Abstract
Peripheral artery disease (PAD) is a prevalent condition that confers substantial morbidity and mortality and remains underdiagnosed as well as undertreated in the overall population. Although PAD prevalence is similar or higher in women compared to men, associations of traditional and non-traditional risk factors with PAD and clinical manifestations of PAD differ by sex and may contribute to delayed or lack of diagnosis in women. Such sex-based differences in the manifestation of PAD may arise from sexual dimorphism in the vascular substrate in health as well as sex variation in the responses to vascular stressors. Despite the availability of proven therapies for improving symptoms and reducing risk of ischemic cardiovascular and limb events among patients with diagnosed PAD, important sex differences in treatment and outcomes have been observed. We provide an overview of current knowledge regarding sex differences in the epidemiology, pathophysiology, clinical presentation, and management of PAD.
Keywords: Cardiovascular Disease, Peripheral vascular disease, women, sex, gender
Introduction
Peripheral artery disease (PAD) generally refers to arterial diseases of the non-coronary vasculature that can arise from atherosclerotic, aneurysmal, inflammatory, or a combination of pathologies.1 To focus on the most prevalent form of peripheral vascular disease, we herein refer to PAD as atherosclerotic narrowing of the peripheral arteries that classically affects the lower extremities. Defined as such, PAD prevalence has not only persisted but in fact doubled over the last three decades, in part due to lengthening life expectancy, rising incidence of risk factors such as obesity and diabetes, and increased screening and detection.2 Importantly, presence versus absence of PAD confers a three-fold greater risk for mortality3,4 and an overall similar or worse risk for ischemic cardiovascular events compared to individuals with only coronary artery disease.5 Notwithstanding these statistics, PAD remains underdiagnosed and, in turn, undertreated when compared to other cardiovascular disease (CVD) conditions. In this context, women are especially vulnerable. Despite similar or higher prevalence of PAD, clinical recognition is often delayed or deferred in women compared to men.2 Even among diagnosed patients, sex-based differences in PAD treatments and outcomes are evident. Therefore, we summarize current knowledge regarding sex differences in the epidemiology, pathophysiology, clinical presentation, and management of PAD – with the aim of increasing awareness and highlighting opportunities to improve outcomes for both women and men with PAD.
Epidemiology
An estimated 236 million adults are diagnosed with PAD, accounting for 5.6% of the worldwide population.6 In the United States, an estimated 4-10% of adults age 40 years or older are affected.7–10 Importantly, PAD incidence substantially increases with age, doubling with each advancing decade of life – affecting 1 in 10 adults aged 70 years or older. With the mean age of the population increasing, the number of PAD cases is rapidly rising: between 2000 to 2015, the prevalence of PAD grew by 45% worldwide.11 Importantly, asymptomatic and under-recognized PAD represent almost 90% of all cases,12 with true disease prevalence likely underestimated.
The gold standard screening modality for PAD is the ankle-brachial index (ABI) and its definition affects epidemiologic estimates of disease.13 Numerous epidemiologic studies have identified a lower range of ABI values in women than in men, which is not accounted for by sex differences in height or other potential confounders.14 Recognizing that sex-specific ranges can obscure population estimates, the Atherosclerosis Risk in Communities study showed that changing the definition of PAD to an ABI <0.85 can eliminate sex differences.15 However, too lower of a threshold can miss important disease. In fact, PAD defined using the <0.90 cutoff has been shown to correspond with ~90% sensitivity and ~80% specificity for 50% diameter stenosis,16 leaving many patients with ABIs in the apparently normal range and under-recognized PAD with associated excess cardiovascular and mortality risk.17–20 Given these challenges, true population-level PAD prevalence remains difficult to accurately estimate for both sexes.
For women, the true prevalence of PAD is likely higher than available estimates for reasons that extend beyond the screening challenges.21 Women commonly manifest atypical claudication symptoms or no symptoms at all.22 Among the 35% of women with ABI <0.90 in the Women’s Health and Aging, only 1 in 6 were aware of having PAD.22 Accordingly, data from the Global Burden of Disease Study suggests that PAD cases in women outnumber those in men across all age groups,2 particularly among younger women in low-income countries, even after controlling for cardiovascular risk factors. The prevalence of PAD has also been observed to be higher among women of non-white compared with white race.7,8,23,24 Of particular interest is the finding of at least similar, if not higher (3 to 29%,) prevalence of PAD in women compared to men despite women having relatively lower historical rates of smoking.25,26 This sex paradox concerning the most substantial modifiable risk factor for PAD underscores the high likelihood of sex dimorphic factors contributing to the pathogenesis of PAD.
Pathophysiology
Sex differences in the clinical manifestation of vascular disease, including PAD, arise from not only sexual dimorphism in the vascular substrate in health but also sex variation in the types and responses to stressors experienced over the life course (Figure 1 and Figure 2). Mounting evidence suggest that sex chromosomes play an important role in the development of vascular and dysfunction atherosclerosis. For example, women with monosomy X (Turner syndrome) have increased LDL cholesterol levels and particle size.27 In reality, however, the majority of sex differences arise from the complex interplay between sex hormones and sex chromosome complement. This concept was elegantly demonstrated in a recent study using male transgenic mice with deletion of Sry from the Y-chromosome. Depending on the presence/absence of testosterone, in male mice infused with AngII, an XY sex chromosome complement promotes diffuse aortic aneurysmal disease, while an XX sex chromosome complement contributes to focal aneurysms.28 Further studies will be required to fully appreciate the complex mechanisms by which sex chromosome complement influences lipid profiles and vascular integrity.
Figure 1. Sex Differences in Responses to Vascular Stressors and Sequelae.
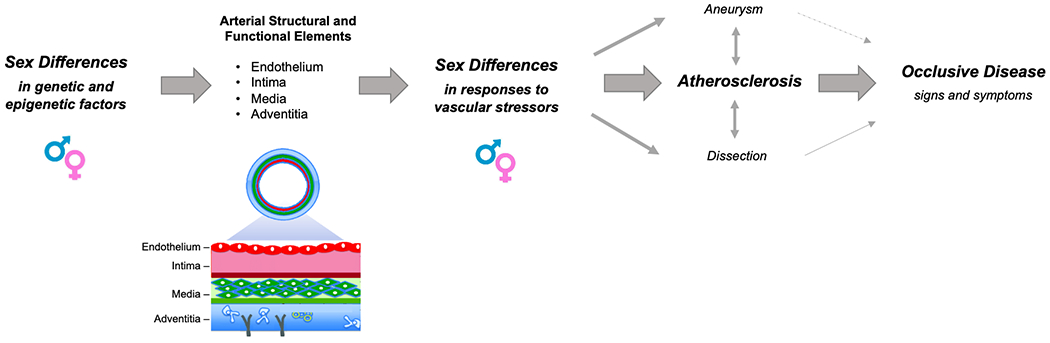
A conventional paradigm proposes that when the same arterial structural and functional substrate is exposed to stressors, sex differences in response to these stressors lead to variations in manifest peripheral vascular disease including the predominance of aortic aneurysm and dissection in men as well as the likely higher prevalence of classic atherosclerotic lower extremity disease in women that also tends to present more as multivessel disease in women compared to men. Emerging evidence suggests that intrinsic sex differences in the arterial substrate, arising from genetic or epigenetic factors, likely also contribute to sexual dimorphism in vascular disease phenotypes.
Figure 2. Sex-Agnostic and Sex-Specific Risk Factors for Peripheral Arterial Disease.
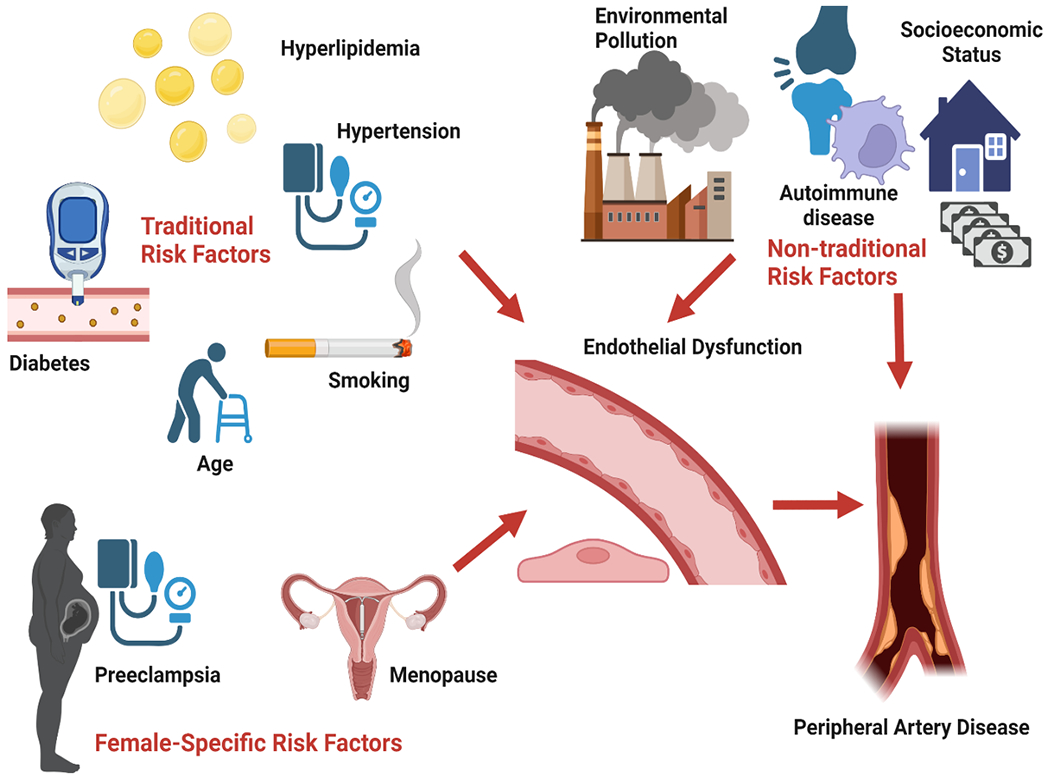
A number of sex-agnostic risk factors for peripheral arterial disease have been identified, with smoking being among the most prominent and potentially contributing a more substantial burden of disease risk in women compared to men, based on analyses of tobacco metabolite associations. The excess burden and multivessel predominance of peripheral arterial disease in women may also arise from a sex-specific predisposition for endothelial dysfunction and, in turn, arterial dysfunction and disease in the setting of age-related estrogen deficiency occurring either with or without a preceding vascular disorder of pregnancy event.
Sex differences in PAD, in particular, are preceded by sex differences in arterial pathophysiology. Endothelial dysfunction is an initial step in the pathogenesis of atherosclerosis and development of PAD.29 A notable feature of endothelial dysfunction is impairment in macro- and micro-vascular endothelium-dependent vasodilation, partly due reduced nitric oxide (NO) bioavailability. In men, the measurable impairment in endothelial function occurs in the 4th decade, whereas in women the impairment occurs about a decade later (around the time of menopause) with subsequent acceleration to similar impairment seen in men.30–32 The sex difference in the age-based onset of impaired endothelial function has been attributed to changes in sex hormones with menopause, particularly estradiol (E2) in women.30–32 Indeed, treatment with E2 improves endothelial function in postmenopausal women,33–36 while surgically or chemically decreasing E2 in premenopausal women induces endothelial dysfunction that is reversed with E2 treatment.37–39 Collectively, these findings suggest that E2 modulates endothelial function in women.
