Abstract
There are now several monoclonal antibody (mAb) therapies (“biologics”) available to treat severe asthma. Omalizumab is an anti-IgE mAb and is licensed in severe allergic asthma. Current evidence suggests it may decrease exacerbations by augmenting deficient antiviral immune responses in asthma. Like all other biologics, clinical efficacy is greatest in those with elevated T2 biomarkers. Three biologics target the interleukin (IL)-5–eosinophil pathway, including mepolizumab and reslizumab that target IL-5 itself, and benralizumab that targets the IL-5 receptor (IL-5R-α). These drugs all reduce the exacerbation rate in those with raised blood eosinophil counts. Mepolizumab and benralizumab have also demonstrated steroid-sparing efficacy. Reslizumab is the only biologic that is given intravenously rather than by the subcutaneous route. Dupilumab targets the IL-4 receptor and like mepolizumab and benralizumab is effective at reducing exacerbation rate as well as oral corticosteroid requirements. It is also effective for the treatment of nasal polyposis and atopic dermatitis. Tezepelumab is an anti-TSLP (thymic stromal lymphopoietin) mAb that has recently completed phase 3 trials demonstrating significant reductions in exacerbation rate even at lower T2 biomarker thresholds.
Many patients with severe asthma qualify for more than one biologic. To date, there are no head-to-head trials to aid physicians in this choice. However, post-hoc analyses have identified certain clinical characteristics that are associated with superior responses to some therapies. The presence of allergic and/or eosinophilic comorbidities, such as atopic dermatitis, nasal polyposis or eosinophilic granulomatosis with polyangiitis, that may additionally benefit by the choice of biologic should also be taken into consideration, as should patient preferences which may include dosing frequency. To date, all biologics have been shown to have excellent safety profiles.
Short abstract
Biologic therapies target T2 inflammatory pathways and elevated FENO and/or blood eosinophil counts are associated with greater clinical efficacy. Choice of drug will depend on individual patient characteristics and preferences. https://bit.ly/3lHOsSQ
Educational aims
To review the major outcome data from phase 3 and real-world studies of biologic therapies for severe asthma.
To understand the key baseline characteristics associated with response to each biologic therapy.
To gain awareness of the practical issues that can impact the choice of biologic therapies in asthma.
Introduction
“Severe asthma” describes asthma that is dependent upon high-dose inhaled corticosteroids (ICS) plus a second controller (and/or systemic corticosteroids) in order to maintain symptom control, or which remains “uncontrolled” despite these therapies [1]. Once comorbidities have been addressed and excluding those patients who are poorly adherent to inhaled therapy, the prevalence of severe asthma is estimated at 3.7% of the asthma population [2]. The severe asthma cohort accounts for a disproportionate share of asthma-related healthcare costs and patients often suffer daily symptoms, missed work/school days, and frequent healthcare utilisation. Severe asthma patients can have multiple exacerbations each year requiring courses of oral corticosteroids (OCS) or may need maintenance oral corticosteroids (mOCS) to control their disease. This exposure to OCS is associated with significant long-term morbidity, including adrenal suppression, osteopenia and osteoporosis, increased risk of type II diabetes, cataracts, and obesity [3]. Previous attempts at steroid-sparing immunosuppression, with therapies such as methotrexate and azathioprine, have no evidence of clinical benefit.
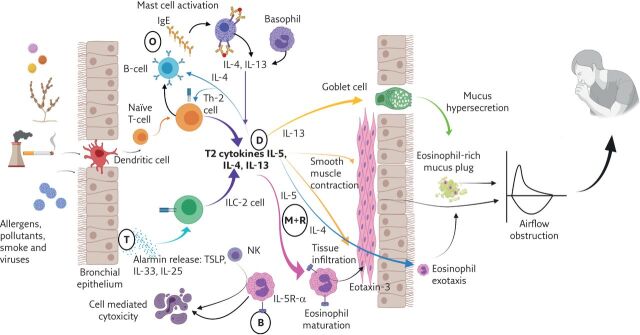
The past decade has seen a renewed focus on the role of the eosinophil and the type 2 (T2) inflammatory cascade in the pathogenesis of asthma. The combination of genetic susceptibility plus exposure to allergens, microbes, pollutants and other triggers cause the airway epithelium to release “alarmins”, including interleukin (IL)-25, IL-33 and thymic stromal lymphopoietin (TSLP), which result in the release of T2 cytokines from cells including the T-helper (Th)2 cell and type 2 innate lymphoid cells (ILC2) [4]. Important T2 cytokines include IL-4 (which drives IgE production from B-cells), IL-5 (associated with the eosinophil), and IL-13 (believed to be the key cytokine for mucus hypersecretion and airway hyperresponsiveness [5]. All the currently available biologic therapies developed for severe asthma target one or more mediators or cells within this pathway (figure 1).
Figure 1.
The role of the T2 inflammatory cascade in asthma pathophysiology and the sites of action of biologic therapies. Allergens, pollutants and cigarette smoke and viral infections trigger the bronchial epithelium to release alarmins (TSLP, IL-33 and IL-25), while dendritic cells drive naïve T-cell maturation to Th-2 phenotypes. ILC-2 and Th-2 cells, alongside activated mast cells and basophils, produce the T2 cytokines: IL-5, IL-4 and IL-13. These lead to: eosinophil growth and maturation (driven by IL-5); smooth muscle contraction, goblet cell hyperplasia, mucus hypersecretion and mucus plugging and eosinophil migration from blood to tissue (predominantly driven by IL-13); and eosinophil airway exotaxis, Th-2 cell population expansion and direction of B-cells to produce IgE (predominantly directed by IL-4). Sites of the biologic therapies are indicated by circles (B: benralizumab; D: dupilumab; M+R: mepolizumab and reslizumab; O: omalizumab; T: tezepelumab). IL-5R-α: IL-5 receptor alpha; NK: natural killer cell. Figure created with BioRender.
These therapies have transformed the management of severe asthma, all showing varying degrees of efficacy in patients with an eosinophilic phenotype as defined by a blood eosinophil count of at least 300 cells·μL−1. The indications for these therapies overlap, which has created a new challenge for physicians who need to decide which drug to prescribe for which patient. The absence of randomised head-to-head trials has meant the absence of coherent national or international guidelines to help navigate this process. This review aims to summarise the data available for each drug and provide a pragmatic framework for decision making. We will examine each biologic in turn and outline the efficacy data from the major phase 3 trials, as well as the long-term and real-world data where available. We will also discuss available data on the baseline clinical characteristics associated with a superior response, and practical aspects that might influence patient and physician choice.
Omalizumab
Omalizumab (“Xolair”) was licensed by the European Medicines Agency (EMA) in 2005 and was the first monoclonal antibody (mAb) approved to treat severe asthma. It binds to free circulating IgE, inhibiting attachment to its receptor (FCεRI) and diminishing downstream effects, including mast cell degranulation and the expression of inflammatory cytokines such as IL-3, IL-4, IL-5, IL-6 and IL-13. Omalizumab inhibits both the early and late phase allergic responses [6] and there is good evidence that it improves the antiviral response in asthma, thereby reducing virus-induced exacerbations [7].
Primary outcomes: exacerbation and OCS reduction
The phase 3 trials of omalizumab focused their inclusion criteria on patients who had evidence of sensitisation to aeroallergens, airway hyperresponsiveness and ongoing asthma symptoms. Omalizumab treatment resulted in a reduction in exacerbations as well as reductions in hospitalisations, emergency department visits and inhaled steroid dose [8–10]. A Cochrane review published in 2014 included data from 10 studies directly addressing annualised exacerbation rate (AER) and reported an overall risk ratio of 0.55 compared with placebo [11]. The reduction in AER is difficult to compare to later biologic trials in asthma, as most omalizumab studies did not specify a minimum pre-treatment AER, ICS dose was often weaned as part of the trial design (increasing the risk of exacerbation) and an exacerbation was not consistently defined [10]. Study populations are therefore heterogeneous. Only one study attempted to limit recruitment to severe asthma patients only by including a requirement for high-dose ICS/long-acting β2-agonist (LABA) combination therapy [12]. However, even in this study the AER of 0.88 in the placebo arm suggests a less than severe cohort were recruited in the end. In the most severe subgroup who required mOCS at baseline, omalizumab was not effective at reducing the exacerbation rate.
