Abstract
Objective:
To examine periodontal changes and the relative quantity of subgingival Porphyromonas gingivalis during orthodontic treatment.
Materials and Methods:
The study subjects were recruited consecutively among malocclusion patients seeking orthodontic treatment. Group A comprised 28 subjects (17.6 ± 5.68 years of age) at the beginning of orthodontic treatment, and group B comprised 20 subjects (17.8 ± 4.49 years of age) at the end of orthodontic treatment. Plaque index (Pl.I), gingival index (GI), and probing pocket depth (PPD) were measured before and after appliance placement in group A and before and after appliance removal in group B. Real-time quantitative polymerase chain reaction was used to quantify P. gingivalis in subgingival plaque at each time point.
Results:
There was a significant increase in Pl.I and GI during the first 3 months of appliance placement but a significant decrease in Pl.I, GI, and PPD during the first 6 months after appliance removal. The carriage and relative quantity of subgingival P. gingivalis were high at the end of orthodontic treatment, and they decreased significantly after appliance removal. The amount of subgingival P. gingivalis after appliance removal (for a period of 6 months) was higher than the amount measured before appliance placement.
Conclusions:
Fixed orthodontic treatment is conducive to dental plaque accumulation and gingival inflammation. In our study, after removal of orthodontic appliances, periodontal health improved, and the carriage and amount of subgingival P. gingivalis decreased. Nevertheless, the amount of subgingival P. gingivalis remained high for 6 months after appliance removal, and this finding might imply a potential risk to periodontal health in certain patients.
Keywords: Fixed orthodontic treatment, Periodontal clinical examination, Porphyromonas gingivalis, Real-time PCR
INTRODUCTION
Fixed orthodontic treatment is the preferred and most common method for treating malocclusion. Fixed appliances such as brackets, bands, or fixed retentions may complicate an optimal oral hygiene, and this may result in accumulation of dental plaque and gingival inflammation.1,2 However, the effect of orthodontic treatment on the periodontal tissues in the long term is questionable. Bone loss may be observed during or immediately after termination of orthodontic treatment.3 A 10-year retrospective study4 concluded that orthodontic treatment during adolescence had no distinct effect upon later periodontal health.
Ample evidence indicates the gram-negative obligate anaerobe Porphyromonas gingivalis as a putative periodontal pathogen in subgingival dental plaque. P. gingivalis plays an important role in the onset and progression of periodontal diseases, and it is implicated as an indicator of periodontal disease.5–9 Gingival changes and oral bacteria in plaque have been studied during orthodontic treatment in adolescents and young adults.10–12 However, changes in periodontal health and the role of P. gingivalis in subgingival plaque at the different stages of orthodontic treatment have not received the same attention.
The aims of the present study were to examine changes in periodontal health in young subjects during orthodontic treatment and to assess the variation, if any, in subgingival P. gingivalis during the treatment.
MATERIALS AND METHODS
Participants
The participants were recruited consecutively among malocclusion patients seeking orthodontic treatment at the Orthodontics Section, First Affiliated Hospital of Dalian Medical University in China. Group A comprised 28 subjects, 22 females and 6 males (17.6 ± 5.68 years of age; range, 12 to 28 years), who were at the beginning of orthodontic treatment. Group B comprised 20 subjects, 13 females and 7 males (17.8 ± 4.49 years of age; range, 12 to 29 years), who were at the completion of orthodontic treatment. The inclusion criteria were the malocclusion angle's Class I, II, and III. The exclusion criteria were decayed teeth, bad oral habits (eg, thumb sucking, nail grinding, and mouth breathing), use of antibiotics or hormones 1 month before the study, concomitant systemic diseases, pregnancy, and lactation. Informed consent forms were signed by either the subjects themselves or their legal guardians.