There are multiple potential mechanisms by which E2 decline may contribute to the endothelial dysfunction that presages atherosclerosis and PAD in women. In healthy women, the decline in E2 with menopause appears to trigger excess reactive oxygen species (ROS) production, subsequently impairing NO-mediated vasodilation.37,39,40 In experimental studies involving women treated with gonadotropin-releasing hormone antagonists to mimic ovarian suppression, vitamin C improves endothelial dependent vasodilation, but the beneficial effect was not present in those also treated with estradiol.39,41 Such findings suggest that estrogen decline may be a key physiological event leading to increased oxidative stress-mediated endothelial dysfunction in women. The modulatory role of sex hormones on oxidative stress-mediated endothelial dysfunction in men are unknown, although evidence suggests oxidative stress contributes to endothelial dysfunction in men with low testosterone.42 Beyond antioxidant effects, estrogen also has anti-inflammatory effects on the vascular endothelium that are particularly evident in a healthy artery.43 Estrogen can antagonize the effects of pro-inflammatory cytokines by inhibiting nuclear factor-kappa-B, consequently reducing endothelial activation and the release of other inflammatory cytokines and ROS.44 Inflammation appears to be a key determinant of endothelial function, as animal and human studies have demonstrated that administration of the TNF-α receptor blocker etanercept enhanced endothelium-dependent vasodilation in ovariectomized animals and in postmenopausal women not treated with estradiol.35,45
The vascular endothelium also releases endothelin-1 (ET-1), a potent vasoconstrictor produced and released from endothelial cells that acts on two receptor subtypes, ETA and ETB, located on the vascular smooth muscle. ETB receptors are also located on the endothelium and mediate vasodilation. Mounting evidence suggest that sex differences may be related to ETB receptors.46 Indeed, ET-1 responsiveness differs between men and women,47,48 preferentially binding to ETB receptors in women.49 More recent data also suggests that ETB receptor function shifts with menopause, favoring vasoconstriction and contributing to overall vascular impairment with age in women.50
Endothelial dysfunction represents an early step in the formation of atherosclerotic plaque by altering the hemostatic mechanisms increasing both the adhesiveness and permeability with respect to leukocytes and platelets.51 These changes produce a procoagulant effect along with inflammation, which if left unabated, start a cascade that includes stimulation and proliferation of smooth muscle cells, as well as an increase in macrophages, lymphocytes, cytokines, and chemokines. Estrogen alters the immune response during atherosclerosis, resulting in sex-specific disease phenotypes.52–54 Eventually, a fibrous cap is formed that overlies a lipid rich plaque with a necrotic core. The vessel accommodates this process by dilating, however once dilation is no longer feasible, the plaque may protrude into the vessel and limit flow.
Data are limited regarding sex differences in the formation of atherosclerosis. Many pre-clinical studies have not reported the sex of the animals, and even when available, sex was not used as a key variable in analyses.54 In limited animal studies, males appear to have more inflamed plaque but smaller plaque volume when compared to females.54 In humans, imaging and histology studies suggest that men develop plaque earlier and have greater plaque burden compared to women, even when controlling for risk factors. However, since many of these studies were performed in patient younger than 60 years of age, it is unclear how increased plaque burden may lead to the “catch up” events seen in women over age 60 years.
Risk Factors
Both traditional and non-traditional risk factors contribute to the development of PAD through various pathophysiologic mechanisms (Figure 2). In addition to genetic factors underlying propensity for PAD,55 a number of non-genetic and environmental exposures have been implicated along with sex variation in their associations with disease outcomes.
Traditional Risk Factors
Smoking is perhaps the most well-recognized of all risk factors. Across epidemiologic studies, prior or current smoking status was a predominant characteristic, especially among individuals with symptomatic PAD. The impact of smoking on PAD development can persist up to 30 years after smoking cessation,56 with both quantity and duration corresponding to greater risk.57 In countries where ~30% of the population are smokers, half of PAD cases are attributed to tobacco use.58 Men with PAD more commonly have a history of smoking than women with PAD.59 The relative effects of smoking on overall CVD risk are especially pronounced in women, and the PAD-specific risk is also at least similar it not potentially also higher in women.60,61 In the Women’s Health Study, past and current smokers had a 3-fold and more than 10-fold greater risk for PAD, respectively, compared to never smokers.
Diabetes is well-recognized as second only to smoking in its attributable risk for PAD. Diabetes is associated with twice the risk of developing PAD62,63 as well as greater risk for the most severe forms of PAD, including chronic limb-threatening ischemia (CLTI) and amputation.64 Women compared to men with diabetes have a greater sex-specific risk for overall atherosclerotic disease.65–68 The evidence for sex-specific diabetes related risk for PAD is less clear. While results from a recent meta-analysis suggested relatively equal diabetes-related risk for PAD by sex,65 a prior Framingham Heart Study analysis found that impaired glucose tolerance posed greater risk for PAD in women than in men.69 It is possible that sex differences in dysglycemia related risk for PAD are more evident earlier in the course of systemic metabolic disease,70 and further studies are needed.
Hypertension is another important risk factor for PAD and, as is the case for other CVD conditions, there is a dose-exposure relationship with worsening blood pressure values being associated with greater risk for PAD in women and men.71,72 In the Women’s Health Study, hypertension was related to a 2–3-fold higher risk of developing symptomatic PAD, with a linear relationship between the degree of hypertension and the risk of PAD.73 Similar increments of elevated blood pressure have been shown to be associated with a greater risk in women than in men for a number of subclinical and clinical CVD outcomes including left ventricular hypertrophy, diastolic dysfunction, coronary heart disease, heart failure, and stroke.74–77 Although similar sex-specific outcomes data for PAD are lacking, there is evidence that hypertension is more common among women than men with PAD.78–80 The relationship between hypertension and claudication symptoms is also more pronounced in women than men.69 While additional investigations are needed, emerging data suggest that effects of elevated blood pressure on the vasculature may be different in females compared to males, in part due to intrinsic dimorphism in arterial physiology, anatomy, or both81 and with downstream effects on arterial pathophysiology that could lead to greater propensity for PAD.
Hyperlipidemia is a well-recognized risk factor for atherosclerotic cardiovascular disease, including PAD.82–84 In the Health Professionals Follow-Up Study, men with hyperlipidemia were found to have higher risk of developing PAD after 25 years later.63 Among patients with PAD, women appear to have a higher prevalence of hyperlipidemia than men.80 However, the lipid panel composition varies by sex, with men tending to have lower high-density lipoprotein levels than women across all age groups. Interestingly, a recent prospective study showed that in young women, elevated levels of small LDL particle concentration rather than total LDL cholesterol were associated with a higher risk for developing PAD. Additional lipid-based analyses may uncover sex specific markers to help clarify risks for PAD.85
Non-Traditional Risk Factors
Non-traditional risk factors may also contribute to PAD, although data are limited. For example, a history of pregnancy complications such as hypertensive disorders of pregnancy are known risk enhancing factors for CVD, including PAD.86–88 Also, patients with autoimmune diseases such as rheumatoid arthritis, the majority of whom are women, are at higher risk for noncardiac vascular disease including PAD.89 Chronic kidney disease (CKD) patients are at higher risk for atherosclerotic disease in general; among CKD patients under the age of 70 years, the incidence of PAD is 50% greater in women than men.90,91 Psychologic risk factors are related to higher cardiovascular disease risk,77 and women with PAD have higher burden of depressive symptoms as compared to men with PAD.92,93 There may also be a link between PAD and bone density, as postmenopausal women with osteoporosis have lower ABIs as compared to women with osteopenia.94
Social Determinants of Health
A growing body of evidence has linked socioeconomic factors with health disparities. Studies have shown an inverse relationship between socioeconomic status and incidence of CVD.95–97 Historically, women have been more vulnerable to healthcare and socioeconomic disparities compared to men.77 This may partially explain the disproportionate excess of CVD in women with lower socioeconomic status98 and the higher absolute number of CV deaths of women as compared to men.99 For PAD in particular, environmental pollution, lower socioeconomic status and lower education level have been associated with higher disease incidence.100–103 Long-term exposure to airborne particulate matter has been related to subclinical atherosclerotic disease, including PAD in population studies.104 There is also an association of long-term traffic exposure, in particular residential proximity to main roads, and increased prevalence of PAD.105,106 Notably, the association was more pronounced in women and inconsistent in men.106 Several studies have also demonstrated that lower income and lower education level are associated with worse PAD outcomes.100,107–109 Among possible causes for this heightened risk is limited access to resources and opportunities that improve overall health while being exposed to chronic psychosocial stress.109
Biomarkers
Given the known limitations of ABI, numerous studies have sought to identify blood biomarkers that might assist with screening, shed light on pathophysiology, and also improve risk estimates. These include markers of inflammation, endothelial dysfunction, angiogenesis, lipid metabolism, oxidative stress, and coagulation.110 Amidst emerging data on sex differences in cardiovascular biomarker associations in general, data specific to PAD are still limited. One study that followed 15,737 adults over two decades in Scotland assessed biomarker predictors of PAD versus coronary artery disease and found that triglycerides, hsCRP, tobacco metabolites, and GGT were more prominently associated with PAD in women whereas fibrinogen, NT-pro-BNP, and 25-OH vitamin D were more prominently associated with PAD in men.61 The extent to which female PAD risk may be more dependent on metabolic and inflammatory pathways, and male risk more related to coagulopathies and other factors requires further investigation.
Hormone Therapy
Relationships between hormone therapy and PAD outcomes are inconsistent. Prospective observational studies report lower prevalence of PAD in women using hormone therapy. In the Rotterdam Study, postmenopausal women taking hormone therapy for ≥1 year had a lower risk of PAD (defined as ABI <0.9).111 Another study similarly demonstrated that hormone therapy was associated with lower PAD risk, despite a higher prevalence of risk factors (i.e., smoking, hypertension and hypercholesterolemia) in women who used hormone therapy.112 In contrast to the apparently protective exposure effect seen in observational studies, prospective data from randomized trials have not suggested benefit. In the Women’s Health Initiative (WHI) estrogen plus progestin and estrogen alone trials113,114 and the Heart and Estrogen/Progestin Replacement (HERS) secondary prevention study, there were no reported differences in PAD outcomes for postmenopausal women treated with hormone therapy compared to placebo.115,116 Rather, a non-significant trend toward risk of peripheral arterial events was seen in the estrogen alone WHI trial.
Importantly, adverse effects of hormone therapy have also been reported in studies of outcomes following PAD interventions. In a study of postmenopausal women undergoing infra-inguinal bypass grafting, hormone therapy use was associated with reduced primary graft patency.117 Similarly, in postmenopausal women undergoing iliac artery stenting, those treated with hormone replacement therapy had lower rates of primary patency than those not using hormone therapy,118 suggesting that adverse outcomes could be related to thromboembolic effects of hormone therapy.117 Accordingly, in premenopausal women, oral contraceptive pill use has been associated with increased PAD risk.119 On balance, the clinical findings to date suggest that while estrogen deficiency may be a predisposing factor for the development of PAD, currently available formulations of hormone replacement are not likely to offer therapeutic benefit at least in the setting of clinically manifest disease.
