Abstract
Intestinal fibrosis is a late-stage phenotype of inflammatory bowel disease (IBD), which underlies most of the long-term complications and surgical interventions in patients, particularly those with Crohn’s disease. Despite these issues, antifibrotic therapies are still scarce, mainly due to the current lack of understanding concerning the pathogenetic mechanisms that mediate fibrogenesis in patients with chronic intestinal inflammation. In the current review, we summarize recent evidence regarding the cellular and molecular factors of innate and adaptive immunity that are considered critical for the initiation and amplification of extracellular matrix deposition and stricture formation. We focus on the role of cytokines by dissecting the pro- vs antifibrotic components of the immune response, while taking into consideration their temporal association to the progressive stages of the natural history of IBD. We critically present evidence from animal models of intestinal fibrosis and analyze inflammation-fibrosis interactions that occur under such experimental scenarios. In addition, we comment on recent findings from large-scale, single-cell profiling of fibrosis-relevant populations in IBD patients. Based on such evidence, we propose future potential targets for antifibrotic therapies to treat patients with IBD.
Keywords: Crohn’s disease, cytokines, fibrosis, immunoregulation, myofibroblasts, ulcerative colitis
Introduction
Fibrosis represents an unfavorable outcome of the fundamental biologic process of tissue response to injury and wound repair. Typically, when a harmful event takes place, the inflicted damage initiates a prompt and vigorous, but eventually, self-limiting response that is executed through the reversible activation of repair pathways.1 In this scenario, any destruction induced by the offending factor(s) is quickly repaired, followed by complete restoration of damaged tissues and restitution of structural integrity and function. Fibrosis corresponds to the opposite end of the repair spectrum, which occurs when such damage-initiated biological sequelae escapes homeostatic control and becomes dysregulated. Loss of regulatory mechanisms manifests as a relentless and unproductive repair effort, with perpetual activation of profibrotic responses and sustained accumulation of extracellular matrix. Eventually, deposition of extensive connective tissue may replace normal local structures, leading to loss of normal organ architecture and function.
Fibrosis is a common outcome in the natural history of inflammatory bowel disease (IBD) and underlies most of its long-term complications, particularly in patients with Crohn’s disease (CD), of whom almost 30% suffer from fibrosis-related morbidity.2 To a much lesser extent, some degree of fibrosis is also present in patients with ulcerative colitis (UC).3 Fibrotic complications almost universally develop at intestinal segments that are affected by inflammation, thus supporting an inflammation-fibrosis sequelae. Nonetheless, a currently unknown and unmet need in IBD patient care is the ability to predict, among CD patients, who will eventually develop strictures. More importantly, despite the recent advances in anti-inflammatory therapies that are available for IBD patients, a clear reduction in the rates of fibrosis-related complications and eventual surgeries has not been demonstrated. Finally, because several patients will present at diagnosis with an already established intestinal scar, there is the demand not only for early prevention of fibrosis but also its successful reversal after the fibrotic lesions become fully established. To address these issues, the pathogenetic mechanisms leading to intestinal fibrosis need to be better characterized and the responsible cellular and molecular participants clearly defined, as eventually these mechanisms will provide the foundation for targeted and more effective therapeutic approaches.4 In the following sections, we will focus on the role of immunological factors, primarily of cytokines, in the pathogenesis of tissue fibrosis in patients with IBD, mainly those suffering from CD.