Fixed Orthodontic Treatment and Oral Hygiene
All of the subjects were treated with straight-wire appliances on directly bonded brackets (Xinya, Hangzhou, China) on incisors, canines, and premolars and orthodontic bands cemented with glass-ionomer cement on the first molars. Professional oral cleaning and oral hygiene instructions on toothbrushing with the Bass technique and correct use of interdental cleaning devices were provided for all subjects 4 weeks before orthodontic treatment. After removal of the fixed appliances the patients were required to wear the Hawley removable retainer at night.
Periodontal Examination
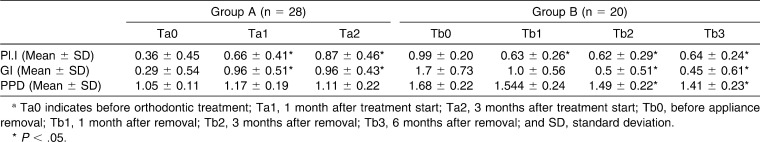
A periodontal examination was performed by three calibrated examiners with a marked periodontal probe (YDM, Tokyo, Japan) at three time points for group A (Ta0 = just before appliance placement, Ta1 = 1 month after appliance placement, Ta2 = 3 months after appliance placement) and at four time points for group B (Tb0 = just before appliance removal, Tb1 = 1 month after appliance removal, Tb2 = 3 months after appliance removal, Tb3 = 6 months after appliance removal). Plaque index (Pl.I),13 gingival index (GI),13 and probing pocket depth (PPD) were registered. Eight teeth from each subject (teeth 15, 13, 11, 21, 33, 31, 41, 45) were selected for periodontal examination, and each tooth was examined on its labial-mesial, labial-middle, and labial-distal sites. The score for each patient was the mean of the measurements on all of the teeth examined.
Subgingival Plaque Sampling
After gently removing supragingival plaque, subgingival plaque samples were taken by inserting a sterile dental curette into the bottom of the gingival crevice during the clinical examination. The samples were collected from the labial-medial and labial-distal surfaces of teeth 31, 32, 41, and 42. The samples from each tooth were pooled into an Eppendorf tube containing 1 mL of sterile saline and stored immediately at −70°C. P. gingivalis strain ATCC 33277 (Sichuan University) was used as a reference strain.
Quantification of P. gingivalis and Total Subgingival Bacteria by Real-Time Polymerase Chain Reaction
The subgingival plaque samples were thawed at room temperature and washed with Tris (pH 8.0). Total DNA was extracted by adding 10 mg/mL lysozyme; incubating in a 37°C water bath for 1 hour; adding 10% sodium dodecyl sulfate and 10 mg/mL proteinase K; incubating in a 56°C water bath for 12 hours; adding an equal volume of phenol/chloroform; and mixing well by inverting the tube until the phases were completely mixed. After centrifuging, the top layer was carefully transferred to a new tube. DNA was precipitated using 95% and 70% cold ethanol and was then dissolved in 20 µL TE buffer. DNA qualification was measured by electrophoresis and spectrophotometer analysis.
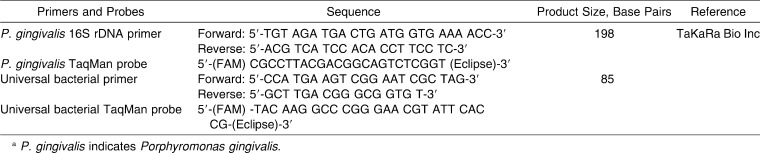
Real-time polymerase chain reaction (PCR) (TaqMan system) was applied to determine both the quantity of P. gingivalis and the quantity of the total bacteria cells presenting in subgingival plaque samples.14,15 In order to eliminate the bias caused by sampling method, a relative quantity of P. gingivalis was used as P. gingivalis number per million total bacteria.14 The primers and probes applied in real-time PCR were designed by TaKaRa Bio Inc, Dalian, China, (Table 1). PCR reaction was set up in a total volume of 25 µL containing Premix Ex Taq (2X) at 12.5 µL, each primer (10 µM) at 1 µL, probe (3 µM) at 1 µL, template DNA at 1 µL, and dH2O at 8.5 µL. Real-time PCR ran at 95°C for 10 seconds; this was followed by 45 cycles at 95°C for 5 seconds and 60°C for 30 seconds, with PCR Thermal Cycler Dice (TaKaRa Inc, Dalian, China).
