation still requires an exploration of the key issues and the related effects between signals.
Bone morphogenetic protein (BMP) is considered to be an important growth factor for bone tissue engineering and a member of the transforming growth factor superfamily (TGF-β)[7]. BMPs promote cell proliferation and differentiation, regulate cell adipogenic differentiation and chemotaxis, and participate in vascularization and organ development in most tissues of vertebrates. BMPs were originally discovered to have the ability to induce MSC to differentiate into chondrocytes and osteoblasts, as well as initiate osteogenic differentiation and participate in the regulation of this entire process[8]. BMP-2, BMP-7, BMP-9 and BMP-6 can induce osteogenic differentiation of MSCs.
As a member of the BMP family with strong osteogenesis and cartilage regenerative potential, BMP-2 has been widely studied by scholars worldwide[9]. At present, it is believed that bone growth factors can accelerate cell proliferation, differentiation, promote vascularization and adhesion and proliferation of osteoblasts, thereby changing osteogenesis and cell product production, which is the key link of bone regeneration[10]. It has been shown that BMP-2 can effectively treat bone defects and nonunion, but the molecular mechanism of BMP-2 promoting bone formation is still unclear. In particular, the interaction of BMP signaling pathways in other osteogenic differentiation cell mediator pathways is relatively rare[11]. Mitochondria provide a place for the life activity of cells and are the main sites for intracellular oxidative phosphorylation and ATP formation. Changes in the mitochondrial function and number are verified to play a vital role in cell proliferation and differentiation. Mitochondrial ROS production and changes in mitochondrial membrane potential directly affect cell survival, so mitochondria are thought to play a key role in the proliferation and apoptosis of cells[12]. In addition, the activity and number of mitochondria have also been confirmed to play a key role in the proliferation and differentiation of the cell[13]. The long-term culture of MSCs and induced osteogenic differentiation are widely used in the study of osteoblast differentiation, but the changes in mitochondrial production during differentiation have not been clearly reported[14].
Therefore, in the present study, we speculated that BMPs and mitochondrial activity interact in the process of MSCs transformation into osteoblast, and can cause the expression of some key factors downstream of this pathway. This study demonstrated that overexpression of BMP-2 can promote osteogenic differentiation. Moreover, the relationship between BMP-2 overexpression and mitochondrial activity was explored. Since the expression of PGC-1α is closely related to mitochondrial activity, the relationship between BMP-2 and PGC-1α was further explored. This study explored the role of BMP-2 in osteoblast differentiation from a new perspective, providing a theoretical and experimental basis for bone defect and repair.
Method and materials
Isolation, culture, and confirmation of MSCs
We designed and carried out the animal experiment according to the ARRIVE checklist (https://www.nc3rs.org.uk/arrive-guidelines)[15,16]. Primary MSCs were isolated as described previously[17]. Briefly, 8 female Sprague Dawley (SD) rats weighing between 280-300 g at 3 months old were purchased from the Model Animal Research Center of Nanjing University. The animals were housed for 7 days in the condition with appropriate humidity and temperature under a 12-hour light/dark circadian rhythm, providing sufficient food and water. The rats were observed every day by two animal behavior specialists to define the condition of the rats for further experiments. Next, the rats were anesthetized by injecting 200 mg/kg of pentobarbital into the abdominal cavity. Determine the animals were fully anesthetized by observing the animal didn’t respond to the squeeze on the animal’s paw or tail. Then the rat’s neck was severed to death. Femur and tibia bone of SD rats were obtained, washed with 75% ethanol for 10-30s D-Hanks solution 5-8 times. Take the bone marrow cavity and washed it twice with α-MEM containing 10% fetal bovine serum (FBS). After centrifugation at 1000 r/min for 5 min, the obtained cells were seeded in culture flasks at 37°C and 5% CO2 saturated humidity incubator. Then the MSCs were seeded in a 6-well plate with a density of 1.0×109/L. Replace half of the medium after 24 h, and total the medium after 48 h. The collected 3th passages MSCs were used in all experiments. The cells were digested, centrifuged at 4°C, and washed with phosphate-buffered saline (PBS), followed by the addition of monoclonal antibodies CD29 and CD45. The cells were then resuspended in 500 µL PBS, and identified MSC cells through flow cytometry (data not shown). Osteogenic differentiation was performed with culture medium containing 50 µg/mL Vitamin C, 10 mmol/L sodium β-glycerophosphate, and 10 µmol/L dexamethasone. All animal experiments were approved by The Xuzhou No.1 People’s Hospital Committee.