Evolution of the Fibrotic Process
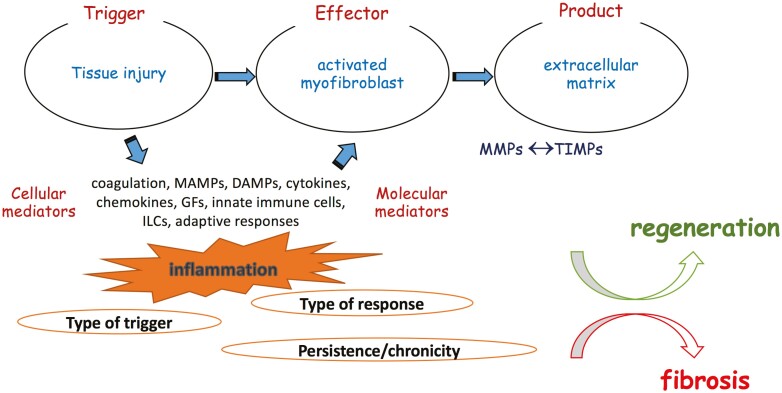
Tissue repair or fibrosis entails 3 fundamental elements: an offensive factor that initiates the pathway (the trigger), the effector cell of the host (the “activated” myofibroblast), and the end product that is deposited in the extracellular space (ie, extracellular matrix [ECM]).5 A fourth essential participant is the intricate network of cellular and molecular inflammatory mediators that are locally recruited and/or amplified in response to tissue damage and that tightly regulate the fibrotic process (Figure 1).
Figure 1.
Immunopathogenesis of intestinal response to injury. The intestinal mucosa is continuously exposed to a variety of potential harmful factors both of external (eg, infections, drugs, environmental toxins) and internal (eg, genetic, inflammatory, autoimmune) origin. The initial injury inflicted by such triggers create a breach in mucosal integrity, which allows entry of bacterial elements in deeper intestinal layers, releases various products of damaged host cells, and activates the coagulation pathway. The end product is a strong local and systemic inflammatory response that is executed via a rich network of cellular and molecular mediators, including cytokines, chemokines, and various constituents of the innate and adaptive arms of immunity. This original inflammatory response induces the enrichment of the local environment with the central cellular effector of fibrogenesis, the activated myofibroblast. Although the origin of myofibroblasts in intestinal fibrosis is still unrevealed, there are several potential sources, such as local fibroblasts and pericytes, circulating fibrocytes, as well as input by epithelial-to-mesenchymal and endothelial-to mesenchymal transition. Once activated, myofibroblasts become potent producers of ECM, which consists of collagens, fibronectin, and several other substances that occupy the intercellular space. This is a tightly regulated dynamic process encompassing a delicate balance between ECM degrading matrix metalloproteinases and their inhibitors. The final outcome is dictated by the type of the initial trigger and the ensuing response and, most importantly, by the transient or persistent nature of myofibroblast-activating stimuli. During homeostatic conditions, repair mechanisms lead to complete tissue regeneration and reestablish the structural integrity of the intestinal wall. In contrast, the chronic persistent inflammation that takes place in IBD constantly fuels profibrotic mechanisms, leading to tissue scarring and resulting in anatomical and functional compromise of the affected areas of the GI tract. Abbreviations: DAMP, damage-associated molecular patterns; ECM, extracellular matrix; IBD, inflammatory bowel disease; MMPs, matrix metalloproteinases; PAMP, pathogen-associated molecular pattern; TIMPs, tissue inhibitors of metalloproteinases.
A plethora of underlying external and/or inherent triggering factors can inflict damage, including infectious agents, toxins, medications, smoke, ischemic disease, and metabolic status. Tissue injury results in the local abundance of exogenous pathogen-associated molecular pattern (PAMP) or endogenous damage-associated molecular patterns (DAMP) ligands that engage their respective pattern recognition receptors (PRRs). The latter are expressed on a wide range of cell types, including both immune and structural cells, which are capable of responding to the former signals. Pattern recognition receptors also allow local cells of the host to cross-talk with constituents of the intestinal microbiota, which has definitively been shown to affect fibrotic pathways by delivering homeostatic signals—or in the event of “dysbiosis,” profibrotic signals. In line with this concept, a recent study reported that mice with selective deletion of the universal adapter protein myeloid differentiation primary response 88 (Myd88) in α-smooth muscle actin (SMA) positive cells were protected from inflammation-induced fibrosis after oral administration of dextran sodium sulfate (DSS).6 Furthermore, a recent in silico analysis showed distinct microbiome signatures in complicated CD that significantly differed from those with pure inflammatory phenotypes, again highlighting the impact of commensal bacteria in the development of intestinal fibrosis.7