Table 1.
Primers and Probes for Real-Time Polymerase Chain Reaction (PCR) Analysesa
Statistical Analysis
SPSS 16.0 for Windows was used for statistical analysis. Analysis of variance was used to analyze the longitudinal data of the periodontal indices and the square root of the relative quantity of subgingival P. gingivalis in each subject; an independent t-test was used for comparing the relative quantity of P. gingivalis between the two groups; and the Spearman test was used for correlation analysis. P ≤ .05 was chosen as the significance level.
RESULTS
Clinical Findings
The measurements of Pl.I, GI, and PPD from two groups of subjects are shown in Table 2. Pl.I, GI, and PPD were low before the commencement of treatment. After the first 3 months of appliance wear, there was a significant increase in Pl.I and GI, but no obvious changes in PPD. By the end of the orthodontic treatment, Pl.I, GI, and PPD were rather high. After 3 to 6 months post–appliance removal, there was a significant decrease in Pl.I, GI, and PPD.
Table 2.
Plaque Index (Pl.I), Gingival Index (GI), and Probing Pocket Depth (PPD) in Patients During Orthodontic Treatmenta
P. gingivalis in Subgingival Plaque
The frequency of patients with subgingival P. gingivalis during orthodontic treatment is shown in Figure 1. During the first 3 months of appliance wear, the frequency with which the patient carried subgingival P. gingivalis decreased. The frequency of patients carrying subgingival P. gingivalis was high at the end of orthodontic treatment, and it dropped after appliance removal.
Figure 1.
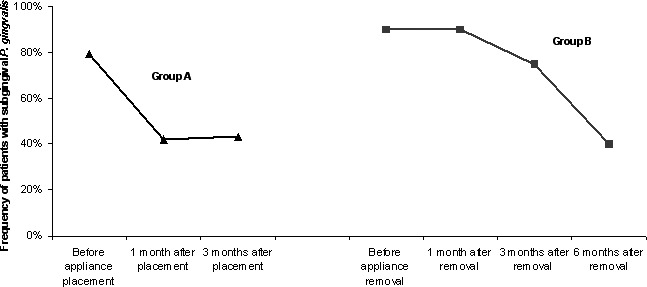
Frequency of patients with subgingival Porphyromonas gingivalis during orthodontic treatment.
The relative quantity of subgingival P. gingivalis in patients following orthodontic treatment is shown in Figure 2. The relative amount of subgingival P. gingivalis varied largely among individuals. Generally, P. gingivalis was detected at a rather low proportion in the total subgingival bacteria during the beginning of orthodontic treatment. The relative amount of subgingival P. gingivalis was high at the end of orthodontic treatment, and this proportion decreased significantly 6 months after appliance removal. Nevertheless, the relative quantity of P. gingivalis after 6 months was higher than the quantity measured before appliance placement.
Figure 2.
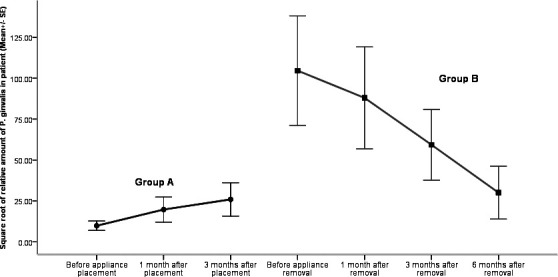
Relative quantity of subgingival Porphyromonas gingivalis in patients during orthodontic treatment.
Association Between the Relative Quantity of P. gingivalis and Periodontal Conditions
In group A, no obvious association was found between the relative quantity of subgingival P. gingivalis and clinical periodontal indices. In group B, there was a significant correlation between the relative quantity of subgingival P. gingivalis and GI.