Outcomes
PAD patients have the highest rates of cardiovascular (CV) death and major CV events among patients with atherosclerotic disease, suggesting that diagnosing PAD is an important marker of overall atherosclerotic burden.120 After 1 year of follow up, PAD patients had 21% risk of major adverse cardiovascular events (MACE) and CV death compared to 15% in patients with CAD or CVA history.120 Similarly, women with PAD have an increased risk of all-cause mortality and MACE as compared to women without PAD.121 Women with an ABI <0.90 have a 3-fold increased risk of all-cause mortality3,122 and this risk is comparable to their male counterparts.3,80,122,123 However, some studies have shown higher risk of mortality in males with PAD as compared to women.124,125 Women also have similar risk of major adverse limb events (MALE) including acute limb ischemia80 and higher rates of above the knee amputation126 as compared to men. Among women with PAD, Black and Native American women also have higher risk of morbidity and mortality relative to White and Hispanic women.8
CLTI is one of the most severe manifestations of PAD and is characterized by chronic inadequate tissue perfusion leading to intractable foot pain at rest and/or tissue necrosis which can lead to limb loss if not treated urgently.127 Among PAD patients, CLTI patients have the highest morbidity and mortality risk, with reports of 20-40% with an amputation within a 1-year period and 20% dead within 6 months.128,129 Although women tend to present with more severe PAD, including CLTI, than men, mortality associated with CLTI is similar in men and women with PAD.128 However, Black race and female gender have been associated with increased odds of receiving an above the knee amputation.126
Clinical Presentation
Clinical presentations vary and symptoms does not necessarily correlate with severity of PAD, as some patients may have atypical symptoms, higher thresholds for pain, or more robust collateral circulation.130 Less than one third of patients have typical intermittent claudication, and the majority have atypical symptoms or are “asymptomatic” with respect to lower extremity symptoms.131 Importantly, PAD, even in the absence of leg symptoms, has been associated with functional decline, including decline in walking endurance.132 Patients with borderline ABI (0.90-0.99) have been shown to have greater mobility loss than patients with normal ABI after 5 years of follow up.133
Differences in symptoms and presentation have been observed between women and men with PAD (Table 1). Women with PAD commonly present with atypical symptoms or are asymptomatic with respect to limb symptoms.121,134–136 In the Women’s Health and Aging study, only 14% of women with PAD had a history of IC, and 64% were asymptomatic.137 Early symptoms are also underrecognized in women as they may be masked by other musculoskeletal conditions prevalent in this population like osteoporosis and osteoarthrosis, in addition to the erroneous belief that PAD is more prevalent in men.138 Compared to men, women are more likely to be older,138–140 to present with more advanced Rutherford class,136,139–141 and to have multivessel atherosclerotic disease identified at revascularization.139,142 Among patients presenting with CLTI, female gender has been shown to be an independent predictor of femoropopliteal disease.142,143
Table 1.
Sex Differences in PAD Presentation and Management
| Sex Differences in PAD | |
|---|---|
| Clinical Presentation | |
| Symptoms | Women more likely to have no or atypical leg symptoms |
| Examination | Women more likely to have greater functional decline and reduced walking parameters and quality of life |
| Diagnostics | Women more likely to present with multilevel disease |
| Management | |
| Hyperlipidemia | Women less often treated with lipid-lowering therapies, and female sex associated with lower odds of achieving target LDL-C |
| Hypertension | Men more likely to be treated with an ACE inhibitor and to achieve target blood pressure |
| Antithrombotic therapy | Lower use of antiplatelet therapy consistently observed among women |
| Supervised exercise therapy (SET) | Small studies suggest less response to SET in women than men, though data are sparse |
| Revascularization | Older data suggest lower use of revascularization in women, but more recent data show similar use between women and men |
Important sex-based differences in physical function have also been observed. Relative to men, women have a faster functional decline144 and are at higher risk for reduced quality of life.145 Relative to men with similar ABI values, women with PAD have poorer functional status, greater ambulatory limitations and reduced lower extremity strength.146 One study showed that women with PAD had 18% lower daily physical activity level than men.147 Another study showed that women walked fewer blocks per week, had lower 6-min walk performance, smaller calf muscle area, lower calf muscle density, and poorer knee extension strength as compared to men with PAD of similar age.144 The Walking Impairment Questionnaire (WIQ) is a tool that measures patient perceived mobility by assessing walking endurance, walking speed and stair-climbing ability in the community and has been validated in men and women with PAD.148 Female sex has been related to lower scores in all the WIQ parameters.149,150
Diagnosis
Several tests can be used for the diagnosis of PAD. The ABI is the diagnostic test of choice, due to its low cost, noninvasiveness, and wide availability.151 Additional testing modalities used to confirm the diagnosis of PAD and define arterial anatomy include duplex ultrasonography, computer tomographic angiography, and magnetic resonance angiography. Invasive angiography remains the gold standard for diagnosing PAD but is generally performed when non-invasive methods are not feasible or inconclusive or when revascularization is planned. Despite observed smaller caliber arteries in women versus men,152 no studies have shown sex-based differences in imaging accuracy for PAD. An important barrier in diagnosing PAD in women remains clinician bias and failure to recognize atypical symptoms.
Management
The goals of managing PAD are to improve functional capacity, preserve limb viability, and reduce MACE and mortality risk.153 As discussed in current guidelines, management options include medical treatments (e.g. lipid-lowering and antithrombotic therapies), exercise therapy, and revascularization.131 However, use of guideline-recommended therapies in the overall PAD population has been consistently low,5,154–157 with variations arising in part from sex disparities.
Lipid-Lowering Therapies
Lipid lowering therapies (LLT) have revolutionized the primary and secondary prevention of CVD, including PAD. LLT has been shown to reduce MACE and limb events, as well as improve function, in patients with PAD,158–165 Furthermore, a relationship between lower LDL levels and lower risk of limb events has been demonstrated,166 suggesting that specifying lipid targets may be important to reduce adverse outcomes in PAD patients.
Despite the relevance of LLT in the management of PAD patients, the use of LLT remains underutilized especially when compared to patients with atherosclerotic disease in other territories.5 Only about 34% of the PAD patients are on a statin,5 and in a recent study, one-quarter of PAD patients were on high intensity statin.167 This suboptimal utilization of LLT is even more prominent in women with PAD as compared to men168,169 with disparities even more pronounced in minority groups such as African American women.170 In a post-hoc analysis of the COMPASS trial of patients with stable atherosclerotic CVD, including PAD, women were less often treated with LLT.171 Among patients with PAD, female sex is associated with lower odds of treatment with any statin172,173 and achieving target LDL-C.167 Among CLTI patients, a high risk subgroup, women are undertreated with statins and revascularization compared to men.128
Anti-Hypertensive Therapies
Anti-hypertensive therapies, in particular angiotensin-converting enzyme (ACE) inhibitors have been associated with improved maximum walking distance in patients with PAD irrespective of sex.174–176 Despite this, PAD patients also tend to be undertreated for hypertension as compared to patients with other known CVD.155,177 Although the incidence of hypertension in women with PAD is higher than in men with PAD, men are more likely to achieve blood pressure control 178 and are 1.3 times more likely to receive an ACE inhibitor than women.173
Antithrombotic Therapy
Antithrombotic therapy is a key component of medical treatment of PAD to reduce the risk for atherothrombotic cardiovascular and limb events,131 and sex-based outcomes have been reported for several relevant trials. The EUCLID trial showed that ticagrelor was not superior to clopidogrel for reducing cardiovascular events in patients with stable PAD.179 In subgroup analysis, women had similar rates of MALE but reduced mortality and MACE events compared with men; no sex modification was seen for the ticagrelor versus clopidogrel effect on MACE.180 The COMPASS trial demonstrated significant reductions in MACE, the primary endpoint, and MALE with low dose rivaroxaban (2.5 mg twice daily) plus aspirin versus aspirin alone in patients with stable atherosclerotic cardiovascular disease, including PAD.181,182 In COMPASS, there was no effect modification by sex, with similar benefits for MACE and similar risk of bleeding associated with combination therapy in women and men.171 The regimen of rivaroxaban 2.5 mg twice daily plus aspirin versus aspirin alone was most recently shown in VOYAGER PAD to reduce the risk of severe limb and cardiovascular events in patients undergoing lower extremity revascularization;183 consistent benefit and risk of bleeding were demonstrated in women and men.183 Additionally, in VOYAGER PAD, rivaroxaban appeared safe and efficacious in patients with impaired renal function, a significant proportion of whom were women.184
A novel antiplatelet agent, vorapaxar, is also approved by the FDA for the treatment of symptomatic PAD based on the results of TRA2P-TIMI 50 trial. In the overall population of patients with stable atherosclerotic cardiovascular disease, vorapaxar versus placebo added to aspirin and a P2Y12 inhibitor reduced MACE; in the subgroup of patients with stable, chronic PAD, vorapaxar did not reduce MACE but did reduce acute limb ischemia and need for peripheral revascularization.185 Although not designed to detect sex-based differences, a post-hoc analysis among patients with impaired renal function (who were more likely to be women) showed similar net clinical benefit for cardiovascular events and bleeding with vorapaxar in this population.186
Studies consistently demonstrate sex-based differences in use of antithrombotic therapy in PAD. Overall, among patients with PAD, men are 1.5 times more likely than women to be treated with antiplatelet agents.173 Even after lower extremity revascularization, a setting associated with higher risk for ischemic limb events, women are less likely to be discharged on evidence based medical therapies from the hospital including antiplatelet therapy, statins, or ACE-inhibitors/ARBs.187 As women tend to be older when diagnosed with PAD, it is thought that frailty and/or bleeding concerns may play a role in decision-making regarding antithrombotic therapies. However, in a study of veteran patients with PAD, young women were also less likely to receive antiplatelet therapy than men (59% vs 78% respectively).188 These findings support the need for further investigation into differential use by sex of this important class of therapies proven to reduce risk of atherothrombotic events in PAD.
