Abstract
Acquired hemophilia is a rare disease resulting from autoantibodies against endogenous factor VIII (FVIII), which associates with bleeding and a high mortality rate. The pathophysiology is still unclear. Recent studies suggest genetic and environmental factors trigger the breakdown of immune tolerance. We report a 77-year-old Taiwanese man presented with multiple ecchymoses and some hemorrhagic blisters three weeks after SARS-CoV-2 mRNA (Moderna) vaccination. Isolated activated partial thromboplastin time (aPTT) prolongation was found. Acquired hemophilia A (AHA) was confirmed by low factor VIII (FVIII) activity and high titer of FVIII inhibitor. The pathohistology of skin biopsy further supported the concomitant diagnosis of bullous pemphigoid. To date, 6 cases of acquired hemophilia A following SARS-CoV-2 mRNA vaccination were reported worldwide. We reviewed and summarized the characteristics of these cases. We also discussed the rare finding of concomitant acquired hemophilia A and bullous pemphigoid. Bullous pemphigoid results from autoantibody against epithelial basement membrane zone of skin. In this article, we proposed possibility of SARS-CoV-2 mRNA vaccine associated autoimmunity against FVIII and epithelial basement membrane zone.
Keywords: Acquired hemophilia A, SARS-CoV-2, mRNA vaccine, Bullous pemphigoid
Introduction
Acquired hemophilia A (AHA) is a rare disease, with an overall incidence of 1.5 per million per year,1 and thus limited data has been reported to support its pathophysiology, disease characteristics, and management. AHA was associated with high morbidity and mortality risks. The estimated all-cause mortality rate is 21%, which is higher in patients aged >65 years.2 Deaths have been associated with bleeding or immunosuppressive therapy (IST) for antibody eliminations.
Recent studies have suggested that the pathophysiology includes the breakdown of immune tolerance due to a combination of genetic and environmental factors.1 AHA management includes bleeding control with replacement therapy of recombinant porcine factor VIII, bypassing agents of activated prothrombin complex concentrate and recombinant activated factor VII, and eradicating factor VIII inhibitor with immunosuppressants, such as high-dose steroids, cyclophosphamide, and rituximab.1 Here, we present a Taiwanese case of newly diagnosed AHA following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination.
Case report
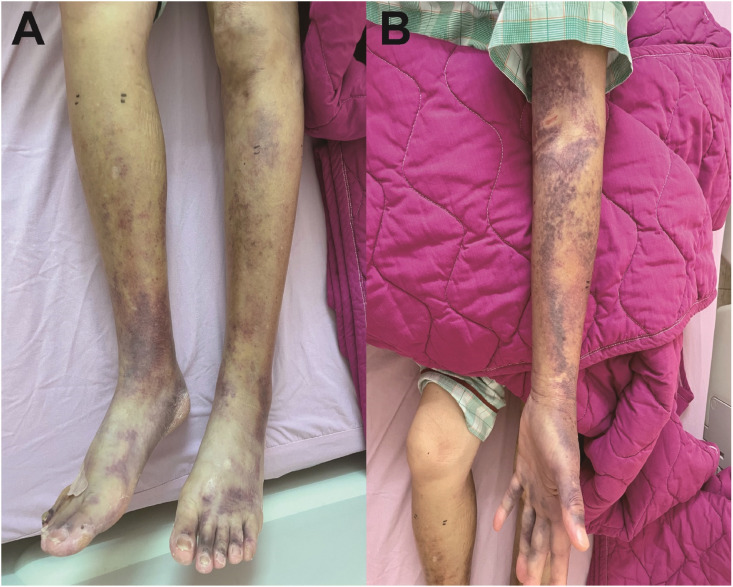
A 77-year-old man presented with multiple ecchymoses at bilateral forearms (Fig. 1 A), and legs (Fig. 1B). He also had hemorrhagic blisters and papules at his hands and trunk three weeks after receiving the second dose of SARS-CoV-2 mRNA (Moderna) vaccine. Skin biopsy was performed, and he visited our hospital due to persistent biopsy wound bleeding.
Figure 1.
