Abstract
Background
Hypertension is common in older adults and its incidence increases with age. We investigated the correlation between physical and cognitive impairment in older adults with frailty and hypertension.
Methods
We recruited frail hypertensive older adults during the COVID-19 pandemic, between March 2021 and December 2021. Global cognitive function was assessed through the Montreal Cognitive Assessment (MoCA), physical frailty assessment was performed following the Fried criteria, and all patients underwent physical evaluation through 5-meter gait speed test.
Results
We enrolled 203 frail hypertensive older adults and we found a significant correlation between MoCA score and gait speed test (r: 0.495; p<0.001) in our population. To evaluate the impact of comorbidities and other factors on our results, we applied a linear regression analysis with MoCA score as a dependent variable, observing a significant association with age, diabetes, chronic obstructive pulmonary disease (COPD), and gait speed test.
Conclusions
Our study revealed for the first time a significant correlation between physical and cognitive impairment in frail hypertensive elderly subjects.
Keywords: Frailty, Cognitive impairment, COVID-19, Hypertension, MoCA, Physical decline
Abbreviations: CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease; AFib, Atrial Fibrillation; MCI, Mild Cognitive Impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; VD, Vascular Dementia; AD, Alzheimer's Disease
1. Background
Frailty is a biological syndrome of decreased physiological reserves with incremented vulnerability to stressors and its prevalence among older adults has been estimated at ∼10% [1]. Hypertension is a prevailing comorbidity in older adults and its incidence increases with age [2,3]. Vascular dementia (VD) and Alzheimer's Disease (AD) are among the main causes of dementia and/or mild cognitive impairment (MCI) in older adults with or without frailty [4]. Despite these shreds of evidence and despite the emerging interest in geriatric conditions, the actual correlation between physical and cognitive impairment in physically frail patients remains unclear.
Cognitive impairment is very common in older populations, and its prevalence increases with age; in fact, dementia is a progressive and irreversible deterioration of cognitive function that is typical of older adults [5]. Similarly, physical impairment is often diagnosed in older adults [6]. Hence, the importance to correlate these geriatric conditions might be useful to set the best pharmacological, clinical, and interventional approach to reduce adverse outcomes and to improve the quality of life of these subjects.
Thus, in our study we investigated the correlation between physical and cognitive impairment in older adults with frailty and hypertension.
2. Methods
2.1. Patients and study design
We investigated the relationship between cognitive and physical impairment in a frail population of hypertensive older adults during the COVID-19 pandemic. We examined frail outpatient subjects (ambulatory care) at ASL Avellino, Italy, between March 2021 and December 2021 (NCT04962841). Inclusion Criteria were: age ≥65 years; frail status; a previous diagnosis of hypertension; Montreal Cognitive Assessment (MoCA) Score <26. Exclusion Criteria were: age <65 years; absence of frail status; absence of hypertension; left ventricular ejection fraction <25%; previous myocardial infarction or previous revascularization with primary percutaneous coronary intervention (PPCI) and/or coronary by-pass grafting.
2.2. Frailty assessment
Physical frailty assessment was performed following the Fried criteria [7,8]; a diagnosis of frailty status was made with at least three of the following five criteria:
-
-
Weight loss (unintentional loss ≥4.5 kg in the past year);
-
-
Weakness (handgrip strength in the lowest 20% quintile at baseline, adjusted for sex and body mass index);
-
-
Exhaustion (poor endurance and energy, self-reported);
-
-
Slowness (walking speed under the lowest quintile adjusted for sex and height);
-
-
Low physical activity level (lowest quintile of kilocalories of physical activity during the past week).
All patients underwent physical evaluation through the 5-meter gait speed test [8]. Gait speed is the most common test to measure the time required to walk a short distance at a comfortable pace. Furthermore, it is one of the most used tests to screen frailty and identify high-risk older adults in need of further evaluation. The gait speed test can quantify impairments in lower-extremity muscle function, neurosensory, and cardiopulmonary function [9], [10], [11], [12]. Furthermore, a recent paper demonstrated that the brain networks are related with gait control and vary with walking speed; hence, gait control in aging requires a distributed network including regions for emotional control that are recruited in challenging walking conditions [13].