The second essential event during tissue repair is the local appearance of activated myofibroblasts. This cell type represents the pivotal effector unit that is appropriately programmed to secrete components of the extracellular material in an effort to heal the wound produced by the offensive agent.8 Myofibroblasts are generally spindle-shaped and bear an intermediate phenotype between fibroblasts and smooth muscle cells.9 The origin of myofibroblasts that are responsible for intestinal fibrosis still remains a matter of debate, as it appears that several local but also systemic sources may supply their pool. Intestinal stromal (including subepithelial) fibroblasts and pericytes that surround the endothelium are local residents that may become myofibroblasts,10 whereas circulating, bone marrow–derived fibrocytes can also be attracted to the inflamed area and give rise to ECM-producers.11 Finally, nonmesenchymal cells may also contribute to fibrosis via epithelial-to-mesenchymal transition (EMT) or endothelial-to-mesenchymal transition (Endo-MT).12
In the final step, extracellular matrix accumulates within the intercellular space in order to replace the damaged and/or missing tissues and restore continuity of the affected organ(s). The extracellular matrix contains both structural (collagen and elastin) and functional (eg, fibronectin, proteoglycans) proteins and has been recently recognized as a dynamic and active participant in maintaining tissue homeostasis—and not solely a mere scaffold for resident cells.13 The balanced quantitative and qualitative composition of ECM is regulated by the opposing functions of tissue matrix metalloproteinases (MMPs) that constantly degrade collagen and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs).14 Furthermore, ECM can interact with its surrounding cells and act as a reservoir for signaling molecules. It should be noted that the composition of ECM can, by itself, serve as an indicator of either a homeostatic or a fibrotic response to tissue injury. In particular, certain subtypes of collagen (COL 1-3) have been associated with the normal repair process, whereas others (COL 4, 6) may signify a pathologic condition, leading to overt fibrosis.15 This is further supported by studies showing redistribution of collagen subtypes in CD towards overexpression of collagens 4-6, which substantially differs from the healthy bowel, which mainly expresses collagens 1 and 3.16
Independent of the causal factor, following the primary tissue damage, an acute inflammatory process is uniformly initiated with coordinated on-site delivery of blood components, both soluble factors and leukocytes. The purpose of such acute inflammation is the elimination of the offensive trigger(s) and orchestration of effective processes to restore local homeostasis. Typically, this is referred to as tissue regeneration and is considered a beneficial type of inflammation that is largely mediated by extravasated neutrophils and tissue macrophages and also involves lipid mediators.4 However, pathologic fibrosis, occasionally denoted as fibroplasia, takes place when the aforementioned repair process becomes dysregulated. This may affect one or more steps of the pathway: the triggering factor may not be removed or the effector cells may be over-reactive due to genetic preconditioning or epigenetic modifications; alternatively, ECM synthesis may overcome degradation. The net result of such pathologic events is the establishment and maintenance of chronic inflammatory activity within the injured tissue, which is dominated by the presence of lymphocytes and their products. Depending on the particular immunophenotype, this state of continuous inflammatory input may eventually lead to perpetual activation of fibrotic pathways, resulting in tissue scarring and permanent damage. At present, almost all clinical and experimental models of intestinal fibrosis are associated with a preceding stage of chronic inflammation. This overlap poses an overwhelming barrier to accurately dissect immunological pathways that mediate inflammation or fibrosis, independent of each other. This obstacle is clearly evident in Crohn’s disease patients with obstructive manifestations, as the latter typically cannot be solely attributed to tissue scarring or ongoing inflammation with wall thickening and luminal narrowing.17 It is obvious that these pathophysiological uncertainties reflect strongly upon therapeutic decisions, as solving the dilemma of pharmacological vs surgical intervention may prove to be a very daunting task in such cases. This predicament becomes more apparent when one considers chronic infection with hepatitis B or C viruses, which offer a typical example of a well-identified triggering factor that leads to unrelenting chronic inflammation and liver fibrosis/cirrhosis if the viruses are not eliminated.18,19 Interestingly, hepatitis B virus (HBV)-related liver fibrosis is almost an exception in the fact that it may be reversible after successful eradication of the virus via treatment with nucleoside analogues. Conceptually, this emphasizes that removing the trigger may be a more effective means of disrupting the progression to fibrosis than targeting chronic inflammation.
