Abstract
Background
Mitochondrial impairment and exaggerated inflammation are hallmarks of sarcopenia. Recently, cell-free mitochondrial DNA (cf-mtDNA) has been in the spotlight as an endogenous danger molecule that can potentially elicit inflammation. Yet, its actual impact on sarcopenia, especially in patients with maintenance hemodialysis (MHD), is still at an early stage of investigation.
Material/Methods
A total of 105 MHD patients were enrolled in this study. The subjects were classified into sarcopenia group (SP) and non-sarcopenia group (NSP) according to the DXA scan and grip strength. Plasma and peripheral blood mononuclear cells (PBMCs) were separated from whole blood. Circulating cf-mtDNA (ccf-mtDNA) was detected using Taq Man RT-qPCR. Cytosolic mtDNA and inflammation- and mitophagy-related genes in PBMCs were quantitated using SYBR Green RT-qPCR. ΔΨm was analyzed using the fluorescent probe JC-1.
Results
ccf-mtDNA content was significantly higher in SP group than in NSP group. Multivariate regression analysis showed a significant correlation of ccf-mtDNA with sarcopenia after adjusting for potential confounders. A similar trend of increased mtDNA was also observed in the mitochondria-free cytoplasm of PBMCs from SP patients, together with higher expression of TLR9 and IL-6 in this group. Next, using PBMCs as surrogates for mitochondria-rich cells, we found that ΔΨm was dramatically decreased in the SP group. In parallel, the mRNA levels of mitophagy-related genes Parkin and LAMP2 were increased in the SP group.
Conclusions
The results obtained demonstrated that ccf-mtDNA, as a potential driver of inflammatory component, may be involved in the pathogenesis of the MHD-related sarcopenia.
Keywords: Inflammation; Mitochondria, Muscle; Renal Dialysis; Sarcopenia
Background
Sarcopenia is a term derived from the Greek “sarx” – meat and “penia” – loss. This concept was first coined in 1988 by Rosenberg and denotes a syndrome characterized by progressive and generalized loss of muscle mass and strength [1]. Sarcopenia is common in chronic kidney disease (CKD) and end-stage renal disease (ESRD) patients, especially after long-term maintenance hemodialysis (MHD) [2,3]. MHD patients with concomitant sarcopenia not only worsen their quality of life, but also increase the risk of adverse health outcomes, including cardiovascular events and overall mortality [4]. Therefore, there is an urgent need to understand the underlying mechanisms of sarcopenia in MHD patients to design novel therapeutics.
Regardless of etiology, the presence of low-grade inflammation has been recognized as a characteristic pathological change in MHD and sarcopenia [5]. Mitochondria are endosymbiotic organelles that carry multiple copies of their own genomes (mitochondrial DNA, mtDNA), encoding proteins required for oxidative phosphorylation and respiratory metabolism [6]. Mitochondria are derived from early endosymbiotic bacteria capable of oxidative phosphorylation, and most of mtDNA contains inflammatogenic unmethylated CpG motifs similar to those in bacterial DNA [7]. In this regard, if mtDNA is released out of the cell and becomes extracellular mtDNA, it can act as a damage-associated molecular pattern (DAMP) molecule and trigger an innate immune response [8].
Accumulated evidence suggests that mtDNA can be easily detected in the cell-free fraction of blood, and its levels are elevated under pathological conditions, such as trauma, sepsis, aging, caner, and immune-mediated disease, which are characterized by a chronic inflammatory status [9–13]. Chen et al. investigated the risk factors for ESRD and found that patients with ESRD exhibited significantly higher ccf-mtDNA contents than the healthy controls [14]. Another study reported that mtDNA release was significantly enhanced in MHD patients, and its level was positively correlated with inflammatory cytokines in vitro [15]. It has previously been demonstrated that high levels of plasma inflammatory substances are associated with sarcopenia, with chronic inflammation playing a role in this disorder [16]. However, the possible contribution of mitochondrial DAMPs to the inflammatory milieu that characterizes muscle wasting disorders has not yet well-explored. This question is worth being pursued as it could help identify novel biological targets for the management of muscle loss, especially in MHD patients, because of the high prevalence of MHD patients with this disease and the lack of effective medications.
Mitochondrial impairment occurs in multiple tissues under uremic conditions, such as muscle[17], heart [18], liver[19], lung[20], endothelial cells[21], and monocytes [22]. When mitochondria are damaged, mtDNA can be released into the extracellular spaces and then recognized by immune cells [23]. Peripheral blood mononuclear cells (PBMCs) are abundantly rich in mitochondria. Recently, tests of PBMCs have been proposed to offer valid information about “general” mitochondrial health [24–26]. Hence, we chose PBMCs as indicators of mitochondrial dysfunction in lieu of tissue biopsy collection to elucidate the release mechanism of ccf-mtDNA.
In the current study, we first sought to determine the association between circulating mtDNA and the risk of sarcopenia in MHD patients. Furthermore, we attempted to decipher the potential molecular mechanisms underlying its release. These findings provide novel mechanistic insights into the linkage between ccf-mtDNA and MHD-related sarcopenia and enable the identification of new therapeutic targets of this disease.
