Abstract
Objective:
To analyze cephalometric features in adults with isolated growth hormone (GH) deficiency (IGHD).
Materials and Methods:
Nine adult IGHD individuals (7 males and 2 females; mean age, 37.8 ± 13.8 years) underwent a cross-sectional cephalometric study, including 9 linear and 5 angular measurements. Posterior facial height/anterior facial height and lower-anterior facial height/anterior facial height ratios were calculated. To pool cephalometric measurements in both genders, results were normalized by standard deviation scores (SDS), using the population means from an atlas of the normal Brazilian population.
Results:
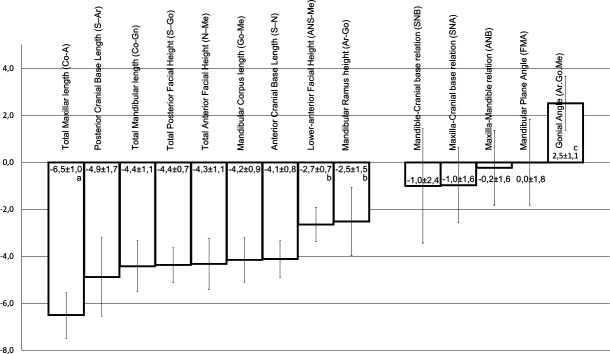
All linear measurements were reduced in IGHD subjects. Total maxillary length was the most reduced parameter (−6.5 ± 1.7), followed by a cluster of six measurements: posterior cranial base length (−4.9 ± 1.1), total mandibular length (−4.4 ± 0.7), total posterior facial height (−4.4 ± 1.1), total anterior facial height (−4.3 ± 0.9), mandibular corpus length (−4.2 ± 0.8), and anterior cranial base length (−4.1 ± 1.7). Less affected measurements were lower-anterior facial height (−2.7 ± 0.7) and mandibular ramus height (−2.5 ± 1.5). SDS angular measurements were in the normal range, except for increased gonial angle (+2.5 ± 1.1). Posterior facial height/anterior facial height and lower-anterior facial height/anterior facial height ratios were not different from those of the reference group.
Conclusions:
Congenital, untreated IGHD causes reduction of all linear measurements of craniofacial growth, particularly total maxillary length. Angular measurements and facial height ratios are less affected, suggesting that lGHD causes proportional blunting of craniofacial growth.
Keywords: Growth hormone, Isolated GH deficiency, Craniofacial growth
INTRODUCTION
Genetic, embryologic, mechanical, and hormonal factors influence craniofacial growth.1–6 Among hormonal factors, growth hormone (GH), thyroid hormone, and adrenocortical and sex steroids have important roles.7,8 GH is produced by somatotroph cells of the anterior pituitary under the influence of two hypothalamic hormones, the stimulatory GH-releasing hormone (GHRH) and the inhibitory somatostatin. GH binds to its receptor, activating the Janus-kinase signal transducers and activators of transcription (JAK-STATs), promoting both direct and indirect (mediated by locally produced and circulating insulin-like growth factor type I [IGF-I]) effects.9 GH can affect craniofacial growth and tooth formation and eruption.7
Craniofacial growth in GH deficiency (GHD) was first studied in 1934,10 with reports of “immature” facial appearance (length and depth of the face inappropriately small for age) resulting in maintenance of a child-like convexity.8,11–17 However, many GHD children lack other pituitary hormones and/or have pituitary or hypothalamic tumors, or they have undergone surgery or radiotherapy. All these factors can influence craniofacial growth. Isolated GHD (IGHD) is rare, with an incidence of 1 in 3480 to 1 in 10,000 live births,18 and is often defined by less strict GH secretion criteria.8,19,20 Therefore, IGHD series often include cases with partial or transient GHD. Hence, the use of this model is questionable.
Furthermore, most previous studies of craniofacial growth in GHD were done in children or young adolescents who could present a delay rather than a deficit in craniofacial growth. Because GHD, no matter the cause, causes delayed tooth eruption,7 this could result in temporary changes before the permanent dentition is complete. Finally, in modern days, most GHD children receive replacement therapy throughout childhood. Ideally, the study of craniofacial growth should be done in adults with severe, congenital, and untreated IGHD. In Itabaianinha County, in the Brazilian state of Sergipe, we have identified18 the largest kindred with congenital IGHD described to date, with 105 IGHD individuals, all carrying the same homozygous inactivating mutation of the GHRH receptor (GHRHR) gene (GHRHR). This mutation (IVS1 + 1G → A) causes complete lack of receptor function, removing the most important stimulus to GH secretion. Affected individuals have very low serum GH and IGF-I21 and present with severe postnatal growth failure (with adult stature 4.5 to 8.6 SD below normal), a high-pitched voice,22 and reduced muscle and bone mass.23,24 Because of the large number of affected subjects, these kindred offer a unique opportunity for studying craniofacial anatomy in adults with severe IGHD. The aim of this work is to analyze cephalometric features in IGHD individuals from these kindred.
