Abstract
Objective:
To quantify the amount of tooth movement and orthodontically induced root resorption (OIRR) in ovariectomized rats.
Materials and Methods:
Five 10-week-old female Wistar rats undergoing ovariectomy (OVX) were investigated as the experimental group, and the other five without ovariectomy served as the control group. Four weeks after ovariectomy, 25-g nickel-titanium closed-coil springs were applied mesially to the maxillary left first molars. Micro–computed tomography was taken at day 0, 1, 3, 7, 14, 21, and 28. At day 28, the molars were extracted. The surface area of root resorption craters, depth, and volume were measured using electron and laser scanning microscopes.
Results:
Tooth movement gradually increased with time throughout 28 days. There was a significant difference in the amount of tooth movement between the control group and the OVX group. For OIRR, the OVX group showed wide and shallow root resorption craters scattered on the mesial root. The deep resorption craters were observed on the distal roots distributed in the cervical, middle, and apical thirds of the roots. Statistically significant differences were found between the control and the OVX groups in the depth and the volume of root resorption craters in the distal roots and the total volume of root resorption craters in all three roots.
Conclusion:
Ovariectomy affected not only tooth movement but also OIRR. Tooth movement in the OVX group was more rapid than the control group. Furthermore, the amount of OIRR in the OVX group was more severe than the control group.
Keywords: OVX, Postmenopause, Orthodontic
INTRODUCTION
During orthodontic treatment, the teeth move individually at different rates, and the amount of tooth movement caused by bone remodeling can be influenced by drug usage and/or systemic factors.1 One of the most common systemic factors is a metabolic bone disorder called osteoporosis.2 Osteoporosis caused by either menopause or ovariectomy results in pathological bone loss.3
Wronski et al.4 observed trabecular bone loss in ovariectomized rats. They found that it was accompanied by accelerated bone resorption. Since tooth movement is involved in bone metabolism, all metabolic and hormonal changes affecting bone turnover may affect tooth movement as well. An increase of bone turnover caused by a reduced level of estrogen in the postmenopausal period affected the bone morphology5,6 and orthodontic treatment, so some research topics about the effects of estrogen on tooth movement were studied.7,8
Micro–computed tomography (micro-CT) has been used to calculate the amount of tooth movement and the initial changes in the periodontal ligament space. The linear and angular measurements are used for superimposition techniques to analyze the effect of different orthodontic force directions and magnitudes.9 For these reasons, micro-CT is a useful three-dimensional method that makes it possible to discover the novel information in orthodontics that could not be approached by a conventional radiographic technique.
Orthodontically induced root resorption (OIRR) is an unavoidable pathologic consequence and one undesirable side effect of orthodontic tooth movement.10 As indicated in an extensive review by Brezniak and Wasserstein,11,12 OIRR has been reported to be related to many factors such as age, sex, and systemic conditions. The techniques that analyze OIRR in animal models are improving. A scanning electron microscope (SEM) is the high visual equipment for studying the surface structure and also could be the image recorder to keep the detail of the mineralized tissue morphology.13 In addition, it is not only the surface appearance of the OIRR that is an interesting topic in orthodontic research but also the OIRR depth and area. The quantification of the depth of root resorption craters is possible using a three-dimensional (3D) laser scanning microscope with depth analysis software. In addition, the root resorption craters can be identified and calculated using area analysis software.14
To date, there have been some reports on the interrelationship between the effect of ovariectomy and tooth movement, but there is no report about the interrelationship between the effect of ovariectomy and the OIRR. Therefore, our study was performed.
MATERIALS AND METHODS
Ten 10-week-old female Wistar rats (SLC, Shizuoka, Japan; body weight, 170–190 g) were used in this study. This study was conducted with the approval from the Animal Welfare Committee of Nagasaki University. The rats were housed in pairs in plastic cages in a colony room and fed a standard pellet diet and water ad libitum. After arrival, the rats were allowed one week for acclimatization prior to the commencement of the experiments. Ten rats were randomly assigned to either the experimental group of five ovariectomized rats (OVX group) or the control group of five nonovariectomized rats.
Bilateral ovariectomy was performed for the OVX group under general anesthesia by intramuscular injection of ketamine hydrochloride at a dose of 87 mg/kg (Ketalar 50, Sankyo Co Ltd, Tokyo, Japan) in combination with xylazine hydrochloride at a dose of 13 mg/kg (Celactal 2%, Bayer-Japan Co Ltd, Tokyo, Japan). A sham operation was also performed for the control group, in which all procedures were the same, except for the removal of the ovaries.