Cilostazol
While the above therapies focus on reducing MACE or MALE in PAD patients, cilostazol is approved by the FDA to help with symptom relief and has been shown to help improve functional capacity.189 Pooled analyses of nine randomized controlled trials showed that cilostazol can increase maximal walking distance and quality of life over 24 weeks in patients with PAD.190,191 Notably, the benefit of cilostazol was significant and similar in men and women in subgroup analysis.190
Exercise Therapy
Supervised exercise therapy (SET) is well known to improve symptoms, exercise capacity, and quality of life189 and, although gender specific data are lacking, small studies suggest benefits of SET are lower in women than men.192–195 Gardner et al. reported 100% of men (both diabetic and non-diabetic) with PAD showed an increase in claudication onset time after the completion of either a supervised or home-based exercise program compared to only 81% of non-diabetic and 37% of diabetic women with PAD.193 Though incompletely understood, this sex disparity may relate to greater baseline limitations in women that manifest as earlier-onset claudication pain, longer time-to-recover calf muscle hemoglobin oxygen saturation after exercise, and more limited daily ambulatory activity.193 Attenuated responses in women with PAD could also be due to diminished effects of exercise training on vascular function. Evidence from exercise training studies in healthy postmenopausal women not taking estrogen-based hormone therapy report that endurance exercise training effects on endothelial function and limb blood flow are diminished or absent compared to middle-age and older men.33,196,197 Further investigations of sex-specific outcomes, particularly in response to exercise, has been identified an AHA/ACC research priority for exercise interventions in individuals with PAD.198
Revascularization
Lower extremity revascularization is an important treatment option for patients with PAD. . Historically, women have undergone lower extremity revascularization for PAD at lower rates than their male counterparts, despite the fact that PAD affects men and women at least equally.136,199 In the 1980s, men were twice more likely than women to be selected for revascularization.200 Fortunately, by 2006-2008, gender was no longer an independent predictor of revascularization for lower extremity PAD.201 Trends in revascularization strategies for women reflect an overall transition to an endovascular-first approach, with rates of endovascular treatment that are nearly equal between men and women.202 In fact, Nationwide Inpatient Sample data from 1998-2006 indicated that, along with yearly increases in endovascular procedures for both sexes, women made up less than half of acute admissions for PAD and were more likely to undergo endovascular as opposed to open surgical revascularization when compared to men.203
Data regarding post-revascularization outcomes by sex show mixed results. Although higher mortality for women after revascularization has been reported,202 most studies do not show a statistically different rate of in-hospital death by sex139,204 with at least equivalent limb salvage rates despite the presence of more multilevel disease in women.142,143 Surgical data yield conflicting results. In comparisons of surgical versus endovascular therapy for aortoiliac disease, women had higher rates of both bypass failure and stent thrombosis in multivariate analysis205 along with poorer patency at all prosthetic graft sizes.206 In randomized data examining autologous vein grafting for CLTI, black women experienced the greatest disparity in overall graft patency.207 Several studies on infra-inguinal bypass for CLTI have shown similar limb salvage rates in women despite lower patency rates,152,208,209 although other studies have shown no effect of gender on patency.210–212 Overall long-term patency rates of surgical bypass are lower in women,209,213 owing at least in part to smaller native arterial diameters than for men of matched age.152
Outcomes data by sex after endovascular peripheral vascular intervention (PVI) are limited, but women generally have higher peri-procedural complication rates without increased rates of major adverse limb events. Large registry data have consistently shown that women are at higher risk of procedural or access site complications than men but not for reintervention, amputation, or death.138,139,214 Despite women presenting with more advanced disease, in a statewide California database, women were shown to have improved amputation-free survival compared to men.215 In contrast, data from a national Korean registry showed that women undergoing PVI had higher rates of peri-procedural complications, myocardial infarction, major amputation, and death compared to men.216 A meta-analysis by Matsi and colleagues reported increased bleeding risk among women undergoing lower extremity PVI.217 Patency outcomes after PVI by sex are less well-characterized. In general, men and women appear to have similar primary patency rates after infra-inguinal PVI141 and femoropopliteal stenting, 218–221 although one study did report poorer secondary patency after femoropopliteal stenting222 and infra-popliteal PVI for CLTI among women versus men.142
Overall, outcomes after revascularization in women are mixed and are likely related to complex interactions between age, risk factors, Rutherford class at presentation, and anatomic factors including presence of microvascular disease.223 Importantly, the lifetime prevalence of PAD is at least equal between men and women, and although women present at older age with more complex, multilevel disease, their post-revascularization outcomes are at least as good as men – when revascularization therapies are offered and applied for women.
Conclusion
Not only is PAD a highly prevalent condition associated with significant morbidity and mortality, it is a condition that affects women as often or more commonly than men. Sex-based differences in pathophysiology and risk factors may contribute to the later-onset and often atypical presentation of women with PAD, in addition to the overall disease burden. Importantly, while underdiagnosis and undertreatment of PAD affects the quality of care and outcomes for all patients, these challenges are especially profound for women – with data consistently demonstrating less frequent use of evidence-based therapies in women compared to men. Studies examining outcomes in PAD among women and men have shown mixed results, likely reflecting the complexity of sex-specific interactions that are relevant to pathophysiology, risk factors, and treatment. Additional efforts are needed to increase awareness of and to better understand sex-based differences in PAD development, diagnosis, and management so that improved outcomes can be achieved in this vulnerable, high-risk population. In particular, sex-specific analyses from translational PAD studies in conjunction with clinical trial findings reported by sex will help to address the persistent unmet need.
Acknowledgments.
We are grateful to Mallory Health, MLS for invaluable assistance with manuscript preparation.
Sources of Funding.
This work was supported in part by National Institutes of Health grants R01-HL134168, R01-HL131532, R01-HL136601, R01-HL146158, R01-HL153963, R01-HL143227, R01-HL142983, R01-HL137771, R15-HL140989, P01-HL137630, R01-AG049762, T32-AG000279, U54-AG062319, U54-HL120163, and U54-AG065141. Further support was provided by the American Heart Association (20SFRN35120118 and 20YVRN35500014), Colorado Clinical and Translational Sciences Institute (UL1 TR001082).
Disclosures.
SC has consulted for Zogenix. SSS has received honoraria from Chiesi, Inc. and Janssen. NH has consulted for NovoNordisk, and Sanifit. CNH receives salary support from CPC Clinical Research, a non-profit academic research organization affiliated with the University of Colorado, that receives research grant/consulting funding from: Abbott, Agios, Alexion Pharma, Alnylam, Amgen, Angionetics, ARCA Biopharma, Array, AstraZeneca, Atentiv, Audentes, Bayer, Better Therapeutics, Brigham and Women’s Hospital, Bristol-Myers Squibb, Cardiol Therapeutics, CellResearch, Cook Medical, Cook, CSL Behring, Eidos Therapeutics, EP Trading Co, Esperion Therapeutics, EverlyWell, Faraday, Fortress Biotech, HDL Therapeutics, Heartflow, Hummingbird Bioscience, Insmed, Janssen, Kowa Research, Lexicon, Merck, Medtronic, Moderna, Novate Medical, NovoNordisk, Pfizer, PhaseBio, PPD Development, Prairie Education and Research, Prothena Ciosciences, Regeneron, Regio Biosciences, Sanifit Therapeutics, Sanofi, Smith and Nephew, Stealth BioTherapeutics, University of Colorado, Worldwide Clinical Trials, Wraser, Yale Cardiovascular Research Group.
Nonstandard Abbreviations and Acronyms:
- ABI
Ankle-brachial index
- ACE
Angiotensin-converting enzyme
- CKD
Chronic kidney disease
- CLTI
Chronic limb-threatening ischemia
- CV
Cardiovascular
- CVD
Cardiovascular disease
- E2
Estradiol
- ET
Endothelin
- ET-1
Endothelin-1
- HERS
Heart and Estrogen/Progestin Replacement
- LLT
Lipid lowering therapies
- MACE
Major adverse cardiovascular events
- MALE
Major adverse limb events
- NO
Nitric oxide
- PAD
Peripheral artery disease
- PVI
Peripheral vascular intervention
- ROS
Reactive oxygen species
- SET
Supervised exercise training
- WHI
Women’s Health Initiative
- WIQ
Walking Impairment Questionnaire
Contributor Information
Maria Pabon, Division of Cardiovascular Medicine, Brigham and Women’s Hospital.
Susan Cheng, Department of Cardiology, Cedars-Sinai Medical Center.
S. Elissa Altin, Division of Cardiology, Yale University School of Medicine.
Sanjum S. Sethi, Columbia Interventional Cardiovascular Care, Division of Cardiology, Columbia University Irving Medical Center.
Michael D. Nelson, Department of Kinesiology, University of Texas at Arlington.
Kerrie L. Moreau, Division of Geriatrics, University of Colorado School of Medicine, and Eastern Colorado Geriatric Research Education and Clinical Center.
Naomi Hamburg, Division of Cardiology, Boston University.
Connie N. Hess, Division of Cardiology, University of Colorado School of Medicine.
References
- 1.Leeper NJ and Hamburg NM. Peripheral Vascular Disease in 2021. Circulation Research. 2021;128:1803–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V and Group G-N-JGBoCDW. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J and Collaboration ABI. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, Darius H, Burghaus I, Trampisch HJ and Group GEToABIS. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–61. [DOI] [PubMed] [Google Scholar]
- 5.Colantonio LD, Hubbard D, Monda KL, Mues KE, Huang L, Dai Y, Jackson EA, Brown TM, Rosenson RS, Woodward M, Muntner P and Farkouh ME. Atherosclerotic Risk and Statin Use Among Patients With Peripheral Artery Disease. J Am Coll Cardiol. 2020;76:251–264. [DOI] [PubMed] [Google Scholar]
- 6.Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR and Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. [DOI] [PubMed] [Google Scholar]
- 7.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR and Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–33. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS and Subcommittee AHACoEaPSCaSS. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 9.Hackler EL, Hamburg NM and White Solaru KT. Racial and Ethnic Disparities in Peripheral Artery Disease. Circ Res. 2021;128:1913–1926. [DOI] [PubMed] [Google Scholar]
- 10.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D and Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–5. [DOI] [PubMed] [Google Scholar]
- 11.Aday AW and Matsushita K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ Res. 2021;128:1818–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoffers HE, Rinkens PE, Kester AD, Kaiser V and Knottnerus JA. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. Int J Epidemiol. 1996;25:282–90. [DOI] [PubMed] [Google Scholar]