Material and Methods
Participants
A cross-sectional study design was conducted in this research. All subjects were recruited from Hemodialysis Center of First Affiliated Hospital of Chengdu Medical College between June 2020 and October 2020. Inclusion criteria for the MHD patients were as follows: (1) age above 40; and (2) patients undergoing regular hemodialysis prescription, 3 times a week, for 4 h per session at least 6 months. The age- and sex-matched healthy controls (HC) were recruited from the same region who took routine health examinations and did not have any type of systemic diseases. The exclusion criteria for all subjects were as follows: (1) active inflammatory diseases within the last 3 months; (2) malignant tumors; (3) immune system diseases; (4) active liver disease;(5) acute cardiovascular and cerebrovascular disease; and (6) inability to complete the measurement of body composition and grip strength due to physical disability. A total of 105 MHD patients were enrolled in our examination program. Referring to the diagnostic criteria established by the Asian Working Group for Sarcopenia (AWGS) [27], participants were divided into 2 groups: MHD patients without sarcopenia (NSP, n=82) and MHD patients with sarcopenia (SP, n=23). The study protocol was approved by the Ethics Committee of First Affiliated Hospital of Chengdu Medical College and adhered to the principles outlined in the Declaration of Helsinki. All participants gave informed written consent.
Sample Size Calculation
Since no previous studies have investigated the role of ccf-mtDNA in kidney disease-related sarcopenia, there was a lack of data to predict the difference in ccf-mtDNA between sarcopenia and no-sarcopenia groups and hence no power calculation was performed prior to the study. The sample size of the validation study was calculated using Power Analysis and Sample Size software (PASS, v 11.0.10). Group sample sizes of 23 (Sarcopenia) and 82 (No-sarcopenia) achieved 95.69% power to reject the null hypothesis of equal means when the ccf-mtDNA mean difference is μ1 (No-sarcopenia) – μ2 (Sarcopenia)=3.72–6.19=−2.5 with standard deviations of 2.5 for No-sarcopenia and 3.7 for Sarcopenia, and with a significance level (alpha) of 0.050 using a two-sided two-sample unequal-variance t-test. Power analyses indicated that the sample size in this study was appropriate.
Assessment of Sarcopenia
Muscle strength was assessed by grip strength, which was measured by a dynamometer (KYTO Digital Hand Dynamometer, Model EH101, China), and low grip strength was defined as <26 kg in men and <18 kg in women. Physical performance was assessed by usual gait speed (m/s) on a 6-m course, and a slow walking speed was defined as slower than 1.0 m/s. Muscle mass was measured through dual-energy X-ray absorptiometry (DXA) (enCORE, General Electric, USA). Skeletal muscle mass index (SMI) was defined by dividing appendicular skeletal muscle mass by height in meters squared (kg/m2). Low muscle mass was defined as SMI <7.0 kg/m2 in men and <5.4 kg/m2 in women. The diagnosis of sarcopenia was based on simultaneous satisfaction of both low muscle strength and mass according to the 2019 AWGS criteria.
Sample Collection
Venous blood samples were drawn from each patient immediately prior to initiation of dialysis. Whole blood was collected into vacutainers and either aliquoted for storage at −80°C or processed into separate cellular and cell-free fractions within 2 hours.
Blood Biochemical Parameters
Blood biochemical examinations, including blood creatinine (Cr), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), fasting blood sugar (FBS), albumin (Ab), hemoglobin (Hb), vitamin D (VitD), calcium (Ca), phosphorus (P), and high-sensitivity C-reactive protein (hs-CRP), were measured in clinical laboratories of the participating hospitals.
Measurement of ccf-mtDNA Copy Number Using TaqMan RT-qPCR
The cell-free DNA was extracted from blood and urine samples using the QIAamp DNA Blood Mini Kit (Qiagen, Germantown, MD, USA), following the protocol of manufacturer. The mtDNA copy number was quantified by amplification of a highly conserved region of mitochondrial cytochrome b (Cytb) gene. The PCR primers and TaqMan probes were designed and synthesized by TsingKe Biological Technology (TsingKe Biotech, Beijing, China).
-
Cytb: sense primer, 5′-CGCTACCTTCACGCCAATG-3′,
antisense primer, 5′-CGATGTGTAGGAAGAGGCAGATAA-3′,
FAM-labeled TAMRA quenched probes, 5′-CGCCTCAATATTC-3′. The linearity of the quantitative assay was assessed using the template cloned into plasmid DNA and serially diluted to prepare a series of calibrators with known concentrations. Then, the absolute values of the mtDNA were determined by calculation from this standard curve, as previously described [28]. Results were presented as mtDNA×105 copies per μl.
Measurement of Mitochondria-Free Cytosolic mtDNA in PBMCs Using SYBR Green RT-qPCR
Mitochondria-free cytosol was prepared first using mitochondrial isolation kits (Thermo Fisher Scientific, Waltham, MA, USA). Cytosolic DNA was collected using a DNeasy Blood & Tissue Kit (Qiagen, Germantown, MD, USA). RT-qPCR was performed to detect mitochondrial DNA Cytb in the cytoplasmic fraction using the following primers:
-
F-5′-ATGACCCCAATACGCAAAAT-3′ and
R-5′-CGAAGTTTCATCATGCGGAG-3′.
The results were normalized using nDNA-encoded 18S-RNA as an internal control and are expressed as relative variation with respect to the control sample, arbitrarily set as 1.