Experimental Tooth Movement
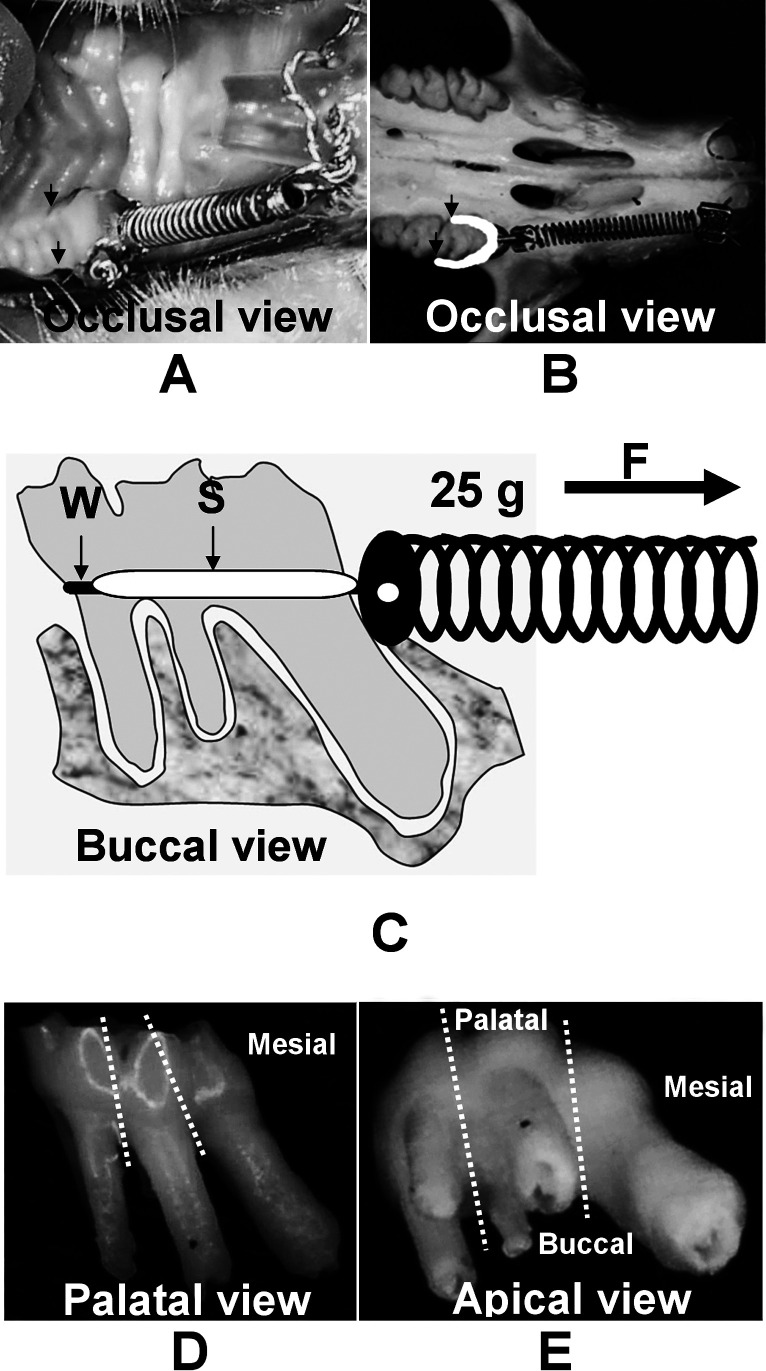
Four weeks after ovariectomy, 25-g nickel-titanium (NiTi) closed-coil springs (Sentalloy, Tomy Inc, Fukushima, Japan) were placed in both the control and OVX groups under general anesthesia. These springs were to move the maxillary left first molar mesially as described previously.9,15 The maxillary right first molars served as the negative controls. After the close-coil springs were set, self-curing resin (Super Bond, Sun Medical, Shiga, Japan) was applied (Figure 1A–C).
Figure 1.
Experimental orthodontic tooth movement in the rat molar. (A) Intraoral photograph. (B) Rat's dry skull showing the appliance in situ. The arrows show the self-cured resin (Super Bond) extending from the disto-buccal line angle to the disto-palatal line angle, and the black mark in (B) was drawn to show the self-cured resin line. (C) A schema shows the appliance design. The arrows show the area of self-cured resin (S) and a twisted ligature wire (W). (D, E) The maxillary right first molar (negative control) with the separation lines is shown.