MATERIALS AND METHODS
A cross-sectional study was performed in IGHD individuals from Itabaianinha. Subjects were invited to participate by an ad posted in the local Dwarves' Association. Thirty-eight adult IGHD individuals volunteered. History and dental records were verified for inclusion criteria: genotype-proven homozygous GHRHR mutation; low IGF-I levels; no previous GH treatment; radiographic evidence of the presence of maxillary and mandibular central incisors, and first or second molars. Ten individuals were excluded because of previous GH treatment, and 19 had lost all molars and/or incisors needing prostheses. This left 9 subjects (7 males and 2 females; mean age, 37.8 ± 13.8 years). Two are second-degree relatives. Figure 1 shows a craniofacial profile of one of the IGHD women.
Figure 1.
Profile of a female patient (age 55) with isolated and untreated growth hormone deficiency.
Somatic and cephalic growth values were converted in height and cephalic perimeter (CP) standard deviation score (SDS) by the formula SDS = (X − Mean) ÷ SD. The normal SDS distribution has a mean of 0 and a standard deviation of ±1. Values of SDS above or below 2 are by definition abnormal. Height SDS values of the IGHD group were −6.6 ± 1.2, ranging from −9.2 to −5.5, and CP SDS values were −3.7 ± 1.0, ranging from −5.8 to −2.6, compared with the mean of local normal subjects.25
Study subjects were transported to Aracaju, 70 miles from Itabaianinha. The Institutional Review Board of the Federal University of Sergipe approved the protocol. Written informed consent was obtained.
Craniofacial Evaluation
Lateral cephalometric radiographs were taken with the head positioned in a cephalostat and oriented to the Frankfort horizontal plane with teeth in the intercuspal position at a radiology clinic. One of the authors, a radiologist with 20 years' experience, traced standardized lateral cephalograms. Twelve landmarks (Figure 2) were digitalized. Nine linear and 5 angular measurements were chosen, according to the protocol of an atlas for the adult Brazilian population.26 Measurements were computed by Ortoview 2.5 software (CIRRUS Informática, São Bernardo do Campo, SP Brazil), and gender-matched SDS values were calculated. Errors in landmark identification were assessed by digitalizing 30 radiographs twice at a weekly interval using seven measures. The P value of the difference between each pair of repetitions was not significant and ranged from .48 to .96. From these measurements, two indices were calculated: posterior facial height/anterior facial height and lower-anterior facial height/anterior facial height ratios.
Figure 2.
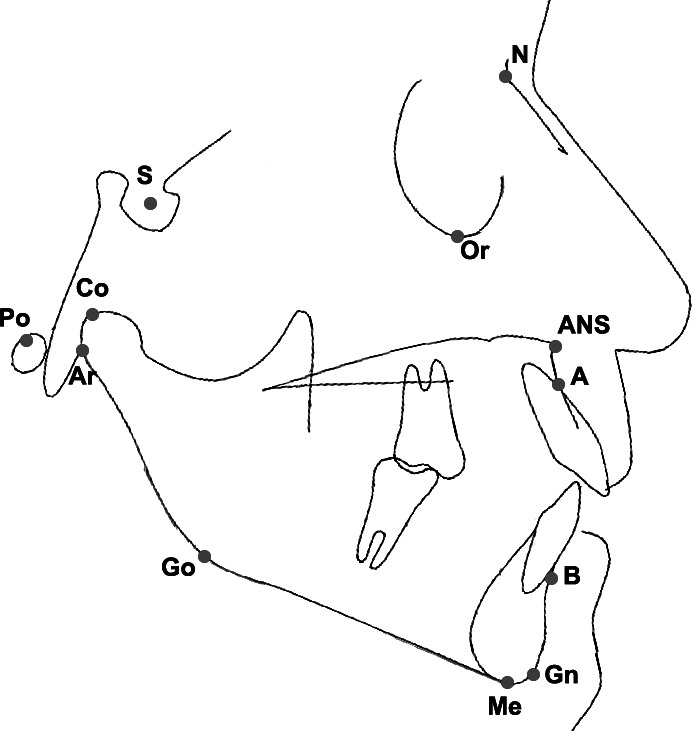
Cephalometric landmarks: A, point A; ANS, anterior nasal spine; Ar, articular; B, point B; Gn, gnathion; Go, gonion; Me, menton; N, nasion; S, sella turcica; Or, orbital; Po, porion; Co, condylion.