DISCUSSION
The present study has described periodontal and microbiological changes during fixed orthodontic treatment in two groups of patients. Group A was followed from the baseline until the 3 months after orthodontic appliance placement. Group B was examined from just before removal of orthodontic appliance to 6 months postremoval. The two groups were comparable with respect to participants' age and gender.
Fixed orthodontic treatment resulted in dental plaque accumulation and gingival inflammation, with a significant increase in Pl.I and GI a short time after orthodontic treatment started (compared to the baseline). The treatment had no obvious effect on PPD. However, this observation might be affected by the short-term (3-month) evaluation period. Just before removal of the orthodontic appliance high scores of Pl.I, GI, and PPD were registered. The increase in plaque accumulation and gingival score during orthodontic treatment is probably a consequence of the plaque retentive effect of the orthodontic appliances, which makes performance of oral hygiene more difficult.12
A critical site for plaque accumulation is the excess composite around the bracket base (as a result of its rough surface and the presence of a distinct gap at the composite enamel surface).16 The placement of brackets should therefore be done under optimal conditions. Six months after orthodontic appliance removal, Pl.I, GI, and PPD decreased significantly among the patients in group B. The orthodontic treatment seemed to have no permanent effects on the oral hygiene situation and gingival inflammation. One must keep in mind that some subjects in this study were in the circum-pubertal age range. It has been shown17 that gingival inflammation and gingival bleeding will increase in children of pubertal age as a result of the hormone changes that occur during puberty. In the literature the influence of orthodontic treatment on periodontal tissues is somewhat unclear. A systematic review on the effect of orthodontic therapy on periodontal health concluded that the effects of orthodontic therapy on gingivitis and attachment loss were inconsistent across studies and that there is an absence of evidence describing positive effects of orthodontic treatment on periodontal health, but many findings indicate that orthodontic therapy may result in small detrimental effects to the periodontium.2 Orthodontic treatment followed by the use of postorthodontic fixed retainers has been found18 to be associated with an increased incidence of gingival recession, increased plaque retention, and increased bleeding on probing. The magnitude of the recession was of low clinical significance.
The subjects in the present study were supplied with a removable retention appliance, and this may be one of the reasons for the better oral hygiene and gingival conditions found. Gingival recession was not registered in our study. Some studies have shown that fixed orthodontic treatment may result in periodontal bone loss within a short period after the placement of bands and brackets12,19,20 and loss of clinical attachment after fixed orthodontic treatment.12,21 In a longitudinal study of alveolar bone loss in orthodontic-treated teenagers over a 4-year period, Aass and Gjermo20 found that the majority of the bone loss observed during or immediately after the termination of orthodontic treatment was transient in nature. In a follow-up study conducted 12–35 years after comprehensive fixed appliance orthodontic treatment during adolescence, Sadowsky and BeGole22 demonstrated that orthodontic treatment in adolescence is not a major factor in determining long-term periodontal health status. They found that no significant amount of either damage or benefit to the periodontal structures could be directly attributed to orthodontic therapy. A controlled clinical study4 of persons who had completed orthodontic therapy at least 10 years previously compared to a group of adults with untreated malocclusion demonstrated that orthodontic treatment during adolescence had no distinct effect upon later periodontal health.