Transfection of BMP-2 and PGC-1α gene into MSCs by adenovirus vector
All adenoviruses used in this study were customized by Shanghai GenePharma. MSCs were cultured as mentioned above. HEK293T cells (ATCC, USA) were seeded in DMEM with a density of 1×105 cells/ml. When the adherent cells reached 60% confluence, used empty recombinant adenovirus green fluorescent protein cDNA (Ad-GFP), recombinant adenovirus BMP-2 cDNA (Ad-BMP-2) expressing BMP-2, and recombinant adenovirus PGC-1α cDNA (Ad-PGC-1α) expressing PGC-1α. After 24 h, the infection of the cells was observed with a fluorescence microscope. When the proportion of fluorescent cells reaches 30%, it continues to culture for 2-5 days; When the proportion of fluorescent cells is between 80-100%, it is mainly shown as round cells with vacuoles, and is accompanied by cell death. When floating cells account for half of the total number of cells, the detached cells were collected in a culture dish. After 1000 rpm centrifuges for 10 minutes, collected the cells and then resuspended them in 500 µL of DMEM. Next, placed in liquid nitrogen for 1-2 min, then placed the tubes containing the cells in 37°C water bath and oscillated continuously. After the cells were lysed, continue to vortex for 1-2 min. Repeat the above steps 4-5 times, centrifuged then at 3000 rpm for 10 min. And MSCs were then transfected with supernatant containing the recombinant adenovirus extract. Inoculate 2 ml of the third-generation MSCs in a 6-well plate at a density of 0.5×105 cells/ml, cultured for 24 h, and then added the corresponding recombinant adenovirus for transfection. There were four transfection groups, namely, Ad-GFP group as Negative control (NC) group, BMP-2 OE group, PGC-1α overexpressed (OE) group, and BMP-2 OE+PGC-1α knockdown (KD) group. The total RNA and total protein were extracted to detect the expression of the target gene and target protein, after the cells were cultured for 30 h.
siRNA preparation and transfection
In this study, the control siRNA and PGC-1α were synthesized by Synthgene (Nanjing, China). Please refer to previous instructions (10.1016 /j.taap.2019.02.016) for the specific method of cell transfection. The sequences used were as follows: PGC-1α sense GCACGCAGUCCUAUUCAUUTT, PGC-1α antisense AAUGAAUAG-GACUGCGUGCTT. According to the manufacturer’s instructions, siRNA was transfected into MSCs using Lipofectamine 2000 (Thermofisher, USA).
Alkaline phosphatase and alizarin red detection
After differentiation for 0, 7 and 14 days, the ALP activity assay was performed according to the previously published protocol[18]. According to the manufacturer’s protocol, the alkaline phosphatase (ALP) activity was measured using the ALP activity kit (Sigma-Aldrich). The Alizarin Red (Sigma-Aldrich) was used to detect mineralization. In short, cells were fixed with 70% ethanol and stained with 2% Alizarin Red (Sigma-Aldrich). Subsequently, it was decolorized with sodium phosphate solution for 30 minutes to quantify the calcium mineral density. Finally, the calcium concentration is determined at the reader 562 nm.
MitoTracker Staining
According to the previously published protocol, check the morphology of mitochondria in MSCs[19]. In brief, MSCs after different treatments were washed twice with PBS, incubated then with 0.01 µmol/L MitoTracker Green FM (M7514; Thermo Fisher Scientific) in the dark place at 37°C for 30 min, then fixed with 4’,6-diamidino-2-phenylindole (DAPI). Finally, observed with a laser confocal scanning microscope (Zeiss LSM Meta 510).
Real-time PCR analysis
Perform total RNA extraction and qRT-PCR analysis according to the manufacturer’s protocol. We used TRIzol reagent (Synthgene, Nanjing, China) to extract total RNA from MSCs at the designated time points. We used Taqman probes (Applied Biosystems, USA) to quantify miRNA. In short, RT primers and AMV reverse transcriptase (Takara, Japan) were used to transcribe total RNA into cDNA. Subsequently, real-time PCR was performed on the Applied Biosystems 7300 sequence detection system using the Taqman PCR kit (Applied Biosystems, USA). The reaction conditions were: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and then 60°C for 1 min. The 2-ΔΔCT method was used for quantitative determination, and the expression of GAPDH was used as an internal control.
Western blot analysis
The western blot procedures were performed according to the previously published protocol[20]. The MSCs were washed with PBS (ice-cold) 2 times, centrifuge was performed at 12000 g for 10 min at 4°C, then lysis was performed using RIPA lysis buffer (Synthgene, China), and then incubated on ice for about 30 min. The cells lysates were centrifuged at 4°C (12,000 g) for another 10 min. Subsequently, determination of protein concentration in supernatant was conducted using a BCA protein Kit (synthgene, China). The protein was then incubated overnight with the following primary antibodies at 4°C: PGC-1α (1:1000, Abcam, USA), BMP-2 (1:1000, Abcam, USA), OCN (1:1000, Abcam, USA), RUNX2 (1:5000, Abcam, USA), Osterix (1:1000, Abcam, USA), GAPDH (1:2000, Abcam, USA). GAPDH served as a loading control. The protein bands were quantified with Image J Software after exposure imaging (1.47V, NIH, USA).