Statistical Analysis
The SDS of these measurements were compared by analysis of variance (ANOVA) with the Tukey test. P < .05 was considered significant.
RESULTS
Figure 3 shows SDS values of linear and angular measures. All linear measurements were reduced in IGHD subjects, with total maxillary length being the most reduced parameter, followed by a cluster of six measurements: posterior cranial base length, total mandibular length, total posterior facial height, total anterior facial height, mandibular corpus length, and anterior cranial base length. The least affected measurements were lower-anterior facial height and mandibular ramus height. SDS angular measurements were in the normal range, except for an increase in the gonial angle. Posterior facial height/anterior facial height (0.638 ± 0.072) and lower-anterior facial height/anterior facial height (0.567 ± 0.035) ratios were not different from those of the reference group (0.667 and 0.574, respectively).
Figure 3.
Standard deviation scores (SDS) for cephalometric features of untreated isolated growth hormone deficiency (IGHD). Values lower than −2 or higher than +2 are by definition abnormal. (a) P < .001 in comparison with the other linear measurements. (b) P < .001 for both lower-anterior facial height and mandibular ramus height in comparison with other linear measurements. (c) P < .001 in comparison with the other angular measurements (analysis of variance [ANOVA] with the Tukey test).
DISCUSSION
This is the first study of craniofacial features in a homogeneous group of adult, untreated individuals with lifetime IGHD due to the same genetic abnormality. All previous reports included children or young adolescents with a mean age of 12 years, therefore far from completion of facial development.8,10–16,18,27–31 Our data show that adult IGHD individuals present reductions in all linear measurements, especially total maxillary length. This finding differs from those of previous reports, which have shown that the most reduced measurements in children were anterior cranial base length,18,19 mandibular corpus length,30 and total mandibular length.31 It is interesting to note that total maxillary length (−6.5 ± 1.0) showed a magnitude of reduction that is compatible with stature reduction (−6.6 ± 1.2), with both measures more reduced than total mandibular length (−4.4 ± 1.1). Although mandibular length has been reported to be very sensitive to GH treatment31 or excess,32 a finding of predominant maxillary length reduction may reflect the nature of maxillary bone structure, preponderantly trabecular (more susceptible to metabolic changes), while mandibular is mainly cortical.
Posterior facial height has been reported to be the most reduced linear dimension in hypopituitary children.15,27 Funatsu et al.8 studied 57 heterogeneous idiopathic GHD patients (4.5 to 16.7 years) and found that anterior cranial base, anterior facial height, and maxillary length were the most reduced dimensions. Notably, in their subjects, GHD was defined by a GH peak <10 ng/mL during provocative tests; therefore, GHD was less severe than in our subjects, whose GH peak is invariably <1 ng/mL.33 Cantu et al.19 showed that in idiopathic GHD children, anterior cranial base length and total mandibular length were the most affected measurements, and that posterior cranial base length and ramus height were the least affected ones. Again, this group had less severe GHD than ours. De Faria et al.31 studied 13 patients with panhypopituitarism and 9 with IGHD. Six of these patients were naïve to GH treatment (mean age, 7.7 years) with a degree of GHD that closely resembled our patients (peak GH < 3.3 ng/mL). Different from our adult individuals, these patients found that total mandibular length was the most reduced dimension, and that anterior facial height was not reduced. However, 13 subjects (including the 6 who were GH-naïve) presented with magnetic resonance imaging (MRI) evidence of ectopic neurohypophysis, and 7 had a transected or thin pituitary stalk, suggesting that the origin of GDH was more likely embryologic34 than genetic. None of these radiologic findings is present in our patients.25 Therefore, differences in age and in causation of GHD could explain these differences.
The reductions in cephalometric measurements that we observed are more marked than those previously reported in GHD: −3 to −2 in pituitary deficiency patients,7 −0.86 to −0.158 and −2.6 to −1.1 in idiopathic GHD,18 and −2.14 to 0.37 in children with severe GHD.32 The extreme severity of GHD in our subjects may have contributed to such a difference.