Most of the available data on mOCS dose reduction in omalizumab comes from real-world, observational trials [13–17]. A systematic review of 42 observational or registry trials reported a mean OCS dose reduction of 68% at 12 months, however with a broad range from −78% to −12% [18]. The large “eXpeRience” registry reported on over 260 patients on baseline OCS and observed that 57% either reduced or stopped mOCS by 1 year of treatment with omalizumab. The mean prednisolone dose at the end of 1 year was still 7.7 mg (compared with 15.5 mg at baseline) [19]. A randomised trial reported more patients reduced or stopped mOCS with omalizumab than with optimal standard care; however, this was an open label study [20]. The true corticosteroid-sparing efficacy of omalizumab in severe asthma remains to be determined.
Secondary outcomes: quality of life, asthma control and FEV1
The initial omalizumab trials used the St George's Respiratory Questionnaire (SGRQ) (validated in COPD) to assess the impact on quality of life, and there was a statistically and clinically significant improvement compared with placebo. Asthma diaries were generally used to assess patient reported asthma control, rather than a validated tool. The methodology used was variable; however, there were improvements in the treatment group compared with placebo and this was mirrored by a reduction in rescue short-acting β2-agonist use in those receiving omalizumab. The impact on forced expiratory volume in 1 s (FEV1) was variable, with only some studies reporting an improvement compared with placebo. An analysis conducted as part of the Cochrane review of omalizumab reported an overall improvement in FEV1 of 2.15% compared with placebo [11]. The minimal clinically important difference (MCID) for FEV1 in asthma is debated, but a recent expert consensus report suggested cut-offs of ≥20% in short trials, or ≥15% for trials ≥1 year duration [21].
Predictors of response
A post-hoc analysis of two large randomised controlled trials (RCTs) found those most likely to benefit from therapy were participants who had required emergency asthma treatment in the year before enrolment, those who were on high-dose ICS and those with a lower FEV1 at baseline [22]; essentially patients with severe asthma. The licence-related criteria for selecting patients who might benefit from omalizumab focus on identifying those with an allergic phenotype; however, neither allergen-specific IgE nor total IgE predicts response to treatment [23] and there are data to suggest omalizumab can reduce the exacerbation rate in non-atopic patients as well [24]. In contrast, the level of the two T2 biomarkers that closely reflect active T2 inflammation, fraction of exhaled nitric oxide (FENO) and the blood eosinophil count, do both relate to efficacy of omalizumab. Specifically, Hanania et al. [25] demonstrated that omalizumab was ineffective in patients with a blood eosinophil count <260 cells·μL−1 and a FENO <20 ppb. This has also been demonstrated in a single RCT [26]. A study by Djukanovic et al. [27] examined airway inflammation in mild-to-moderate asthma patients treated with omalizumab and observed a reduction in eosinophil counts, which may in part explain the observed relationship between eosinophilia and efficacy. Additionally, elevated T2 biomarkers at the time of omalizumab cessation was shown to be a predictor of future exacerbation [28]. This latter study is particularly helpful in determining whether a trial off omalizumab is likely to be successful or not.
Real-world data and length of treatment
The benefit of omalizumab as an add-on therapy in severe asthma has been confirmed by numerous “real-life” observational trials, summarised in a meta-analysis by Alhossan et al. [29]. The largest real-world study of omalizumab showed modest efficacy: in a Japanese cohort of over 3000 patients with severe allergic asthma, 42% experienced two or more exacerbations despite omalizumab (compared with 63% prior to treatment) and 28% of the cohort continued to experience four or more exacerbations (compared with 48% prior to treatment). The ongoing exacerbation frequency highlights the limitations of this treatment [30].
While extension trials have confirmed the continued efficacy of omalizumab to 1 year, the optimal treatment length for omalizumab is not known. The Xolair Persistency of Response after Long-term Therapy (XPORT) study randomised patients who had been treated with omalizumab for at least 5 years to either continue the drug or to have placebo for a further year. 48% of those in the placebo arm had zero exacerbations in the year, suggesting omalizumab could be safely withdrawn. However, importantly there were no data available on AER prior to starting omalizumab and more patients were exacerbation-free in the treatment arm (67%) [28].
Comorbidities
The treatment of chronic rhinosinusitis with nasal polyposis (CRwNP) with omalizumab has been evaluated in two phase 3 trials and resulted in improved endoscopic nasal obstruction scores, nasal congestion scores (patient reported) and disease specific quality of life scores (SNOT-22) [31]. It has recently been licensed for this indication as an add-on therapy to intranasal steroids. It is also licensed for use in chronic spontaneous urticaria.
Practical considerations
Omalizumab is only licensed in allergic asthma, which means patients need a positive skin prick test or in vitro reactivity to a perennial aeroallergen to qualify for the drug. The EMA licensing also states the requirement for FEV1 <80% predicted, ongoing symptoms and “multiple” exacerbations despite high-dose ICS/LABA. The UK National Institute for Health and Care Excellence (NICE), which considers the cost effectiveness as well as clinical effectiveness of treatments for the National Health Service, quantifies the number of exacerbations required as ≥4 severe exacerbations in the last year (or the need for OCS to maintain asthma control). There is no requirement to demonstrate an eosinophilic phenotype, despite data demonstrating a lack of efficacy without one.
Omalizumab is given by subcutaneous injection 2–4 weekly, with dose and frequency determined by weight and serum IgE. If the serum IgE is >1500 international units (IU) omalizumab is not licensed for use, and this level decreases as weight increases (e.g. a patient of ≥90 kg would need a serum IgE of ≤600 IU). It is notable that the major clinical trials only included patients with an IgE of 30–700 IU. Pre-filled syringes of omalizumab come as 75 mg and 150 mg, therefore those on the highest dose require four subcutaneous injections every 2 weeks, which may be burdensome for patients when compared to a single injection every 4 or 8 weeks with mepolizumab or benralizumab, for example. The main side-effects from the clinical trial programme were injection site reactions, arthralgia/pain, fatigue, and dizziness. Anaphylaxis is reported in ∼0.1% [32].
Mepolizumab
Mepolizumab (“Nucala”) was the first mAb licensed for severe eosinophilic asthma that targeted the IL-5 pathway. IL-5 is the critical cytokine for the development, migration and survival of eosinophils [33] and mepolizumab has been demonstrated to significantly reduce both blood [34] and airway eosinophil numbers [35].
Primary outcomes: exacerbation and OCS reduction
The phase 3 MENSA trial demonstrated a reduction in exacerbation rate of 52% with mepolizumab treatment when compared with placebo [36]. Inclusion criteria included an eosinophilic phenotype (with blood eosinophils of ≥150 cells·μL−1 at screening, or ≥300 cells·μL−1 in the past 12 months) and frequent exacerbations (≥2 exacerbations in the year prior to commencing mepolizumab). As well as reducing exacerbations mepolizumab has demonstrated efficacy in reducing OCS dose in those dependent on daily OCS to maintain asthma control. The SIRIUS trial recruited subjects with severe eosinophilic asthma dependent on daily OCS and blood eosinophils of ≥300 cells·μL−1 in the past year, or ≥150 cells·μL−1 in the OCS dose optimisation phase. At 24 weeks, 14% of subjects treated with mepolizumab were able to completely stop prednisolone and overall, a median reduction of 50% was achieved. In the placebo arm, the median reduction was zero; however, a third of subjects were able to reduce their prednisolone dose by at least 50% despite only receiving placebo, highlighting a failure to optimise systemic corticosteroid exposure in these patients prior to randomisation [37]. Importantly, despite the overall reduction in OCS, exacerbations were reduced (AER 1.44 in the mepolizumab arm versus 2.12 in the placebo arm).
Secondary outcomes: quality of life, asthma control and FEV1
In MENSA, asthma control as measured by the five-question asthma control questionnaire (ACQ5) improved by 0.4, just short of the MCID of 0.5. This trial also used the SGRQ and demonstrated an increase of 7 points versus placebo (meeting the MCID for mild–moderate COPD). There was a small improvement in FEV1 of ∼100 mL.
Predictors of response
Additional analysis of data from MENSA demonstrated that baseline eosinophil count and AER were the only covariates that influenced efficacy. In those with high blood eosinophils of ≥500 cells·μL−1 there was a 79% reduction in exacerbation rate versus placebo, as well as gains in pre-bronchodilator FEV1 of 132 mL and ACQ5 of 0.75.
Response to mepolizumab does not appear related to IgE levels, the presence of atopy, or co-eligibility of patients for omalizumab [38, 39]. Real-world characteristics associated with improved response include nasal polyposis, lower baseline OCS dose and lower body mass index (BMI) [40]. In the context of eosinophilic asthma, FENO level does not appear to predict response [39, 41].