Mitochondrial complex activity determination
Mitochondrial complex activity determination was performed according to the previously published protocol[18]. In short, the cells were collected and resuspended in 1.0 mL hypotonic buffer (2.5 mmol/L MgCl2, 10 mmol/L Tris base, 10 mmol/L NaCl, pH 7.5), and homogenize was performed on ice using glass homogenizer (Fisher Scientific, Pittsburgh, PA, USA). After homogenization, centrifuge it at 1300 × g for 5 min at 4°C. After that, centrifuged supernatant at 17000 × g for 15 min at 4°C, and then the mitochondrial pellet was resuspended in 100 µL isotonic buffer (70 mmol/L sucrose, 210 mmol/L mannitol, 1 mmol/L EDTA•2Na, 5 mmol/L Tris base, pH7.5). According to the previously described method determined the activities of nicotinamide adenine dinucleotide (NADH)-ubiquinone reductase (complex I) and succinate-Co Q oxidoreductase (complex II)[21,22]. The specific protocol is as follows:
Assays for nicotinamide adenine dinucleotide (NADH)-ubiquinone reductase (complex I) activity: accurately draw 2 ml of enzyme complex I reaction buffer [10 mM Tris-HCl Ph 8.0], add 10µl of mitochondrial protein, then add 80 AM 2,3-dimethoxy-5-methyl-6-decyl-1, 4-benzoquinone (DB), 1mg/ml BSA, 2mM NaN3 and 2Ag/ml antimycin (antimycin A). After mixed the mixture and incubated for 5 min at 30°C, the NADH (200 AM) absorbance changes were monitored at 340 nm.
Assays for succinate-Co Q oxidoreductase (complex II) activity: accurately draw 2ml of enzyme complex II reaction buffer [50 mM potassium phosphate solution (configuration ratio: K2HPO4:KH2PO4=4:1), pH 7.4], add 10µl mitochondrial protein and incubated. Then add 50 AM DCPIP, 2 mM NaN3, 2 Ag/ml rotenone, 2 Ag/ml antimycin A, 25AM coenzyme Q0. After mixed well, add 10µl 20AM sodium succinate, measure the change of DCPIP at 600 nm.
Statistical analysis
SPSS 18.0 (SPSS, inc.) was used for data analysis. All experiments were repeated three times, and the data were presented as the means ± SD. one-way ANOVA and post hoc Dunnett’s T3 test were used to compare the differences among and between groups. p<0.05 was considered significant.
Results
Overexpression of BMP-2 can promote osteogenic differentiation of MSCs
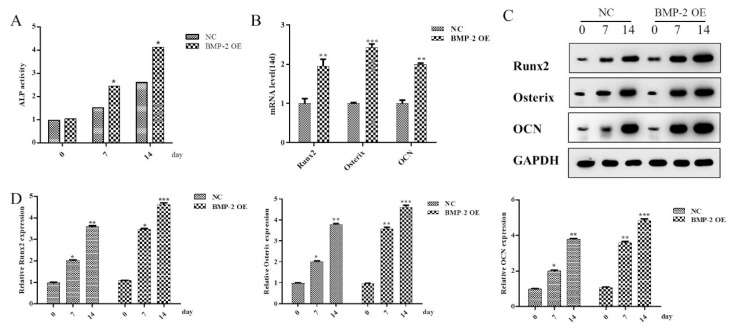
In order to explore whether BMP-2 has a regulatory effect on the osteogenic differentiation of MSCs, we overexpressed and knockdown BMP-2 separately (Figure 1A). Since ALP is a landmark indicator of osteogenic differentiation of MSCs, in this study, ALP activity was detected at 0d, 7d, and 14d after osteogenic differentiation of MSCs. As a result, it was found that compared with the NC group, the ALP activity of the BMP-2 overexpression group was significantly increased on the 7th day, and the difference was more pronounced on the 14th day (Figure 1B). The mRNA expression levels of osteogenic differentiation-related proteins were detected by RT-qPCR on day 14, and it was found that the mRNA expression levels of Runx2, Osterix, and OCN in the BMP-2 overexpression group were significantly increased, compared with the NC group (Figure 1C). The Western blot analysis showed the protein expression levels of osteogenic differentiation-related proteins (Runx2, Osterix and OCN) at 0d, 7d, and 14d after MSCs osteogenic differentiation (Figure 1D). Results revealed that with the increase of MSCs osteogenic differentiation days, the protein expressions of Runx2, Osterix, and OCN also increased, and the increase of the BMP-2 overexpression group was more obvious than that of the NC group (Figure 1E).