Under general anesthesia as described previously, a micro-CT (Rigaku Co, Tokyo, Japan) was taken at day 0 (before and immediately after the orthodontic appliance was applied). This process was repeated at day 1, 3, 7, 14, 21, and 28 (before and immediately after the orthodontic appliance was removed) in the same animal. At day 28, the rats were sacrificed by CO2 inhalation. Tooth movement was measured by the image reconstruction using i-VIEW software (J. Morita MFG Corp, Kyoto, Japan) from the micro-CT images by the appropriate cross-section using landmark points identified on the first molar's crown and roots. The distance from the distal contact point of the maxillary left first molar and the mesial contact point of the maxillary left second molar was investigated to represent the distance of tooth movement. In addition, the micro-CT images of the rats were used for the superimposition technique using commercial 3D medical image analysis software (RATOC, Ratoc System Engineering, Tokyo, Japan).
Orthodontically Induced Root Resorption
After the experimental tooth movements, the rats were sacrificed and the maxillary left first molars were extracted. In addition, the maxillary right first molars in which all of the other procedures were exactly the same were used as the negative control. The extracted molars were submerged in 1% sodium hypochlorite for 10 minutes to eliminate the periodontal ligament remnants. The five roots of the maxillary left first molar were divided into three parts using a diamond disc (Figure 1D,E). Only the mesial and the distal roots (disto-buccal and disto-palatal) were used in this study. The mesial surfaces of the roots were scanned using an SEM (TM-1000, Hitachi, Tokyo, Japan). The area of the resorption craters was measured by means of commercial software (Mimics program, Materialise, Leuven, Belgium), and the depth of the root resorption craters was evaluated with a 3D laser scanning microscope (VK-8500, Keyence, Kyoto, Japan) followed by Scion Imaging software (Scion Corp, Frederick, Md).
The same investigator performed all measurements, and all measurements were repeated three times. The mean value was used as the final measurement.
Statistical Analysis
Statistical analysis was performed using SPSS software (version 16.0, SPSS, Chicago, Ill). The Mann-Whitney test was used to compare the amount of tooth movement and the severity of root resorption between groups.
RESULTS
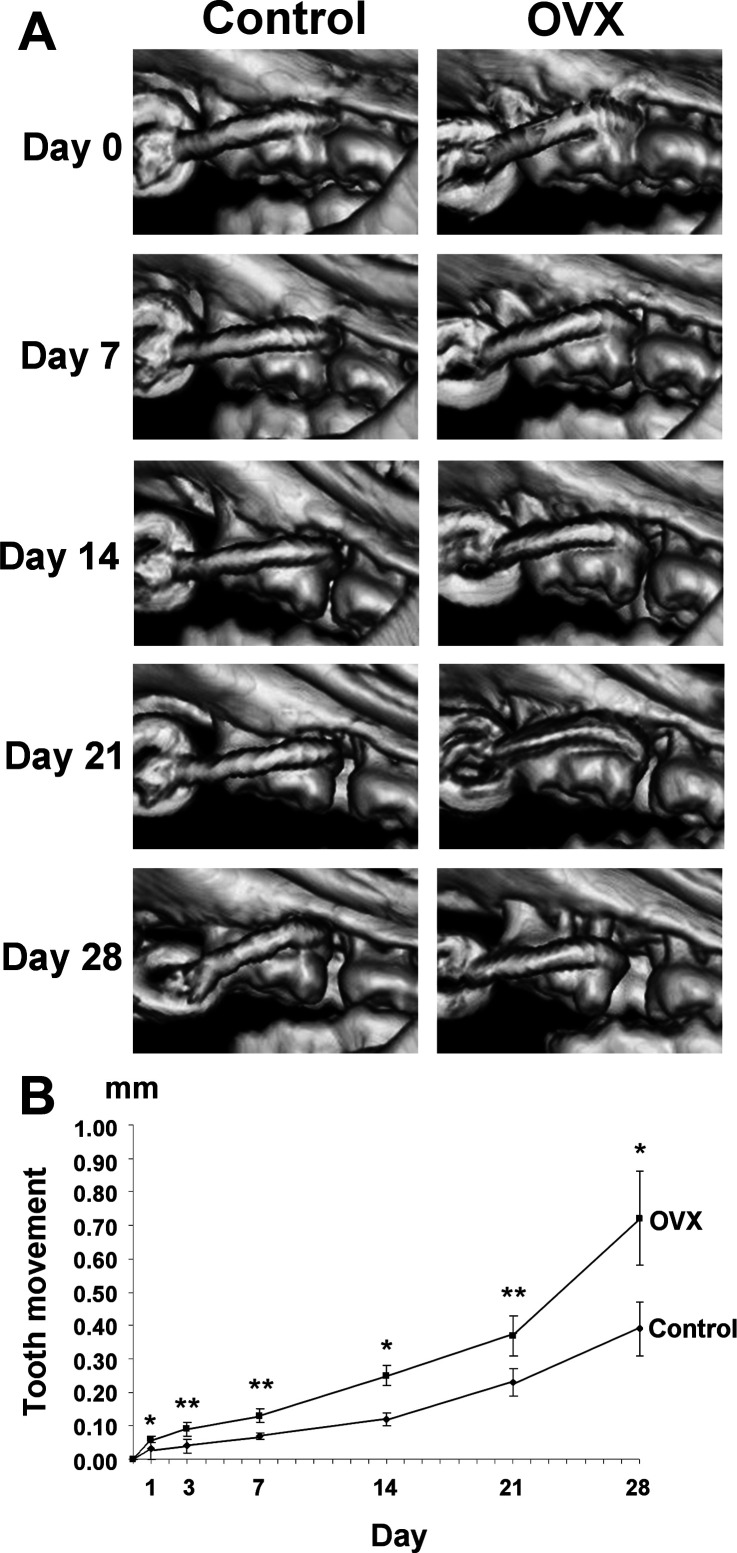
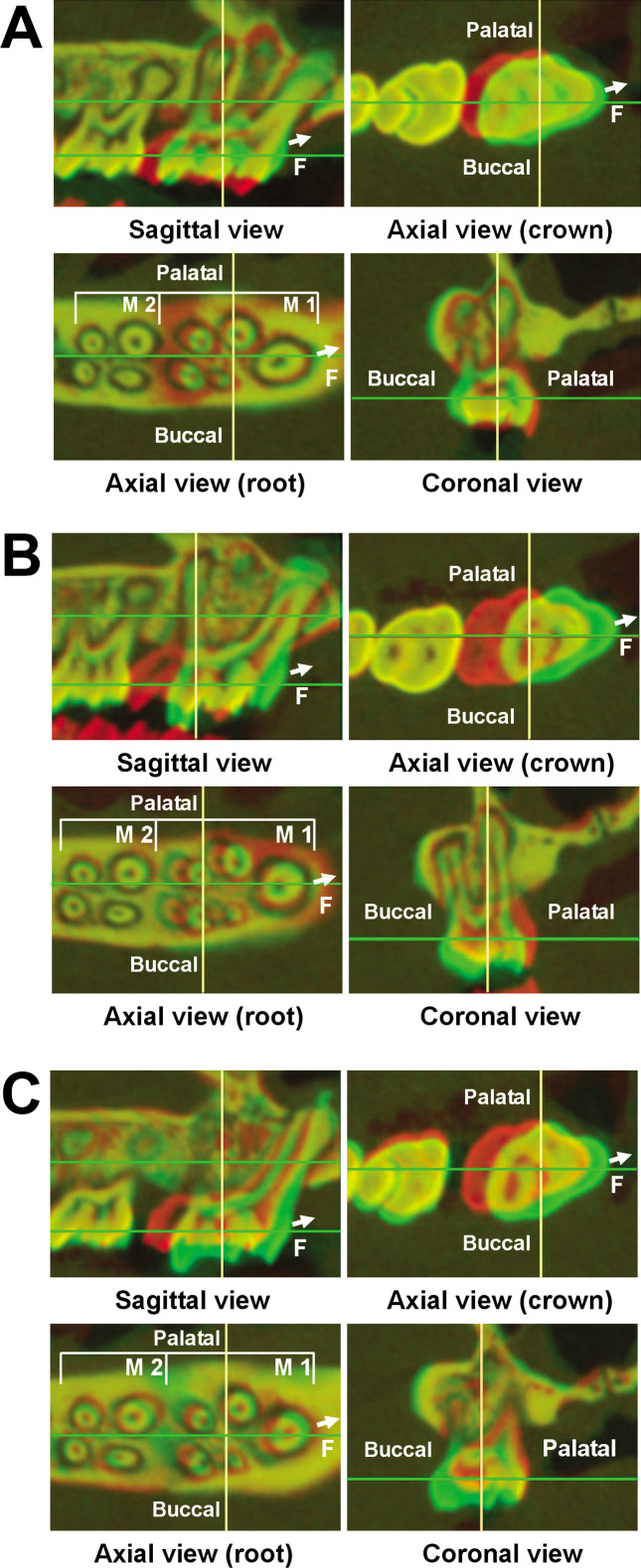
Experimental tooth movement, quantified by using 3D micro-CT images at different time points, showed that the tooth movement in the OVX group was greater than in the control group throughout the experimental periods (Figure 2A,B; Table 1). In addition, the superimposition images from sagittal, axial (crown and root levels), and coronal views showed the direction of tooth movement (Figure 3A–C). The amount of tooth movement in the OVX group was obviously more rapid than in the control group.