Experimental Models for the Study sf Intestinal Fibrosis
In Vitro Systems
Myofibroblast culture.
In vitro models of intestinal fibrosis have been developed mainly for the study of myofibroblasts, the principal effector cell during fibrogenesis. Myofibroblasts can be isolated from surgical or endoscopic specimens and are unique among all other intestinal cell types, inasmuch they can be sustained in primary cell cultures without the need of supportive specialized media, hormones, or other trophic, differentiating, or polarizing factors. This minimizes the risk for phenotypic alterations; hence, their in vitro properties reliably reproduce in vivo function. Cultured myofibroblasts maintain their αSMA+/Vimentin+/Desmin weakly+ phenotype and can divide more than 10 times, possibly limited by the shortening of chromosome telomeres. Myofibroblasts in culture attach to extracellular matrix proteins such as collagen, polymers mimicking the tertiary structure of collagen such as poly-L- or D-lysine, or positively charged rough surfaces such as cell culture plastics. Such in vitro cultures have proved to be significant tools to study the responses of myofibroblasts to various stimuli. The addition of factors secreted by other cells can also provide indirect information regarding the involvement of myofibroblasts in cell-cell interactions. As a typical example, conditioned medium from cultures of epithelial cells that were prestimulated with pro-inflammatory cytokines enhance subepithelial myofibroblast (SEMF) migration and production of collagen, MMPs, and inflammatory mediators, supporting the role of a cross-talk between SEMFs and overlying epithelial cells in fibrogenesis during intestinal inflammation.20,21 Differences between myofibroblasts derived from healthy or stenotic tissue from IBD patients can also be studied. Sabatino et al studied primary myofibroblasts from mucosa overlying strictured or nonstrictured areas from individual patients with CD and found higher expression of various profibrotic elements in the former.22 In other studies, myofibroblasts from patients with CD showed higher basal collagen production and pro-inflammatory cytokine expression.20,21
The effects of mechanical factors on myofibroblast properties can also be traced in culture by adjusting the stiffness of the supporting matrix.23 The pathophysiological concept is that accumulation of ECM components increases tissue stiffness, which further activates ECM-producing myofibroblasts, leading to perpetuation of fibrogenic mechanisms and culminating to scar formation. By increasing the matrix stiffness in myofibroblast cultures, the microenvironment of stricturing CD can be recapitulated and studied in vitro. Using this approach, Johnson et al showed that when grown on stiff matrices, human colonic fibroblast Ccd-18Co cells increased their proliferation rate and acquired an accelerated profibrotic phenotype with upregulated expression of αSMA, fibrogenic and proinflammatory genes, and decreased MMP production.24 Furthermore, de Bryn et al recently showed that normal myofibroblasts exert a compensatory activation of MMPs when embedded in a high pressure substrate, a mechanism that was reversed in myofibroblasts from intestinal areas with CD-associated strictures.25
The functional characteristics of myofibroblasts in monolayer cultures and their modifications via various stimuli can also be demonstrated via the wound healing and migration assay.26 This technique employs the creation of a physical gap within the myofibroblast monolayer and the sequential observation of the gap closure through the cell migration, which gives a readout of myofibroblast’s fibrotic properties. By applying different stimulatory conditions, the effects of exogenous factors in this process can also be studied.
Human intestinal organoids
The main drawback of primary cultures is that myofibroblasts are expanded in monolayers and, thus, lack neighbor cells and cell-cell interactions that usually take place in vivo and regulate the total functionality of intestinal tissue. This gap is partially filled by the use of intestinal organoids. Organoids are 3D structures that are cultured in vitro and composed of functional, live cells that can self-renew and spatially organize, thus, recapitulating the assembly of the corresponding tissue. Such structures can either be reconstituted from human tissue cultures or induced from embryonal or pluripotent stem cells by use of specific culture conditions that prompt the differentiation into specific cell types.27 Therefore, intestinal (ie, from small intestine) or colonic (ie, from large intestine) organoids contain both epithelium and mesenchyme.28 The translational relevance of organoids can be further enhanced by the use of scaffold systems that reproduce the in vivo condition even more closely.29 Recently developed organoid-on-a-chip platforms multiply the research potential by allowing the study of more complex features of the human intestine, including nutrient support, signaling gradients, and host-bacterial interaction.30 Taken together, these developing techniques facilitate the study of intestinal myofibroblasts within the perspective of their natural microenvironment and constitute a great advance relative to monolayer cultures. For example, Giuffrida et al have introduced a 3D decellularized human intestinal scaffold system that was obtained from surgical specimens.31 The scaffold that contained a preserved composition of ECM proteins was subsequently engineered with primary human intestinal myofibroblasts. The authors showed that engrafted myofibroblasts demonstrated diverge genotype and dissimilar responses to profibrotic factors in comparison with myofibroblasts in standard 2D cultures on plastic dishes.