Figure 1.
Overexpression of BMP-2 can promote osteogenic differentiation of MSCs. (A) The expression of BMP-2 in BMP-2 OE and KD groups were detected by Western blot. Histogram results in (A). *p<0.05 and **p<0.01 comparing to NC group. (B) The ALP activity in MSCs in differentiation days was detected. *p<0.05 comparing to value in NC group. (C) RT-qPCR was used to detect the mRNA expression of Runx2, Osterix, and OCN in MSCs at 14th day. **p<0.01 and ***p<0.001 comparing to NC group. (D) The expression of Runx2, Osterix, and OCN in MSCs in differentia-tion days was detected by Western blot. (E) Histogram summarizing results in (D). *p<0.05, **p<0.01 and ***p<0.001. Data are shown as mean ± SEM (n=3).
Overexpression of BMP-2 promotes mitochondrial production and activity in MSCs
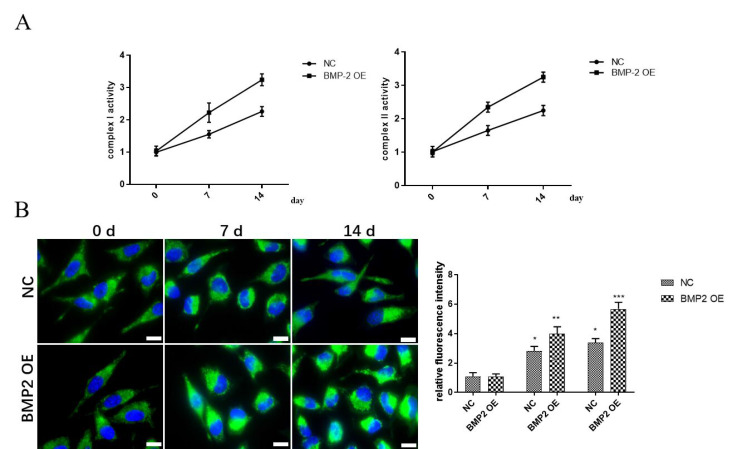
To explore the relationship between BMP-2 and mitochondria status during osteogenic differentiation of MSCs, the activities of mitochondrial respiratory chain complexes I and II were detected at 0d, 7d, and 14d after osteogenic differentiation of MSCs. Mitochondrial respiratory chain complex activity is one of the indicators of mitochondrial activity. Results revealed that the activities of mitochondrial respiratory chain complexes I and II in BMP-2 overexpression group were significantly increased with the increase of differentiation days in the MSCs (Figure 2A). At the same time, we used confocal microscopy to observe the mitochondria at 0d, 7d, and 14d after MSCs osteogenic differentiation. Results revealed that the mitochondrial fluorescence intensity of the BMP-2 overexpression group was significantly increased with the increase of differentiation days in the MSCs, compared with the NC group (Figure 2B).
Figure 2.
The effect of overexpression of BMP-2 on mitochondria during osteo-genic differentiation of MSCs. (A) The activities of mitochondrial respiratory chain complexes I and II in MSCs in differentiation days were detected. (B) The view of mitochondrial morphology in MSCs in differentiation days was detected by confocal mi-croscopy. Data are shown as mean ± SEM (n=3). Asterisks indicate significant differences from the control (*, p<0.05; **, p<0.01; ***, p<0.001) (scale bar 50 µm).