A. Ecchymosis involving bilateral legs, feet and ankles. Fig. 1B. Ecchymosis involving left forearm.
The patient was hospitalized due to progression of the ecchymoses and persistent bleeding from the skin biopsy site. Physical examination showed no relevant findings except for the ecchymoses. Laboratory examinations showed normocytic anemia (hemoglobin 9.4 g/dL [normal range 13–18 g/dL], mean corpuscular volume 91 fL [normal range 81–98 fL]) and normal platelet count (200 × 103/μL [normal range 143–349 × 103/μL]). Coagulation profile showed severe prolongation of the activated partial thromboplastin time (aPTT) (97.3 s, [normal range 29.3–40.1 s]), and normal prothrombin time (PT) (11.9 s, [normal range 9.4–12.5 s]). aPTT mixing study prolonged with incubation. Diluted Russel viper venom time (dRVVT) did not show lupus anticoagulants. The activity of von Willebrand factor function was elevated (230%). Factor VIII (FVIII) activity lowered to 0.6%, and FVIII inhibitor titer was 71.6 Bethesda units (BU). Factor IX activity was 117.6%. Anti-platelet factor 4 antibody titer was weak positive (0.745 optical density (OD) value, [normal range <0.4OD]). Acquired hemophilia A was diagnosed, and prednisolone was soon started at a dose of 1 mg/kg/day. Two doses of FVIII inhibitor bypassing activity (FEIBA) of recombinant factor VII activated (rFVIIa) at the dose of 90 mcg/kg were given due to persistent bleeding of the biopsy wound. After rFVIIa treatment, the progression of the ecchymoses halted, and the wound bleeding stopped. There was no major bleeding after the start of prednisolone treatment. However, aPTT prolongation did not improve after one week of prednisolone treatment. Oral cyclophosphamide at a dose of 100 mg/day was added on. After four weeks of cyclophosphamide treatment, aPTT prolongation improved. FVIII activity increased to 9% and the titer of FVIII inhibitor was lowered to 49 BU.
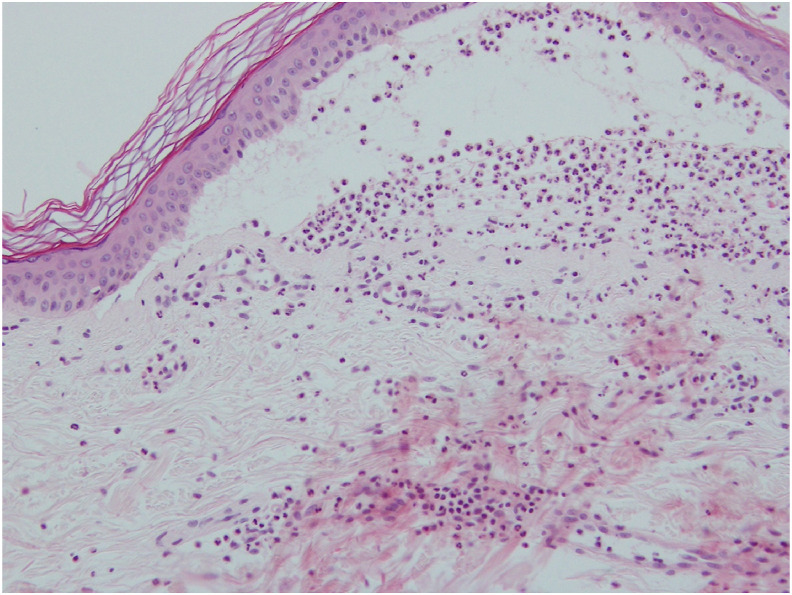
The microscopic findings of the skin biopsy showed sub-epidermal blistering with fibrin and abundant neutrophils (Fig. 2 ). Further direct immunofluorescence study revealed linear deposition of IgG and C3 at the dermis–epidermis junction. Indirect immunofluorescence (IIF) test was negative of anti-ICS and anti-BMZ antibodies. On the basics of the clinical presentation and pathological findings, a diagnosis of bullous pemphigoid was made.
Figure 2.
Microscopic findings of the blister with H&E stain indicating there is subepidermal bullae with primarily neutrophils at dermal–epidermal junction.
Discussion
In 2021, six cases of AHA that were possibly triggered by SARS-CoV-2 vaccination were reported (Table 1 .). These six cases shared several common clinical characteristics. First, the vaccines administered were SARS-CoV-2 mRNA vaccines, either Pfizer-BioNTech or Moderna. Second, the response rate of treatment was higher and time to remission was shorter in vaccine-associated AHA compared to non-vaccine-associated AHA.1 No established management of vaccine-associated AHA was recommended, thus the treatment followed the previously reported guidance. All cases achieved remission within four weeks of IST, except for one case which died from gallbladder rupture. The case in this report showed decreased factor FVIII inhibitor levels after a 4-week cyclophosphamide and 5-week prednisolone treatment. Third, all cases were exhibited AHA, and there were no vaccine-associated acquired hemophilia B cases reported so far. However, some differences were observed among these cases. The onset of bleeding tendency varied from four days to three weeks after vaccination, either first or second dose, and the factor VIII inhibitor titer was diverse, ranging 1.01–110 BU/ml.