P. gingivalis is a member of the normal microbiota of the oral cavity, which comprise more than 700 different species of microorganisms. P. gingivalis has been detected in 25% of the healthy subjects and in 79% of a periodontitis group by a specific PCR assay.5 However, isolation of this oral anaerobe has proven difficult in a healthy oral cavity.23 P. gingivalis has been suggested27 as a possible etiologic agent for periodontal lesions, infections, and adult periodontal diseases24–26 through its production of a number of virulence factors (eg, collagenase, hemolysins, proteases, endotoxin, and fatty acids). In the present study, real-time PCR was the technique used to identify and quantify P. gingivalis in subgingival plaque. Real-time PCR is a relatively new molecular technique used to assay a small amount of template DNA from periodontal pathogens. It has been used for identification of P. gingivalis and represents a sensitive, efficient, and reliable approach in P. gingivalis detection.28–30
The present study showed that the frequency of subjects with subgingival P. gingivalis decreased during the first 3 months after placement of orthodontic appliances. This finding agrees with that of another study31 that found a statistically significant decrease in the number of patients with P. gingivalis and P. nucleatum in plaque samples taken up to 6 months after application of fixed appliances. Despite an increase in Pl.I and GI, the relative quantity of subgingival P. gingivalis remained stable after subjects wore the orthodontic appliances. The explanation may be that the placement of fixed appliances initially disrupts supra- and subgingival dental plaque that causes subgingival plaque in a new dynamic state, where aerobic bacteria start to boost and anaerobes reduce. This disruption may have a long-lasting effect on plaque composition.32 Speer et al.33 attributed the reduction in subgingival aerobes and anaerobes under fixed appliance therapy to metal corrosion and the release of primarily nickel ions, which have a toxic effect on bacteria.
At the completion of the orthodontic treatment both the frequency of P. gingivalis carrier and the amount of subgingival P. gingivalis were high. The increasing P. gingivalis could be the result of abundant dental plaque on the tooth surface and increasing gingival inflammation. In this episode, the booming facultative and aerobic microorganisms consume oxygen and provide nutrients, enzymes, and coadhesions that are favorable to the colonization of anaerobic bacteria.34 Gingival inflammation enhances the flowing of gingival crevicular fluids that supply plasma proteins, such as albumin, lactoferrin, and lysozyme, which are essential for the growth of proteolytic anaerobes.34 It has been shown7,34 that the microflora associated with gingivitis is diverse with Actinomyces species, capnophilic, and obligate anaerobic gram-negative bacteria being predominant. After removal of the orthodontic appliance, both P. gingivalis carriage and the relative amount of subgingival P. gingivalis were reduced significantly in 6 months. This reduction correlated with the decrease of gingival inflammation, and such correspondence was also found in other studies25,35 that showed a significant reduction in P. gingivalis following an improvement in periodontal health. Kawada et al.25 reported a positive correlation between the number of P. gingivalis and pocket depth, while others have shown5,9,36 that P. gingivalis occurred with greater frequency and at higher levels at sites of disease activity.
Despite a significant decrease in the relative quantity of subgingival P. gingivalis after appliance removal, the P. gingivalis quantity 6 months after appliance removal was still higher than the quantity measured before appliance placement. This indicates that there might be a potential risk to periodontal health from orthodontic therapy in certain people, which needs to be further verified by a long-term prospective study on one group of subjects.
CONCLUSIONS
Fixed orthodontic treatment is conducive to dental plaque accumulation and gingival inflammation. Accordingly, after removal of the orthodontic appliances, the periodontal condition improved.
The carriage and the amount of P. gingivalis in subgingival plaque were high at the end of orthodontic treatment, while they decreased after orthodontic appliance removal. Nevertheless, the quantity of subgingival P. gingivalis remained high at the end of the treatment compared to the quantity measured before treatment.
Acknowledgments
The study was supported by grant 20062153 from the Liaoning Natural Science Foundation and grant 2008E13SF220 from the Dalian Scientific Technology Foundation, China.