BMP-2 promotes osteogenic differentiation of MSCs through up-regulating the expression of PGC-1α
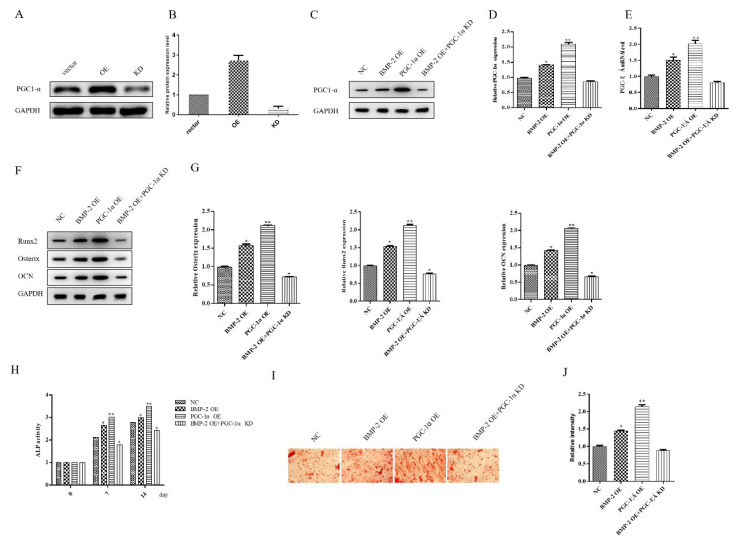
To explore the relationship between BMP-2 and PGC-1α during MSCs osteogenic differentiation, we first overexpressed and knocked down PGC-1α separately. Then, we knocked down PGC-1α while overexpressing BMP-2. The protein expression of PGC-1α was detected by western blot (Figure 3A-D). Compared with the NC group, overexpression of BMP-2 or PGC-1α increased the PGC-1α expression, and the latter increased more significantly. Overexpression of BMP-2 and knockdown of PGC-1α counteracted the effect of overexpression of BMP-2 to promote PGC-1α expression (Figure 3C-D). The qPCR analysis result of PGC-1α mRNA expression was consistent with protein results (Figure 3E). To further validate the role of BMP-2 in regulating PGC-1α in the osteogenic differentiation of MSCs, Western blot results showed that overexpression of BMP-2 and PGC-1α enhanced the expression of osteogenic differentiation-related proteins (Runx2, Osterix, and OCN) compared with the NC group. Overexpression of BMP-2 while knocking down PGC-1α resulted in a significant decreased expression of related proteins (Figure 3 F-G). We also tested the enzyme activity of ALP. The activity of ALP was examined at 0d, 7d, and 14d after MSCs osteogenic differentiation of MSCs. The overexpression of BMP-2 and PGC-1α enhanced ALP activity, and the latter increased more significantly compared with the NC group. When BMP-2 was overexpressed, knockdown of PGC-1α significantly inhibited ALP activity (Figure 3H). The results of alizarin red staining to detect osteogenic differentiation of MSCs was consistent with the ALP enzyme activity (Figure 3I-J). Taking together all of the above results, these studies have confirmed that BMP-2 can enhance mitochondrial activity by up-regulating PGC-1α to promote osteogenic differentiation of MSCs.
Figure 3.
BMP-2 promotes osteogenic differentiation of MSCs through up-regulating the expression of PGC-1α. (A) Western blot was used to detect the expression of PGC-1α in PGC-1α OE and KD groups. (B) Histogram summarizing results in (A). *p<0.05 and **p<0.01 comparing to NC group. (C) The expression of PGC-1α in different groups (including BMP-2 OE, PGC-1α OE and BMP-2 OE+ PGC-1α KD groups) were detected by Western blot. (D) Histogram summarizing results in (C). *p<0.05 and **p<0.01 comparing to NC group. (E) RT-qPCR detects the mRNA expression of PGC-1α in different groups. *p<0.05 and **p<0.01 comparing to NC group. (F) The expression of Runx2, Osterix, and OCN in MSCs in differentiation days were detected by Western blot. (G) Histogram summarizing results in (F). *p<0.05 and **p<0.01 comparing to NC group. (H) ALP activity of MSCs in differentiation days was detected. *p<0.05 and **p<0.01 comparing to value in NC group at the same time. (I) The alizarin red staining of MSCs in differentiation 14 days was detected. (J) Histogram summarizing results in (I). *p<0.05 and **p<0.01 comparing to NC group. Data are shown as mean ± SEM (n=3) (scale bar, 200 nm).
BMP-2 can up-regulate PGC-1α to promote mitochondrial production and activity of MSCs
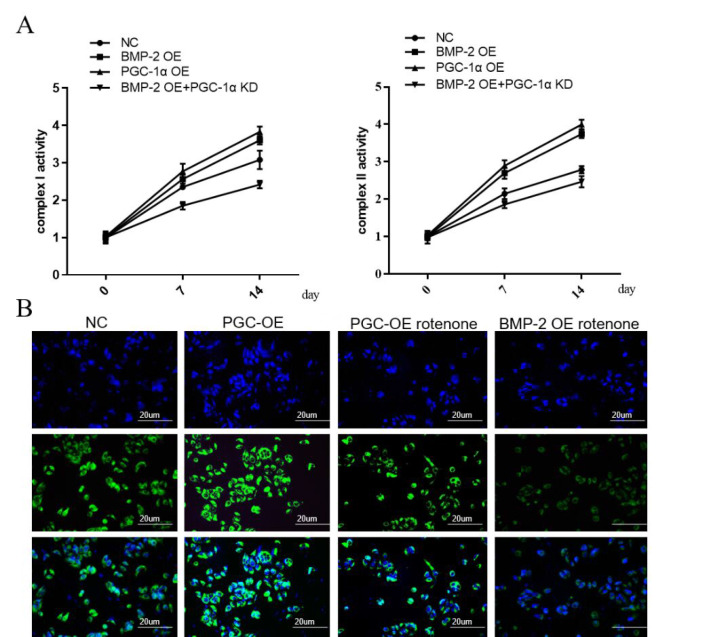
Since the expression of PGC-1α is closely related to mitochondrial activity, the relationship between BMP-2 and PGC-1α was further explored. The activities of mitochondrial respiratory chain complexes I and II were examined at 0d, 7d, and 14d after osteogenic differentiation of MSCs. Overexpression of BMP-2 and PGC-1α resulted in increased complex activity compared to the NC group, whereas overexpression of BMP-2 while knocking down PGC-1α inhibited complex activity (Figure 4A). At the same time, we used confocal microscopy to observe the mitochondria. To further clarify the functions of PGC-1α and BMP-2 in mitochondria, we treated the overexpression of PGC-1α or BMP-2 with the addition of the mitochondrial inhibitor rotenone. The results showed that the addition of mitochondrial inhibitors could eliminate the increase in mitochondrial activity induced by overexpression of PGC-1α or BMP-2 (Figure 4B).