Measurement of Inflammation- and Mitophagy-Related Genes in PBMCs Using SYBR Green RT-qPCR
RNA was extracted from purified PBMCs using RNeasy (Baifeite, Chengdu, China) according to the manufacturer’s recommendations. The following primers were used for qPCR amplification: Toll-like receptor 9 (TLR9):
-
F-5′-GGACCTCGAGTGTGAAGCAT-3′ and
5′-TGGAGCTCACAGGGTAGGAA-3′;
Cytosolic cyclic GMP-AMP synthase (cGAS):
-
F-5′-GAAGCAACTACGACTAAAGCCATT-3′ and
R-5′-TTCGATGTGAGAGAAGGATAGCC-3′;
Nucleotide-binding oligomerization domain (NOD)-like receptor 3 (NLRP3):
-
F-5′-TGTTCAGCTTTGTCCTCGGT-3′ and
R-5′-ACGTGAGGTTGCAGTTGTCT-3′;
Tumor necrosis factor alpha (TNF-α):
-
F-5′-CAACCTCCTCTCTGCCATCAAG-3′ and
R-5′-CAGGGCAATGATCCCAAAGTA-3′;
Interleukin-1 beta (IL-1β):
-
F-5′-GCACGATGCACCTGTACGAT-3′ and
R-5′-GCCCAAGGCCACAGGTATTT-3′;
Interleukin-6 (IL-6):
-
F-5′-TGACAACTCATCTCATTGCACCTGTACGAT-3′ and
R-5′-GCCCAAGGCCACAGGTATTT-3′;
Parkin:
-
F-5′-TACGTGCACAGACGTCAGGAG-3′ and
R-5′-GACAGCCAGCCACACAAGGC-3′;
PTEN induced putative kinase 1 (PINK1):
-
F-5′-TACGTGCACAGACGTCAGGAG-3′ and
R-5′-GACAGCCAGCCACACAAGGC-3′;
Autophagic receptors like microtubule-associated protein light chain 3 (LC3):
-
F-5′-GAAGATGTCCGACTTATTCGAGAG-3′ and
R-5′-ACTCTCATACACCTCTGAGATTGG-3′;
Beclin1:
-
F-5′-ATCCTGGACCGTGTCACCATCCAGG-3′ and
R-5′-GTTGAGCTGAGTGTCCAGCTGG-3′;
Lysosome-associated membrane protein-2 (LAMP2):
-
F-5′-CGTTCTGGTCTGCCTAGTCC-3′ and
R-5′-CAGTGCCATGGTCTGAAATG-3′;
GAPDH:
-
F-5′-GGAGTCCACTGGCGTCTTCA-3′ and
R-5′-GTCATGAGTCCTTCCACGATACC-3′.
Data were normalized to expression levels of the housekeeping gene GAPDH. The relative changes in the SP groups were expressed as a percentage of the NSP groups (arbitrarily set at 1).
Measurement of Mitochondrial Membrane Potential (ΔΨm) Using Flow Cytometry
PBMCs were isolated from whole blood using the Ficoll-Hypaque density gradient separation technique, and then the PBMCs were suspended in PBS at a final concentration of ~105 cells/ml for flow cytometry. The JC-1 dye (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide) (Beyotime, Shanghai, China) was used for ΔΨm assessment in PBMCs. JC-1 forms J-aggregates within healthy mitochondria that exhibits red fluorescence (emission, 590 nm) in polarized ΔΨm. In cells with altered mitochondrial function, JC-1 only forms monomers exhibiting green fluorescence (emission, 527 nm) in the cytoplasm in depolarized ΔΨm. The changes in ΔΨm were recorded by flow cytometry for the determination of cells with green fluorescence. All staining was performed following the manufacturer’s instructions and was analyzed using fluorescence-activated cell sorting (FACS) software.
Statistical Analyses
Data are expressed as the mean±standard error (SEM) or frequency, as appropriate. The distribution of the data was tested using the Kolmogorov–Smirnov test. Normally distributed data were analyzed using an independent t-test. Nonnormally distributed data were analyzed using the Mann-Whitney test. Spearman’s tests were applied to determine the associations between continuous variables. Categorical data between 2 groups were compared using the chi-square test (χ2 test) with Fisher’s exact test. Logistic regression was used to describe and explain the relationship between dependent binary variables and independent variables. Values of p<0.05 were considered statistically significant. All statistical analyses were conducted using SPSS 18.0.
Results
Characteristics and Laboratory Data of MHD Patients with and without Sarcopenia
The study recruited 105 subjects with MHD that were categorized into sarcopenia group (SP, n=23) and non-sarcopenia group (NSP, n=82). The general characteristics and laboratory data of the 2 groups with respect to their sarcopenia status are presented in Table 1. SP patients had a longer duration of dialysis, higher ccf-mtDNA content, and lower BMI, HS, SMI, VitD, and P than NSP patients. There was no difference with respect to age, sex, Cr, SBP, DBP, TC, TG, LDL-C, FBS, Ab, Hb, Ca or hs-CRP between the 2 groups.
Table 1.
Characteristics and laboratory data of MHD patients with and without sarcopenia.