In children born small for gestational age, all linear craniofacial measurements are decreased, except for lower-anterior facial height, resulting in a triangular face associated with a wide cranial base and an increased mandibular plane.29 This differs from IGHD, a model of postnatal growth failure characterized by proportional reduction in lower-anterior facial height and anterior facial height. Indeed, posterior facial height/anterior facial height and lower-anterior facial height/anterior facial height ratios were not different in our individuals from those in the reference group.
Our study has a limitation: All subjects belong to the same pedigree and therefore may share other genetic trait(s) that may affect craniofacial growth. Regrettably, no published data on other forms of genetic IGHD are available. Subjects with GH resistance (“Laron dwarfs”) have been reported to have a reduction in all linear measures, particularly total mandibular length, mandibular corpus length, total cranial base, and total maxillary length,28 but cannot be compared with our subjects because of the age difference (mean age, 12.2 years).
Although maxillary and mandibular lengths were reduced, the maxilla-cranial base relation (SNA), mandible-cranial base relation (SNB), and maxilla-mandible relation (ANB) were in the normal range, indicating proportionality of craniofacial measurements. GH seems to affect the dimensions of the maxilla and mandible more than those of the middle face.
The mandibular plane angle (FMA) was similar to that in the reference group, without the steep mandibular plane previously reported in GHD.18 This difference can be explained by the pattern of facial height reduction, with similar posterior facial height/anterior facial height ratio between our IGHD and reference groups.
We also found reduced anterior and posterior cranial base lengths. The growth of this region depends on the spheno-occipital synchondrosis, with cartilaginous joints reminiscent of nonossified bones in the cranial base and involved in endochondral ossification. These regions are probably dependent from an intact GH/IGF-I axis.4,6,15
The gonial angle was increased in IGHD individuals, similar to the increase observed in Laron's patients,28 and in some14 but not all hypopituitary patients.8 The degree of the gonial angle is influenced by the function and activity of the masticator muscles,14 particularly the elevator muscles of the mandible. Subjects with increased masticator muscle activity, such as bruxism, have a reduced gonial angle.2 The opposite occurs in subjects with myotonic dystrophy.2 Therefore, the reduction of muscle mass seen in our subjects23,24 could contribute to the increased gonial angle.
Our findings could establish a practical cephalometric approach to diagnosis and treatment of GHD. With knowledge of the benefits of GH for profile and occlusion,31 SDS parameters could help dentists and physicians define the order of treatment (hormonal vs orthodontic). A practical cephalometric assessment expressed in SDS can facilitate diagnosis in dubious GHD cases,35 differentiating true GHD from the many cases of false positivity seen in GH stimulation tests.
CONCLUSIONS
Congenital, lifetime, untreated IGHD reduces all linear craniofacial measurements, mostly total maxillary length. This may be the most characteristic feature of IGHD.
The angular measures and facial height ratios are less markedly affected, suggesting proportionality in reduction of craniofacial parameters. The doll facies, reported in the past in GHD, includes reductions in cephalic perimeter, posterior and anterior facial height, and maxillary dimensions.
A cephalometry protocol using SDS could be a useful tool for confirming IGHD and following adequacy of treatment.
Acknowledgments
We thank the study subjects. We acknowledge the help of Professor José Roberto Lauris for statistical analyses, Ivanilde Lemos for secretarial assistance, and the “Centro de Imagem em Odontologia de Sergipe” for technical support. None of the authors received remuneration for this work.