2.3. Cognitive evaluation
Global cognitive function was assessed through the MoCA test [8,14], which is freely available and can be used, reproduced, and distributed by Universities, Foundations, Health Professionals, Hospitals, and Public Health Institutes. We preferred the MoCA test to the Mini-Mental State Examination (MMSE) because the first one has been proved to be more specific to evaluate cognitive domains (attention, concentration, memory, language, calculation, orientation, and executive functions) and is widely considered the gold standard test to detect mild cognitive impairment (MCI) [15,16]. Additionally, MMSE scores have been shown to be influenced by demographic variables such as age and years of education: subjects with higher education levels have better results than subjects with lower levels, and older adults show worst performances in MMSE scores that are age-dependent [17]. Cognitive impairment was defined by MoCA Score <26, as previously reported [15,18].
2.4. Ethical considerations
The study was carried out following the tenets of the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by the Bioethics Committee of Campania Nord. All patients, or their legal representatives, provided informed consent and were informed that they could withdraw from the study at any stage.
2.5. Statistical analysis
The sample size was calculated using G*POWER software, assuming a power of 80% and a two-sided alpha level of 0.05, we found that 140 patients were needed to evaluate the primary endpoint of the study. Descriptive statistics, including frequencies and percentages, were used to describe the categorical variables analyses; continuous variables were expressed as mean ± standard deviation (SD). Multiple regression models have been used to explore the relationship between MoCA Score and several covariates. All calculations were computed using SPSS 26. A two-sided p value <0.05 was considered statistically significant.
3. Results
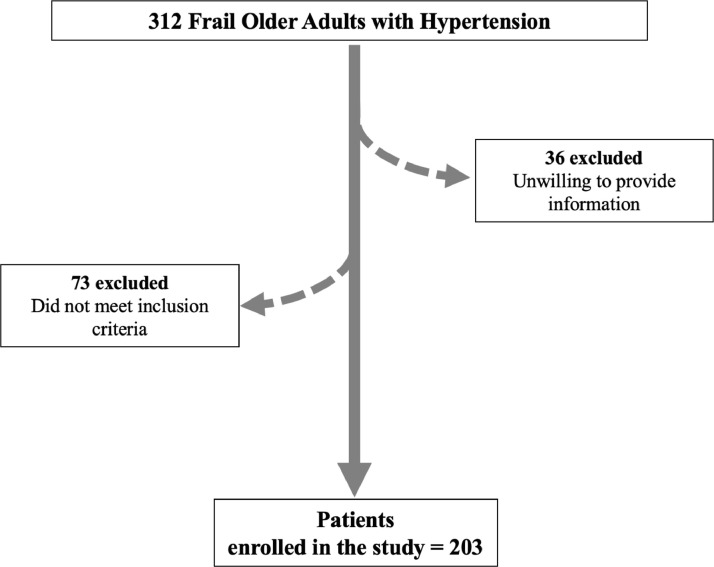
312 consecutive patients were evaluated during the COVID-19 pandemic, of which 73 presented no frailty status and 36 were unwilling to provide clinical information (Figure 1 ). Therefore, 203 frail older adults were successfully enrolled. All frail patients were administered a MoCA test and performed a 5-meter gait speed test at the beginning of the study. The mean age, sex distribution, BMI, and comorbidities are reported in Table 1 . The use of diuretics, angiotensin-converting enzyme inhibitors, beta-blockers, and calcium channel blockers is reported in Table 1, as well.
Fig. 1.
Study flow chart.
Table 1.
Clinical characteristics of our population.
| Parameter | Values |
|---|---|
| N | 203 |
| Sex (F) | 118 (58.5) |
| Age (years) | 81.1±7.1 |
| BMI (kg/m2) | 28.8±1.5 |
| SBP (mmHg) | 129.5±11.1 |
| DBP (mmHg) | 79.3±6.5 |
| Heart rate (bpm) | 81.2±7.4 |
| Comorbidities | |
| Diabetes, n (%) | 119 (59.0) |
| Hyperlipidemia, n (%) | 143 (70.5) |
| COPD, n (%) | 66 (32.5) |
| CKD, n (%) | 83 (41.0) |
| Osteoarthritis, n (%) | 81 (40.0) |
| AFib, n (%) | 47 (23.2) |
| Laboratory Analyses | |
| Plasma glucose (mg/dl) | 143.4±18.8 |
| Creatinine (mg/dl) | 1.0±0.2 |
| Global Cognitive Evaluation | |
| MoCA | 18.7±4.2 |
| Fried Criteria | |
| Weight Loss, n (%) | 84 (41.5) |
| Exhaustion, n (%) | 143 (70.5) |
| Low Physical Activity, n (%) | 67 (33.0) |
| Slowness, n (%) | 171 (84.0) |
| Weakness, n (%) | 134 (66.0) |
| Active Treatments | |
| βAR-blockers, n (%) | 144 (71.0) |
| ACE-inhibitors, n (%) | 160 (79.0) |
| Angiotensin Receptor blockers, n (%) | 39 (19.2) |
| Calcium Channel blockers, n (%) | 25 (12.3) |
| Diuretics, n (%) | 42 (20.7) |
Data are expressed as mean ± SD or raw number and percentages. AFib: atrial fibrillation; AR: adrenergic receptors; BMI: body mass index; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DBP: diastolic blood pressure; SBP: systolic blood pressure.