| Variable | NSP (n=82) | SP (n=23) | t/χ2/Z | p |
|---|---|---|---|---|
| Age (years) | 55.40±1.58 | 59.78±3.14 | −1.282 | 0.203 |
| Gender (M/F) | 45/37 | 14/9 | 0.262 | 0.609 |
| Dialysis duration (years) | 4.56±0.32 | 6.32±0.82 | −1.976 | 0.048 |
| BMI (kg/m2) | 23.09±0.54 | 20.26±0.72 | −2.553 | 0.011 |
| HS (kg) | 24.71±0.53 | 17.73±1.20 | −4.733 | <0.01 |
| SMI (kg/m2) | 7.93±0.20 | 5.07±0.26 | −6.240 | <0.01 |
| Cr (μmol/L) | 900.32±53.17 | 876.37±61.06 | −0.221 | 0.825 |
| SBP (mmHg) | 147.88±4.23 | 148.69±9.59 | −0.391 | 0.696 |
| DBP (mmHg) | 90.57±2.72 | 83.70±5.55 | 1.161 | 0.248 |
| TC (mmol/L) | 3.78±0.17 | 4.07±0.39 | −0.662 | 0.508 |
| TG (mmol/L) | 2.59±0.25 | 1.93±0.36 | −1.364 | 0.173 |
| LDL-C (mmol/L) | 2.87±0.16 | 2.95±0.29 | −0.046 | 0.963 |
| FBS (mmol/L) | 7.76±0.44 | 7.91±0.74 | −0.666 | 0.505 |
| Ab (g/L) | 28.26±1.14 | 27.18±2.34 | −0.504 | 0.615 |
| Hb (g/L) | 88.08±2.73 | 89.87±3.35 | −0.328 | 0.744 |
| VitD (ng/mL) | 30.00±1.01 | 25.13±1.93 | 2.259 | 0.026 |
| Ca (mmol/L) | 2.03±0.04 | 1.96±0.08 | −0.608 | 0.543 |
| P (mmol/L) | 1.88±0.11 | 1.42±0.17 | −2.038 | 0.042 |
| hs-CRP (mg/L) | 6.73±0.58 | 7.56±1.28 | −0.535 | 0.593 |
| ccf-mtDNA (105 copies/uL) | 3.72±0.27 | 6.19±0.77 | −3.452 | <0.01 |
BMI – body mass index; HS – handgrip strength; SMI – skeletal muscle index; Cr – creatinine; SBP – systolic blood pressure; DBP – diastolic blood pressure; TC – total cholesterol; TG – triglyceride; LDL-C – low-density lipoprotein cholesterol; FBS – fasting blood sugar; Ab – albumin; Hb – hemoglobin; VitD – vitamin D; Ca – calcium; P – phosphorus; hs-CRP – high-sensitivity C-reactive protein; ccf-mtDNA – circulating cell-free mitochondrial DNA.
Association Between ccf-mtDNA and the Occurrence of Sarcopenia in MHD Patients
MtDNA copy number was quantified by amplification of a highly conserved region of mtDNA-encoded CytB gene. As shown in Table 1, the level of ccf-mtDNA was significantly higher in SP group than in NSP group. However, we wondered if the higher levels of ccf-mtDNA in subjects with sarcopenia were a consequence of other parameters, as dialysis duration, BMI, HS, SMI, VitD and P were also significantly different between the 2 groups. Statistically significant variables were included in the multivariate logistic regression model. As the diagnostic criteria for sarcopenia include HS and SMI, these 2 variables were excluded from further analyses. Of note, after adjusting for these confounders, dialysis duration, BMI, and ccf-mtDNA remained predictive of sarcopenia; however, VitD and P were no longer associated with sarcopenia (Table 2). As reduced BMI is well known to be a risk factor for sarcopenia in CKD patients [29], our data strongly suggest that longer dialysis duration and higher ccf-mtDNA levels represent newly identified risk factors for the MHD-related sarcopenia.
Table 2.
Univariate and multivariate logistic regression analysis for the occurrence of sarcopenia.
| Variable | Univariate regression analysis | Multivariate regression analysis | ||
|---|---|---|---|---|
| OR | p-values | OR | p-values | |
| Dialysis duration | 1.164 | 0.028 | 1.218 | 0.016 |
| BMI | 0.877 | 0.015 | 0.872 | 0.032 |
| VitD | 0.943 | 0.030 | 0.954 | 0.152 |
| Phosphorus | 0.545 | 0.044 | 0.739 | 0.369 |
| ccf-mtDNA | 1.286 | <0.01 | 1.236 | 0.013 |
A Similar Trend Of Increased mtDNA Was Also Observed in the Mitochondria-Free Cytoplasm of PBMCs From SP Patients
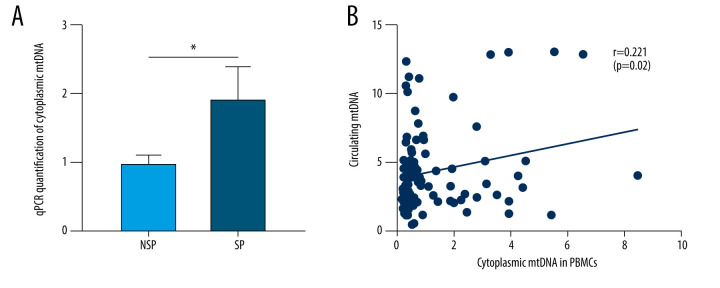
As a DAMP molecule, circulatory mtDNA can be easily engulfed by immune cells, thus fueling the secretion of proinflammatory cytokines [30]. Monocytes are a major source of the proinflammatory response factors. To determine whether this uptake is also present in SP patients, the mtDNA content in the cytoplasm of PBMCs was assessed. As expected, the content of cytoplasmic mtDNA in PBMCs of SP patients was significantly increased (Figure 1A). Correlation analysis revealed a positive correlation between PBMCs cytoplasmic mtDNA and circulating mtDNA (Figure 1B). Next, it will be interesting to investigate whether the cytosol-accumulated mtDNA initiates the subsequent inflammatory response.
Figure 1.