- 13.Force UPST. Screening for Peripheral Artery Disease and Cardiovascular Disease Risk Assessment With the Ankle-Brachial Index: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:177–183. [DOI] [PubMed] [Google Scholar]
- 14.Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC Jr. and Manolio TA. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA). J Vasc Surg. 2007;45:319–27. [DOI] [PubMed] [Google Scholar]
- 15.Zheng ZJ, Rosamond WD, Chambless LE, Nieto FJ, Barnes RW, Hutchinson RG, Tyroler HA and Heiss G. Lower extremity arterial disease assessed by ankle-brachial index in a middle-aged population of African Americans and whites: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Prev Med. 2005;29:42–9. [DOI] [PubMed] [Google Scholar]
- 16.Dachun X, Jue L, Liling Z, Yawei X, Dayi H, Pagoto SL and Yunsheng M. Sensitivity and specificity of the ankle--brachial index to diagnose peripheral artery disease: a structured review. Vasc Med. 2010;15:361–9. [DOI] [PubMed] [Google Scholar]
- 17.Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, Pittrow D, von Stritzky B, Tepohl G and Trampisch HJ. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr., White CJ, White J, White RA, Antman EM, Smith SC Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL and Riegel B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–312. [DOI] [PubMed] [Google Scholar]
- 19.Vogt MT, McKenna M, Anderson SJ, Wolfson SK and Kuller LH. The relationship between ankle-arm index and mortality in older men and women. J Am Geriatr Soc. 1993;41:523–30. [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S and Sharrett AR. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. [DOI] [PubMed] [Google Scholar]
- 21.Collins TC, Suarez-Almazor M, Bush RL and Petersen NJ. Gender and Peripheral Arterial Disease. The Journal of the American Board of Family Medicine. 2006;19:132–140. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MM, Fried L, Simonsick E, Ling S and Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women’s health and aging study. Circulation. 2000;101:1007–12. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita K, Sang Y, Ning H, Ballew SH, Chow EK, Grams ME, Selvin E, Allison M, Criqui M, Coresh J, Lloyd-Jones DM and Wilkins JT. Lifetime Risk of Lower-Extremity Peripheral Artery Disease Defined by Ankle-Brachial Index in the United States. J Am Heart Assoc. 2019;8:e012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman AB, Sutton-Tyrrell K and Kuller LH. Lower-extremity arterial disease in older hypertensive adults. Arterioscler Thromb. 1993;13:555–62. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP and Higgins JA. Epidemiology of peripheral arterial disease in women. J Epidemiol. 2003;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornelius ME, Wang TW, Jamal A, Loretan CG and Neff LJ. Tobacco Product Use Among Adults — United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;69:1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gravholt CH, Juul S, Naeraa RW and Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol. 1998;51:147–58. [DOI] [PubMed] [Google Scholar]
- 28.Alsiraj Y, Thatcher SE, Blalock E, Fleenor B, Daugherty A and Cassis LA. Sex Chromosome Complement Defines Diffuse Versus Focal Angiotensin II-Induced Aortic Pathology. Arterioscler Thromb Vasc Biol. 2018;38:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davignon J and Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:Iii27–32. [DOI] [PubMed] [Google Scholar]
- 30.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J and Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–6. [DOI] [PubMed] [Google Scholar]
- 31.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S and Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28:576–82. [DOI] [PubMed] [Google Scholar]
- 32.Moreau KL, Hildreth KL, Meditz AL, Deane KD and Kohrt WM. Endothelial Function Is Impaired across the Stages of the Menopause Transition in Healthy Women. J Clin Endocrinol Metab. 2012;97:4692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreau KL, Stauffer BL, Kohrt WM and Seals DR. Essential Role of Estrogen for Improvements in Vascular Endothelial Function With Endurance Exercise in Postmenopausal Women. Journal of Clinical Endocrinology & Metabolism. 2013;98:4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau KL, Meditz A, Deane KD and Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol. 2012;302:H1211–8.3311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreau KL, Deane KD, Meditz AL and Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis. 2013;230:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK and Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27:1782–7. [DOI] [PubMed] [Google Scholar]
- 37.Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S and Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101:2258–63. [DOI] [PubMed] [Google Scholar]
- 38.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF and Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. American Journal of Physiology - Heart and Circulatory Physiology. 2008;294:H1630–H1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau KL, Hildreth KL, Klawitter J, Blatchford P and Kohrt WM. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience. 2020;42:1699–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Donnell E, Goodman JM and Harvey PJ. Clinical review: Cardiovascular consequences of ovarian disruption: a focus on functional hypothalamic amenorrhea in physically active women. J Clin Endocrinol Metab. 2011;96:3638–48. [DOI] [PubMed] [Google Scholar]
- 41.Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S and Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101:2258–63. [DOI] [PubMed] [Google Scholar]
- 42.Babcock MC, DuBose LE, Witten TL, Stauffer BL, Hildreth KL, Schwartz RS, Kohrt WM and Moreau KL. Oxidative stress and inflammation are associated with age-related endothelial dysfunction in men with low testosterone. J Clin Endocrinol Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novella S, Heras M, Hermenegildo C and Dantas AP. Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol. 2012;32:2035–42. [DOI] [PubMed] [Google Scholar]
- 44.Ghisletti S, Meda C, Maggi A and Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25:2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arenas IA, Armstrong SJ, Xu Y and Davidge ST. Chronic Tumor Necrosis Factor-{alpha} Inhibition Enhances NO Modulation of Vascular Function in Estrogen-Deficient Rats. Hypertension. 2005;46:76–81. [DOI] [PubMed] [Google Scholar]
- 46.Kitada K, Ohkita M and Matsumura Y. Pathological Importance of the Endothelin-1/ET(B) Receptor System on Vascular Diseases. Cardiol Res Pract. 2012;2012:731970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kellogg DL Jr., Liu Y and Pergola PE. Selected contribution: Gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol (1985). 2001;91:2407–11; discussion 2389–90. [DOI] [PubMed] [Google Scholar]
- 48.Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP and Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol. 2010;298:R261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ergul A, Shoemaker K, Puett D and Tackett RL. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther. 1998;285:511–7. [PubMed] [Google Scholar]
- 50.Wenner MM, Sebzda KN, Kuczmarski AV, Pohlig RT and Edwards DG. ETB receptor contribution to vascular dysfunction in postmenopausal women. Am J Physiol Regul Integr Comp Physiol. 2017;313:R51–R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross R Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 52.Arnold AP, Cassis LA, Eghbali M, Reue K and Sandberg K. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arterioscler Thromb Vasc Biol. 2017;37:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fairweather D Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol. 2014;8:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Man JJ, Beckman JA and Jaffe IZ. Sex as a Biological Variable in Atherosclerosis. Circ Res. 2020;126:1297–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klarin D, Lynch J, Aragam K, Chaffin M, Assimes TL, Huang J, Lee KM, Shao Q, Huffman JE, Natarajan P, Arya S, Small A, Sun YV, Vujkovic M, Freiberg MS, Wang L, Chen J, Saleheen D, Lee JS, Miller DR, Reaven P, Alba PR, Patterson OV, DuVall SL, Boden WE, Beckman JA, Gaziano JM, Concato J, Rader DJ, Cho K, Chang KM, Wilson PWF, O’Donnell CJ, Kathiresan S, Tsao PS and Damrauer SM. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat Med. 2019;25:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding N, Sang Y, Chen J, Ballew SH, Kalbaugh CA, Salameh MJ, Blaha MJ, Allison M, Heiss G, Selvin E, Coresh J and Matsushita K. Cigarette Smoking, Smoking Cessation, and Long-Term Risk of 3 Major Atherosclerotic Diseases. J Am Coll Cardiol. 2019;74:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huxley RR, Yatsuya H, Lutsey PL, Woodward M, Alonso A and Folsom AR. Impact of age at smoking initiation, dosage, and time since quitting on cardiovascular disease in african americans and whites: the atherosclerosis risk in communities study. Am J Epidemiol. 2012;175:816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willigendael EM, Teijink JA, Bartelink ML, Kuiken BW, Boiten J, Moll FL, Büller HR and Prins MH. Influence of smoking on incidence and prevalence of peripheral arterial disease. J Vasc Surg. 2004;40:1158–65. [DOI] [PubMed] [Google Scholar]
- 59.Sigvant B, Lundin F, Nilsson B, Bergqvist D and Wahlberg E. Differences in presentation of symptoms between women and men with intermittent claudication. BMC Cardiovasc Disord. 2011;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hiramoto JS, Katz R, Weisman S and Conte M. Gender-specific risk factors for peripheral artery disease in a voluntary screening population. Journal of the American Heart Association. 2014;3:e000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tunstall-Pedoe H, Peters SAE, Woodward M, Struthers AD and Belch JJF. Twenty-Year Predictors of Peripheral Arterial Disease Compared With Coronary Heart Disease in the Scottish Heart Health Extended Cohort (SHHEC). J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA and Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 63.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA and Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geiss LS, Li Y, Hora I, Albright A, Rolka D and Gregg EW. Resurgence of Diabetes-Related Nontraumatic Lower-Extremity Amputation in the Young and Middle-Aged Adult U.S. Population. Diabetes Care. 2019;42:50–54. [DOI] [PubMed] [Google Scholar]
- 65.Chase-Vilchez AZ, Chan IHY, Peters SAE and Woodward M. Diabetes as a risk factor for incident peripheral arterial disease in women compared to men: a systematic review and meta-analysis. Cardiovasc Diabetol. 2020;19:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters SA, Huxley RR and Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973–80. [DOI] [PubMed] [Google Scholar]
- 67.Peters SA, Huxley RR and Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–51. [DOI] [PubMed] [Google Scholar]
- 68.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S and Investigators I. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–40. [DOI] [PubMed] [Google Scholar]
- 69.Kannel WB and McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33:13–8. [DOI] [PubMed] [Google Scholar]
- 70.Conen D, Rexrode KM, Creager MA, Ridker PM and Pradhan AD. Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women: a prospective study. Circulation. 2009;120:1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW and Investigators RR. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–9. [DOI] [PubMed] [Google Scholar]