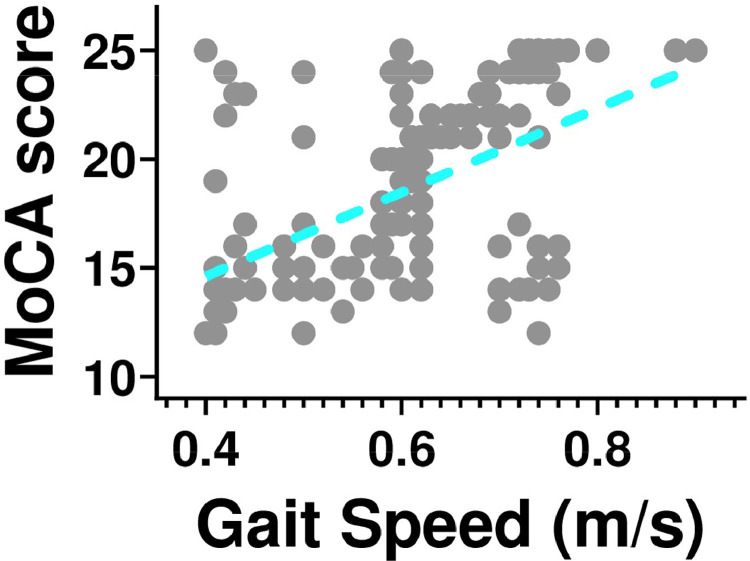
We found a significant correlation between MoCA score and gait speed test (r: 0.495; p<0.001, Figure 2 ).
Fig. 2.
Correlation between physical decline, assessed through 5-meter gait speed, and Montreal Cognitive Assessment (MoCA) score in our population of hypertensive frail elderly patients (r: 0.495; p<0.001).
To evaluate the influence of comorbidities and other factors on our results, we performed a multivariate linear regression analysis with MoCA score as a dependent variable (Table 2 ). We observed a significant impact of age, diabetes, COPD, and 5-meter gait speed test (p<0.001).
Table 2.
Multivariate linear regression analysis in all patients using MoCA score as a dependent variable.
| P | 95.0% CI for B | |||||
|---|---|---|---|---|---|---|
| B | Standard Error | Lower Bound | Upper Bound | |||
| Age | -0.185 | 0.040 | <0.001 | -0.264 | -0.106 | |
| BMI | -0.072 | 0.138 | 0.603 | -0.343 | 0.200 | |
| SBP | 0.023 | 0.029 | 0.428 | -0.035 | 0.081 | |
| DBP | -0.019 | 0.036 | 0.596 | -0.090 | 0.052 | |
| HR | 0.028 | 0.027 | 0.300 | -0.025 | 0.080 | |
| Diabetes | -2.789 | 0.495 | <0.001 | -3.765 | -1.813 | |
| Hyperlipidemia | -0.408 | 0.480 | 0.397 | -1.354 | 0.539 | |
| COPD | -1.750 | 0.488 | <0.001 | -2.712 | -0.788 | |
| CKD | -0.399 | 0.571 | 0.485 | -1.525 | 0.726 | |
| Osteoarthritis | 1.123 | 0.574 | 0.052 | -0.008 | 2.255 | |
| AFib | -0.581 | 0.545 | 0.288 | -1.655 | 0.494 | |
| Gait speed test | 16.899 | 2.161 | <0.001 | 12.635 | 21.163 | |
AFib: atrial fibrillation; AR: adrenergic receptors; BMI: body mass index; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure.