Figure 2.
Results of tooth movement. (A) Comparison of tooth movement between the control and the ovariectomy (OVX) group using the three-dimensional micro–computed tomography images at day 0, 7, 14, 21, and 28. (B) A graph of tooth movement at day 0, 1, 3, 7, 14, 21, and 28 between the control group and the OVX group. * P < .05; ** P < .01 (Mann-Whitney test).
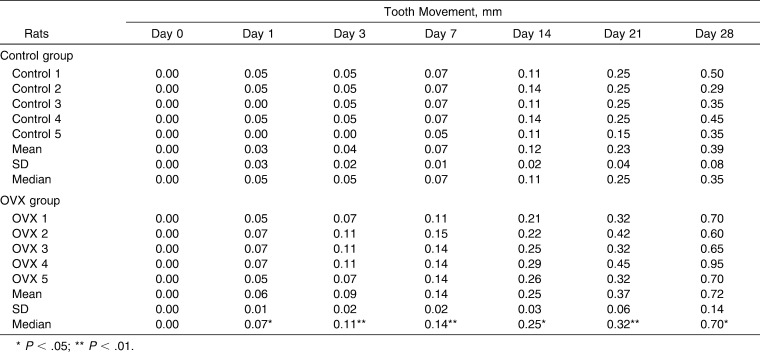
Table 1.
Experimental Tooth Movement Comparison Between the Control and the Ovariectomy (OVX) Groups at Day 0, 1, 3, 7, 14, 21, and 28 on the Mesial Root (M), the Disto-buccal Root (DB), and the Disto-palatal Root (DP)
Figure 3.
Superimposition of the computed tomography images. (A) A control rat at day 0 (red) and day 28 (green) in the sagittal, axial, and coronal views is shown. The overlapped area is shown in yellow. The arrows show the orthodontic force direction. (B) An ovariectomy (OVX) rat at day 0 (red) and day 28 (green) in the sagittal, axial, and coronal views is shown. (C) A control rat (red) and an OVX rat (green) at day 28 in the sagittal, axial, and coronal views are shown.
The amount of tooth movement gradually increased from day 0 until the end of experiments in both the control group and the OVX group. The initial tooth movement was 0.03 ± 0.03 mm in the control group and 0.06 ± 0.01 mm in the OVX group (Figure 2B). The tooth movement results are detailed in Table 1. There were statistically significant differences in the amount of tooth movement between the control group and the OVX group (P < .01 at day 3, 7, and 21; P < .05 at day 1, 14, and 28).