A similar trend of increased mtDNA was also observed in the mitochondria-free cytoplasm of PBMCs from SP patients. (A) Mitochondria-free mtDNA in PBMCs cytoplasm was measured using RT-qPCR targeting the Cytb region of mtDNA. * p<0.05 compared to NSP. (B) Spearman’s rank correlation analyses were performed between PBMCs cytosolic mtDNA and circulating mtDNA in MHD patients. Each dot represents data from one subject.
PBMCs Exhibit Increased Gene Expression Associated with Proinflammatory Activities in SP Patients
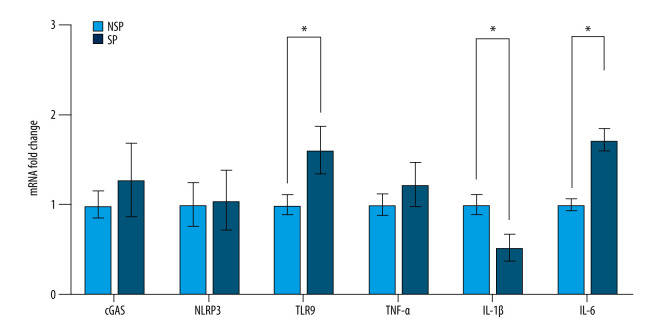
Previous research has shown that mtDNA recognition is established by pattern-recognition receptors (PRRs), such as TLR9 [31], NLRP3 [32] and cGAS-STING [33], which are essential for initiating the adaptive immune response. Based on this concept, we analyzed the mRNA levels of molecules downstream of mtDNA in PBMCs and found that the mRNA levels of TLR9 and IL-6 in SP group were significantly higher than that in the NSP group, whereas IL-1β levels were significantly lower in the SP group. No significant differences in cGAS, NLRP3 or TNF-α were observed between the 2 groups (Figure 2). As inflammation has been postulated to play a significant role in the development of sarcopenia, we speculated that the mtDNA-induced inflammation is likely one of the important causes for the MHD-related sarcopenia.
Figure 2.
PBMCs exhibit increased gene expression associated with proinflammatory activities in SP patients. The mRNA levels of cGAS, NLRP3, TLR9, TNF-α, IL-1β and IL-6 in PBMCs were detected using RT-qPCR. Data are normalized to GAPDH and are expressed relative to the NSP group. * p<0.05 compared to NSP.
Mitochondria Appear to be Impaired More Severely in Patients with Sarcopenia
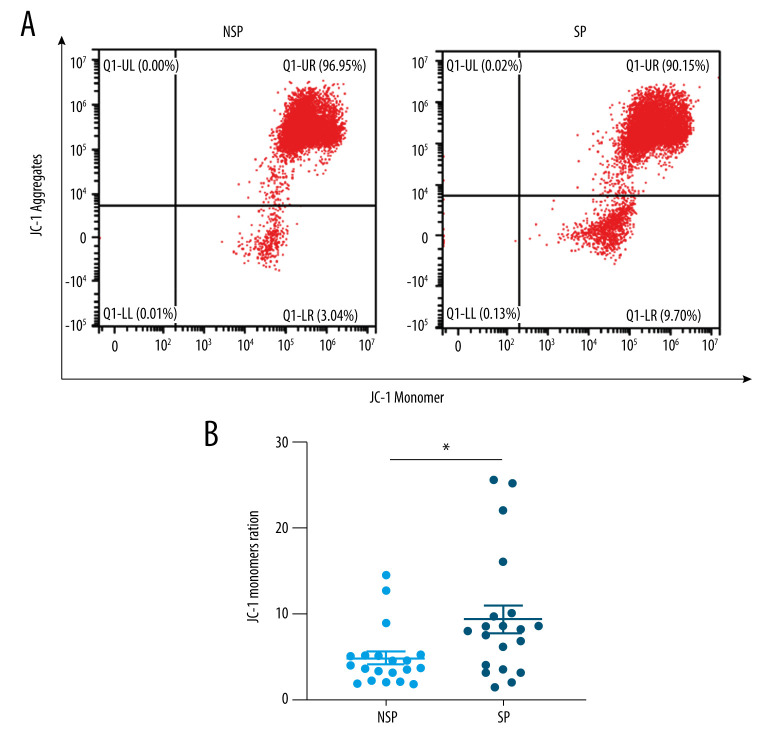
The depletion of ΔΨm, reactive oxygen species generation, increased membrane permeability, and calcium overload are characteristics of mitochondrial injury. To investigate the degree of mitochondrial damage, we assessed the loss of ΔΨm using JC-1. Since we were unable to acquire informed consent to obtain tissues from patients, we chose PBMCs, a mitochondria-rich source, as indicators of mitochondrial dysfunction in lieu of tissue biopsy collection. Notably, FACS analysis showed that ΔΨm was significantly decreased in the SP group compared to the NSP group (Figure 3A, 3B). This finding suggests that mitochondria might be impaired more severely in the tissues and cells of patients with sarcopenia, even in easily obtainable circulating PBMCs.
Figure 3.
(A, B) Mitochondria appear to be impaired more severely in patients with sarcopenia. Flow cytometry analysis showing the loss of ΔΨm in PBMCs from SP (n=20) and NSP (n=20) groups. * p<0.05 compared to NSP.