Animal models of fibrosis
A major obstacle for understanding the pathogenetic mechanisms of intestinal fibrosis in IBD is that its early stages cannot be tracked and patients usually present when a fully established, purely fibrotic stenosis is present. This also precludes early intervention and prevention of the process in its earliest stages. Animal models of intestinal fibrosis attempt to bridge this gap because they allow scientists to dissect the various mechanisms that link initial tissue injury to permanent fibrous formation. A detailed presentation of these models is beyond the scope of this review and can be found elsewhere32; only a brief overview will be included herein (Table 1). It should be noted that, with rare exceptions, all established murine models that display a fibrosis phenotype were primarily developed as a means to study intestinal inflammation, with fibrosis being a secondary outcome.
Table 1.
Commonly used animal models of intestinal fibrosis.
| Designation | Descriptor | Technical Characteristics | Strengths and Drawbacks |
|---|---|---|---|
| DSS | Chemical | Repeated intermittent administration of DSS in the drinking water leads to inflammation and fibrosis
Colonic localization |
Simple and easily reproducible
Model of epithelial injury and repair but not chronic inflammation Questionable relevance to IBD |
| TNBS | Chemical | Repeated intrarectal administration of TNBS/ethanol
Colitis induction and severity may be unpredictable, depending on chemical batch and the animal strain Colonic localization |
T-cell dependent mucosal injury at the colon
Early/Th1 vs late/Th2-Th17 immunophenotype, which is compatible with the current paradigm for inflammation-induced fibrosis Pathological lesions include transmural inflammation Chemical injury/hapten utilization unrelated to IBD pathogenesis |
| PG-PS | Microbial | Subserosal injection of bacterial-derived PG-PS polymers in rats
Cecal or small intestinal localization |
Chronic granulomatous inflammation with significant fibrosis
technically demanding artificial model |
| Fecal injection | Microbial | Intramural injection of filtered fecal suspension in rats
Colonic localization |
Focal colitis
Transmural fibrosis with stricture formation Bacterial invasion may be relevant in IBD technically demanding |
| Salmonella Infection | Microbial | Pre-treatment with antibiotics, followed by ingestion of Salmonella Colonic localization |
Infection with Salmonella in humans leads neither to chronic inflammation nor fibrosis |
| tgf-b1-Tg | Genetic | Overexpression of TGFβ via rectal instillation of an adenoviral vector, following ethanol-induced disruption of epithelial lining
Colonic localization |
Focal distribution of fibrosis
Stricture formation TGFβ possibly relevant in IBD-associated fibrosis |
| tl1a-Tg | Genetic | Myeloid- or lymphoid-specific overexpression of TL1A
Colonic and small intestinal localization |
TL1A/DR3 is an important mucosal cytokine system
Neutralization of TL1A exerts antifibrotic effect Fibrosis is affected by signals from the microbiota Mild inflammatory and fibrotic changes |
| mcp-1 | Genetic | Intramural injection of an adenoviral vector carrying MCP-1
Colonic localization |
TGFβ1 and collagen deposition
Increase in collagen type 3:I ratio (similar to CD) Fibrosis is absent in RAG–/– mice Only MCP-1-dependent fibrogenesis is examined |
| SAMP1/Yit | Spontaneous | No chemical, immunological or genetic manipulation
Small intestinal (terminal ileum) localization |
Unbiased, spontaneous nature
Pathology closely mimics Crohn’s disease Inflammation to fibrosis evolution traceable Precise immunopathogenetic mechanisms still unknown |
The chemically induced models are mainly represented by the DSS and trinitrobenzene sulfonic acid (TNBS) models of colitis.33,34 In both models, the triggering factor is known, and this facilitates the study of the initial events that are implicated in postinjury repair, which is typically mediated by innate immunity. In the classical protocols, however, full recovery of the intestine takes place; hence, the development of fibrosis requires modification of the protocols. In particular, short-term treatment with a high concentration of DSS generally causes acute inflammation, but administering either a longer course of low-dose DSS or cyclical re-administration of relatively high doses can reproduce a more chronic colitis with development of fibrosis.35 Similarly, a single dose of TNBS via intrarectal instillation generates an acute inflammatory response that then primes an antigen-specific T-cell response but does not lead to fibrosis unless repeated administration of the chemical is applied, leading to a change in immunophenotype from T helper 1 (Th1)- to Th2/Th17-dominance, and with subsequent collagen deposition and fibrosis.36 Despite their widespread use, chemical models also have major drawbacks, which include their questionable relevance to human disease, as well as incomplete penetrance and mild severity of fibrosis.