- 72.Bavry AA, Anderson RD, Gong Y, Denardo SJ, Cooper-Dehoff RM, Handberg EM and Pepine CJ. Outcomes Among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the INternational VErapamil-SR/Trandolapril STudy. Hypertension. 2010;55:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powell TM, Glynn RJ, Buring JE, Creager MA, Ridker PM and Pradhan AD. The relative importance of systolic versus diastolic blood pressure control and incident symptomatic peripheral artery disease in women. Vasc Med. 2011;16:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L and Investigators IS. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 75.Wenger NK, Arnold A, Bairey Merz CN, Cooper-DeHoff RM, Ferdinand KC, Fleg JL, Gulati M, Isiadinso I, Itchhaporia D, Light-McGroary K, Lindley KJ, Mieres JH, Rosser ML, Saade GR, Walsh MN and Pepine CJ. Hypertension Across a Woman’s Life Cycle. J Am Coll Cardiol. 2018;71:1797–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K and Devereux RB. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51:1109–14. [DOI] [PubMed] [Google Scholar]
- 77.Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, Guerrero M, Kunadian V, Lam CSP, Maas A, Mihailidou AS, Olszanecka A, Poole JE, Saldarriaga C, Saw J, Zuhlke L and Mehran R. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. 2021;397:2385–2438. [DOI] [PubMed] [Google Scholar]
- 78.Wisman PP, Tangelder MJ, van Hattum ES, de Borst GJ and Moll FL. Young women with PAD are at high risk of cardiovascular complications. Eur J Vasc Endovasc Surg. 2012;43:441–5. [DOI] [PubMed] [Google Scholar]
- 79.Pawlik A, Januszek R, Ruzsa Z, Óriás V, Kleczyński P, Wojtasik-Bakalarz J, Arif S, Nyerges A, Chyrchel M, Stanek A, Dudek D and Bartuś S. Gender differences and long-term clinical outcomes in patients with chronic total occlusions of infrainguinal lower limb arteries treated from retrograde access with peripheral vascular interventions. Adv Med Sci. 2020;65:197–201. [DOI] [PubMed] [Google Scholar]
- 80.Haine A, Kavanagh S, Berger JS, Hess CN, Norgren L, Fowkes FGR, Katona BG, Mahaffey KW, Blomster JI, Patel MR, Jones WS, Rockhold FW, Hiatt WR, Baumgartner I and Trial ISCaIotE. Sex-Specific Risks of Major Cardiovascular and Limb Events in Patients With Symptomatic Peripheral Artery Disease. J Am Coll Cardiol. 2020;75:608–617. [DOI] [PubMed] [Google Scholar]
- 81.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN and Cheng S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020;5:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ridker PM, Stampfer MJ and Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. [DOI] [PubMed] [Google Scholar]
- 83.Rizzo M, Pernice V, Frasheri A and Berneis K. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis. 2008;197:237–41. [DOI] [PubMed] [Google Scholar]
- 84.Toth PP, Philip S, Hull M and Granowitz C. Hypertriglyceridemia is associated with an increased risk of peripheral arterial revascularization in high-risk statin-treated patients: A large administrative retrospective analysis. Clin Cardiol. 2019;42:908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S and Pradhan AD. Lipoprotein Particle Profiles, Standard Lipids, and Peripheral Artery Disease Incidence. Circulation. 2018;138:2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J and Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ben-Ami S, Oron G, Ben-Haroush A, Blickstein D, Hod M and Bar J. Primary atherothrombotic occlusive vascular events in premenopausal women with history of adverse pregnancy outcome. Thromb Res. 2010;125:124–7. [DOI] [PubMed] [Google Scholar]
- 88.Weissgerber TL, Turner ST, Bailey KR, Mosley TH, Kardia SL, Wiste HJ, Miller VM, Kullo IJ and Garovic VD. Hypertension in pregnancy is a risk factor for peripheral arterial disease decades after pregnancy. Atherosclerosis. 2013;229:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang KP, Liang KV, Matteson EL, McClelland RL, Christianson TJ and Turesson C. Incidence of noncardiac vascular disease in rheumatoid arthritis and relationship to extraarticular disease manifestations. Arthritis Rheum. 2006;54:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang GJ, Shaw PA, Townsend RR, Anderson AH, Xie D, Wang X, Nessel LC, Mohler ER, Sozio SM, Jaar BG, Chen J, Wright J, Taliercio JJ, Ojo A, Ricardo AC, Lustigova E, Fairman RM, Feldman HI, Ky B and Investigators CS. Sex Differences in the Incidence of Peripheral Artery Disease in the Chronic Renal Insufficiency Cohort. Circ Cardiovasc Qual Outcomes. 2016;9:S86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eraso LH, Fukaya E, Mohler ER, Xie D, Sha D and Berger JS. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jelani QU, Mena-Hurtado C, Burg M, Soufer R, Gosch K, Jones PG, Spertus JA, Safdar B and Smolderen KG. Relationship Between Depressive Symptoms and Health Status in Peripheral Artery Disease: Role of Sex Differences. J Am Heart Assoc. 2020;9:e014583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramirez JL, Zahner GJ, Arya S, Grenon SM, Gasper WJ, Sosa JA, Conte MS and Iannuzzi JC. Patients with depression are less likely to go home after critical limb revascularization. J Vasc Surg. 2021;74:178–186.e2. [DOI] [PubMed] [Google Scholar]
- 94.Ness J and Aronow WS. Comparison of prevalence of atherosclerotic vascular disease in postmenopausal women with osteoporosis or osteopenia versus without osteoporosis or osteopenia. Am J Cardiol. 2006;97:1427–8. [DOI] [PubMed] [Google Scholar]
- 95.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A and Brindle P. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manrique-Garcia E, Sidorchuk A, Hallqvist J and Moradi T. Socioeconomic position and incidence of acute myocardial infarction: a meta-analysis. J Epidemiol Community Health. 2011;65:301–9. [DOI] [PubMed] [Google Scholar]
- 97.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, Mieres JH, Ferdinand KC, Mensah GA and Sperling LS. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Backholer K, Peters SAE, Bots SH, Peeters A, Huxley RR and Woodward M. Sex differences in the relationship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epidemiol Community Health. 2017;71:550–557. [DOI] [PubMed] [Google Scholar]
- 99.Mosca L, Barrett-Connor E and Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kröger K, Dragano N, Stang A, Moebus S, Möhlenkamp S, Mann K, Siegrist J, Jöckel KH, Erbel R and Group HNRSI. An unequal social distribution of peripheral arterial disease and the possible explanations: results from a population-based study. Vasc Med. 2009;14:289–96. [DOI] [PubMed] [Google Scholar]
- 101.Collins TC, Slovut DP, Newton R, Johnson WD, Larrivee S, Patterson J, Johnston JA and Correa A. Ideal cardiovascular health and peripheral artery disease in African Americans: Results from the Jackson Heart Study. Prev Med Rep. 2017;7:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serra R, Abramo A, Ielapi N, Procopio S and Marino P. Environmental Pollution and Peripheral Artery Disease. Risk Manag Healthc Policy. 2021;14:2181–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ward-Caviness CK, Kraus WE, Blach C, Haynes CS, Dowdy E, Miranda ML, Devlin R, Diaz-Sanchez D, Cascio WE, Mukerjee S, Stallings C, Smith LA, Gregory SG, Shah SH, Neas LM and Hauser ER. Associations Between Residential Proximity to Traffic and Vascular Disease in a Cardiac Catheterization Cohort. Arterioscler Thromb Vasc Biol. 2018;38:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, Astor B and Keeler J. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:667–75. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y, Wellenius GA, Hickson DA, Gjelsvik A, Eaton CB and Wyatt SB. Residential Proximity to Traffic-Related Pollution and Atherosclerosis in 4 Vascular Beds Among African-American Adults: Results From the Jackson Heart Study. Am J Epidemiol. 2016;184:732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoffmann B, Moebus S, Kröger K, Stang A, Möhlenkamp S, Dragano N, Schmermund A, Memmesheimer M, Erbel R and Jöckel KH. Residential exposure to urban air pollution, ankle-brachial index, and peripheral arterial disease. Epidemiology. 2009;20:280–8. [DOI] [PubMed] [Google Scholar]
- 107.Carson AP, Rose KM, Catellier DJ, Kaufman JS, Wyatt SB, Diez-Roux AV and Heiss G. Cumulative socioeconomic status across the life course and subclinical atherosclerosis. Ann Epidemiol. 2007;17:296–303. [DOI] [PubMed] [Google Scholar]
- 108.Ferguson HJ, Nightingale P, Pathak R and Jayatunga AP. The influence of socio-economic deprivation on rates of major lower limb amputation secondary to peripheral arterial disease. Eur J Vasc Endovasc Surg. 2010;40:76–80. [DOI] [PubMed] [Google Scholar]
- 109.Pande RL and Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ Cardiovasc Qual Outcomes. 2014;7:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cooke JP and Wilson AM. Biomarkers of peripheral arterial disease. J Am Coll Cardiol. 2010;55:2017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Westendorp IC, in’t Veld BA, Grobbee DE, Pols HA, Meijer WT, Hofman A and Witteman JC. Hormone replacement therapy and peripheral arterial disease: the Rotterdam study. Arch Intern Med. 2000;160:2498–502. [DOI] [PubMed] [Google Scholar]
- 112.Rockman CB, Maldonado TS, Jacobowitz GR, Adelman MA and Riles TS. Hormone Replacement Therapy is Associated With a Decreased Prevalence of Peripheral Arterial Disease in Postmenopausal Women. Annals of Vascular Surgery. 2012;26:411–418. [DOI] [PubMed] [Google Scholar]
- 113.Hsia J, Criqui MH, Rodabough RJ, Langer RD, Resnick HE, Phillips LS, Allison M, Bonds DE, Masaki K, Caralis P and Kotchen JM. Estrogen Plus Progestin and the Risk of Peripheral Arterial Disease. Circulation. 2004;109:620–626. [DOI] [PubMed] [Google Scholar]
- 114.Hsia J, Criqui MH, Herrington DM, Manson JE, Wu L, Heckbert SR, Allison M, McGrae McDermott M, Robinson J and Masaki K. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. American Heart Journal. 2006;152:170–176. [DOI] [PubMed] [Google Scholar]
- 115.Hsia J, Simon JA, Lin F, Applegate WB, Vogt MT, Hunninghake D and Carr M. Peripheral Arterial Disease in Randomized Trial of Estrogen With Progestin in Women With Coronary Heart Disease. Circulation. 2000;102:2228–2232. [DOI] [PubMed] [Google Scholar]
- 116.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N and Group ftHR. Cardiovascular Disease Outcomes During 6.8 Years of Hormone TherapyHeart and Estrogen/Progestin Replacement Study Follow-up (HERS II). JAMA. 2002;288:49–57. [DOI] [PubMed] [Google Scholar]
- 117.Timaran CH, Stevens SL, Grandas OH, Piercy KT, Freeman MB and Goldman MH. Influence of hormone replacement therapy on graft patency after femoropopliteal bypass grafting. Journal of Vascular Surgery. 2000;32:506–518. [DOI] [PubMed] [Google Scholar]
- 118.Timaran CH, Stevens SL, Grandas OH, Freeman MB and Goldman MH. Influence of hormone replacement therapy on the outcome of iliac angioplasty and stenting. Journal of Vascular Surgery. 2001;33:85–92. [DOI] [PubMed] [Google Scholar]
- 119.Van Den Bosch MA, Kemmeren JM, Tanis BC, Mali WP, Helmerhorst FM, Rosendaal FR, Algra A and Van Der Graaf Y. The RATIO study: oral contraceptives and the risk of peripheral arterial disease in young women. J Thromb Haemost. 2003;1:439–44. [DOI] [PubMed] [Google Scholar]
- 120.Steg PG, Bhatt DL, Wilson PW, D’Agostino R, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S and Investigators RR. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–206. [DOI] [PubMed] [Google Scholar]
- 121.Vogt MT, Cauley JA, Newman AB, Kuller LH and Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465–9. [PubMed] [Google Scholar]
- 122.Dreyer RP, van Zitteren M, Beltrame JF, Fitridge R, Denollet J, Vriens PW, Spertus JA and Smolderen KG. Gender differences in health status and adverse outcomes among patients with peripheral arterial disease. J Am Heart Assoc. 2014;4:e000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hussain MA, Lindsay TF, Mamdani M, Wang X, Verma S and Al-Omran M. Sex differences in the outcomes of peripheral arterial disease: a population-based cohort study. CMAJ Open. 2016;4:E124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morrell J, Zeymer U, Baumgartner I, Limbourg T, Röther J, Bhatt DL, Steg PG and Investigators RR. Differences in management and outcomes between male and female patients with atherothrombotic disease: results from the REACH Registry in Europe. Eur J Cardiovasc Prev Rehabil. 2011;18:270–7. [DOI] [PubMed] [Google Scholar]