Real-world data
There is a substantial body of real-world data supporting the efficacy seen in the phase 3 programme [40, 42, 43]. These data are very important as the subjects recruited to the controlled studies appeared to be less severe than initially intended. For example, in the placebo arms of the phase 3 MENSA and phase 3b MUSCA studies of mepolizumab, only 13% and 6%, respectively, of subjects randomised to placebo had an AER >2.
Real-world data also suggest that response can be accurately assessed earlier than 1 year. A retrospective analysis of 99 patients treated with mepolizumab defined response as a reduction in exacerbation rate of at least 50% or, for those on mOCS, a reduction in prednisolone dose of at least 50%. At 16 weeks, 81% of patients had the same responder status as when assessed after 1 year of treatment, rising to 93% of patients by 24 weeks [40].
Length of treatment
In an extension study to early phase work, Haldar et al. [44] followed-up participants for 12 months after they discontinued mepolizumab. Exacerbations increased 3–6 months after discontinuation and after 12 months there was no significant difference between those who had been treated with mepolizumab and those who had placebo [44]. “COMET” randomised patients who had received mepolizumab for at least 3 years to either continue mepolizumab or to placebo for a further year. Those who stopped mepolizumab had a significantly shorter time to first exacerbation and a decrease in asthma control (measured by ACQ5) when compared with those who continued mepolizumab [45]. An open label extension study (“COLUMBIA”) has confirmed a sustained response to mepolizumab up to 4.5 years of treatment [46].
Comorbidities
Eosinophilic granulomatosis with polyangiitis
In addition to severe eosinophilic asthma, Mepolizumab is licensed for treatment of eosinophilic granulomatosis with polyangiitis (EGPA) in the USA, and as of September 2021 has also been approved by the EMA. A RCT of 136 patients with EGPA compared mepolizumab (300 mg every 4 weeks, three times the dose in severe eosinophilic asthma) with placebo [47]. This demonstrated that those treated with mepolizumab had more weeks in clinical remission, with 32% in remission at both 36 and 48 weeks of treatment, compared with only 3% in the placebo arm. Although close to 50% of those treated with mepolizumab had a relapse during the 48 weeks of treatment, this was still 50% lower than the placebo relapse rate.
CRwNP
Mepolizumab has recently received US Food and Drug Administration and EMA approval for use in CRwNP. A large RCT of patients with recurrent, severe bilateral nasal polyposis, refractory to medical and surgical treatment demonstrated significant reductions in endoscopic nasal scores and nasal obstruction symptoms with mepolizumab compared with placebo [48]. In addition, fewer of those treated with mepolizumab required a course of oral steroids during the year (25% versus 37% in the placebo arm) or surgery (9% of those treated with mepolizumab, versus 23% of those on placebo).
Practical considerations
Mepolizumab is given as a fixed 100 mg dose every 4 weeks, subcutaneously. It has recently been made available in a pre-filled syringe for self-administration. The commonest side-effects with mepolizumab are headache, backache and injection site reactions; however, overall there are very good safety data available up to 4.5 years [46, 49].
Reslizumab
Reslizumab (“Cinqaero”) is a recombinant humanised IgG4 mAb that, like mepolizumab, binds IL-5 thereby reducing blood and airway eosinophils. Two duplicate phase 3 trials of weight-based intravenous reslizumab were published in 2015 by Castro et al. [50]. These trials selected patients with blood eosinophils of ≥400 cells·μL−1 and with one or more exacerbation requiring OCS in the past year. When compared with placebo, there was a 50–59% reduction in annual exacerbation rate in the treatment arm and a statistically significant improvement in both FEV1 and ACQ, although this did not meet the MCID. Efficacy was not demonstrated in those with eosinophil counts of <400 cells·μL−1 [51]. The efficacy of reslizumab in reducing mOCS use in asthma has not been formally evaluated.
A recent trial of a subcutaneous formation was not successful [52]. Despite very good efficacy in severe eosinophilic asthma, the need to give reslizumab intravenously is a major practical consideration, as it is inconvenient for patients and costly to deliver. Given the availability of both mepolizumab and benralizumab, which are subcutaneous injections and can be self-delivered, the number of patients receiving reslizumab is consequently relatively small. As such, long-term and real-life data on reslizumab are more limited. Nonetheless, the weight-based dosing has been an attractive option for patients in whom the fixed 100 mg s.c. mepolizumab dose is suspected to be insufficient. In addition, there is reported efficacy of reslizumab in EGPA, although this is limited to small numbers of patients in open label and observational studies [53, 54].
Benralizumab
Benralizumab (“Fasenra”) targets the IL-5 pathway through ligation to the α subunit of the IL-5 receptor (IL-5R-α), expressed on eosinophils and basophils. This induces antibody-dependent cell mediated cytotoxicity, resulting in apoptosis of cells on which IL-5R-α is expressed [55, 56]. Circulating blood eosinophils rapidly fall within 4 h of the first dose and are usually undetectable shortly after [57]. Eosinophil numbers in sputum and bronchial mucosal and submucosal biopsies are also markedly reduced [58]. As benralizumab induces an almost complete eosinopenic state, use of this therapy has allowed the opportunity to explore the importance of the eosinophil in severe asthma in a way that was not previously possible.
Primary outcomes
Two large phase 3 trials in severe eosinophilic asthma (SIROCCO [59] and CALIMA [60]) recruited patients with two or more exacerbations in the past year stratified by blood eosinophil counts (≥300 cells·μL−1 and <300 cells·μL−1). In those with blood eosinophils <300 cells·μL−1, there was a 17–40% reduction in exacerbation rate compared with placebo, compared to a 28–51% reduction in those with eosinophils ≥300 cells·μL−1. The difference in exacerbation reduction versus placebo reflected a very large placebo response in CALIMA. However, the actual AER on treatment in both studies was ∼0.6. A phase 3b study, ANDHI, demonstrated an overall reduction of 49% in AER compared with placebo at 24 weeks, increasing to 59% in those with baseline blood eosinophils ≥300 cells·μL−1 [61].
The ZONDA study enrolled OCS-dependent patients and demonstrated a 50% reduction in OCS dose compared with placebo. Over 50% of participants in the treatment arms achieved 100% reduction by 28 weeks and the reduction in exacerbations was an impressive 70% [62]. More recently, the open-label PONENTE study of 598 OCS-dependent patients treated with benralizumab reported that over 80% were able to eliminate use or achieved a dosage of 5 mg or less if the reason for stopping the reduction was adrenal insufficiency rather than asthma [63]. To date, PONENTE is the largest OCS sparing study of its kind.
Secondary outcomes
SIROCCO and CALIMA included ACQ and asthma quality of life questionnaire (AQLQ) as patient-reported outcome measures. There were statistically significant improvements, but these narrowly missed the MCID threshold of 0.5 compared with placebo. There were small improvements in FEV1 of ∼100 mL. The ANDHI trial evaluated quality of life using the SGRQ rather than AQLQ and demonstrated an improvement of −8.1 (MCID of 4), with an improvement evident from week four. It also reported a slightly larger improvement in FEV1 of 160 mL, with a noticeable separation between placebo and treatment arms by week four [61].
Predictors of response
A post-hoc analysis of pooled data from SIROCCO/CALIMA identified high baseline exacerbation rate and higher blood eosinophils as associated with an enhanced response to benralizumab [64]. The presence of nasal polyposis (itself associated with eosinophilic inflammation and severe disease), low baseline forced vital capacity (FVC) (<65% predicted) and dependence on OCS were also associated with a better response [65]. ANDHI also reported greater reductions in AER in those with blood eosinophils of ≥300 cells·μL−1 at baseline, adult-onset disease and nasal polyposis [61]. Real-world data have similarly shown an association between enhanced response and high baseline eosinophils and the presence of nasal polyposis [66]. A differential response has not been shown to be related to baseline FENO level [41], IgE [67] or co-eligibility for omalizumab treatment [39].
Real-world data and length of treatment
Large real-world cohorts now confirm the clinical effectiveness of benralizumab in reducing AER and mOCS requirement as well as reducing symptom scores and improving quality of life metrics [66]. The BORA extension trial has demonstrated continued efficacy and safety of benralizumab out to 2 years [68]. In this study, more than 70% of patients in their second year of benralizumab treatment remained exacerbation free. A recent report of adolescents who had completed 3 years of benralizumab as part of the extension trial confirmed continued efficacy and safety [69], whilst the MELTEMI study has provided evidence of continued efficacy along with reassuring safety data out to 5 years [70].