The conceptual justification of microbial models arises from converging lines of evidence showing that the presence of intestinal microbiota is a prerequisite for the development of chronic inflammation and possibly fibrosis in the intestines. Inflammation does not occur in germ-free animals and responds to treatment with antibiotics, whereas genome-wide association studies have repeatedly highlighted the relevance of immune-bacterial interactions and intestinal dysbiosis in the pathogenesis of IBD. A number of animal models of IBD, therefore, use specific microbes or microbial components to generate inflammation and subsequent fibrosis. One such model involves the subserosal injection of purified, sterile, bacterial-derived peptidoglycan-polysaccharide (PG-PS) polymers into the cecal or small bowel wall of rats during laparotomy. This induces transmural enterocolitis that initiates acute inflammatory infiltration, followed by chronic granulomatous inflammation, with significant fibrosis. Interestingly, mesenchymal cells that bear the characteristics of myofibroblasts accumulate in the periphery of granulomas.37,38
A similar model is the intramural injection of filtered fecal suspension in rats, which creates severe focal colitis alongside prominent transmural fibrosis with colonic strictures.39 Although both models produce impressive intestinal fibrosis, major difficulties are often encountered, including the demanding technique of administration and nonapplicability to mice. On the other hand, chronic infection with Salmonella typhimurium generates inflammation and fibrosis in infected mice.40 Early—but not late—eradication of the microorganism with antibiotics prevents fibrosis in this model, once again pointing out that the timely removal of the trigger may be the most efficient antifibrotic measure.41 The major drawback of this model is that Salmonella infection is not associated with fibrogenesis in humans.
The presence of fibrosis has been consistently sought after in several of the genetically manipulated models of colitis; nonetheless, it represents a rare phenotype. As transforming growth factor (TGF)β1 signaling is considered critical during fibrogenesis, the effect of local colonic overexpression of TGFβ by enema delivery of an adenoviral vector was studied.42 Aided by ethanol-induced disruption of epithelial integrity, this model includes an initial diffuse, ulcerative, innate-type of inflammation that is followed by a more localized chronic, transmural inflammation. The latter is accompanied by ECM matrix deposition, thickening of both muscle layers, and increased presence of myofibroblast-type cells. Eventually, the majority of mice develop colonic strictures with prestenotic dilatation. The chemokine macrophage chemoattractant protein (MCP-1) has been shown to play an important role in the development of fibrosis in the lung, kidney, liver, and pancreas.43 Intramural injection of an adenoviral vector carrying MCP-1 in the rectum of B6 mice leads to early upregulation of TGFβ1 and collagen deposition, along with a shift in the collagen type 3:1 ratio, which is reminiscent of stricturing CD. This fibrotic response is mediated by lymphocytes, as it is missing in recombination-activating gene (RAG)2–/– mice. Finally, the development of fibrosis has been reported in mice that overexpress tumor necrosis factor (TNF)-like cytokine 1A (TL1A)/TNF superfamily15 (TNFSF15). The rationale for this approach have been human studies reporting that TL1A and its functional receptor, death-domain receptor 3 (DR3), are upregulated in both active CD and UC, whereas IBD-specific, protective, and at-risk polymorphisms have been reported for TNFSF15 (TL1A) and TNFRSF6B (which encodes for the TL1A-binding, decoy receptor 3 [DcR3]) genes.44,45 In mouse studies, it has been reported that either global or selective (myeloid or lymphoid) overexpression of TL1A (eg, TL1A-Tg mice) result in increased collagen deposition with mild fibrosis in both the small intestine and colon, which is further intensified following challenge with DSS and is associated with elevations in interleukin (IL)-17 expression.46 Interestingly, fibrosis can be reversed upon treatment with a neutralizing TL1A antibody.47 Given the significance of the TL1A/DR3 system in experimental inflammation and IBD, the TL1A-Tg model should be considered one of the most relevant models of fibrosis, at present.