Figure 4.
BMP-2 can up-regulate PGC-1α to promote mitochondrial production and activity of MSCs. (A) The activities of mitochondrial respiratory chain complexes I and II were detected at 0d, 7d, and 14d after osteogenic differentiation of MSCs. (B) The view of mitochondrial morphology in MSCs at 0d, 7d, and 14d was detected by confocal microscopy. Data are shown as mean ± SEM (n=3). Asterisks indicate signif-icant differences from the control (**, p<0.01; ***, p<0.001) (scale bar, 20 µm).
Discussion
In bone tissue engineering and bone regenerative medicine, the research on the regulation of MSC-oriented osteogenic differentiation has become a widespread concern of scholars worldwide. Osteoclasts are often induced on multidifferentiated cells using osteogenic growth factors and osteogenic gene transfection as osteoinductive agents[23,24]. Bone growth factor accelerates cell proliferation and differentiation, promotes vascularization and osteoblast proliferation and changes osteogenesis. The formation of cell products becomes the key link of bone regeneration. Bone growth factor can promote the repair or regeneration of tissues and organs. It plays very important role in construction of tissue-engineered human tissue[25].
At present, researchers worldwide pay attention to the signaling pathway that regulates bone metabolism and promotes bone healing to find effective drug targets for the treatment of bone diseases such as bone defects and nonunion. The BMP signaling pathway is a relatively well-researched signaling pathway, which has significant effects on bone repair and remodeling. BMPs have a strong role in inducing osteogenesis and promoting cell proliferation and metabolism and are currently a hotspot in bone regenerative medicine and tissue engineering research[26]. BMP-2 has been used clinically because of its strong osteogenic activity and has achieved good results in bone reconstruction and repair cases[27,28]. The BMP pathway expresses specific osteogenic factors such as Msx2, Runx2, Osterix (Osx), and osteocalcin (OCN)[29]. In our study, we detected ALP activity in BMP-2 overexpressed (OE) MSCs after 0, 7, 14 days of osteogenic differentiation. Our results showed that the ALP activity of the BMP-2 OE group increased significantly on the 7th day, and the difference was more pronounced on the 14th day, compared with the NC group. This result initially indicated the important role of BMP-2 in the osteogenic differentiation of MSCs. We then measured the mRNA and protein expression levels of Runx2, Osterix, and OCN. The results showed that as the number of days of osteogenic differentiation of MSCs increased, the mRNA and protein expressions of Runx2, OCN and Osterix were also increased, and the increases in overexpression of BMP-2 group was more obvious than that of NC group. All these results demonstrated that BMP-2 is involved in the regulation of osteogenic differentiation of MSCs. The aforementioned results elucidate that BMP-2 is involved in the regulation of MSCs osteogenic differentiation.
Then we further explored how BMP-2 participated in regulating the osteogenic differentiation of MSCs. Mitochondria play a particularly important role in the body’s homeostasis. Mitochondria synthesis and decomposition affect ATP production and cellular functional status[30]. The PGC-1α is an important regulator mitochondrial production, and its transcriptional costimulatory action activates the expression of many transcription factors involved in mitochondrial components and mitochondrial respiration. PGC-1α has been reported to be highly expressed in tissues with high energy demands including skeletal muscle, adipose tissue, and heart, etc[31-33]. Recent studies have found that the PGC-1α mediates the differentiation of MSCs into brown adipocytes[34]. However, PGC-1α mediates the effect of osteogenic differentiation of MSCs is still unclear. In our study, Mitotracker Green staining revealed a significant increase in mitochondrial production during MSC osteogenic differentiation. Further detection of respiratory chain-related complex activity revealed a significant increase in cell mitochondrial complex activity, confirming that the increase in cell mitochondrial activity is related to the osteogenic differentiation of cells after overexpression of BMP-2. Since there is a close correlation between the expression of PGC-1α and mitochondrial activity, we explored the relationship between BMP-2 and PGC-1α. The study found that after overexpression of BMP-2, PGC-1α also overexpressed, and mitochondrial activity increased, promoting osteogenic differentiation. However, when BMP-2 is overexpressed while interfering with the expression of PGC-1α, the expression level of PGC-1α is restored, and mitochondrial activity is not increased, and the corresponding osteogenic differentiation is also maintained at a normal level. Therefore, it was concluded that BMP-2 regulates mitochondrial activity by up-regulating PGC-1α to promote osteogenic differentiation of MSCs.