Comorbidities
Benralizumab is not currently licensed as a treatment for CRwNP, although there is evidence to suggest efficacy for this indication. A post-hoc analysis of the ANDHI trial found significant improvement in SNOT-22 scores in those treated with benralizumab versus placebo [71], and this has also been reported in a real-world analysis [72]. A phase 3 trial examining the efficacy of benralizumab in CRwNP (OSTRO) has recently been published and reported a reduction in nasal blockage score (patient reported) and nasal polyp score (determined by nasoendoscopy). There was also an improvement in disease-specific quality of life (SNOT-22) versus placebo, but this did not reach statistical significance [73]. OSTRO did not require evidence of eosinophilic disease for enrolment, and a further trial of benralizumab in eosinophilic CRwNP (ORCHID, ClinicalTrials.gov identifier: NCT04157335) is currently recruiting.
The use of benralizumab to treat EGPA is currently being evaluated in a large RCT comparing the efficacy of benralizumab to mepolizumab (ClinicalTrials.gov identifier: NCT04157348). At present there is a promising pilot study (open label, prospective) [74], as well as real-world data [75] suggesting a significant steroid-sparing effect in EGPA.
Practical considerations
Benralizumab is a fixed dose subcutaneous injection, given 4-weekly for the first three doses, and then 8-weekly thereafter. This is the least frequent dosing of any of the current biologic options for severe asthma. Benralizumab is available as a pre-filled auto-inject syringe for self-administration. The main side-effects reported in the phase 3 clinical trials were injection site reactions (2–3%) and nasopharyngitis, although these occurred at similar rates in the treatment and placebo groups.
Concerns about the health implications of the effective “knock-out” of a highly evolutionarily conserved cell like the eosinophil have been raised. This most commonly relates to a possible homeostatic role for the eosinophil in host-defence against both helminth and other infections, as well as in tumour surveillance [76]. However, despite over 8000 patients being exposed to benralizumab across clinical development programmes in asthma, COPD and other conditions, there have, so far, been no signals of harm including no increased infection or malignancy rates [77]. MELTEMI confirmed ongoing safety at 5 years following initiation of benralizumab with rates of malignancy and serious infections at less than 2% [70].
Dupilumab
Dupilumab (“Dupixent”) is a mAb targeted against the α subunit of the IL-4 receptor, a ligand for both IL-4 and IL-13. Dupilumab thereby decreases signalling of both of these key T2 cytokines. It was first licensed for use in atopic dermatitis [78, 79], but has subsequently demonstrated efficacy in severe eosinophilic asthma.
Exacerbation and OCS reduction
The LIBERTY ASTHMA QUEST trial enrolled 1902 asthma patients irrespective of their blood eosinophils or FENO with one or more exacerbations in the past year. As the subjects could be on either medium or high-dose ICS, it was a less severe cohort than the anti-IL-5/IL-5R-α biologic trials [80]. Compared with placebo, there was an overall reduction in AER of 48%. A statistically significant benefit was only seen in those with a blood eosinophil count of ≥300 cells·μL−1, in whom the AER fell by almost two-thirds. Clinical efficacy was also seen in those with a FENO of ≥25 ppb. Although there is reported efficacy in subjects with a blood eosinophil count >150 cells·μL−1 this effect size was driven by the patients with an eosinophil count of ≥300 cells·μL−1 and there was no statistically significant benefit in subjects with a blood eosinophil count <300 cells·μL−1.
Dupilumab has also demonstrated efficacy in reducing mOCS use in asthma. The LIBERTY ASTHMA VENTURE trial [81] showed an overall median prednisolone dose reduction of 50% versus placebo and 69% reduced their dose below 5 mg·day−1, compared with 33% of the placebo group. This trial suffered from a very marked placebo response, but did nonetheless show excellent corticosteroid-sparing efficacy. There was a significant reduction in corticosteroid dose in both low (<300 cells·μL−1) and high (≥300 cells·μL−1) blood eosinophil groups, although the greatest benefit was again seen in those subjects with higher baseline blood eosinophil counts.
FEV1 and patient-reported outcome measures
In LIBERTY QUEST [80], dupilumab treatment resulted in an overall improvement of 130–140 mL in pre-bronchodilator FEV1 versus placebo at 12 weeks; however, much of this result was driven by the T2 high groups. For example, in those with baseline FENO ≥50 ppb there was a 300–390 mL improvement versus placebo, compared to 120–190 mL in the FENO ≥25 ppb to <50 ppb group, and only 30–50 mL in the FENO <25 ppb group. Although there was an initial rise in FEV1 in the placebo group (presumably due to improved ICS/LABA adherence), there was then a decline over time of ∼40 mL per year, which did not occur in the treatment group. AQLQ and ACQ both improved, more so in the high blood eosinophil subgroup, but still less than the MCID when compared with placebo.
Predictors of response
As outlined above, the biggest reductions in exacerbation rate and improvements in FEV1 were seen in those with blood eosinophils ≥300 cells·μL−1 or FENO ≥25 ppb. A post-hoc analysis of QUEST stratified patients according to baseline exacerbation rate and the biggest improvements in exacerbation rate, FEV1 and ACQ were in those with more exacerbations at baseline [82]. Improvements were seen irrespective of whether participants were on medium-dose or high-dose ICS at baseline [83].
Real-world and long-term data
To date, there are no substantial real-world data on the use of dupilumab in asthma. The publication of an extension trial to 96 weeks (“LIBERTY ASTHMA TRAVERSE”, ClinicalTrials.gov identifier: NCT02134028) is awaited.
Comorbidities
Atopic dermatitis
Atopic dermatitis can have a significant negative impact on quality of life, both due to the symptoms themselves and the impact the disease has on appearance. For patients with moderate-to-severe atopic dermatitis, dupilumab treatment results in a reduction in objective signs of the disease as well as subjective symptoms, quality of life scores and depression and anxiety scores compared with placebo [78]. Considering this, for severe asthma patients with significant coexisting atopic dermatitis we would choose dupilumab as first-line therapy over other biologics.
CRwNP
Two large phase 3 RCTs have assessed the effectiveness of dupilumab as an add-on therapy to nasal corticosteroids in CRwNP. These reported objective improvements in nasal polyp size, as well as statistically and clinically significant improvements in quality of life. These improvements were greatest in the subgroup with coexistent asthma [84] and were larger than the improvements seen with omalizumab [31] and mepolizumab [48]; however, an indirect comparison is limited by differing inclusion criteria and severity of the nasal polyposis at baseline. In addition, the omalizumab studies, POLYP 1 and 2 trials, were 24 weeks, rather than 52 weeks in duration. Dupilumab is currently licensed in some countries for CRwNP that has failed either surgery or OCS.
Practicalities
Dupilumab has a somewhat broader indication than the anti-IL-5 biologics, as its European licence only requires evidence of T2-high inflammation (raised blood eosinophils and/or raised FENO) [85]. In the USA, it is also licensed for use in OCS-dependent asthma regardless of baseline blood eosinophils. Dupilumab is given 2-weekly by subcutaneous injection at a fixed dose and is available as a pre-filled auto-inject syringe.
The most common side-effects in clinical trials were injection site reactions (16.8%). This is the highest rate amongst biologic therapies. Akin to the theoretical concern about eosinophil depletion with benralizumab has been a concern regarding hypereosinophilia with dupilumab. A rise in peripheral blood eosinophils ≥3000 cells·μL−1 was observed in 4% of those treated in LIBERTY ASTHMA QUEST. This was associated with clinical symptoms in four patients (out of 1264 in the combined treatment arms). The average blood eosinophil count returned to baseline by 24 weeks of therapy. Although this is a relatively new therapy in severe asthma, there are longer-term safety data available following its use in the treatment of atopic dermatitis. In patients with atopic dermatitis treated with dupilumab, a high incidence of conjunctivitis has been reported [86].