Significant Upregulation of the Mitophagy-Related Genes in Patients with Sarcopenia
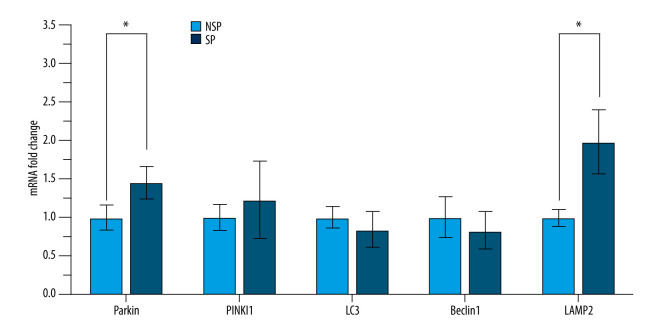
It is known that mitophagy represents a “double-edged sword” in the mtDNA release mechanism. To gain insight into the role of mitophagy in this process, we measured the expression of mitophagy-related genes in PBMCs using RT-qPCR. As shown in Figure 4, the mRNA levels of Parkin and LAMP2 in the SP group were significantly higher than those in the NSP group, while mRNA levels of PINK1, LC3 and Beclin1 were not significantly changed. These results suggest that mitophagy may be enhanced in patients with sarcopenia, enabling mtDNA leakage into the extracellular spaces.
Figure 4.
Significant upregulation of the mitophagy-related genes in patients with sarcopenia. Expression of Parkin, PINK1, LC3, Beclin1 and LAMP2 in PBMCs was detected by RT-qPCR. Data are normalized to GAPDH and are expressed relative to the NSP group. * p<0.05 compared to NSP.
Discussion
Inflammation is widely recognized as a “hallmark of sarcopenia”. Recently, mtDNA has been in the spotlight as an endogenous danger molecule that can potentially elicit inflammation [30]. However, its actual impact on sarcopenia, especially in MHD patients have yet to be investigated. The first striking observation is that the high copy number of circulating mtDNA was significantly associated with a higher risk of sarcopenia in MHD patients, and that the increase in plasma mtDNA might contribute to the onset and maintenance of proinflammatory status. Furthermore, we analyze the state of mitochondria and the activation of mitophagy and propose a potential mechanism for regulating the emergence of cf-mtDNA.
Many chronic diseases such as cardiovascular disease, cancer, chronic kidney disease, diabetes, and neurodegenerative diseases, among others, are initiated or worsened by systemic inflammation [34,35]. Nevertheless, evidence from recent works suggests that plasma levels of proinflammatory cytokines increase with the disease progresses independently of chronic antigenic stimulation, such as infections [36]. It is therefore possible that stimuli different from “classical” antigens derived from bacteria and viruses can also contribute to the inflammatory phenomenon. In this regard, it has been reported that intramitochondrial components, including mitochondrial DNA, cardiolipin, and formyl peptides, can be released extracellularly, enter the blood flow, and act as DAMP agents to activate membrane or cytoplasmic PRRs, such as TLR9 [31], NLRP3 [32] and cGAS-STING mediated pathways [33] and cause inflammation [12]. Thus, mitochondria not only participate in danger signaling inside the cell, but are also a major source of molecule able to activate an innate immune response. Recently, it has been shown that plasma levels of mtDNA are increased during pathological conditions, such as trauma, sepsis, aging, caner, kidney diseases and immune-mediated diseases, which are characterized by a chronic inflammatory status [9–13,15]. However, the possible contribution of mtDNA to the inflammatory milieu that characterizes muscle wasting disorders remains to be explored.
Muscle atrophy (sarcopenia) is a muscle wasting syndrome associated with several pathological conditions in humans such as aging, diabetes, cancer and kidney diseases, and the presence of sarcopenia worsens outcome [37]. Among the possible pathogenic mechanisms of sarcopenia, mitochondrial dysfunction has been actively investigated. Indeed, mitochondrial impairment and systemic inflammation are hallmarks of sarcopenia. The existence of crosstalk between inflammation and mitochondrial dysfunction in muscle wasting represents a novel and potentially relevant field of research. One study focused on the interface between inflammation and mitochondrial DAMPs in skeletal muscle at a cellular level. This investigation made use of Opa1-deficient muscle cell and reported that mtDNA signal co-localized with TLR9 and drove muscle inflammation in this context [31]. In the current study, we observed higher levels of ccf-mtDNA in the SP group. Univariate analysis and multivariate regression analyses both revealed that ccf-mtDNA levels were independently associated with the occurrence of sarcopenia in MHD patients. Thus, it could be implied that ccf-mtDNA can be employed as a predictive marker for MHD-related sarcopenia. It has previously been shown that the “out of place” mtDNA can be easily engulfed by immune cells and acts as a DAMP [30]. Accordingly, a similar trend of increased mtDNA was also observed in the mitochondria-free cytoplasm of PBMCs from SP patients, together with higher expression of TLR9 and IL-6 in this group. The above results support our hypothesis that high levels of circulating mtDNA, as those observed in sarcopenia subjects, polarize adaptive immune responses by activating monocytes, macrophages and possibly other antigen-presenting cells. However, it is noteworthy that such an increase was not present for cGAS, NLRP3, IL-1β or TNF-α in PBMCs, suggesting that alternative mechanisms might exist, which warrant further investigation.
Mitophagy is the selective degradation of damaged mitochondria by autophagy, contributing to maintenance of a healthy population of mitochondria. Moderate mitophagy is primarily responsible for the elimination of mitochondria with damaged mtDNA [38–40]. Nevertheless, excessive mitophagy triggers mitochondria damage, resulting in mitochondrial membrane fracturing and mtDNA leakage into extracellular fluid [41,42]. Hence, mitophagy acts as a “double-edged sword” in the process of mtDNA release. PBMCs are abundantly rich in mitochondria. ΔΨm is considered an important parameter of mitochondria function and the loss of ΔΨm is regarded as a hallmark of mitochondrial impairment. Recently, tests of PBMCs have been proposed to offer valid information about “general” mitochondrial health [24–26]. We found that ΔΨm was dramatically decreased in PBMCs from SP patients. Furthermore, RT-qPCR analysis showed a significant increase in Parkin and LAMP2 mRNA levels in SP group compared to NSP group. The combination of the data presented in this paper paint a picture of severely compromised mitochondria in patients with sarcopenia, and that the excessive induction of mitophagy may destroy the mitochondria and lead to the release of mtDNA into the blood circulation.