4. Discussion
The management of frail patients is a health problem of increasing importance worldwide. A recent study determined that markers of physical performance are linked to the current cognitive status and modestly related to cognitive decline [19]. Nevertheless, the authors of that study did not report data on hypertension in the baseline characteristics of their population. Comorbidities such as hypertension play a key role in increasing the risk of mortality, hospitalization, and disability [20]; hence, the main goal to reduce frailty rates and cognitive decline should focus on the care of underlying chronic diseases that are prevalent in elders [21,22]. Indeed, high blood pressure affects ~75% of people aged over 70 and frailty adds further complexity to the management of blood pressure modifications in later life [23]. In this scenario, our study evidenced a significant correlation between physical and cognitive impairment in frail hypertensive patients. This result is especially interesting because physical capacity and global cognitive function might influence each other in this population; thus, clinical assessment should be implemented. Interestingly, the majority of our patients were women, which is in agreement with the reports of the REPOSI Study on elderly people [24].
Notably, since our data were collected during the COVID-19 pandemic, we need to reckon that this aspect could have influenced cognitive and physical function in frailty, as recently shown by us and others [25], [26], [27], [28], [29], [30].
To further confirm our results, we applied a multivariate linear regression analysis to verify the impact of some covariates, using MoCA as a dependent variable. This analysis revealed a significant impact of diabetes, age, COPD, and 5-meter gait speed test (Table 2).
Several limitations warrant consideration: first, the sample size is relatively small, however, we had performed an a priori power analysis and the estimated sample size was 140 patients; second, the results would have benefited from a follow-up. Dedicated studies investigating the mechanisms underlying these effects are necessary, and could involve for instance endothelial dysfunction, inflammation, and oxidative stress; however, these experiments are beyond the scope of this study.
Conclusions
Taken together, our results indicate that there is a significant correlation between physical and cognitive impairment in frail hypertensive elderly subjects.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Bioethics Committee of Campania Nord (#001443). All patients or legal representatives provided written informed consent and were informed that they could withdraw from the study at any stage. The study was carried out following the tenets of the Declaration of Helsinki and Good Clinical Practice guidelines.
Consent for publication
Not applicable.
Availability of data and materials
Data are available from the First author upon reasonable request.
Funding
The Santulli’s Laboratory is supported in part by the National Institutes of Health (NIH: R01-HL159062, R01-DK123259, R01-HL146691, R01-DK033823, and T32-HL144456 to G.S.), by the Irma T. Hirschl and Monique Weill-Caulier Trusts (to G.S.), and by the Diabetes Action Research and Education Foundation (to G.S.).
Authors' contributions
Dr. Mone had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy for the data analysis. Study concept and design: PM, AP, GS. Acquisition, analysis, and interpretation of data: PM, AP, AdD, VB, AM, SF, and PDB. Drafting of the manuscript: PM, AP, GS. Critical revision of the manuscript for important intellectual content: AP, MM, ADL, GS. Statistical analysis: PM, AdD, and GS. Administrative, technical, or material support: PM, AP, MM, ADL, GS. Study supervision: PM, GS.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank the study participants.
References
- 1.Dent E, Kowal P, EO Hoogendijk. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Buford TW. Hypertension and aging. Ageing Res Rev. 2016;26:96–111. doi: 10.1016/j.arr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anker D, Santos-Eggimann B, Santschi V, Del Giovane C, Wolfson C, Streit S, et al. Screening and treatment of hypertension in older adults: less is more? Public Health Rev. 2018;39:26. doi: 10.1186/s40985-018-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer's disease: a cross-sectional analysis of data from the rush memory and aging project. Lancet Neurol. 2019;18(2):177–184. doi: 10.1016/S1474-4422(18)30371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, RB Wallace. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Mone P, Lombardi A, Gambardella J, Pansini A, Macina G, Morgante M, et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022 doi: 10.2337/dc21-2434. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(10):1083–1089. doi: 10.1093/gerona/glr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mone P, Gambardella J, Pansini A, Martinelli G, Minicucci F, Mauro C, et al. Cognitive dysfunction correlates with physical impairment in frail patients with acute myocardial infarction. Aging Clin Exp Res. 2021 doi: 10.1007/s40520-021-01897-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol Ser A, Biol Sci Med Sci. 2013;68(1):39–46. doi: 10.1093/gerona/gls174. [DOI] [PubMed] [Google Scholar]