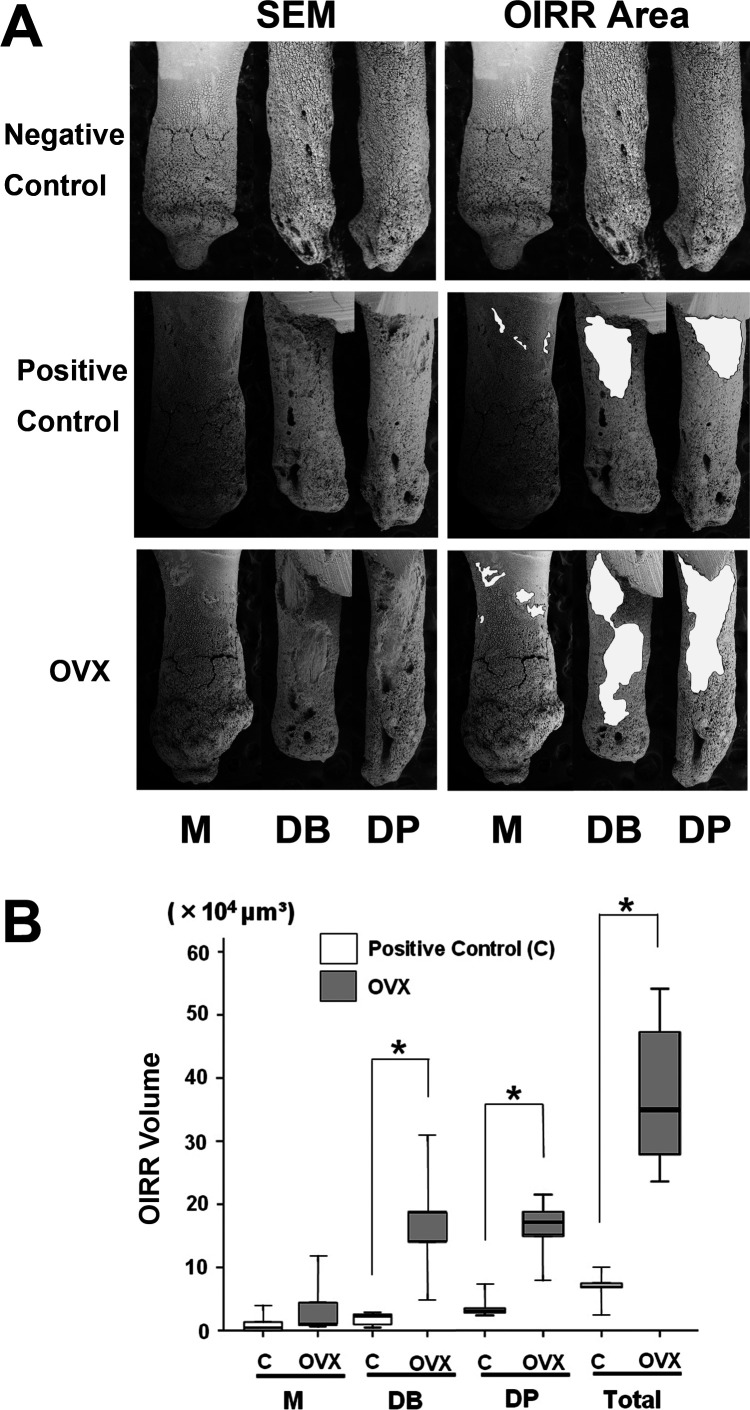
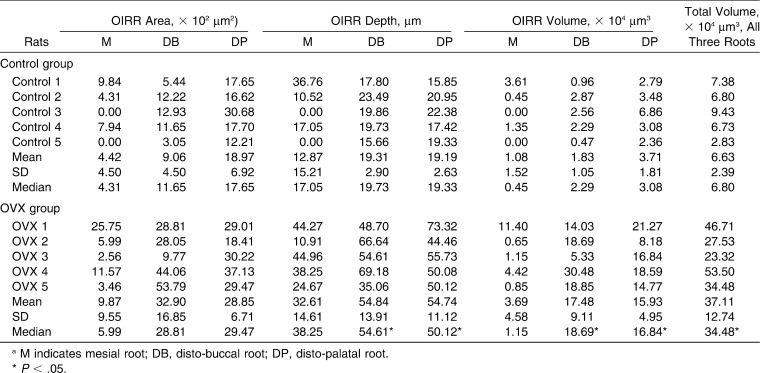
SEM images of the mesial, disto-buccal, and disto-palatal roots in both the control group and the OVX group are shown in Figure 4A. Most negative control roots were covered by undamaged cementum with a characteristic smooth surface. Three types of craters were clearly identified in the experimental group: isolated lacunae, wide and shallow resorption pits, and deep resorption craters. Small isolated lacunae were mainly seen scattered on the cervical half of the mesial surface of the mesial roots in both the positive control group and the OVX group. Wide craters were observed on the disto-buccal and disto-palatal roots covering the cervical and middle portions of the roots in the control group. On the other hand, wide and deep resorption craters were observed in the disto-buccal and disto-palatal roots not only at the cervical and middle portions of the roots but also at the apical portion in the OVX group. There were significant differences (P < .05) between the positive control group and the OVX group in the depth and the volume of OIRR in the disto-buccal and disto-palatal roots and the total OIRR volume in all three roots. However, there was no significant difference between the control and the OVX group when the depth and the volume of OIRR in the mesial roots were analyzed. In addition, no significant difference was found in the OIRR area in all the roots (Figure 4B; Table 2).
Figure 4.