Tezepelumab
Tezepelumab is a mAb targeting the epithelial alarmin TSLP. TSLP is released by epithelial cells in response to pro-inflammatory stimuli and is related to both T2 cytokine expression and disease severity in asthma [87, 88]. It sits higher up the inflammatory cascade than IL-5, IL-4 and IL-13, and as such, TSLP blockade has the theoretical potential for broader effects than the mAbs discussed thus far. A mechanistic placebo-controlled study of tezepelumab, “CASCADE”, recruited patients on moderate or high dose ICS plus another controller and conducted bronchoscopies at baseline and end of treatment, along with measures of bronchial hyperresponsiveness and airway remodelling. Tezepelumab treatment resulted in a reduction in submucosal eosinophils irrespective of baseline blood eosinophils and a decrease in airway hyperresponsiveness; however, there was no effect on other inflammatory cells in the submucosa or significant improvements in the airway remodelling outcomes [89].
Primary outcomes
The phase 3 trial for tezepelumab, “NAVIGATOR”, has recently been published [90]. Inclusion criteria included two or more severe exacerbations in the past 12 months and at least moderate dose ICS plus an additional controller medication. There was no requirement for raised blood eosinophils; however, the trial population was monitored to recruit approximately 50% with blood eosinophils of ≥300 cells·μL−1 and 40% with ≥3 exacerbations in the previous 12 months. Pre-planned subgroup analyses were carried out according to blood eosinophils and FENO. Overall, treatment with tezepelumab led to a reduction in exacerbation rate of 56% versus placebo (rate ratio (RR) 0.44). Unsurprisingly, the greatest reductions were seen in those with the strongest T2 signal (higher blood eosinophils and higher FENO). Those with blood eosinophils of <150 cells·μL−1 still had a substantial benefit (RR of 0.47) if they had a FENO ≥25 ppb. The only biomarker subgroup with no efficacy was the dual biomarker low group (blood eosinophils <150 cells·μL−1 and low FENO <25 ppb). A recent post-hoc analysis of the phase 2 trial PATHWAY confirmed response was unrelated to atopic status [91].
The SOURCE trial enrolled patients with OCS-dependent asthma and randomised them to tezepelumab or placebo [92]. The results have yet to be formally published; however, the investigators have announced the trial did not meet the primary endpoint of reducing daily OCS whilst maintaining asthma control [93].
Secondary outcomes
There were statistically significant improvements in FEV1, ACQ and AQLQ with tezepelumab treatment, which were seen as early as week 2; however, these results were mainly driven by those with blood eosinophils of ≥300 cells·μL−1. In this subgroup, there were significant improvements compared with placebo, meeting the MCIDs for FEV1 (+230 mL), ACQ6 (−0.5) and AQLQ (+0.5). There are no extension, long-term, or real-world data yet available for tezepelumab.
Practicalities
Tezepelumab is not yet licensed for use; however, in the clinical trials it was given at a set dose of 210 mg, 2-weekly by subcutaneous injection. There have been no significant safety issues to date, with only a small increase in injection site reactions compared with placebo; however, there are no published data beyond 52 weeks. An extension trial, “DESTINATION”, is in progress (ClinicalTrials.gov identifier: NCT03706079).
Additional considerations
Pregnancy and breastfeeding
mAbs, such as asthma biologics, are known to cross the placenta, increasing proportionally as the pregnancy progresses, so it is hypothesised that the greatest risk would be in the second and third trimester [94]. At present there are only limited data to help physicians and women to make an informed choice around whether to start or continue biologic therapy if they are planning to, or become, pregnant. The only substantive human data published concern omalizumab. Data from a registry of 191 pregnant women taking omalizumab (“EXPECT”) were published in 2019, and more recently these data have been compared with a diseased matched cohort, which did not suggest any increased risk of spontaneous abortion or fetal death [95]. Although registries are in place for the other asthma biologics, no data from these have yet been published.
It should be noted that pregnant women with severe asthma have an increased risk of adverse perinatal outcomes, including an increased risk of prematurity and decreased fetal growth [96], and OCS are also known to increase the risk of pre-eclampsia. The known risk of poorly controlled asthma must therefore be weighed against the relatively unknown risk of biologic therapy.
Adrenal insufficiency
Effective mOCS reduction and withdrawal reveals underlying adrenal insufficiency in patients treated with anti-IL-5 mAbs in around 75% of cases [97]. It is, therefore, important to monitor early-morning cortisol as OCS is weaned towards physiological levels. Patients who are suspected to have adrenal axis suppression that does not normalise on slowly weaning the OCS dose should be referred for a more formal review and short synacthen test by endocrinology services.
Choosing a biologic in severe eosinophilic asthma
Which biologic first?
The availability of biologic therapies, whether limited by health insurance, prescribing guidelines or other factors, will be the first factor dictating choice. With that consideration, for the patient on mOCS we would limit the choice to those with proven steroid-sparing efficacy in controlled trials: mepolizumab, benralizumab or dupilumab. If there remains more than one option, we then discuss with the patient their priorities for treatment and consider existing characteristics that predict response. Do they have a comorbidity, such as CRwNP or atopic dermatitis, that is impairing their quality of life? Would frequent dosing be difficult for them? Similar considerations are warranted for patients not on mOCS, but in this context omalizumab is also a consideration, particularly in younger patients with child-onset disease and those with clinically significant and treatment-resistant allergic comorbidities.
A summary of information to inform this two-step process is given in tables 1 and 2. Good practice is to review patients throughout the first 6 months of treatment and to make a more formal assessment of response at 6 months, to ensure a switch to an alternate biologic is not inappropriately delayed.
Table 1.
Indications and dosing of asthma biologics
| Drug | Asthma indication (EMA) | NICE guidance (UK) | Dosing (adults) | Additional licensed indications |
|
Omalizumab (“Xolair”) |
Add-on therapy in patients aged 6+ years with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function (FEV1 <80%, this is not required if aged 6–11 years) as well as frequent symptoms and who have had multiple severe asthma exacerbations despite daily high-dose ICS, plus a LABA | Optimised standard therapy Confirmed severe persistent allergic IgE-mediated asthma ≥4 exacerbations in past year or continuous OCS |
Based on weight and serum IgE (75–600 mg), 2–4 weekly Subcutaneous injection Auto-injector pre-filled syringe available (75 mg and 150 mg) |
Chronic spontaneous urticaria CRwNP |
|
Mepolizumab (“Nucala”) |
Add-on treatment of patients with severe refractory eosinophilic asthma in adults, adolescents and children aged 6+ years | Followed optimised treatment plan (i.e. adherent), plus either: BE ≥300 cells·μL−1 with ≥4 exacerbations in past year requiring OCS or continuous OCS equivalent to ≥5 mg·day−1 for previous 6 months or BE ≥400 cells·μL−1 with ≥3 exacerbations in past year requiring OCS or continuous OCS equivalent to ≥5 mg·day−1 for previous 6 months Review at 12 months |
100 mg every 4 weeks Subcutaneous injection Auto-injector pre-filled syringe available |
EGPA (300 mg dose) CRwNP |
|
Reslizumab (“Cinqair”) |
Add-on therapy in adult patients with severe eosinophilic asthma inadequately controlled despite high-dose ICS plus another medicinal product for maintenance treatment | Asthma inadequately controlled despite ICS plus one other drug and BE ≥400 cells·μL−1 with ≥3 exacerbations in past year requiring OCS Review at 12 months |
Weight-based dosing (3 mg·kg−1) every 4 weeks Intravenous infusion |
None |
|
Benralizumab (“Fasenra”) |
Add-on maintenance treatment in adult patients with severe eosinophilic asthma inadequately controlled despite high-dose ICS plus LABA | ICS and LABA, followed optimised treatment plan (i.e. adherent), plus either: BE ≥300 cells·μL−1 with ≥4 exacerbations in past year requiring OCS or continuous OCS equivalent to ≥5 mg·day−1 for previous 6 months or BE ≥400 cells·μL−1 with ≥3 exacerbations in past year requiring OCS or continuous OCS equivalent to ≥5 mg·day−1 for previous 6 months Review at 12 months |
30 mg every 4 weeks for 3 doses then 8-weekly (most infrequent of any biologic) Subcutaneous injection Pre-filled syringe, auto-injector available |
None |
|
Dupilumab (“Dupixent”) |
Add-on maintenance treatment in patients with moderate-to-severe asthma aged 12 years and older with an eosinophilic phenotype or with OCS-dependent asthma | Not yet published, expected 2021 | 300 mg every 2 weeks Subcutaneous injection |
Atopic dermatitis CRwNP |
|
Tezepelumab (Amgen/AstraZeneca) |
Not yet approved Phase 3 data as add-on treatment in severe asthma aged 12 years or older (though note small number adolescents enrolled) suggests efficacy in eosinophilic phenotype (high BE or FENO) |
N/A | 210 mg every 4 weeks Subcutaneous injection |
None |
BE: blood eosinophils; N/A: not available. Blood eosinophils of 100 cells·μL−1 (units used in USA) are equivalent to blood eosinophils of 0.1×109 L−1 (units used in UK).