- 12.Mone P, Pansini A. Gait speed test and cognitive decline in frail women with acute myocardial infarction. Am J Med Sci. 2020;360(5):484–488. doi: 10.1016/j.amjms.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Allali G, Montembeault M, Brambati SM, Bherer L, Blumen HM, Launay CP, et al. Brain structure covariance associated with gait control in aging. J Gerontol A Biol Sci Med Sci. 2019;74(5):705–713. doi: 10.1093/gerona/gly123. [DOI] [PubMed] [Google Scholar]
- 14.Mone P, Gambardella J, Pansini A, de Donato A, Martinelli G, Boccalone E, et al. Cognitive impairment in frail hypertensive elderly patients: role of hyperglycemia. Cells. 2021;10(8) doi: 10.3390/cells10082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto TCC, Machado L, Bulgacov TM, Rodrigues-Junior AL, Costa MLG, Ximenes RCC, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's Disease (AD) in the elderly? Int Psychogeriatr. 2019;31(4):491–504. doi: 10.1017/S1041610218001370. [DOI] [PubMed] [Google Scholar]
- 16.Dong Y, Sharma VK, Chan BP, Venketasubramanian N, Teoh HL, Seet RC, et al. The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci. 2010;299(1-2):15. doi: 10.1016/j.jns.2010.08.051. -8. [DOI] [PubMed] [Google Scholar]
- 17.Limongi F, Noale M, Bianchetti A, Ferrara N, Padovani A, Scarpini E, et al. The instruments used by the Italian centres for cognitive disorders and dementia to diagnose mild cognitive impairment (MCI) Aging Clin Exp Res. 2019;31(1):101–107. doi: 10.1007/s40520-018-1032-8. [DOI] [PubMed] [Google Scholar]
- 18.Pansini A, Lombardi A, Morgante M, Frullone S, Marro A, Rizzo M, et al. Hyperglycemia and physical impairment in frail hypertensive older adults. Front Endoc. 2022;13:831556. doi: 10.3389/fendo.2022.831556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooghiemstra AM, Ramakers I, Sistermans N, Pijnenburg YAL, Aalten P, Hamel REG, et al. Gait speed and grip strength reflect cognitive impairment and are modestly related to incident cognitive decline in memory clinic patients with subjective cognitive decline and mild cognitive impairment: findings from the 4C study. J Gerontol A Biol Sci Med Sci. 2017;72(6):846–854. doi: 10.1093/gerona/glx003. [DOI] [PubMed] [Google Scholar]
- 20.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 21.Howrey BT, Al Snih S, Middleton JA, Ottenbacher KJ. Trajectories of frailty and cognitive decline among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2020;75(8):1551–1557. doi: 10.1093/gerona/glz295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vetrano DL, Foebel AD, Marengoni A, Brandi V, Collamati A, Heckman GA, et al. Chronic diseases and geriatric syndromes: the different weight of comorbidity. Eur J Intern Med. 2016;27:62–67. doi: 10.1016/j.ejim.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Masoli JAH, Delgado J. Blood pressure, frailty and dementia. Exp Gerontol. 2021;155 doi: 10.1016/j.exger.2021.111557. [DOI] [PubMed] [Google Scholar]
- 24.Corrao S, Santalucia P, Argano C, Djade CD, Barone E, Tettamanti M, et al. Gender-differences in disease distribution and outcome in hospitalized elderly: data from the REPOSI study. Eur J Intern Med. 2014;25(7):617–623. doi: 10.1016/j.ejim.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Mone P, Gambardella J, Wang X, Jankauskas SS, Matarese A, Santulli G. miR-24 targets the transmembrane glycoprotein neuropilin-1 in human brain microvascular endothelial cells. Noncoding RNA. 2021;7(1) doi: 10.3390/ncrna7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gambardella J, Coppola A, Izzo R, Fiorentino G, Trimarco B, Santulli G. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit Care. 2021;25(1):306. doi: 10.1186/s13054-021-03731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visser M, Schaap LA, HAH Wijnhoven. Self-reported impact of the COVID-19 pandemic on nutrition and physical activity behavior in Dutch older adults living independently. Nutrients. 2020;12(12) doi: 10.3390/nu12123708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinohara T, Saida K, Tanaka S, Murayama A. Do lifestyle measures to counter COVID-19 affect frailty rates in elderly community dwelling? Protocol for cross-sectional and cohort study. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozupone M, La Montagna M, Di Gioia I, Sardone R, Resta E, Daniele A, et al. Social frailty in the COVID-19 pandemic era. Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.577113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woolford SJ, D'Angelo S, Curtis EM, Parsons CM, Ward KA, Dennison EM, et al. COVID-19 and associations with frailty and multimorbidity: a prospective analysis of UK Biobank participants. Aging Clin Exp Res. 2020;32(9):1897–1905. doi: 10.1007/s40520-020-01653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the First author upon reasonable request.