Table 1.
The reported cases of acquired hemophilia A following SARS-CoV-2 mRNA vaccines.
| Age | Sex | Co-morbidities | Vaccine | Onset | Hemophilia A or B | FVIII:C (%) | FVIII inhibitor Titer (BU/ml) | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 13 | 69 | Man | DM, HTN, prostate cancer | Pfizer-BioNTech SARS-CoV-2 mRNA | 9 days after first dose | A | 1% | 80 | Prednisolone | FVIII increased to 5% after 4 weeks |
| Case 24 | 67 | Man | None | Pfizer-BioNTech SARS-CoV-2 mRNA | 19 days after second dose | A | Not detected | 110 | FEIBA, prednisolone, rFVIIa, rituximab | FVIII inhibitor decreased to 8BU after around 14 days |
| Case 35 | 76 | Woman | Asthma, Raynaud's phenomenon | Moderna SARS-CoV-2 mRNA | 4 days after second dose | A | <3% | 11.2 | IVIG + high dose steroid | Total recovery after 25 days of treatment |
| Case 46 | 85 | Man | Not mentioned | Moderna SARS-CoV-2 mRNA | 1 week after first dose | A | Not detected | 2.2 | rFVIIa, APCC, prednisolone 100 mg/day, rituximab | Died after gallbladder rupture and active arterial bleeding three and half weeks after diagnosis |
| Case 56 | 86 | Woman | Moderate to severe aortic stenosis, third-degree AV block. | Moderna SARS-CoV-2 mRNA | 3 weeks after second dose | A | 23% | 1.01 | rFVIIa, APCC, prednisolone 1 mg/kg | FVIII:C increased to 178% 17 days after oral prednisolone treatment |
| Case 66 | 72 | Woman | Multiple comorbidities, including arterial disease | Moderna SARS-CoV-2 mRNA | 2 weeks after first dose | A | Not detected | 12.4 | rFVIIa, tranexamic acid, prednisolone 100 mg/day, rituximab 375mg/m2 weekly for 4 weeks | After third dose of rituximab, FVIII activity increased to 5%, FVIII inhibitor decreased to 5.6BU/ml |
DM: diabetes mellitus; HTN; hypertension; BU: Bethesda unit; FEIBA: factor VIII inhibitor bypassing activity (FEIBA); rFVIIa: recombinant factor VII activated; IVIG: intravenous immunoglobulin; AV: atrioventricular.
Two mechanisms of AHA have been proposed in several reports, including antigenic mimicry and non-specific activation of quiescent autoreactive T and B cells.7 However, the exact pathogenesis mechanism remains unclear. Besides SARS-CoV-2 mRNA vaccine-associated AHA, two cases of AHA following influenza vaccination have been reported.8 , 9 The first case was a 72-year-old female, who developed AHA eight days after the seasonal influenza vaccination, and the other was a 66-year-old female, who developed AHA 20 days after H1N1 influenza vaccination. AHA recovery was observed after a high-dose corticosteroid treatment, but the time to recovery varied from 3 weeks to 2 months. It is noteworthy that influenza and SARS-CoV-2 mRNA vaccine antigens differ, and thus further research for understanding the underlying pathophysiology of vaccine-associated AHA is needed.
Interestingly, our case was concomitantly diagnosed with acquired hemophilia and bullous pemphigoid after the second dose of mRNA vaccine was administered. Since both AHA and bullous pemphigoid are autoimmune diseases, treatment involved IST. The pathogenesis of bullous pemphigoid is autoantibody-mediated damage to the epithelial basement membrane zone of the skin, which causes cutaneous bullae and erosive mucosal lesions. The antigenic targets of these autoantibodies are bullous pemphigoid antigen (BP) 180 (also known as type XVII collagen) and BP230. Few previously published articles that primarily focus on case reports have suggested that there are some shared epitopes between factor VIII, BP230, and BP180. However, the study by Patel et al. (2006) did not find any obvious homologous sequences through the protein database.10 Whether this is due to a post-translational modification of the proteins leading to minimal possibility of exposure to the same binding domain as autoantibodies or due to incidentally triggered cascade of immune reactions has to be further explored. Multiple studies have indicated that medications and/or vaccines play a role in inducing bullous pemphigoid,11 but without a definite pathogenesis. Further, autoantibody identification might be crucial for the case described in this study for establishing a clearer relationship between acquired hemophilia and bullous disease.