Table 2.
Summary of clinical trial outcomes and practical considerations
| Omalizumab | Mepolizumab | Reslizumab | Benralizumab | Dupilumab | Tezepelumab ¶ | |
| Biomarkers associated with reduction in exacerbation rate | ||||||
| Blood eosinophils, cells·μL−1 | ≥260# | ≥300 | ≥400 | ≥300 | ≥300 | ≥150 |
| FENO, ppb | ≥19.5 | Not associated | Not associated | Not associated | ≥25 | ≥25 |
| Outcomes | ||||||
| Exacerbation rate reduction+ | 25%§ | ∼50% | ∼40% | ∼50% | ∼70% | ∼70% |
| mOCS reduction | ++ | ++ | ++ | |||
| Quality of life improvement | + | + | + | + | + | ++ |
| FEV1 improvementƒ | +/− | + | +/− | + | ++ | ++ |
| Treatment of comorbidities | ||||||
| CRwNP## | ++ | ++ | + | ++¶¶ | ||
| Atopic dermatitis | ++ | |||||
| Chronic urticaria | + | |||||
| EGPA | ++ | + | + | |||
| Practical considerations | ||||||
| Frequency | 2–4 weekly | 4 weekly | 4 weekly | 8 weekly | 2 weekly | 4 weekly |
| Route | s.c. variable dose++ | s.c. fixed dose | i.v. | s.c. fixed dose | s.c. fixed dose | s.c. fixed dose |
Where possible, information in this table relates to phase 3 trial populations with blood eosinophils ≥300 cells·μL−1 and using current licensed doses. Quality of life relates to AQLQ only. #: see Hanania et al. [25]. ¶: the data presented relate to the subgroup with blood eosinophils ≥300 cells·μL−1; this population had ≥3 exacerbations in the previous 12 months, which is higher than the other phase 3 studies. +: exacerbation rate is difficult to compare across biologics due to differing study populations (e.g. moderate-to-severe asthma), different inclusion criteria (e.g. eosinophil count threshold) and size of placebo responses. §: see Normansell et al. [11]. ƒ: FEV1 improvement: +/− indicates unclear or inconsistent results; + indicates improvement of ∼100 mL to <200 mL; ++ indicates improvement of ≥200 mL. ##: for CRwNP: + indicates significant improvement in SNOT-22 score or severity of disease; ++ indicates improvement in both. ¶¶: improvement with dupilumab larger magnitude than with mepolizumab/omalizumab. ++: this may result in more than one injection to achieve the correct dose.
What if first-line therapy fails?
Some patients continue to exacerbate or are unable to wean mOCS despite treatment with a biologic therapy. The MEX trial investigated exacerbations whilst on mepolizumab treatment and found approximately half of the exacerbations were associated with eosinophilic sputum [98]. The phase 2b dosing study of mepolizumab, DREAM, demonstrated that there was a dose related reduction in sputum eosinophils, with no statistically significant difference in sputum eosinophil count in the lowest dose mepolizumab arm compared with placebo [99]. This was not associated with worse clinical outcomes; however, it is recognised that the sputum eosinophil count is associated with exacerbation risk, and this raises the question as to whether some patients might benefit from more potent suppression of airway eosinophilia than the licensed 100 mg dose of mepolizumab. In this case, a switch to either reslizumab or benralizumab, which offers greater suppression of eosinophilia, may give a superior response [100, 101].
Decreased adherence to ICS can also play a role in a suboptimal response to biologic therapy. In a real-world analysis of 91 mOCS-dependent severe asthma patients treated with mepolizumab those who had poor adherence to ICS experienced significantly more exacerbations and less reduction in prednisolone dose than those with good adherence [102], although this was not the case with benralizumab [103], potentially due to its more complete eosinophil depletion.
The other half of exacerbations in the MEX trial (mepolizumab) were non-eosinophilic and likely to be infection related, i.e. they were likely to have neutrophilic sputum, a raised C-reactive protein and be treated with antibiotics. In these instances, appropriate antibiotic therapy followed by consideration of a maintenance antibiotic (if the infections are frequent), such as azithromycin, is likely to be of more benefit than a switch in biologic.
In the phase 3 studies of benralizumab, ∼10% of subjects developed serum neutralising anti-drug antibodies during treatment with benralizumab [59, 60]. The authors reported that they could not detect any change in efficacy in this subgroup. There is no commercially available assay for detecting benralizumab; however, a detectable blood eosinophil count can be used as a surrogate marker. In a real-world analysis of 130 patients treated with benralizumab, 18 patients had a suboptimal response and of these five had detectable eosinophils, suggesting that neutralising anti-drug antibodies could be a mechanism of drug failure in a minority of patients [66].
Conclusion
The care of patients with severe asthma has been transformed over recent years, with six biologic therapies reporting positive results in phase 3 trials. As the number of available treatment options expands, decision making has become ever more complex. Without the benefit of head-to-head trials, knowing which biologic to initiate first in a patient eligible for multiple options can be daunting. However, it is important to recognise that based on currently available data, for most patients with severe eosinophilic asthma the majority of these therapies are likely to be effective.
Key points
Biologic therapies target type 2 inflammatory pathways that are central to exacerbation pathogenesis in asthma.
Elevated blood eosinophil counts ± FENO are associated with greater clinical efficacy with all the asthma biologics.
All biologic therapies have been shown to reduce exacerbation frequency in controlled trials.
Mepolizumab, benralizumab and dupilumab are the only biologics to achieve significant reductions in daily OCS use in large controlled trials.
Specific allergic and eosinophilic comorbidities, as well as patient preference and practicalities of drug delivery, will influence biologic choice.
Self-evaluation questions
1. Which of the biologics have proven steroid-sparing efficacy in asthma?
2. Baseline FENO is a useful predictor of response to which biologic therapies?
3. Which biologic is given intravenously?
4. Which biologic has the most infrequent dosing?
5. Match the drugs to the correct target:
| Drug | Target |
| Benralizumab | |
| Tezepelumab | IgE |
| Omalizumab | IL-5 |
| Reslizumab | IL-5R-α |
| Dupilumab | IL-4/13R |
| Mepolizumab | TSLP |
Suggested answers
1. Mepolizumab, benralizumab and dupilumab.
2. Omalizumab, dupilumab and tezepelumab.
3. Reslizumab.
4. Benralizumab.
5. Benralizumab: IL-5R-α; tezepelumab: TSLP; omalizumab: IgE; reslizumab: IL-5; dupilumab: IL-4/13R; mepolizumab: IL-5.
Footnotes
Conflict of interest: J.E. Kavanagh reports receiving support for travel to the ERS Congress 2018 from Teva.
Conflict of interest: D.J. Jackson reports receiving speaker fees, advisory board fees and congress travel support from GSK, AZ, Sanofi, Chiesi, Napp, and Teva, outside the submitted work.
Conflict of interest: A.P. Hearn has nothing to disclose.