REFERENCES
- 1.Huggare JÅ, Rönning O. V. Growth of the cranial vault: influence of intracranial and extracranial pressures. Acta Odontol Scand. 1995;53:192–195. doi: 10.3109/00016359509005971. [DOI] [PubMed] [Google Scholar]
- 2.Kiliaridis S. Masticatory muscle influence on craniofacial growth. Acta Odontol Scand. 1995;53:196–202. doi: 10.3109/00016359509005972. [DOI] [PubMed] [Google Scholar]
- 3.Kjaer I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand. 1995;53:135–143. doi: 10.3109/00016359509005963. [DOI] [PubMed] [Google Scholar]
- 4.Ronning O. Basicranial synchondroses and the mandibular condyle in craniofacial growth. Acta Odontol Scand. 1995;53:162–166. doi: 10.3109/00016359509005966. [DOI] [PubMed] [Google Scholar]
- 5.Persson M. The role of sutures in normal and abnormal craniofacial growth. Acta Odontol Scand. 1995;53:152–161. doi: 10.3109/00016359509005965. [DOI] [PubMed] [Google Scholar]
- 6.Thilander B. Basic mechanisms in craniofacial growth. Acta Odontol Scand. 1995;53:144–151. doi: 10.3109/00016359509005964. [DOI] [PubMed] [Google Scholar]
- 7.Pirinen S. Endocrine regulation of craniofacial growth. Acta Odontol Scand. 1995;53:179–185. doi: 10.3109/00016359509005969. [DOI] [PubMed] [Google Scholar]
- 8.Funatsu M, Sato K, Mitani H. Effects of growth hormone on craniofacial growth. Angle Orthod. 2006;76:970–977. doi: 10.2319/011905-17. [DOI] [PubMed] [Google Scholar]
- 9.Martari M, Salvatori R. Diseases associated with growth hormone releasing hormone receptor (GHRHR) mutations in tao Y-X. Prog Mol Biol Transl Sci. 2009;88:57–84. doi: 10.1016/S1877-1173(09)88003-4. [DOI] [PubMed] [Google Scholar]
- 10.Schour I, Brodie A. G, King E. Q. The hypophysis and the teeth. IV. Dental changes in a hypopituitary condition: a case report. Angle Orthod. 1934;4:285–304. [Google Scholar]
- 11.Markus M. B, Goosman S. D, Einhorn N. H, Lerner J. Facial development in hypopituitary dwarfism. Am J Orthod Oral Surg. 1942;23:334–350. [Google Scholar]
- 12.Cohen M. M, Wagner R. Dental development in pituitary dwarfism. J Dent Res. 1948;27:445–458. doi: 10.1177/00220345480270040201. [DOI] [PubMed] [Google Scholar]
- 13.Bevis R. R, Hayles A. B, Isaacson R. J, Sather A. H. Facial growth response to human growth hormone in hypopituitary dwarfs. Angle Orthod. 1977;47:193–205. doi: 10.1043/0003-3219(1977)047<0193:FGRTHG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Edler R. J. Cephalometric parameters in hypopituitary patients. Brit J Orthod. 1979;6:19–22. doi: 10.1179/bjo.6.1.19. [DOI] [PubMed] [Google Scholar]
- 15.Pirinen S, Majurin A, Lenko H. L, Koski K. Craniofacial features in patients with deficient and excessive growth hormone. J Craniofac Genet Dev Biol. 1994;14:144–152. [PubMed] [Google Scholar]
- 16.Kjellberg H, Beiring M, Albertsson W. K. Craniofacial morphology, dental occlusion, tooth eruption, and dental maturity in boys of short stature with or without growth hormone deficiency. Eur J Oral Sci. 2000;108:359–367. doi: 10.1034/j.1600-0722.2000.108005359.x. [DOI] [PubMed] [Google Scholar]
- 17.Segal D. G, Pescovitz O. H, Schaefer G. B, DiMeglio L. A. Craniofacial and acral growth responses in growth hormone-deficient children treated with growth hormone. J Pediatr. 2004;144:437–443. doi: 10.1016/j.jpeds.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Salvatori R, Hayashida C. Y, Aguiar-Oliveira M. H, et al. Familial dwarfism due to a novel mutation of the growth hormone releasing hormone receptor gene. J Clin Endocrinol Metab. 1999;84:917–923. doi: 10.1210/jcem.84.3.5599. [DOI] [PubMed] [Google Scholar]
- 19.Cantu G, Buschang P. H, Gonzalez J. L. Differential growth and maturation in idiopathic growth-hormone-deficient children. Eur J Orthod. 1997;19:131–139. doi: 10.1093/ejo/19.2.131. [DOI] [PubMed] [Google Scholar]
- 20.Kjellberg H, Albertsson W. K. A longitudinal study of craniofacial growth in idiopathic short stature and growth hormone-deficient boys treated with growth hormone. Eur J Orthod. 2007;29:243–250. doi: 10.1093/ejo/cjm005. [DOI] [PubMed] [Google Scholar]