REFERENCES
- 1.Wennstrom J. L. Mucogingival considerations in orthodontic treatment. Semin Orthod. 1996;2:46–54. doi: 10.1016/s1073-8746(96)80039-9. [DOI] [PubMed] [Google Scholar]
- 2.Bollen A. M, Cunha-Cruz J, Bakko D. W, Huang G. J, Hujoel P. P. The effects of orthodontic therapy on periodontal health: a systematic review of controlled evidence. J Am Dent Assoc. 2008;139:413–422. doi: 10.14219/jada.archive.2008.0184. [DOI] [PubMed] [Google Scholar]
- 3.Aass A. M, Albandar J, Aasenden R, Tollefsen T, Gjermo P. Variation in prevalence of radiographic alveolar bone loss in subgroups of 14-year-old schoolchildren in Oslo. J Clin Periodontol. 1988;15:130–133. doi: 10.1111/j.1600-051x.1988.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 4.Polson A. M, Subtelny J. D, Meitner S. W, Polson A. P, Sommers E. W, Iker H. P, Reed B. E. Long-term periodontal status after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1988;93:51–58. doi: 10.1016/0889-5406(88)90193-x. [DOI] [PubMed] [Google Scholar]
- 5.Griffen A. L, Becker M. R, Lyons S. R, Moeschberger M. L, Leys E. J. Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol. 1998;36:3239–3242. doi: 10.1128/jcm.36.11.3239-3242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socransky S. S, Haffajee A. D, Cugini M. A, Smith C, Kent R. L., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 7.Tanner A. C, Maiden M. F, Zambon J. J, Thoren G. S, Kent R. L., Jr Rapid chair-side DNA probe assay of Bacteroides forsythus and Porphyromonas gingivalis. J Periodontal Res. 1998;33:105–117. doi: 10.1111/j.1600-0765.1998.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 8.Morinushi T, Lopatin D. E, Van Poperin N, Ueda Y. The relationship between gingivitis and colonization by Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in children. J Periodontol. 2000;71:403–409. doi: 10.1902/jop.2000.71.3.403. [DOI] [PubMed] [Google Scholar]
- 9.Dahlen G. Microbiological diagnostics in oral diseases. Acta Odontol Scand. 2006;64:164–168. doi: 10.1080/00016350500520318. [DOI] [PubMed] [Google Scholar]
- 10.Paolantonio M, Pedrazzoli V, di Murro C, di Placido G, Picciani C, Catamo G, De Luca M, Piaccolomini R. Clinical significance of Actinobacillus actinomycetemcomitans in young individuals during orthodontic treatment. A 3-year longitudinal study. J Clin Periodontol. 1997;24:610–617. doi: 10.1111/j.1600-051x.1997.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 11.Leung N. M, Chen R, Rudney J. D. Oral bacteria in plaque and invading buccal cells of young orthodontic patients. Am J Orthod Dentofacial Orthop. 2006;130:698.e11–18. doi: 10.1016/j.ajodo.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Ristic M, Vlahovic Svabic M, Sasic M, Zelic O. Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod Craniofac Res. 2007;10:187–195. doi: 10.1111/j.1601-6343.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 13.Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 14.Lyons S. R, Griffen A. L, Leys E. J. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J Clin Microbiol. 2000;38:2362–2365. doi: 10.1128/jcm.38.6.2362-2365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki N, Yoshida A, Nakano Y. Quantitative analysis of multi-species oral biofilms by TaqMan Real-Time PCR. Clin Med Res. 2005;3:176–185. doi: 10.3121/cmr.3.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sukontapatipark W, el-Agroudi M. A, Selliseth N. J, Thunold K, Selvig K. A. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod. 2001;23:475–484. doi: 10.1093/ejo/23.5.475. [DOI] [PubMed] [Google Scholar]
- 17.Mombelli A, Gusberti F. A, van Oosten M. A, Lang N. P. Gingival health and gingivitis development during puberty. A 4-year longitudinal study. J Clin Periodontol. 1989;16:451–456. doi: 10.1111/j.1600-051x.1989.tb01674.x. [DOI] [PubMed] [Google Scholar]
- 18.Levin L, Samorodnitzky-Naveh G. R, Machtei E. E. The association of orthodontic treatment and fixed retainers with gingival health. J Periodontol. 2008;79:2087–2092. doi: 10.1902/jop.2008.080128. [DOI] [PubMed] [Google Scholar]