Results of orthodontically induced root resorption (OIRR). (A) Scanning electron microscope images (magnification 50×) of the mesial root (M), the disto-buccal root (DB), and the disto-palatal root (DP) of the maxillary left first molar in the negative control, the positive control, and the OVX groups are shown. The areas of OIRR are painted in white on the right. (B) Box plots of OIRR volume in the mesial root (M), the disto-buccal root (DB), the disto-palatal root (DP), and the total OIRR volume in all three roots. * P < .05 (Mann-Whitney test).
Table 2.
Area, Depth, Volume, and Total Volume in All Three Roots of OIRR in the Control and the OVX Groupsa
Nevertheless, the results showed that the OIRR depth in the OVX group was obviously deeper than in the control group in all the roots. Furthermore, the OIRR area also demonstrated similar results, that is, the OIRR area in the OVX group was distinctively larger than the control group in all the roots.
DISCUSSION
In our experiment, we used 3D methods to quantify experimental tooth movement and also OIRR. The maxillary left first molars in the OVX group moved more rapidly than the control group throughout the experimental periods, day 1, 3, 7, 14, 21, and 28 (Table 1). Several studies16,17 reported that experimental tooth movement in a rat model was divided into three phases: the first phase, initial tooth movement; the second phase, delayed tooth movement; and the last phase, a linear increment of tooth movement. In the present study, our results were in agreement with the tooth movement phases that have been classically advocated. However, it is important to mention that a significant difference between the control rats and the OVX rats was found even on day 1. The teeth moved after 1 day of force application: 0.03 ± 0.03 mm in the control group and 0.06 ± 0.01 mm in the OVX group (Table 1). This small amount of tooth movement might be the result of the initial compression within the periodontal ligament space after force application. We also found that tooth movement from day 0 until day 7 showed a gradual increase, and after day 7, tooth movement showed a steeper slope that continued until day 21. The peak velocity of tooth movement was observed between day 21 and day 28 in both groups (Figure 2B).
In ovariectomized rats, osteogenesis and also chondrogenesis are decreased because estrogen deficiency alters the production of osteoinductive proteins such as osteogenin and bone morphogenetic protein, which results in the disruption of bone matrix formation.18 This finding suggests that the effect of ovariectomy was related to the bone turnover rate caused by the reduction of estrogen levels in the OVX group.5,6 In the present study, tooth movement in the ovariectomized group was more rapid than in the nonovariectomized group. The ovariectomy might have increased bone turnover and led to the acceleration of tooth movement. In addition, Wronski et al.4 found that ovariectomy induced osteopenia and increased the indices of bone resorption and formation in the rat tibia at day 14 after ovariectomy, and the maximal increase in the bone resorption parameters in the femur occurred up to a few months postovariectomy. Some studies19,20 that evaluated the hormonal effect on tooth movement reported that the rate of experimental tooth movement was increased in the osteoporotic alveolar bone due to a systemic-osteoporotic hormonal imbalance.
Several investigators attempted to clarify the precise cellular mechanisms to explain the theory of root resorption. A recent study found that root resorption was involved not only in osteoclastogenesis but also in odontoclastogenesis via the OPG/RANK/RANKL system. This system includes the balance between OPG and RANKL on the tension and the compression side of the tooth during orthodontic tooth movement.21 These factors may have been modulated by the loss of estrogen by ovariectomy and possibly increased osteoclastogenesis.22 From these results, the OIRR in the OVX group may have been more severe than in the control group. Thus, the present study might indicate that ovariectomy affects OIRR as well as tooth movement through the hormonal system by the alteration of bone metabolism via some biomarkers and biological pathways.
Further investigations are needed to elucidate the relationship between orthodontic treatment and the hormonal changes that occur in women. Demand of orthodontic treatment for adult patients is increasing. Therefore, it is important to investigate how age change affects orthodontic treatment and how possible pharmacological interactions are related to them.
CONCLUSIONS
Initial tooth movement was observed one day after orthodontic force was applied, and tooth movement in the OVX group was more rapid than in the control group throughout the experimental periods.
OIRR in the OVX group was deeper and more severe than in the control group.