In conclusion, we reported a rare case of acquired hemophilia A (AHA) and bullous pemphigoid following the second dose of SARS-CoV-2 mRNA vaccination. Bleeding events were noted in our case, and hemostasis was achieved by bypassing agent of recombinant factor VII activated (rFVIIa). After five weeks of immunosuppressive therapy (IST) with prednisolone and cyclophosphamide, factor VIII (FVIII) activity increased and the titer of FVIII inhibitor was lowered. Bullous pemphigoid was well controlled by IST too. In these reported cases including ours, we highlight possible association between SARS-CoV-2 mRNA vaccine and autoimmunity. Though the definite correlation between SARS-CoV-2 mRNA vaccine and autoimmune diseases including AHA and bullous pemphigoid is not confirmed, this case raises the awareness of these rare side effects. Early recognition of these rare autoimmune phenomenon following vaccine injection facilitates immediate medical management. Once the risk factors of vaccine-related autoimmunity can be identified in the future, the complications may be completely avoided.
Author contributions
P.A. Fu and C.W. Chen drafted the manuscript. Y.T. Hsu and K.C. Wei revised the manuscript critically for important intellectual content. P.A. Fu, C.W Chen, Y.T. Hsu, K.C. Wei, P.C. Lin and T.Y. Chen all read and approved the final manuscript.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Kruse-Jarres R., Kempton C.L., Baudo F., Collins P.W., Knoebl P., Leissinger C.A., et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92(7):695–705. doi: 10.1002/ajh.24777. [DOI] [PubMed] [Google Scholar]
- 2.Bitting R.L., Bent S., Li Y., Kohlwes J. The prognosis and treatment of acquired hemophilia: a systematic review and meta-analysis. Blood Coagul Fibrinolysis. 2009;20(7):517–523. doi: 10.1097/MBC.0b013e32832ca388. [DOI] [PubMed] [Google Scholar]
- 3.Radwi M., Farsi S. A case report of acquired hemophilia following COVID-19 vaccine. J Thromb Haemostasis. 2021;19(6):1515–1518. doi: 10.1111/jth.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farley S., Ousley R., Van Wagoner N., Bril F. Thrombosis and Haemostasis; 2021. Autoimmunity after coronavirus disease 2019 (COVID-19) vaccine: a case of acquired hemophilia A. [DOI] [PubMed] [Google Scholar]
- 5.Portuguese A.J., Sunga C., Kruse-Jarres R., Gernsheimer T., Abkowitz J. Autoimmune-and complement-mediated hematologic condition recrudescence following SARS-CoV-2 vaccination. Blood Adv. 2021;5(13):2794–2798. doi: 10.1182/bloodadvances.2021004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cittone M.G., Battegay R., Condoluci A., Terzi di Bergamo L., Fernandes E., Galfetti E., et al. The statistical risk of diagnosing coincidental acquired hemophilia A following anti-SARS-CoV-2 vaccination. J Thromb Haemostasis. 2021;19(9):2360–2362. doi: 10.1111/jth.15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wraith D.C., Goldman M., Lambert P.-H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(9396):1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 8.Moulis G., Pugnet G., Bagheri H., Courtellemont C., Huart A., Chauveau D., et al. Acquired factor VIII haemophilia following influenza vaccination. Eur J Clin Pharmacol. 2010;66(10):1069–1070. doi: 10.1007/s00228-010-0852-z. [DOI] [PubMed] [Google Scholar]
- 9.Pirrotta M., Bernardeschi P., Fiorentini G. A case of acquired haemophilia following H1N1 vaccination. Haemophilia. 2011;5(17):815–829. doi: 10.1111/j.1365-2516.2011.02493.x. [DOI] [PubMed] [Google Scholar]
- 10.Patel R.S., Harman K.E., Nichols C., Burd R.M., Pavord S. Acquired haemophilia heralded by bleeding into the oral mucosa in a patient with bullous pemphigoid, rheumatoid arthritis, and vitiligo. Postgrad Med. 2006;82(963):e3. doi: 10.1136/pgmj.2005.036483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stavropoulos P.G., Soura E., Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014 Sep;28(9):1133–1140. doi: 10.1111/jdv.12366. [DOI] [PubMed] [Google Scholar]