There were several limitations in our study that need to be acknowledged. First, due to the retrospective cross-sectional study design, a cause-and-effect relationship needs to be established in future studies. Second, although we observed the upregulation of mitophagy-related genes in SP patients, mitophagy may be induced as an adaptive rather than toxic response, which deserves further investigation. Third, we used PBMCs as an alternative cellular source, as no biopsies were performed. Therefore, more experiments are required to unequivocally determine the source of ccf-mtDNA.
Conclusions
In summary, we found that the higher copy number of cell-free mtDNA in peripheral blood is significantly associated with a higher risk of sarcopenia in MHD patients. A schematic diagram of the proposed pathway is shown in Figure 5. From a future perspective, the identification of the role of mtDNA could be of importance not only to identify possible new markers of diseases, but also in designing new therapeutic strategies against circulating mtDNA or its receptors to reduce a harmful immune activation.
Figure 5.
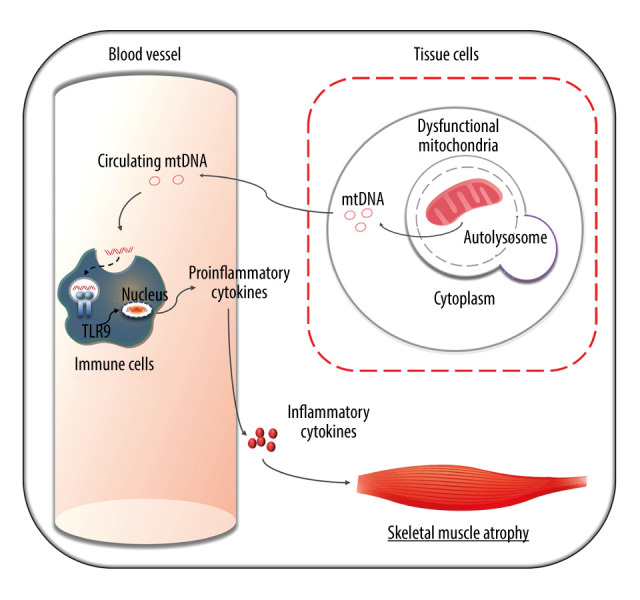
A schematic representation of mitophagy-mediated mtDNA release and inflammatory responses in MHD patients with sarcopenia. Mitochondrial dysfunction exists in a subset of patients undergoing hemodialysis. Excessive mitophagy may destroy the mitochondria, causing leakage of mtDNA into the extracellular fluid. mtDNA can be recognized by TLR9 in immune cells. TLR9 interacts with downstream signaling, leading to the transcriptional activation of proinflammatory cytokines. Proinflammatory cytokines then mature and transform into inflammatory cytokines, amplifying the inflammatory cascade, which ultimately results in skeletal muscle inflammation and sarcopenia.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This work was supported by Sichuan Provincial Cadre Health Research Project funded by the Sichuan Provincial Health Commission (Grant No. 2021-211)
References
- 1.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Sharma D, Hawkins M, Abramowitz MK. Association of sarcopenia with eGFR and misclassification of obesity in adults with CKD in the United States. Clin J Am Soc Nephrol. 2014;9:2079–88. doi: 10.2215/CJN.02140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bross R, Chandramohan G, Kovesdy CP, et al. Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis. 2010;55:885–96. doi: 10.1053/j.ajkd.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cigarrán S, Pousa M, Castro MJ, et al. Endogenous testosterone, muscle strength, and fat-free mass in men with chronic kidney disease. J Ren Nutr. 2013;23:e89–95. doi: 10.1053/j.jrn.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Marchant V, Tejera-Muñoz A, Marquez-Expósito L, et al. IL-17A as a potential therapeutic target for patients on peritoneal dialysis. Biomolecules. 2020;10:1361. doi: 10.3390/biom10101361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling F, Yoshida M. Rolling-circle replication in mitochondrial DNA inheritance: Scientific evidence and significance from yeast to human cells. Genes. 2020;11:514. doi: 10.3390/genes11050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moya GE, Rivera PD, Dittenhafer-Reed KE. Evidence for the role of mitochondrial DNA release in the inflammatory response in neurological disorders. Int J Mol Sci. 2021;22:7030. doi: 10.3390/ijms22137030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons JD, Lee YL, Mulekar S, et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258:591–96. doi: 10.1097/SLA.0b013e3182a4ea46. discussion 596–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu X, Yao Y, Wu G, Lv T, Luo L, Song Y. The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PLoS One. 2013;8:e72834. doi: 10.1371/journal.pone.0072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajizadeh S, DeGroot J, TeKoppele JM, et al. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5:R234–40. doi: 10.1186/ar787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinti M, Cevenini E, Nasi M, et al. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging”. Eur J Immunol. 2014;44:1552–62. doi: 10.1002/eji.201343921. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Feng M, Guan W. Mitochondrial DNA sensing by STING signaling participates in inflammation, cancer and beyond. Int J Cancer. 2016;139:736–41. doi: 10.1002/ijc.30074. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhao Y, Wen S, et al. Associations of mitochondrial haplogroups and mitochondrial DNA copy numbers with end-stage renal disease in a Han population. Mitochondrial DNA A, DNA Mapp Seq Anal. 2017;28:725–31. doi: 10.1080/24701394.2016.1177038. [DOI] [PubMed] [Google Scholar]
- 15.Cao H, Ye H, Sun Z, et al. Circulatory mitochondrial DNA is a pro-inflammatory agent in maintenance hemodialysis patients. PLoS One. 2014;9:e113179. doi: 10.1371/journal.pone.0113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Perumal E, Bi X, Wang Y, Ding W. Potential mechanisms of uremic muscle wasting and the protective role of the mitochondria-targeted antioxidant Mito-TEMPO. Int Uurol Nephrol. 2020;52:1551–61. doi: 10.1007/s11255-020-02508-9. [DOI] [PubMed] [Google Scholar]