Acknowledgments
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Ghoneima A. A, Allam E. S, Zunt S. L, Windsor L. J. Bisphosphonates treatment and orthodontic considerations. Orthod Craniofac Res. 2010;13:1–10. doi: 10.1111/j.1601-6343.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- 2.Dempster D. W, Lindsay R. Pathogenesis of osteoporosis. Lancet. 1993;341:797–801. doi: 10.1016/0140-6736(93)90570-7. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay R, Hart D. M, Aitken J. M, MacDonald E. B, Anderson J. B, Clarke A. C. Long-term prevention of postmenopausal osteoporosis by oestrogen: evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976;1:1038–1041. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- 4.Wronski T. J, Cintrón M, Doherty A. L, Dann L. M. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology. 1988;123:681–686. doi: 10.1210/endo-123-2-681. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh Y. D, Devlin H, McCord F. The effect of ovariectomy on the healing tooth socket of the rat. Arch Oral Biol. 1995;40:529–531. doi: 10.1016/0003-9969(94)00197-j. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Ejiri S, Toyooka E, Kohno S, Ozawa H. Effects of ovariectomy on trabecular structures of rat alveolar bone. J Periodontal Res. 2002;37:161–165. doi: 10.1034/j.1600-0765.2002.01601.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyajima K, Nagahara K, Iizuka T. Orthodontic treatment for a patient after menopause. Angle Orthod. 1996;66:173–180. doi: 10.1043/0003-3219(1996)066<0173:OTFAPA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Yamashiro T, Takano-Yamamoto T. Influences of ovariectomy on experimental tooth movement in the rat. J Dent Res. 2001;80:1858–1861. doi: 10.1177/00220345010800091701. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales C, Hotokezaka H, Arai Y, et al. An in vivo 3D micro-CT evaluation of tooth movement after the application of different force magnitudes in rat molar. Angle Orthod. 2009;79:703–714. doi: 10.2319/071308-366.1. [DOI] [PubMed] [Google Scholar]
- 10.Brezniak N, Wasserstein A. Orthodontically induced inflammatory root resorption. Part I: the basic science aspects. Angle Orthod. 2002;72:175–179. doi: 10.1043/0003-3219(2002)072<0175:OIIRRP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: part 1. Literature review. Am J Orthod Dentofacial Orthop. 1993;103:62–66. doi: 10.1016/0889-5406(93)70106-X. [DOI] [PubMed] [Google Scholar]
- 12.Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: part 2. Literature review. Am J Orthod Dentofacial Orthop. 1993;103:138–146. doi: 10.1016/S0889-5406(05)81763-9. [DOI] [PubMed] [Google Scholar]
- 13.Boyde A, Jones S. J. Scanning electron microscopy of cementum and Sharpey fibre bone. Z Zellforsch Mikrosk Anat. 1968;92:536–548. doi: 10.1007/BF00336664. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales C, Hotokezaka H, Darendeliler M. A, Yoshida N. Repair of root resorption 2 to 16 weeks after the application of continuous forces on maxillary first molars in rats: a 2- and 3-dimensional quantitative evaluation. Am J Orthod Dentofacial Orthop. 2010;137:477–485. doi: 10.1016/j.ajodo.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian J. H, Darendeliler M. A, Yoshida N. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 2008;78:502–509. doi: 10.2319/052007-240.1. [DOI] [PubMed] [Google Scholar]
- 16.Reitan K, Kvam E. Comparative behavior of human and animal tissue during experimental tooth movement. Angle Orthod. 1971;41:1–14. doi: 10.1043/0003-3219(1971)041<0001:CBOHAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Storey E. The nature of tooth movement. Am J Orthod. 1973;63:292–314. doi: 10.1016/0002-9416(73)90353-9. [DOI] [PubMed] [Google Scholar]
- 18.Cesnjaj M, Stavljenic A, Vukicevic S. Decreased osteoinductive potential of bone matrix from ovariectomized rats. Acta Orthop Scand. 1991;62:471–475. [PubMed] [Google Scholar]
- 19.Ashcraft M. B, Southard K. A, Tolley E. A. The effect of corticosteroid-induced osteoporosis on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1992;102:310–319. doi: 10.1016/0889-5406(92)70046-D. [DOI] [PubMed] [Google Scholar]
- 20.Midgett R. J, Shaye R, Fruge J. F., Jr The effect of altered bone metabolism on orthodontic tooth movement. Am J Orthod. 1981;80:256–262. doi: 10.1016/0002-9416(81)90289-x. [DOI] [PubMed] [Google Scholar]
- 21.Tyrovola J. B, Spyropoulos M. N, Makou M, Perrea D. Root resorption and the OPG/RANKL/RANK system: a mini review. J Oral Sci. 2008;50:367–376. doi: 10.2334/josnusd.50.367. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz M. C. Cytokines and estrogen in bone: antiosteoporotic effects. Science. 1993;260:626–627. doi: 10.1126/science.8480174. [DOI] [PubMed] [Google Scholar]