Abstract
Objective
Fast and reliable detection of infection is a key to control the SARS-CoV-2 pandemic. Lateral flow antigen tests (LFATs) are inexpensive, easy to use, but have to be verified, as they are rather unspecific and can produce both, false positive and false negative results. Our objective was to combine the speed of LFAT for SARS-CoV-2 with the reliability of qPCR tests.
Methods
A serial dilution of a patient sample positive for SARS-CoV-2 was prepared and added to LFAT wells from two manufacturers. After evaluation, the devices were opened, the strips removed and extracted in a solution. Amplification was performed using point of care PCR systems (cobas® Liat®, ID NOW™) or on a LightCycler after extraction by MagNAPure 96.
Results
The nucleic acid amplification systems yielded higher sensitivity to LFAT. Thus, all samples determined positive by LFAT from the serial dilution were also positive in the subsequent amplification reactions. Sensitivity using extracted eluates was 10–100 times higher.
Significance
The usage of LFAT is highly recommended for single samples in emergency dental or emergency clinical settings, for smaller cohorts, or even for larger population screening, as it is inexpensive and fast. Positive results can be conveniently verified directly from the test devices using either point of care test equipment or more complex laboratory equipment thus making a major impact on efficient management of infections and isolations.
Keywords: Dental clinical safety, Lateral flow antigen test (LFAT), Rapid antigen tests, SARS-CoV-2, Coronavirus, QPCR, Test strategy, Cobas Liat, ID Now
1. Introduction
A fast and reliable detection of infection is an essential element to control the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) pandemic. Therefore various efforts have been made to reduce the time from specimen collection to laboratory-confirmed diagnosis [1].
Quantitative polymerase chain reaction (qPCR) has been established as the gold standard for the diagnosis for SARS-CoV-2. The disadvantage of this method is that it can only be done in a laboratory environment. qPCR exhibits high sensitivity and specificity and allows the handling of thousands of samples, depending on the available equipment of the laboratory.
Such assays are performed in batch format and it takes several hours to receive the result for a single run.
In contrast, machines like the cobas® Liat® (Roche) or the ID NOW™ (Abbott) combine fast nucleic acid extraction and PCR or isothermal amplification in a single cartridge and allow for a random access start of a single sample. Therefore the time to result can be reduced dramatically and in principle the assays can be performed close to the sampling place (point of care; POC). However, only a limited number of samples can be analyzed using such machines.
Another disadvantage of both, batch and single sample techniques, especially the latter one, is that they are quite costly.
As an alternative lateral flow antigen tests (LFAT) are widely used. A LFAT is usually a hand-held device with an absorbent pad (specimen well) at one end and a result window at the other. Inside the device is a nitrocellulose test strip where antigen lines change colors in the presence of SARS-CoV-2 proteins (antigens). The main advantage of these tests is that the test result is rapidly available, are easy to use and do not require the handling and processing by laboratory and trained personnel. They are inexpensive and provide a result within about 15 min [2]. Thus, they are frequently used in large scale screening e.g. at the work place, clinics, medical practices, in schools, sport events, concerts but also for testing at home. They form an essential part in the various test strategies proposed to control the pandemic [2]. However, these tests are criticized to be inaccurate. LFAT can produce false positive results in some cases even close to 50% [3], and due to their lower sensitivity in comparison to many PCR assays, there is a possibility for false negative results [4], [5], [6].
In case of a positive or an ambiguous test result of a LFAT, confirmative tests are recommended using nucleic acid amplification techniques [6], usually qPCR tests. This procedure is time consuming as it may require a new test appointment, travel to the test center, sample taking by trained personnel and the processing in a laboratory. Critical time gets lost and the procedure is at least inconvenient for the patient and increases risk for infecting others.
Due to the high demand for qPCR testing during pandemic waves, there is a delay until the result can be reported. However, educational events, such as the training of (dental) medical students, must continue. Especially dental medical students have the problem that the distance to the nasopharyngeal zone of the patient cannot be kept. Therefore it is crucial to test this group and obtain a rapid result. This could easily be accomplished by LFAT at the entrance of a practice/hospital. An enormous improvement of this procedure would be, if single positive LFATs could be verified by nucleic acid amplification directly from the LFAT device. This would offer the following advantages. First of all, only a limited number of samples (the putative positives) would have to be tested by nucleic acid amplification. This would save important resources. Second, no retesting would be required. This in turn would save resources for trained staff, for protective clothing, and for setting up a second test facility. The person would be isolated and not allowed to enter the facility (e.g. practice, hospital) until a qPCR result is available.