Briefly, BMP-2 and mitochondrial activity exhibit complex and important interactions during osteogenic differentiation of MSCs, which together regulate the osteogenic differentiation and bone formation of cells. This study demonstrated that overexpression of BMP-2 promoted osteogenic differentiation and further explored the relationship between BMP-2, mitochondrial activity, and PGC-1α. This study confirmed a new pathway of BMP-2 in promoting osteogenic differentiation of MSCs; specifically, BMP-2 regulates mitochondrial activity by regulating PGC-1α to promote osteogenic differentiation of MSCs. Our study provides theoretical basis and new ideas for the treatment of bone metabolism and bone injury. In addition, due to the limitation of experimental conditions, electron microscopy cannot be used to count the number of mitochondria in our study. Efforts should be made to overcome the experimental limitations and provide valuable information for the future research.
Funding
This work was supported by grants from the Fundamental Research Funds for the Central Universities 2020QN78.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Shapiro G, Lieber R, Gazit D, Pelled G. Recent Advances and Future of Gene Therapy for Bone Regeneration. Curr Osteoporos Rep. 2018;16(4):504–511. doi: 10.1007/s11914-018-0459-3. [DOI] [PubMed] [Google Scholar]
- 2.Morikawa M, Koinuma D, Mizutani A, Kawasaki N, Holmborn K, Sundqvist A, Tsutsumi S, Watabe T, Aburatani H, Heldin CH, et al. BMP Sustains Embryonic Stem Cell Self-Renewal through Distinct Functions of Different Kruppel-like Factors. Stem Cell Reports. 2016;6(1):64–73. doi: 10.1016/j.stemcr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veriter S, Andre W, Aouassar N, Poirel HA, Lafosse A, Docquier PL, Dufrane D. Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different “Hospital Exemption”Clinical Applications. PLoS One. 2015;10(10):e0139566. doi: 10.1371/journal.pone.0139566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deschaseaux F, Pontikoglou C, Sensebe L. Bone regeneration: the stem/progenitor cells point of view. J Cell Mol Med. 2010;14(1-2):103–115. doi: 10.1111/j.1582-4934.2009.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zigdon-Giladi H, Rudich U, Michaeli Geller G, Evron A. Recent advances in bone regeneration using adult stem cells. World J Stem Cells. 2015;7(3):630–640. doi: 10.4252/wjsc.v7.i3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Tan N, Zhou Y, Zhou X, Chen H, Wei H, Chen J, Xu X, Zhang S, Yang G, et al. Semaphorin 3A Shifts Adipose Mesenchymal Stem Cells towards Osteogenic Phenotype and Promotes Bone Regeneration In Vivo. Stem Cells Int 2016. 2016:2545214. doi: 10.1155/2016/2545214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katagiri T, Watabe T. Bone Morphogenetic Proteins. Cold Spring Harb Perspect Biol. 2016;8(6) doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Gomez P, Anselmo NP, Mira H. BMPs as therapeutic targets and biomarkers in astrocytic glioma. Biomed Res Int 2014. 2014:549742. doi: 10.1155/2014/549742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatesan J, Anil S, Kim SK, Shim MS. Chitosan as a vehicle for growth factor delivery: Various preparations and their applications in bone tissue regeneration. Int J Biol Macromol. 2017;104(Pt B):1383–1397. doi: 10.1016/j.ijbiomac.2017.01.072. [DOI] [PubMed] [Google Scholar]
- 10.Gkiatas I, Lykissas M, Kostas-Agnantis I, Korompilias A, Batistatou A, Beris A. Factors affecting bone growth. Am J Orthop (Belle Mead NJ) 2015;44(2):61–67. [PubMed] [Google Scholar]
- 11.Wu M, Chen G, Li YP. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Bliek AM. Cell Biology of the Mitochondrion. Genetics. 2017;207(3):843–871. doi: 10.1534/genetics.117.300262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta S. Mitochondria and the aging process. Nihon Rinsho. 2016;74(9):1456–1460. [PubMed] [Google Scholar]