References
- 1.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma . Difficult-to-treat and severe asthma in adolescent and adult patients. 2019. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf
- 3.Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy 2018; 11: 193–204. doi: 10.2147/JAA.S176026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol 2015; 15: 57–65. doi: 10.1038/nri3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity 2019; 50: 975–991. doi: 10.1016/j.immuni.2019.03.018 [DOI] [PubMed] [Google Scholar]
- 6.Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med 1997; 155: 1828–1834. doi: 10.1164/ajrccm.155.6.9196082 [DOI] [PubMed] [Google Scholar]
- 7.Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol 2015; 136: 1476–1485. doi: 10.1016/j.jaci.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001; 108: 184–190. doi: 10.1067/mai.2001.117880 [DOI] [PubMed] [Google Scholar]
- 9.Holgate S, Chuchalin A, Hebert J, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 2004; 34: 632–638. doi: 10.1111/j.1365-2222.2004.1916.x [DOI] [PubMed] [Google Scholar]
- 10.Soler M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18: 254–261. doi: 10.1183/09031936.01.00092101 [DOI] [PubMed] [Google Scholar]
- 11.Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014; 1: CD003559. doi: 10.1002/14651858.CD003559.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011; 154: 573–582. doi: 10.7326/0003-4819-154-9-201105030-00002 [DOI] [PubMed] [Google Scholar]
- 13.Molimard M, Buhl R, Niven R, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir Med 2010; 104: 1381–1385. doi: 10.1016/j.rmed.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 14.Rottem M. Omalizumab reduces corticosteroid use in patients with severe allergic asthma: real-life experience in Israel. J Asthma 2012; 49: 78–82. doi: 10.3109/02770903.2011.637598 [DOI] [PubMed] [Google Scholar]
- 15.Braunstahl G-J, Chlumský J, Peachey G, et al. Reduction in oral corticosteroid use in patients receiving omalizumab for allergic asthma in the real-world setting. Allergy Asthma Clin Immunol 2013; 9: 47. doi: 10.1186/1710-1492-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsaounou P, Buhl R, Brusselle G, et al. Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma. Respir Med 2019; 150: 51–62. doi: 10.1016/j.rmed.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 17.Pelaia C, Calabrese C, Barbuto S, et al. Omalizumab lowers asthma exacerbations, oral corticosteroid intake and blood eosinophils: results of a 5-year single-centre observational study. Pulm Pharmacol Ther 2019; 54: 25–30. doi: 10.1016/j.pupt.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 18.MacDonald KM, Kavati A, Ortiz B, et al. Short- and long-term real-world effectiveness of omalizumab in severe allergic asthma: systematic review of 42 studies published 2008–2018. Expert Rev Clin Immunol 2019; 15: 553–569. doi: 10.1080/1744666X.2019.1574571 [DOI] [PubMed] [Google Scholar]
- 19.Braunstahl G-J, Chen C-W, Maykut R, et al. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med 2013; 107: 1141–1151. doi: 10.1016/j.rmed.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 20.Siergiejko Z, Świebocka E, Smith N, et al. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Curr Med Res Opin 2011; 27: 2223–2228. doi: 10.1185/03007995.2011.620950 [DOI] [PubMed] [Google Scholar]
- 21.Bonini M, Di Paolo M, Bagnasco D, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. Eur Respir Rev 2020; 29: 190137. doi: 10.1183/16000617.0137-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bousquet J, Wenzel S, Holgate S, et al. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest 2004; 125: 1378–1386. doi: 10.1378/chest.125.4.1378 [DOI] [PubMed] [Google Scholar]
- 23.Tabatabaian F, Ledford DK. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy 2018; 11: 53–61. doi: 10.2147/JAA.S107982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia G, Magnan A, Chiron R, et al. A proof-of-concept, randomized, controlled trial of omalizumab in patients with severe, difficult-to-control, nonatopic asthma. Chest 2013; 144: 411–419. doi: 10.1378/chest.12-1961 [DOI] [PubMed] [Google Scholar]
- 25.Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187: 804–811. doi: 10.1164/rccm.201208-1414OC [DOI] [PubMed] [Google Scholar]
- 26.Busse W, Spector S, Rosén K, et al. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol 2013; 132: 485–486.e11. doi: 10.1016/j.jaci.2013.02.032 [DOI] [PubMed] [Google Scholar]
- 27.Djukanovic R, Wilson SJ, Kraft M, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 2004; 170: 583–593. doi: 10.1164/rccm.200312-1651OC [DOI] [PubMed] [Google Scholar]
- 28.Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol 2017; 140: 162–169.e2. doi: 10.1016/j.jaci.2016.08.054 [DOI] [PubMed] [Google Scholar]
- 29.Alhossan A, Lee CS, MacDonald K, et al. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract 2017; 5: 1362–1370.e2. doi: 10.1016/j.jaip.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 30.Adachi M, Kozawa M, Yoshisue H, et al. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Respir Med 2018; 141: 56–63. doi: 10.1016/j.rmed.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 31.Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020; 146: 595–605. doi: 10.1016/j.jaci.2020.05.032 [DOI] [PubMed] [Google Scholar]
- 32.Cox L, Platts-Mills TA, Finegold I, et al. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology joint task force report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol 2007; 120: 1373–1377. doi: 10.1016/j.jaci.2007.09.032 [DOI] [PubMed] [Google Scholar]
- 33.Sanderson C. Interleukin-5, eosinophils, and disease. Blood 1992; 79: 3101–3109. doi: 10.1182/blood.V79.12.3101.bloodjournal79123101 [DOI] [PubMed] [Google Scholar]
- 34.Smith DA, Minthorn EA, Beerahee M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin-5 monoclonal antibody. Clin Pharmacokinet 2011; 50: 215–227. doi: 10.2165/11584340-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 35.Flood-Page PT, Menzies-Gow AN, Kay AB, et al. Eosinophil's role remains uncertain as anti–interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 2003; 167: 199–204. doi: 10.1164/rccm.200208-789OC [DOI] [PubMed] [Google Scholar]
- 36.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 37.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 38.Ortega H, Chupp G, Bardin P, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J 2014; 44: 239–241. doi: 10.1183/09031936.00220413 [DOI] [PubMed] [Google Scholar]
- 39.Hearn AP, Hug OD, Somani ZA, et al. Real world effectiveness of anti-IL-5/5R therapies is independent of co-eligibility for anti-IgE therapy. Eur Respir J 2021; 57: 2100166. doi: 10.1183/13993003.00166-2021 [DOI] [PubMed] [Google Scholar]
- 40.Kavanagh JE, d'Ancona G, Elstad M, et al. Real-world effectiveness and the characteristics of a ‘super-responder’ to mepolizumab in severe eosinophilic asthma. Chest 2020; 158: 491–500. doi: 10.1016/j.chest.2020.03.042 [DOI] [PubMed] [Google Scholar]
- 41.Hearn AP, Kavanagh J, d'Ancona G, et al. The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract 2021; 9: 2093–2096.e1. doi: 10.1016/j.jaip.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 42.Harvey ES, Langton D, Katelaris C, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J 2020; 55: 1902420. doi: 10.1183/13993003.02420-2019 [DOI] [PubMed] [Google Scholar]
- 43.Harrison T, Canonica GW, Chupp G, et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J 2020; 56: 2000151. doi: 10.1183/13993003.00151-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haldar P, Brightling CE, Singapuri A, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol 2014; 133: 921–923. doi: 10.1016/j.jaci.2013.11.026 [DOI] [PubMed] [Google Scholar]
- 45.Moore WC, Kornmann O, Humbert M, et al. Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study). Eur Respir J 2022; 59: 2100396. doi: 10.1183/13993003.00396-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khatri S, Moore W, Gibson PG, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol 2019; 143: 1742–1751.e7. doi: 10.1016/j.jaci.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 47.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017; 376: 1921–1932. doi: 10.1056/NEJMoa1702079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021; 9: 1141–1153. doi: 10.1016/S2213-2600(21)00097-7 [DOI] [PubMed] [Google Scholar]
- 49.Khurana S, Brusselle GG, Bel EH, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther 2019; 41: 2041–2056.e5. doi: 10.1016/j.clinthera.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 50.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 51.Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest 2016; 150: 799–810. doi: 10.1016/j.chest.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 52.Bernstein JA, Virchow JC, Murphy K, et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid-dependent asthma: results from two phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2020; 8: 461–474. doi: 10.1016/S2213-2600(19)30372-8 [DOI] [PubMed] [Google Scholar]
- 53.Kent BD, d'Ancona G, Fernandes M, et al. Oral corticosteroid-sparing effects of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. ERJ Open Res 2020; 6: 00311-2019. doi: 10.1183/23120541.00311-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manka LA, Guntur VP, Denson JL, et al. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann Allergy Asthma Immunol 2021; 126: 696–701.e1. doi: 10.1016/j.anai.2021.01.035 [DOI] [PubMed] [Google Scholar]