Thus, e.g., a (dental) student (or patient) positive in the LFAT could receive the verification or rejection of the positive result by nucleic acid amplification at short notice. Dependent of the result, the students/patients could then participate in the training of treatment, or self-isolate. The educational trainings for the (dental) students could still take place, and the treatment for the patients could be guaranteed. If the (dental) student or patient had to wait several hours or even days for the qPCR report, the treatment or training would have to be postponed, even in case of a false positive LFAT.
The objective of this study was to investigate if the used positive or questionable positive LFAT device itself could be utilized for verification by nucleic acid amplification.
2. Methods and material
2.1. Viral load determination
A sample positive for SARS-CoV-2, established via qPCR, and identified as delta by variant-specific PCR, was diluted to contain 109 copies/ml, based on a quantified standard (INSTAND e.V, Düsseldorf, Germany), then further diluted serially in 1:10 steps in 0.9% NaCl.
2.2. LFAT analysis of dilutions
The dilutions were added to the extraction buffers of the LFAT kits (1:10) and analyzed with the respective LFAT from two providers (SD Biosensors Roche Diagnostics, Mannheim, Germany and Clinitest ® Siemens Healthcare, Erlangen, Germany) according to the manufacturer’s instructions. In brief, 3 drops of the extraction buffer were added to the specimen well of the device, and incubated at room temperature for 15 min, until a result was determined. The tests were read independently by three experienced persons.
2.3. Nucleic acid analysis of LFAT
The plastic cover of the LFAT devices was removed and the containing strips were incubated for 30 min in 2 ml extraction solution (5x phosphate buffered saline (PBS); pH adjusted by adding 20 µl of 1 M NaOH to 2 ml 5xPBS).
Aliquots from the extraction solution were analyzed on both the cobas® Liat® (Roche) and the ID NOW™ (Abbott, Wiesbaden, Germany) systems with the specific reagents for SARS-CoV-2 according to the manufacturer’s instructions. For the cobas® Liat® test 200 µl extraction solution were transferred into the testing device. For the ID NOW™ test 200 µl of the extraction solution were added to the sample receiver. Both the cobas® Liat® test and the ID NOW™ test showed the results after about 10–20 min
Simultaneously, viral RNA was extracted from the extraction solution using the MagnaPure 96 (Roche). The DNA and viral nucleic acid small volume kit (Roche) was used according to the manufacturer’s instructions on a MagNAPure 96 system. Elution was done in 50 µl. Extracted nucleic acids were analyzed for SARS-CoV-2 using the RIDA®gene SARS-CoV-2 qPCR assay (R-Biopharm, Darmstadt, Germany) on the LightCycler® 480II system (Roche). 5 µl of the RNA was used in a 25 µl PCR. The test results for this procedure are available after approximately 3 h.
2 negative samples were included in each of qPCR/LAMP PCR experiment.
3. Results
3.1. Sensitivity of the LFATs
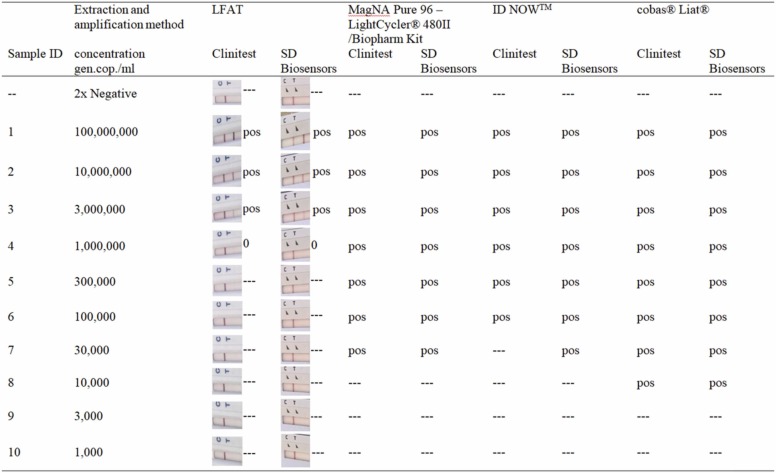
The LFATs, both the SD Biosensors and Clinitest® assays, showed a robust positive signal for the three samples with the highest concentration of SARS-CoV-2, with a faint band visible for sample 7 (1000,000 genomic copies / ml; Table1).
3.2. PCR testing of the extraction solution
Washing of the test strips and subsequent testing with the MagNAPure96 – LightCycler® 480II method, the cobas® Liat® or the ID NOW™ yielded in all cases a positive test result with test strips that were determined positive. In addition, the ID NOW™ detected the extracted strips positive until a viral load of 100,000 copies/ml in all cases, MagNAPure 96-LightCycler® 480II up to a viral load of 30,000 copies/ml and the cobas® Liat® up to a viral load of 10,000 copies/ml ( Fig. 1).