- 14.Rodriguez-Carballo E, Gamez B, Ventura F. p38 MAPK Signaling in Osteoblast Differentiation. Front Cell Dev Biol. 2016;4:40. doi: 10.3389/fcell.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18(7):e3000411. doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao AG, Zhou YC, Hu ZJ, Lu BB. Ipriflavone promotes osteogenesis of MSCs derived from osteoporotic rats. European Review for Medical and Pharmacological Sciences. 2018;22(14):4669–4676. doi: 10.26355/eurrev_201807_15527. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Feng Z, Wang X, Zeng M, Liu J, Han S, Xu J, Chen L, Cao K, Long J, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018;25(2):229–240. doi: 10.1038/cdd.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Hong Y, He H, Jiang G, You W, Liang X, Fu Q, Han S, Lian Q, Zhang Y. FGF21 Mediates Mesenchymal Stem Cell Senescence via Regulation of Mitochondrial Dynamics. Oxid Med Cell Longev. 2019;2019:4915149. doi: 10.1155/2019/4915149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng XP, Huang L, Liu ZH. miRNA-133a attenuates lipid accumulation via TR4-CD36 pathway in macrophages. Biochimie. 2016;127:79–85. doi: 10.1016/j.biochi.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Vyatkina G, Bhatia V, Gerstner A, Papaconstantinou J, Garg N. Impaired mitochondrial respiratory chain and bioenergetics during chagasic cardiomyopathy development. Biochim Biophys Acta. 2004;1689(2):162–173. doi: 10.1016/j.bbadis.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Jarreta D, Orús J, Barrientos A, Miró O, Roig E, Heras M, Moraes CT, Cardellach F, Casademont J. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc Res. 2000;45(4):860–865. doi: 10.1016/s0008-6363(99)00388-0. [DOI] [PubMed] [Google Scholar]
- 23.Martin V, Bettencourt A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mater Sci Eng C Mater Biol Appl. 2018;82:363–371. doi: 10.1016/j.msec.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Lu H, Liu Y, Guo J, Wu H, Wang J, Wu G. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. Int J Mol Sci. 2016;17(3):334. doi: 10.3390/ijms17030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novais A, Lesieur J, Sadoine J, Slimani L, Baroukh B, Saubamea B, Schmitt A, Vital S, Poliard A, Helary C, et al. Priming Dental Pulp Stem Cells from Human Exfoliated Deciduous Teeth with Fibroblast Growth Factor-2 Enhances Mineralization Within Tissue-Engineered Constructs Implanted in Craniofacial Bone Defects. Stem Cells Transl Med. 2019;8(8):844–857. doi: 10.1002/sctm.18-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzahrani MM, Anam E, AlQahtani SM, Makhdom AM, Hamdy RC. Strategies of enhancing bone regenerate formation in distraction osteogenesis. Connect Tissue Res. 2018;59(1):1–11. doi: 10.1080/03008207.2017.1288725. [DOI] [PubMed] [Google Scholar]
- 27.Kim EC, Yoon SJ, Noh K, Lee DW. Dual Effect of Curcumin/BMP-2 Loaded in HA/PLL Hydrogels on Osteogenesis In Vitro and In Vivo. J Nanosci Nanotechnol. 2017;17(1):143–152. doi: 10.1166/jnn.2017.12380. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Guo S, Zhang H. Synergistic Effects of Controlled-Released BMP-2 and VEGF from nHAC/PLGAs Scaffold on Osteogenesis. Biomed Res Int 2018. 2018:3516463. doi: 10.1155/2018/3516463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowery JW, Rosen V. The BMP Pathway and Its Inhibitors in the Skeleton. Physiol Rev. 2018;98(4):2431–2452. doi: 10.1152/physrev.00028.2017. [DOI] [PubMed] [Google Scholar]
- 30.Guerrero-Beltran CE, Bernal-Ramirez J, Lozano O, Oropeza-Almazan Y, Castillo EC, Garza JR, Garcia N, Vela J, Garcia-Garcia A, Ortega E, et al. Silica nanoparticles induce cardiotoxicity interfering with energetic status and Ca(2+) handling in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2017;312(4):H645–H661. doi: 10.1152/ajpheart.00564.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 32.St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278(29):26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 33.Waldman M, Bellner L, Vanella L, Schragenheim J, Sodhi K, Singh SP, Lin D, Lakhkar A, Li J, Hochhauser E, et al. Epoxyeicosatrienoic Acids Regulate Adipocyte Differentiation of Mouse 3T3 Cells, Via PGC-1αActivation, Which Is Required for HO-1 Expression and Increased Mitochondrial Function. Stem Cells Dev. 2016;25(14):1084–1094. doi: 10.1089/scd.2016.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang PI, Chen YC, Chen LH, Juan CC, Ku HH, Wang ST, Chiou SH, Chiou GY, Chi CW, Hsu CC, et al. PGC-1αmediates differentiation of mesenchymal stem cells to brown adipose cells. J Atheroscler Thromb. 2011;18(11):966–980. doi: 10.5551/jat.7401. [DOI] [PubMed] [Google Scholar]