- 125.Sigvant B, Lundin F and Wahlberg E. The Risk of Disease Progression in Peripheral Arterial Disease is Higher than Expected: A Meta-Analysis of Mortality and Disease Progression in Peripheral Arterial Disease. Eur J Vasc Endovasc Surg. 2016;51:395–403. [DOI] [PubMed] [Google Scholar]
- 126.Lefebvre KM and Chevan J. The persistence of gender and racial disparities in vascular lower extremity amputation: an examination of HCUP-NIS data (2002–2011). Vasc Med. 2015;20:51–9. [DOI] [PubMed] [Google Scholar]
- 127.Farber A and Eberhardt RT. The Current State of Critical Limb Ischemia: A Systematic Review. JAMA Surg. 2016;151:1070–1077. [DOI] [PubMed] [Google Scholar]
- 128.Mentias A, Vaughan-Sarrazin M, Saad M and Girotra S. Sex Differences in Management and Outcomes of Critical Limb Ischemia in the Medicare Population. Circ Cardiovasc Interv. 2020;13:e009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Varu VN, Hogg ME and Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–41. [DOI] [PubMed] [Google Scholar]
- 130.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D and Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. [DOI] [PubMed] [Google Scholar]
- 131.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D and Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:e71–e126. [DOI] [PubMed] [Google Scholar]
- 132.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ and Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61. [DOI] [PubMed] [Google Scholar]
- 133.McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, Sharma L and Criqui MH. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study). J Am Coll Cardiol. 2009;53:1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vogt MT, Cauley JA, Kuller LH and Nevitt MC. Functional status and mobility among elderly women with lower extremity arterial disease: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 1994;42:923–9. [DOI] [PubMed] [Google Scholar]
- 135.McDermott MM, Mehta S and Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159:387–92. [DOI] [PubMed] [Google Scholar]
- 136.Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E and Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–91. [DOI] [PubMed] [Google Scholar]
- 137.McDermott MM, Ferrucci L, Simonsick EM, Balfour J, Fried L, Ling S, Gibson D and Guralnik JM. The ankle brachial index and change in lower extremity functioning over time: the Women’s Health and Aging Study. J Am Geriatr Soc. 2002;50:238–46. [DOI] [PubMed] [Google Scholar]
- 138.Vouyouka AG, Egorova NN, Salloum A, Kleinman L, Marin M, Faries PL and Moscowitz A. Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. J Vasc Surg. 2010;52:1196–202. [DOI] [PubMed] [Google Scholar]
- 139.Jackson EA, Munir K, Schreiber T, Rubin JR, Cuff R, Gallagher KA, Henke PK, Gurm HS and Grossman PM. Impact of sex on morbidity and mortality rates after lower extremity interventions for peripheral arterial disease: observations from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Am Coll Cardiol. 2014;63:2525–2530. [DOI] [PubMed] [Google Scholar]
- 140.Ramkumar N, Suckow BD, Brown JR, Sedrakyan A, Cronenwett JL and Goodney PP. Sex-Based Assessment of Patient Presentation, Lesion Characteristics, and Treatment Modalities in Patients Undergoing Peripheral Vascular Intervention. Circ Cardiovasc Interv. 2018;11:e005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DeRubertis BG, Vouyouka A, Rhee SJ, Califano J, Karwowski J, Angle N, Faries PL and Kent KC. Percutaneous intervention for infrainguinal occlusive disease in women: equivalent outcomes despite increased severity of disease compared with men. J Vasc Surg. 2008;48:150–7; discussion 157–8. [DOI] [PubMed] [Google Scholar]
- 142.McCoach CE, Armstrong EJ, Singh S, Javed U, Anderson D, Yeo KK, Westin GG, Hedayati N, Amsterdam EA and Laird JR. Gender-related variation in the clinical presentation and outcomes of critical limb ischemia. Vasc Med. 2013;18:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ortmann J, Nüesch E, Traupe T, Diehm N and Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. 2012;55:98–104. [DOI] [PubMed] [Google Scholar]
- 144.McDermott MM, Ferrucci L, Liu K, Guralnik JM, Tian L, Kibbe M, Liao Y, Tao H and Criqui MH. Women with peripheral arterial disease experience faster functional decline than men with peripheral arterial disease. J Am Coll Cardiol. 2011;57:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Collins TC, Suarez-Almazor M, Bush RL and Petersen NJ. Gender and peripheral arterial disease. J Am Board Fam Med. 2006;19:132–40. [DOI] [PubMed] [Google Scholar]
- 146.McDermott MM, Greenland P, Liu K, Criqui MH, Guralnik JM, Celic L and Chan C. Sex differences in peripheral arterial disease: leg symptoms and physical functioning. J Am Geriatr Soc. 2003;51:222–8. [DOI] [PubMed] [Google Scholar]
- 147.Gardner AW. Sex differences in claudication pain in subjects with peripheral arterial disease. Med Sci Sports Exerc. 2002;34:1695–8. [DOI] [PubMed] [Google Scholar]
- 148.McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH and Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998;28:1072–81. [DOI] [PubMed] [Google Scholar]
- 149.Jain A, Liu K, Ferrucci L, Criqui MH, Tian L, Guralnik JM, Tao H and McDermott MM. The Walking Impairment Questionnaire stair-climbing score predicts mortality in men and women with peripheral arterial disease. J Vasc Surg. 2012;55:1662–73.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Han DK, Faries PL, Chung C, Weaver MV, Tadros RO, Ting W and Vouyouka AG. Intermediate Outcomes of Femoropopliteal Stenting in Women: 3-Year Results of the DURABILITY II Trial. Ann Vasc Surg. 2016;30:110–7. [DOI] [PubMed] [Google Scholar]
- 151.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D, Disease AHACoPV, Prevention CoEa, Cardiology CoC, Nursing CoC and Council on Cardiovascular Radiology and Intervention aCoCSaA. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- 152.AhChong AK, Chiu KM, Wong M and Yip AW. The influence of gender difference on the outcomes of infrainguinal bypass for critical limb ischaemia in Chinese patients. Eur J Vasc Endovasc Surg. 2002;23:134–9. [DOI] [PubMed] [Google Scholar]
- 153.Bonaca MP, Hamburg NM and Creager MA. Contemporary Medical Management of Peripheral Artery Disease. Circulation Research. 2021;128:1868–1884. [DOI] [PubMed] [Google Scholar]
- 154.Saratzis A, Jaspers NEM, Gwilym B, Thomas O, Tsui A, Lefroy R, Parks M, Htun V, Mera Z, Thatcher A, Bosanquet D, Forsythe R, Benson R, Dattani N, Dovell G, Lane T, Shalhoub J, Sidloff D, Visseren FLJ, Dorresteijn JAN, Richards T and Collaborators VaERNV. Observational study of the medical management of patients with peripheral artery disease. Br J Surg. 2019;106:1168–1177. [DOI] [PubMed] [Google Scholar]
- 155.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM and Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. [DOI] [PubMed] [Google Scholar]
- 156.Pande RL, Perlstein TS, Beckman JA and Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoeks SE, Scholte op Reimer WJ, van Gestel YR, Schouten O, Lenzen MJ, Flu WJ, van Kuijk JP, Latour C, Bax JJ, van Urk H and Poldermans D. Medication underuse during long-term follow-up in patients with peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2009;2:338–43. [DOI] [PubMed] [Google Scholar]
- 158.Arya S, Khakharia A, Binney ZO, DeMartino RR, Brewster LP, Goodney PP and Wilson PWF. Association of Statin Dose With Amputation and Survival in Patients With Peripheral Artery Disease. Circulation. 2018;137:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aung PP, Maxwell HG, Jepson RG, Price JF and Leng GC. Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database Syst Rev. 2007:CD000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Henke PK, Blackburn S, Proctor MC, Stevens J, Mukherjee D, Rajagopalin S, Upchurch GR, Stanley JC and Eagle KA. Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage, and mortality. J Vasc Surg. 2004;39:357–65. [DOI] [PubMed] [Google Scholar]
- 161.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC, Goto S, Ohman EM, Elbez Y, Sritara P, Baumgartner I, Banerjee S, Creager MA, Bhatt DL and Investigators RR. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mondillo S, Ballo P, Barbati R, Guerrini F, Ammaturo T, Agricola E, Pastore M, Borrello F, Belcastro M, Picchi A and Nami R. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med. 2003;114:359–64. [DOI] [PubMed] [Google Scholar]
- 163.Feringa HH, Karagiannis SE, van Waning VH, Boersma E, Schouten O, Bax JJ and Poldermans D. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45:936–43. [DOI] [PubMed] [Google Scholar]
- 164.Mohler ER, Hiatt WR and Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–6. [DOI] [PubMed] [Google Scholar]
- 165.Ramos R, García-Gil M, Comas-Cufí M, Quesada M, Marrugat J, Elosua R, Sala J, Grau M, Martí R, Ponjoan A, Alves-Cabratosa L, Blanch J and Bolíbar B. Statins for Prevention of Cardiovascular Events in a Low-Risk Population With Low Ankle Brachial Index. J Am Coll Cardiol. 2016;67:630–640. [DOI] [PubMed] [Google Scholar]
- 166.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgozoglu L, Somaratne R, Sever PS, Pedersen TR and Sabatine MS. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137:338–350. [DOI] [PubMed] [Google Scholar]
- 167.Hess CN, Cannon CP, Beckman JA, Goodney PP, Patel MR, Hiatt WR, Mues KE, Orroth KK, Shannon E and Bonaca MP. Effectiveness of Blood Lipid Management in Patients With Peripheral Artery Disease. J Am Coll Cardiol. 2021;77:3016–3027. [DOI] [PubMed] [Google Scholar]
- 168.Miller M, Byington R, Hunninghake D, Pitt B and Furberg CD. Sex bias and underutilization of lipid-lowering therapy in patients with coronary artery disease at academic medical centers in the United States and Canada. Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT) Investigators. Arch Intern Med. 2000;160:343–7. [DOI] [PubMed] [Google Scholar]
- 169.Schrott HG, Bittner V, Vittinghoff E, Herrington DM and Hulley S. Adherence to National Cholesterol Education Program Treatment goals in postmenopausal women with heart disease. The Heart and Estrogen/Progestin Replacement Study (HERS). The HERS Research Group. JAMA. 1997;277:1281–6. [PubMed] [Google Scholar]
- 170.Schroff P, Gamboa CM, Durant RW, Oikeh A, Richman JS and Safford MM. Vulnerabilities to Health Disparities and Statin Use in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liang Y, Zhu J, Liu L, Anand SS, Connolly SJ, Bosch J, Guzik TJ, O’Donnell M, Dagenais GR, Fox KA, Shestakovska O, Berkowitz SD, Muehlhofer E, Keller L, Yusuf S, Eikelboom JW and Investigators C. Efficacy and safety of rivaroxaban plus aspirin in women and men with chronic coronary or peripheral artery disease. Cardiovasc Res. 2021;117:942–949. [DOI] [PubMed] [Google Scholar]
- 172.McDermott MM, Guralnik JM, Greenland P, Pearce WH, Criqui MH, Liu K, Taylor L, Chan C, Sharma L, Schneider JR, Ridker PM, Green D and Quann M. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation. 2003;107:757–61. [DOI] [PubMed] [Google Scholar]
- 173.Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O and Wahlberg E. Risk factor profiles and use of cardiovascular drug prevention in women and men with peripheral arterial disease. Eur J Cardiovasc Prev Rehabil. 2009;16:39–46. [DOI] [PubMed] [Google Scholar]
- 174.Thomas Manapurathe D, Krishna SM, Dewdney B, Moxon JV, Biros E and Golledge J. Effect of blood pressure lowering medications on leg ischemia in peripheral artery disease patients: A meta-analysis of randomised controlled trials. PLoS One. 2017;12:e0178713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shahin Y, Barnes R, Barakat H and Chetter IC. Meta-analysis of angiotensin converting enzyme inhibitors effect on walking ability and ankle brachial pressure index in patients with intermittent claudication. Atherosclerosis. 2013;231:283–90. [DOI] [PubMed] [Google Scholar]
- 176.Hirsch AT and Duprez D. The potential role of angiotensin-converting enzyme inhibition in peripheral arterial disease. Vasc Med. 2003;8:273–8. [DOI] [PubMed] [Google Scholar]