Fig. 1.
Comparison of different nucleic acid amplification systems on extracted lateral flow antigen test (LFAT) for SARS-CoV-2. The third and fourth columns show the LFAT results without extraction with the respective interpretation.
4. Discussion
It could be demonstrated that the extraction of nucleic acid from LFAT devices and subsequent nucleic acid analysis is a feasible diagnostic option to fight the spread of SARS-CoV-2.
Our study showed that by extracting the LFAT strips, sufficient nucleic acid can be made available for further analysis. The LFAT strips used are representative for the LFATs available in the market regarding their design and material [7]. It can therefore be assumed that the described method is applicable for a variety of LFATs.
It is of note that all of the three methods for testing extracted solutions from LFAT devices, i.e. MagNAPure96 – LightCycler® 480II, cobas® Liat® and ID NOW™, showed a higher sensitivity than the LFAT itself. The cobas® Liat® system exhibited the best performance, detecting concentrations as low as 10,000 genomic copies/ml extracted from LFAT devices.
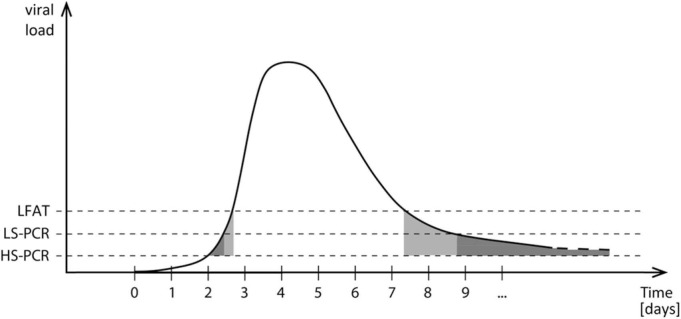
To control the epidemic, it is of crucial importance to detect individuals carrying a high viral load, which is in line with a high contagiousness. Usually, the viral load shows a relatively steep increase at the beginning of infection ( Fig. 2). Different qPCR methods exhibit different sensitivities depending on sample volume, volume of eluate after purification and amount of template used in the amplification. Fig. 2 shows the results for different assay types during the course of infection. Viral loads above the dashed lines are scored positive with the respective assay. A high sensitivity PCR (HS-PCR) has the advantage of detecting infected person already in the initial phase of the disease. However, the time slot for a positive result in this phase is quite narrow, as indicated by the grey shaded area between the time points t = 2 and t = 3. Therefore, the likelihood that an infection with SARS-CoV-2 is missed by a lower sensitive PCR (LS-PCR) or a LFAT seems not be a major problem for diagnostics. The decrease of viral load in the late phase of infection is slower and a low viral load can be present for quite a long time. During this period of infection, there is low to no contagiousness anymore combined with only mild or without symptoms. At this late stage, a HS-PCR analysis yields positive results in many cases and cut-offs have to be established, which is not a simple task. Fig. 2 indicates that this problem is not as prominent for LS-PCR and LFATs.
Fig. 2.
Schematic overview of viral load and detectability. HS-PCR is a high sensitivity (HS) qPCR system, utilizing a high sample volume in low elution volume, LS-PCR is a lowered sensitivity (LS) qPCR system using less sample volume, LFAT resembles the sensitivity of lateral flow antigen tests.
Recent data suggest, that the time point, when LFATs start to turn positive at the beginning of an infection with SARS-CoV-2, strongly correlates with the time point, when the virus becomes culturable, and thus contagious [8]. This also seems to hold true for the late phase of infection, thus, negative antigen tests indicate the absence of contagiousness. Therefore screening of larger cohorts by LFATs can be recommended.
False positive LFAT remain a concern in daily routine testing. First of all there is a number of false positive samples, that can reach up 50% of positive results altogether [3]. Second, false positive patients might be sent to a COVID-19 ward and get infected there [9]. Last, there is a chance of faking positive tests using soft drinks [10].
We have shown here, that in order to avoid false positives, positive or questionable tests can be verified directly from the LFAT device, without need for a new sample. The dried LFAT can be easily transported in any kind of transport device without the danger of spills, leakage or similar problems that can occur during transport, that are covered by the general rules of transportation [11]. Our new procedure provides a major improvement for physicians. Usually, a physician will perform a SARS-CoV-2 LFAT from patients with respiratory symptoms before allowing him to come to consultation. The patient has to wait for the result in the waiting room together with other patients. If it turns out positive, however, there was a risk for other patients in the waiting room. The physician has to take another swab sample from a likely SARS-CoV-2 positive and contagious patient to be sent to the laboratory. Precaution protocols have to be followed for that a second time.