- 55.Ghazi A, Trikha A, Calhoun WJ. Benralizumab – a humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity – a novel approach for the treatment of asthma. Expert Opin Biol Ther 2012; 12: 113–118. doi: 10.1517/14712598.2012.642359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol 2010; 125: 1344–1353.e2. doi: 10.1016/j.jaci.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 57.Moran AM, Ramakrishnan S, Borg CA, et al. Blood eosinophil depletion with mepolizumab, benralizumab, and prednisolone in eosinophilic asthma. Am J Respir Crit Care Med 2020; 202: 1314–1316. doi: 10.1164/rccm.202003-0729LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 2013; 132: 1086–1096.e5. doi: 10.1016/j.jaci.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 60.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 388: 2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 61.Harrison TW, Chanez P, Menzella F, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med 2021; 9: 260–274. doi: 10.1016/S2213-2600(20)30414-8 [DOI] [PubMed] [Google Scholar]
- 62.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl J Med 2017; 376: 2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 63.Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med 2022; 10: 47–58doi: 10.1016/S2213-2600(21)00352-0. [DOI] [PubMed] [Google Scholar]
- 64.FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med 2018; 6: 51–64. doi: 10.1016/S2213-2600(17)30344-2 [DOI] [PubMed] [Google Scholar]
- 65.Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J 2018; 52: 1800936. doi: 10.1183/13993003.00936-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest 2021; 159: 496–506. doi: 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 67.Jackson DJ, Humbert M, Hirsch I, et al. Ability of serum IgE concentration to predict exacerbation risk and benralizumab efficacy for patients with severe eosinophilic asthma. Adv Ther 2020; 37: 718–729. doi: 10.1007/s12325-019-01191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busse WW, Bleecker ER, FitzGerald JM, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med 2019; 7: 46–59. doi: 10.1016/S2213-2600(18)30406-5 [DOI] [PubMed] [Google Scholar]
- 69.Busse WW, Bleecker ER, FitzGerald JM, et al. Benralizumab for adolescent patients with severe, eosinophilic asthma: safety and efficacy after 3 years of treatment. J Allergy Clin Immunol 2021; 148: 266–271.e2. doi: 10.1016/j.jaci.2021.02.009 [DOI] [PubMed] [Google Scholar]
- 70.Korn S, Bourdin A, Chupp G, et al. Integrated safety and efficacy among patients receiving benralizumab for up to 5 years. J Allergy Clin Immunol Pract 2021; 9: 4381–4392.e4. doi: 10.1016/j.jaip.2021.07.058 [DOI] [PubMed] [Google Scholar]
- 71.Canonica GW, Harrison TW, Chanez P, et al. Benralizumab improves symptoms of patients with severe, eosinophilic asthma with a diagnosis of nasal polyposis. Allergy 2022; 77: 150–161doi: 10.1111/all.14902. [DOI] [PubMed] [Google Scholar]
- 72.Nolasco S, Crimi C, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a real-world multicenter study. J Allergy Clin Immunol Pract 2021; 9: 4371–4380.e4. doi: 10.1016/j.jaip.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 73.Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: a randomized, placebo-controlled trial. J Allergy Clin Immunol 2021; in press [ 10.1016/j.jaci.2021.08.030]. [DOI] [PubMed] [Google Scholar]
- 74.Guntur VP, Manka LA, Denson JL, et al. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract 2021; 9: 1186–1193.e1. doi: 10.1016/j.jaip.2020.09.054 [DOI] [PubMed] [Google Scholar]
- 75.Nanzer AM, Dhariwal J, Kavanagh J, et al. Steroid-sparing effects of benralizumab in patients with eosinophilic granulomatosis with polyangiitis. ERJ Open Res 2020; 6: 00451-2020. doi: 10.1183/23120541.00451-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jacobsen EA, Jackson DJ, Heffler E, et al. Eosinophil knockout humans: uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu Rev Immunol 2021; 39: 719–757. doi: 10.1146/annurev-immunol-093019-125918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson DJ, Korn S, Mathur SK, et al. Safety of eosinophil-depleting therapy for severe, eosinophilic asthma: focus on benralizumab. Drug Saf 2020; 43: 409–425. doi: 10.1007/s40264-020-00926-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335–2348. doi: 10.1056/NEJMoa1610020 [DOI] [PubMed] [Google Scholar]
- 79.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287–2303. doi: 10.1016/S0140-6736(17)31191-1 [DOI] [PubMed] [Google Scholar]
- 80.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 81.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378: 2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 82.Corren J, Katelaris CH, Castro M, et al. Effect of exacerbation history on clinical response to dupilumab in moderate-severe uncontrolled asthma. Eur Respir J 2021; 58: 2004498. doi: 10.1183/13993003.04498-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bourdin A, Papi AA, Corren J, et al. Dupilumab is effective in type 2-high asthma patients receiving high-dose inhaled corticosteroids at baseline. Allergy 2021; 76: 269–280. doi: 10.1111/all.14611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394: 1638–1650. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 85.European Medicines Agency . CHMP assessment report on extension of marketing authorisation and an extension of indication variation: Dupixent. Procedure No. EMEA/H/C/004390/X/0004/G. 2019. www.ema.europa.eu/en/documents/variation-report/dupixent-h-c-4390-x-0004-g-epar-assessment-report-extension_en.pdf
- 86.Popiela MZ, Barbara R, Turnbull AMJ, et al. Dupilumab-associated ocular surface disease: presentation, management and long-term sequelae. Eye (Lond) 2021; 35: 3277–3284. doi: 10.1038/s41433-020-01379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ziegler SF, Roan F, Bell BD, et al. The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol 2013; 66: 129–155. doi: 10.1016/B978-0-12-404717-4.00004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ying S, O'Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 2005; 174: 8183–8190. doi: 10.4049/jimmunol.174.12.8183 [DOI] [PubMed] [Google Scholar]
- 89.Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9: 1299–1312. doi: 10.1016/S2213-2600(21)00226-5 [DOI] [PubMed] [Google Scholar]
- 90.Menzies-Gow A, Colice G, Griffiths JM, et al. NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res 2020; 21: 266. doi: 10.1186/s12931-019-1261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Emson C, Corren J, Sałapa K, et al. Efficacy of tezepelumab in patients with severe, uncontrolled asthma with and without nasal polyposis: a post hoc analysis of the phase 2b PATHWAY study. J Asthma Allergy 2021; 14: 91–99. doi: 10.2147/JAA.S288260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wechsler ME, Colice G, Griffiths JM, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res 2020; 21: 264. doi: 10.1186/s12931-020-01503-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.AstraZeneca . Update o n SOURCE Phase III trial for tezepelumab in patients with severe, oral corticosteroid-dependent asthma. Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/update-on-source-phase-iii-trial-for-tezepelumab-in-patients-with-severe-oral-corticosteroid-dependent-asthma.html Date last accessed: 14 September 2021; date last updated: 22 December 2020.
- 94.Jenner B, Nelson-Piercy C. Biological and immunosuppressive respiratory therapy in pregnancy. In: Lapinsky S, Plante L, eds. Respiratory Disease in Pregnancy. Cambridge, Cambridge University Press, 2020; pp. 200–209. doi: 10.1017/9781108163705.022 [DOI] [Google Scholar]
- 95.Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015; 135: 407–412. doi: 10.1016/j.jaci.2014.08.025 [DOI] [PubMed] [Google Scholar]
- 96.Kemppainen M, Lahesmaa-Korpinen A-M, Kauppi P, et al. Maternal asthma is associated with increased risk of perinatal mortality. PLoS One 2018; 13: e0197593. doi: 10.1371/journal.pone.0197593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nanzer AM, Chowdhury A, Raheem A, et al. Prevalence and recovery of adrenal insufficiency in steroid-dependent asthma patients receiving biologic therapy. Eur Respir J 2020; 56: 1902273. doi: 10.1183/13993003.02273-2019 [DOI] [PubMed] [Google Scholar]
- 98.McDowell PJ, Diver S, Yang F, et al. A prospective observational study of the Iinflammatory profile of Mepolizumab EXacerbations in participants with severe refractory eosinophilic asthma (the MEX study). Lancet Respir Med 2021; 9: 1174–1184. doi: 10.1016/S2213-2600(21)00004-7 [DOI] [PubMed] [Google Scholar]
- 99.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 100.Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med 2018; 197: 38–46. doi: 10.1164/rccm.201707-1323OC [DOI] [PubMed] [Google Scholar]
- 101.Kavanagh JE, Hearn AP, d'Ancona G, et al. Benralizumab after sub-optimal response to mepolizumab in severe eosinophilic asthma. Allergy 2021; 76: 1890–1893. doi: 10.1111/all.14693 [DOI] [PubMed] [Google Scholar]
- 102.d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to inhaled corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J 2020; 55: 1902259. doi: 10.1183/13993003.02259-2019 [DOI] [PubMed] [Google Scholar]
- 103.d'Ancona G, Kavanagh JE, Dhariwal J, et al. Adherence to inhaled corticosteroids and clinical outcomes following a year of benralizumab therapy for severe eosinophilic asthma. Allergy 2021; 76: 2238–2241. doi: 10.1111/all.14737 [DOI] [PubMed] [Google Scholar]