- 19.Boyd R. L, Baumrind S. Periodontal considerations in the use of bonds or bands on molars in adolescents and adults. Angle Orthod. 1992;62:117–126. doi: 10.1043/0003-3219(1992)062<0117:PCITUO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Aass A. M, Gjermo P. Changes in radiographic bone level in orthodontically treated teenagers over a 4-year period. Community Dent Oral Epidemiol. 1992;20:90–93. doi: 10.1111/j.1600-0528.1992.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 21.Alexander S. A. Effects of orthodontic attachments on the gingival health of permanent second molars. Am J Orthod Dentofacial Orthop. 1991;100:337–340. doi: 10.1016/0889-5406(91)70071-4. [DOI] [PubMed] [Google Scholar]
- 22.Sadowsky C, BeGole E. A. Long-term effects of orthodontic treatment on periodontal health. Am J Orthod. 1981;80:156–172. doi: 10.1016/0002-9416(81)90216-5. [DOI] [PubMed] [Google Scholar]
- 23.Aas J. A, Paster B. J, Stokes L. N, Olsen I. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Socransky S. S, Haffajee A. D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 25.Kawada M, Yoshida A, Suzuki N, Nakano Y, Saito T, Oho T, Koga T. Prevalence of Porphyromonas gingivalis in relation to periodontal status assessed by real-time PCR. Oral Microbiol Immunol. 2004;19:289–292. doi: 10.1111/j.1399-302X.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 26.Tanner A. C, Izard J. Tannerella forsythia, a periodontal pathogen entering the genomic era. Periodontol 2000. 2006;42:88–113. doi: 10.1111/j.1600-0757.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 27.Holt S. C, Ebersole J. L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 28.Boutaga K, van Winkelhoff A. J, Vandenbroucke-Grauls C. M, Savelkoul P. H. Comparison of real-time PCR and culture for detection of Porphyromonas gingivalis in subgingival plaque samples. J Clin Microbiol. 2003;41:4950–4954. doi: 10.1128/JCM.41.11.4950-4954.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morillo J. M, Lau L, Sanz M, Herrera D, Silva A. Quantitative real-time PCR based on single copy gene sequence for detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. J Periodontal Res. 2003;38:518–524. doi: 10.1034/j.1600-0765.2003.00684.x. [DOI] [PubMed] [Google Scholar]
- 30.Jervoe-Storm P. M, Koltzscher M, Falk W, Dorfler A, Jepsen S. Comparison of culture and real-time PCR for detection and quantification of five putative periodontopathogenic bacteria in subgingival plaque samples. J Clin Periodontol. 2005;32:778–783. doi: 10.1111/j.1600-051X.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 31.Ristic M, Vlahovic Svabic M, Sasic M, Zelic O. Effects of fixed orthodontic appliances on subgingival microflora. Int J Dent Hyg. 2008;6:129–136. doi: 10.1111/j.1601-5037.2008.00283.x. [DOI] [PubMed] [Google Scholar]
- 32.Scheie A. A, Arneberg P, Krogstad O. Effect of orthodontic treatment on prevalence of Streptococcus mutans in plaque and saliva. Scand J Dent Res. 1984;92:211–217. doi: 10.1111/j.1600-0722.1984.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 33.Speer C, Pelz K, Hopfenmuller W, Holtgrave E. A. Investigations on the influencing of the subgingival microflora in chronic periodontitis. A study in adult patients during fixed appliance therapy. J Orofac Orthop. 2004;65:34–47. doi: 10.1007/s00056-004-0333-z. [DOI] [PubMed] [Google Scholar]
- 34.Marsh P. D. Plaque mediated diseases. In: Marsh P. D, Martin M. V, editors. Oral Microbiology 5th ed. Vol. 120 Edinburgh, London, UK: Churchill Livingstone; 2009. [Google Scholar]
- 35.Mineoka T, Awano S, Rikimaru T, Kurata H, Yoshida A, Ansai T, Takehara T. Site-specific development of periodontal disease is associated with increased levels of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in subgingival plaque. J Periodontol. 2008;79:670–676. doi: 10.1902/jop.2008.070398. [DOI] [PubMed] [Google Scholar]
- 36.Socransky S. S, Haffajee A. D, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the sampled site. J Clin Periodontol. 1991;18:766–775. doi: 10.1111/j.1600-051x.1991.tb00070.x. [DOI] [PubMed] [Google Scholar]