Using our approach, the patient can leave the rooms, only be let in again, if the test is negative. If the LFAT is positive, no second sample has to be taken from the patient, the patient can go home, self-isolate, and wait for the confirmation or rejection of the LFAT result by the laboratory.
Our approach can also be applied on a negative LFAT for patients with a high probability of infection due to e.g. close contact to another positive person in combination with typical symptoms. Even negative test strips could turn out positive, as testing of the strips extraction solution shifts the sensitivity into the range, where usually only qPCR tests can yield a positive result.
Using the cobas® Liat® or the ID NOW™ system it takes about 10–20 min after extraction from the LFAT device to get a result. Fast confirmation is important in clinics or outpatient practices, when a patient has to undergo emergency treatment while his infection status is ambiguous. Especially dental clinical procedures pose a special risk, as aerosols can be widely spread from the nasopharyngeal zone. If the clinic is equipped accordingly the test could be done directly after the LFAT without transport to a laboratory. Faster information helps to take precaution measures, if necessary [12]. However, the more expensive testing by nucleic acid amplification can be reduced to testing of the questionable or positive LFATs only.
In a high prevalence setting or a screening, the described method is especially useful, as a large number of people can be screened with the LFATs. The putative positives can be analyzed in combination with a higher throughput system (e.g. the MagNAPure96 system) to verify the positives or to discharge the false positives from isolation.
Thus, we here demonstrated a cost efficient screening possibility of a larger number of samples e.g. daily testing of students that includes confirmation of positives, without the need to test all persons by qPCR. Thus, screening the way described here is less expensive and faster, compared to only testing by qPCR. Only positive or questionable tests need to be retested. A fresh sample does not have to be taken, the used LFAT devices are easy to transport and the test procedure avoids further exposure of contagious individuals to others. Here we provide a novel tool serving as basis for a test strategy for SARS-CoV-2 to manage infections and isolations.
5. Conclusion
Confirmation of positive or questionable LAFT results directly from the LAFT device by nucleic acid amplification assays is a major improvement in SARS-CoV-2 diagnostics.
Patents
Ludwig Czibere, Jürgen Durner and Marc Becker have a patent application pending: Used rapid test strip as sample material for a follow-up test (Country: DE 20210315 2021 Patent Number: DE2021–10202110618).
References
- 1.Durner J., Burggraf S., Czibere L., Fleige T., Madejska A., Watts D.C., et al. Fast and simple high-throughput testing of COVID 19. Dent Mater. 2020;36:e141–e142. doi: 10.1016/j.dental.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeling R.W., Olliaro P.L., Boeras D.I., Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis. 2021;21:e290–e295. doi: 10.1016/S1473-3099(21)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kretschmer A., Kossow A., Grune B., Schildgen O., Mathes T., Schildgen V. False positive rapid antigen tests for SARS-CoV-2 in the real-world and their economic burden. J Infect. 2021 doi: 10.1016/j.jinf.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jegerlehner S., Suter-Riniker F., Jent P., Bittel P., Nagler M. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings. Int J Infect Dis. 2021;109:118–122. doi: 10.1016/j.ijid.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinnes J. COVID-19 rapid antigen testing strategies require careful evaluation. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinnes J., Deeks J.J., Berhane S., Taylor M., Adriano A., Davenport C., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3 doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sajid M., Kawde A.N., Daud M. Designs, formats and applications of lateral flow assay: A literature review. J Saudi Chem Soc. 2015;19:689–705. [Google Scholar]
- 8.Ben K., Alex M., Mariya K., Alison B., Niluka G., Jie Z., et al. Nat Portf. 2022 doi: 10.21203/rs.3.rs-1121993/v1. [DOI] [Google Scholar]
- 9.Itoh K., Kawamitsu T., Osaka Y., Sato K., Suzuki Y., Kiriba C., et al. False positive results in severe acute respiratory coronavirus 2 (SARS-CoV-2) rapid antigen tests for inpatients. J Infect Chemother. 2021;27:1089–1091. doi: 10.1016/j.jiac.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velavan T.P., Pallerla S.R., Kremsner P.G. How to (ab)use a COVID-19 antigen rapid test with soft drinks? Int J Infect Dis. 2021;111:28–30. doi: 10.1016/j.ijid.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nybo M., Cadamuro J., Cornes M.P., Gomez Rioja R., Grankvist K. Sample transportation - an overview. Diagn (Berl) 2019;6:39–43. doi: 10.1515/dx-2018-0051. [DOI] [PubMed] [Google Scholar]
- 12.Durner J., Beikler T., Watts D.C., Becker M., Draenert M.E. SARS-CoV-2 and regular patient treatment - from the use of rapid antigen testing up to treatment specific precaution measures. Head Face Med. 2021;17:39. doi: 10.1186/s13005-021-00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]