- 177.Cea-Soriano L, Fowkes FGR, Johansson S, Allum AM and García Rodriguez LA. Time trends in peripheral artery disease incidence, prevalence and secondary preventive therapy: a cohort study in The Health Improvement Network in the UK. BMJ Open. 2018;8:e018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cacoub PP, Abola MT, Baumgartner I, Bhatt DL, Creager MA, Liau CS, Goto S, Röther J, Steg PG, Hirsch AT and Investigators RR. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Atherosclerosis. 2009;204:e86–92. [DOI] [PubMed] [Google Scholar]
- 179.Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS, Blomster J, Millegard M, Reist C, Patel MR, Committee ETS and Investigators. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N Engl J Med. 2017;376:32–40. [DOI] [PubMed] [Google Scholar]
- 180.Haine A, Kavanagh S, Berger JS, Hess CN, Norgren L, Fowkes FGR, Katona BG, Mahaffey KW, Blomster JI, Patel MR, Jones WS, Rockhold FW, Hiatt WR, Baumgartner I, International Steering C and Investigators of the ET. Sex-Specific Risks of Major Cardiovascular and Limb Events in Patients With Symptomatic Peripheral Artery Disease. J Am Coll Cardiol. 2020;75:608–617. [DOI] [PubMed] [Google Scholar]
- 181.Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Störk S, Zhu J, Lopez-Jaramillo P, O’Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD, Vanassche T, Avezum AA, Chen E, Branch K, Leong DP, Bangdiwala SI, Hart RG and Yusuf S. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229. [DOI] [PubMed] [Google Scholar]
- 182.Anand SS, Caron F, Eikelboom JW, Bosch J, Dyal L, Aboyans V, Abola MT, Branch KRH, Keltai K, Bhatt DL, Verhamme P, Fox KAA, Cook-Bruns N, Lanius V, Connolly SJ and Yusuf S. Major Adverse Limb Events and Mortality in Patients With Peripheral Artery Disease: The COMPASS Trial. J Am Coll Cardiol. 2018;71:2306–2315. [DOI] [PubMed] [Google Scholar]
- 183.Bonaca MP, Bauersachs RM, Anand SS, Debus ES, Nehler MR, Patel MR, Fanelli F, Capell WH, Diao L, Jaeger N, Hess CN, Pap AF, Kittelson JM, Gudz I, Mátyás L, Krievins DK, Diaz R, Brodmann M, Muehlhofer E, Haskell LP, Berkowitz SD and Hiatt WR. Rivaroxaban in Peripheral Artery Disease after Revascularization. N Engl J Med. 2020;382:1994–2004. [DOI] [PubMed] [Google Scholar]
- 184.Hsia J, Szarek M, Anand S, Patel MR, Debus S, Berkowitz SD, Muehlhofer E, Haskell LP, Bauersachs RM and Bonaca MP. Rivaroxaban in Patients With Recent Peripheral Artery Revascularization and Renal Impairment: The VOYAGER PAD Trial. J Am Coll Cardiol. 2021;78:757–759. [DOI] [PubMed] [Google Scholar]
- 185.Bonaca MP, Scirica BM, Creager MA, Olin J, Bounameaux H, Dellborg M, Lamp JM, Murphy SA, Braunwald E and Morrow DA. Vorapaxar in patients with peripheral artery disease: results from TRA2{degrees}P-TIMI 50. Circulation. 2013;127:1522–9, 1529e1-6. [DOI] [PubMed] [Google Scholar]
- 186.Correa S, Bonaca MP, Scirica BM, Murphy SA, Goodrich EL, Morrow DA and O’Donoghue ML. Efficacy and safety of more potent antiplatelet therapy with vorapaxar in patients with impaired renal function. J Thromb Thrombolysis. 2019;47:353–360. [DOI] [PubMed] [Google Scholar]
- 187.Altin SE, Castro-Dominguez YS, Kennedy KF, Orion KC, Lanksy AJ, Abbott JD and Aronow HD. Predictors of Underutilization of Medical Therapy in Patients Undergoing Endovascular Revascularization for Peripheral Artery Disease. JACC Cardiovasc Interv. 2020;13:2911–2918. [DOI] [PubMed] [Google Scholar]
- 188.Lee MT, Mahtta D, Ramsey DJ, Liu J, Misra A, Nasir K, Samad Z, Itchhaporia D, Khan SU, Schofield RS, Ballantyne CM, Petersen LA and Virani SS. Sex-Related Disparities in Cardiovascular Health Care Among Patients With Premature Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2021;6:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D and Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1465–1508. [DOI] [PubMed] [Google Scholar]
- 190.Pande RL, Hiatt WR, Zhang P, Hittel N and Creager MA. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc Med. 2010;15:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP and Stansby G. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014:CD003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dörenkamp S, Mesters I, de Bie R, Teijink J and van Breukelen G. Patient Characteristics and Comorbidities Influence Walking Distances in Symptomatic Peripheral Arterial Disease: A Large One-Year Physiotherapy Cohort Study. PLoS One. 2016;11:e0146828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gardner AW, Parker DE, Montgomery PS and Blevins SM. Diabetic women are poor responders to exercise rehabilitation in the treatment of claudication. J Vasc Surg. 2014;59:1036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gommans LN, Scheltinga MR, van Sambeek MR, Maas AH, Bendermacher BL and Teijink JA. Gender differences following supervised exercise therapy in patients with intermittent claudication. J Vasc Surg. 2015;62:681–8. [DOI] [PubMed] [Google Scholar]
- 195.Manfredini R, Lamberti N, Manfredini F, Straudi S, Fabbian F, Rodriguez Borrego MA, Basaglia N, Carmona Torres JM and Lopez Soto PJ. Gender Differences in Outcomes Following a Pain-Free, Home-Based Exercise Program for Claudication. J Womens Health (Larchmt). 2019;28:1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pierce GL, Eskurza I, Walker AE, Fay TN and Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond). 2011;120:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Parker BA, Kalasky MJ and Proctor DN. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. European Journal of Applied Physiology. 2010;110:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Treat-Jacobson D, McDermott MM, Bronas UG, Campia U, Collins TC, Criqui MH, Gardner AW, Hiatt WR, Regensteiner JG and Rich K. Optimal Exercise Programs for Patients With Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e10–e33. [DOI] [PubMed] [Google Scholar]
- 199.Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, Hiatt WR, Karas RH, Lovell MB, McDermott MM, Mendes DM, Nussmeier NA, Treat-Jacobson D, Disease AHACoPV, Nursing CoC, Intervention CoCRa, Anesthesia CoCSa, Cardiology CoC and Prevention CoEa. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2012;125:1449–72. [DOI] [PubMed] [Google Scholar]
- 200.Feinglass J, McDermott MM, Foroohar M and Pearce WH. Gender differences in interventional management of peripheral vascular disease: evidence from a blood flow laboratory population. Ann Vasc Surg. 1994;8:343–9. [DOI] [PubMed] [Google Scholar]
- 201.Amaranto DJ, Abbas F, Krantz S, Pearce WH, Wang E and Kibbe MR. An evaluation of gender and racial disparity in the decision to treat surgically arterial disease. J Vasc Surg. 2009;50:1340–7. [DOI] [PubMed] [Google Scholar]
- 202.Egorova N, Vouyouka AG, Quin J, Guillerme S, Moskowitz A, Marin M and Faries PL. Analysis of gender-related differences in lower extremity peripheral arterial disease. J Vasc Surg. 2010;51:372–8 e1; discussion 378–9. [DOI] [PubMed] [Google Scholar]
- 203.Rowe VL, Weaver FA, Lane JS and Etzioni DA. Racial and ethnic differences in patterns of treatment for acute peripheral arterial disease in the United States, 1998–2006. J Vasc Surg. 2010;51:21S–26S. [DOI] [PubMed] [Google Scholar]
- 204.Hultgren R, Olofsson P and Wahlberg E. Sex-related differences in outcome after vascular interventions for lower limb ischemia. J Vasc Surg. 2002;35:510–6. [DOI] [PubMed] [Google Scholar]
- 205.Ballard JL, Bergan JJ, Singh P, Yonemoto H and Killeen JD. Aortoiliac stent deployment versus surgical reconstruction: analysis of outcome and cost. J Vasc Surg. 1998;28:94–101; discussion 101–3. [DOI] [PubMed] [Google Scholar]
- 206.Green RM, Abbott WM, Matsumoto T, Wheeler JR, Miller N, Veith FJ, Money S and Garrett HE. Prosthetic above-knee femoropopliteal bypass grafting: five-year results of a randomized trial. J Vasc Surg. 2000;31:417–25. [PubMed] [Google Scholar]
- 207.Nguyen LL, Hevelone N, Rogers SO, Bandyk DF, Clowes AW, Moneta GL, Lipsitz S and Conte MS. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009;119:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Magnant JG, Cronenwett JL, Walsh DB, Schneider JR, Besso SR and Zwolak RM. Surgical treatment of infrainguinal arterial occlusive disease in women. J Vasc Surg. 1993;17:67–76; discussion 76–8. [PubMed] [Google Scholar]
- 209.Duffy RP, Adams JE, Callas PW, Schanzer A, Goodney PP, Ricci MA, Cronenwett JL, Bertges DJ and Vascular Study Group of New E. The influence of gender on functional outcomes of lower extremity bypass. J Vasc Surg. 2014;60:1282–1290 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Eugster T, Gurke L, Obeid T and Stierli P. Infrainguinal arterial reconstruction: female gender as risk factor for outcome. Eur J Vasc Endovasc Surg. 2002;24:245–8. [DOI] [PubMed] [Google Scholar]
- 211.Ballotta E, Gruppo M, Lorenzetti R, Piatto G, DaGiau G and Toniato A. The impact of gender on outcome after infrainguinal arterial reconstructions for peripheral occlusive disease. J Vasc Surg. 2012;56:343–52. [DOI] [PubMed] [Google Scholar]
- 212.Belkin M, Conte MS, Donaldson MC, Mannick JA and Whittemore AD. The impact of gender on the results of arterial bypass with in situ greater saphenous vein. Am J Surg. 1995;170:97–102. [DOI] [PubMed] [Google Scholar]
- 213.Enzler MA, Ruoss M, Seifert B and Berger M. The influence of gender on the outcome of arterial procedures in the lower extremity. Eur J Vasc Endovasc Surg. 1996;11:446–52. [DOI] [PubMed] [Google Scholar]
- 214.Ferranti KM, Osler TM, Duffy RP, Stanley AC, Bertges DJ and Vascular Study Group of New E. Association between gender and outcomes of lower extremity peripheral vascular interventions. J Vasc Surg. 2015;62:990–7. [DOI] [PubMed] [Google Scholar]
- 215.Hedayati N, Brunson A, Li CS, Baker AC, Pevec WC, White RH and Romano PS. Do Women Have Worse Amputation-Free Survival Than Men Following Endovascular Procedures for Peripheral Arterial Disease? An Evaluation of the California State-Wide Database. Vasc Endovascular Surg. 2015;49:166–74. [DOI] [PubMed] [Google Scholar]
- 216.Choi KH, Park TK, Kim J, Ko YG, Yu CW, Yoon CH, Lee JH, Min PK, Koh YS, Chae IH, Choi D, Choi SH and Investigators KV. Sex Differences in Outcomes Following Endovascular Treatment for Symptomatic Peripheral Artery Disease: An Analysis From the K- VIS ELLA Registry. J Am Heart Assoc. 2019;8:e010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Matsi PJ and Manninen HI. Complications of lower-limb percutaneous transluminal angioplasty: a prospective analysis of 410 procedures on 295 consecutive patients. Cardiovasc Intervent Radiol. 1998;21:361–6. [DOI] [PubMed] [Google Scholar]
- 218.Dearing DD, Patel KR, Compoginis JM, Kamel MA, Weaver FA and Katz SG. Primary stenting of the superficial femoral and popliteal artery. J Vasc Surg. 2009;50:542–7. [DOI] [PubMed] [Google Scholar]
- 219.Ihnat DM, Duong ST, Taylor ZC, Leon LR, Mills JL Sr., Goshima KR, Echeverri JA and Arslan B. Contemporary outcomes after superficial femoral artery angioplasty and stenting: the influence of TASC classification and runoff score. J Vasc Surg. 2008;47:967–74. [DOI] [PubMed] [Google Scholar]
- 220.Lenti M, Cieri E, De Rango P, Pozzilli P, Coscarella C, Bertoglio C, Troiani R and Cao P. Endovascular treatment of long lesions of the superficial femoral artery: results from a multicenter registry of a spiral, covered polytetrafluoroethylene stent. J Vasc Surg. 2007;45:32–9. [DOI] [PubMed] [Google Scholar]
- 221.Pulli R, Dorigo W, Pratesi G, Fargion A, Angiletta D and Pratesi C. Gender-related outcomes in the endovascular treatment of infrainguinal arterial obstructive disease. J Vasc Surg. 2012;55:105–12. [DOI] [PubMed] [Google Scholar]
- 222.Stavroulakis K, Donas KP, Torsello G, Osada N and Schonefeld E. Gender-related long-term outcome of primary femoropopliteal stent placement for peripheral artery disease. J Endovasc Ther. 2015;22:31–7. [DOI] [PubMed] [Google Scholar]
- 223.Gardner AW, Montgomery PS, Blevins SM and Parker DE. Gender and ethnic differences in arterial compliance in patients with intermittent claudication. J Vasc Surg. 2010;51:610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