- 21.Aguiar-Oliveira M. H, Gill M. S, Barreto E. S, et al. Effect of severe growth hormone (GH) deficiency due to a mutation in the GH-releasing hormone receptor on insulin-like growth factor (IGFs), IGF-binding proteins and ternary complex formation throughout life. J Clin Endocrinol Metab. 1999;84:4118–4126. doi: 10.1210/jcem.84.11.6133. [DOI] [PubMed] [Google Scholar]
- 22.Barreto V. M, D'Avila J. S, Sales N. J, Gonçalves M. I, Seabra J. D, Salvatori R, Aguiar-Oliveira M. H. Laryngeal and vocal evaluation in untreated growth hormone deficient adults. Otolaryngol Head Neck Surg. 2009;140:37–42. doi: 10.1016/j.otohns.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Barretto E. S. A, Gill M. S, Freitas M. E. S, Magalhaes M. M. G, Souza A. H. O, Aguiar-Oliveira M. H, Clayton P. E. Serum leptin and body composition in children with familial GH deficiency (GHD) due to a mutation in the growth hormone-releasing hormone (GHRH) receptor. Clin Endocrinol (Oxf) 1999;51:559–564. doi: 10.1046/j.1365-2265.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 24.Barreto-Filho J. A. S, Alcântara M. R. S, Salvatori R, et al. Familial isolated growth hormone deficiency is associated with increased systolic blood pressure, central obesity and dyslipidemia. J Clin Endocrinol Metab. 2002;87:2018–2023. doi: 10.1210/jcem.87.5.8474. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira H. A, Salvatori R, Kraus M. P, Oliveira C. R. P, Silva P. C. R, Aguiar-Oliveira M. H. Magnetic resonance imaging study of pituitary morphology in subjects homozygous and heterozygous for a null mutation of the GHRH receptor gene. Eur J Endocrinol. 2003;148:427–432. doi: 10.1530/eje.0.1480427. [DOI] [PubMed] [Google Scholar]
- 26.Martins D. R, Janson G. R. P, Almeida R. R, Pinzam A, Freitas M. R. Atlas de Crescimento Craniofacial. São Paulo: Santos; 1998. [Google Scholar]
- 27.Spiegel R. N, Sather A. H, Hayles A. B. Cephalometric study of children with various endocrine diseases. Am J Orthod. 1971;59:362–375. doi: 10.1016/0002-9416(71)90232-6. [DOI] [PubMed] [Google Scholar]
- 28.Konfino R, Pertzelan A, Laron Z. Cephalometric measurements of familial dwarfism and high plasma immunoreactive growth hormone. Am J Orthod. 1975;68:196–201. doi: 10.1016/0002-9416(75)90208-0. [DOI] [PubMed] [Google Scholar]
- 29.VanErum R, Mulier M, Carels C, Verbeke G, de Zegher F. Craniofacial growth in short children born small for gestational age: effect of growth hormone treatment. J Dent Res. 1997;76:1579–1586. doi: 10.1177/00220345970760091001. [DOI] [PubMed] [Google Scholar]
- 30.VanErum R, Mulier G, Carels C, deZegher F. Craniofacial growth and dental maturation in short children born small for gestational age: effect of growth hormone treatment—own observations and review of the literature. Horm Res. 1998;50:141–146. doi: 10.1159/000023262. [DOI] [PubMed] [Google Scholar]
- 31.de Faria M. E. J, Carvalho L. R, Rossetto S. M, Amaral T. S, Berger K, Arnhold I. J. P, Mendonca B. B. Analysis of craniofacial and extremity growth in patients with growth hormone deficiency during growth hormone therapy. Horm Res. 2009;71:173–177. doi: 10.1159/000197875. [DOI] [PubMed] [Google Scholar]
- 32.Herrmann B. L, Mortsch F, Berg C, Weischer T, Mohr C, Mann K. Acromegaly: a cross-sectional analysis of the oral and maxillofacial pathologies. Exp Clin Endocrinol Diabetes. 2010 Jul 23 doi: 10.1055/s-0030-1255020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Salvatori R, Serpa M. G, Parmigiani G, et al. GH response to hypoglycemia and clonidine in the GH-releasing hormone resistance syndrome. J Endocrinol Invest. 2006;29:805–808. doi: 10.1007/BF03347374. [DOI] [PubMed] [Google Scholar]
- 34.Osorio M. G, Marui S, Jorge A. A, et al. Pituitary magnetic resonance imaging and function in patients with growth hormone deficiency with and without mutations in GHRH-R, GH-1, or PROP-1 genes. J Clin Endocrinol Metab. 2002;87:5076–5084. doi: 10.1210/jc.2001-011936. [DOI] [PubMed] [Google Scholar]
- 35.Loche S, Bizzarri C, Maghnie M, Faedda A, Tzialla C, Autelli M, Casini M. R, Cappa M. Results of early re-evaluation of growth hormone secretion in short children with apparent growth hormone deficiency. J Pediatr. 2002;140:445–449. doi: 10.1067/mpd.2002.122729. [DOI] [PubMed] [Google Scholar]