- 18.Taylor D, Bhandari S, Seymour AM. Mitochondrial dysfunction in uremic cardiomyopathy. Am J Physiol Renal Physiol. 2015;308:F579–87. doi: 10.1152/ajprenal.00442.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigand KM, Schirris TJJ, Houweling M, et al. Uremic solutes modulate hepatic bile acid handling and induce mitochondrial toxicity. Toxicol In Vitro. 2019;56:52–61. doi: 10.1016/j.tiv.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Chang JF, Liang SS, Thanasekaran P, et al. Translational medicine in pulmonary-renal crosstalk: therapeutic targeting of p-Cresyl sulfate triggered nonspecific ROS and chemoattractants in dyspneic patients with uremic lung injury. J Clin Med. 2018;7:266. doi: 10.3390/jcm7090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Z, Lu F, Zheng Y, et al. Grape seed proanthocyanidin extract protects human umbilical vein endothelial cells from indoxyl sulfate-induced injury via ameliorating mitochondrial dysfunction. Renal Fail. 2016;38:100–8. doi: 10.3109/0886022X.2015.1104609. [DOI] [PubMed] [Google Scholar]
- 22.Ravi S, Mitchell T, Kramer P, Chacko B, Darley-Usmar VM. Mitochondria in monocytes and macrophages-implications for translational and basic research. Int J Biochem Cell Biol. 2014;53:202–7. doi: 10.1016/j.biocel.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindqvist D, Wolkowitz OM, Picard M, et al. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology. 2018;43:1557–64. doi: 10.1038/s41386-017-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer PA, Ravi S, Chacko B, et al. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–10. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czajka A, Ajaz S, Gnudi L, et al. Altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBioMedicine. 2015;2:499–512. doi: 10.1016/j.ebiom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose S, Carvalho E, Diaz EC, et al. A comparative study of mitochondrial respiration in circulating blood cells and skeletal muscle fibers in women. Am J Physio Endocrinol Metab. 2019;317:e503–12. doi: 10.1152/ajpendo.00084.2019. [DOI] [PubMed] [Google Scholar]
- 27.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Chiu RW, Chan LY, Lam NY, et al. Quantitative analysis of circulating mitochondrial DNA in plasma. Clin Chem. 2003;49:719–26. doi: 10.1373/49.5.719. [DOI] [PubMed] [Google Scholar]
- 29.Souza VA, Oliveira D, Barbosa SR, et al. Sarcopenia in patients with chronic kidney disease not yet on dialysis: Analysis of the prevalence and associated factors. PLoS One. 2017;12:e0176230. doi: 10.1371/journal.pone.0176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17:363–75. doi: 10.1038/nri.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Nuevo A, Díaz-Ramos A, Noguera E, et al. Mitochondrial DNA and TLR9 drive muscle inflammation upon Opa1 deficiency. EMBO J. 2018;37:e96553. doi: 10.15252/embj.201796553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong Z, Liang S, Sanchez-Lopez E, et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560:198–203. doi: 10.1038/s41586-018-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bode C, Fox M, Tewary P, et al. Human plasmacytoid dentritic cells elicit a Type I Interferon response by sensing DNA via the cGAS-STING signaling pathway. Eur J Immunol. 2016;46:1615–21. doi: 10.1002/eji.201546113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas SK. Does the Interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. 2011;75:2739–48. doi: 10.1253/circj.cj-11-1184. [DOI] [PubMed] [Google Scholar]
- 36.Rea IM, Gibson DS, McGilligan V, et al. Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sukhanov S, Semprun-Prieto L, Yoshida T, et al. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci. 2011;342:143–47. doi: 10.1097/MAJ.0b013e318222e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–30. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sliter DA, Martinez J, Hao L, et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561:258–62. doi: 10.1038/s41586-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Moore TM, Drew BG, et al. Estrogen receptor a controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci Transl Med. 2020;12:eaax8096. doi: 10.1126/scitranslmed.aax8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing R, Hu ZK, Lin F, et al. Mitophagy-mediated mtDNA release aggravates stretching-induced inflammation and lung epithelial cell injury via the TLR9/MyD88/NF-κB pathway. Front Cell Dev Biol. 2020;8:819. doi: 10.3389/fcell.2020.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimasuay KG, Schaunaman N, Martin RJ, et al. Parkin, an E3 ubiquitin ligase, enhances airway mitochondrial DNA release and inflammation. Thorax. 2020;75:717–24. doi: 10.1136/thoraxjnl-2019-214158. [DOI] [PubMed] [Google Scholar]