Currently, the SAMP1/YitFc (SAMP) strain is the only spontaneous model of experimental ileitis and subsequent fibrosis.48 A clear advantage of this mouse is that it offers the opportunity to investigate the natural course of disease over time: prior to the onset of gut inflammation, throughout acute and chronic mucosal inflammatory events, and during the formation and progression of intestinal fibrotic lesions. At the same time, however, the spontaneous nature of SAMP ileitis also indicates that the underlying pathogenetic mechanisms of inflammation and fibrosis are, similarly to IBD, largely unknown. The SAMP mice represent a model of Th1/Th2-driven chronic enteritis, with Th1 events occurring early and further increasing as disease severity progresses. In contrast, Th2 responses become dominant later on and coincide with hypertrophy of the ileal muscularis propria, extensive collagen deposition, and frank stricture formation with prestenotic dilatation.49 The mRNA expression of Col1a1, Col3a1, insulin-like grown factor-1, and connective tissue growth factor are dramatically increased in the ilea of SAMP vs the parental control strain.50
High Dimensionality Analysis
In recent years, significant progress has been accomplished regarding our ability to analyze the mRNA and protein composition at the single-cell level, even when the starting point is a complex cellular community, such as the intestinal mucosa.51 This is facilitated with bioinformatics analysis, which currently allows for the identification of detailed molecular signatures and comparison between single cells that—until now—could only be analyzed as a total population based on the expression some universal markers. Such methodology may prove very important for the accurate characterization of the stromal cell component at the intestinal wall, given the highly diverse origins of ECM-producing myofibroblasts. In one of the earliest studies in this filed, Higuchi et al showed that the transcriptional profile of fibroblasts is dependent on the organ and anatomical site of origin; hence, intestinal fibroblasts are characterized by unique gene expression signatures that encompass genes involved in transcriptional regulation, signaling ligands, and extracellular matrix remodeling.52
A more recent study fingerprinted mesenchymal cells from healthy controls and patients with IBD by use of unbiased single-cell profiling of over 16,500 colonic mesenchymal cells.53 The authors reported the existence of 4 distinct subtypes of fibroblasts based on their transcriptomic and functional signatures that were different from pericytes and myofibroblasts. Importantly, they identified a subepithelial population that was dysregulated in patients with colitis. A novel colitis-associated activated mesenchymal cell was demonstrated, which correlated to disease severity and expressed TNF superfamily member 14 (TNFSF14), fibroblastic reticular cell-associated genes, IL-33, and Lysyl oxidases.53 The possibility of communication between this colitis-specific stromal cell and the epithelium was also raised. Additional studies, using high dimensionality approaches have brought about important roles for the bioactive vitamin D metabolite 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), and the Wnt/β-catenin pathway factor, Wnt3A, as critical regulators of colonic fibroblasts.54
Immunoregulation of Intestinal Fibrosis
The chronic inflammatory response that takes place in IBD is orchestrated by a plethora of overlapping and redundant molecular modules, among which cytokines are the most extensively studied. The traditional dogma of IBD immunopathogenesis proposed a simplified “CD/Th1 vs UC/Th2” paradigm, which was strongly challenged by the discovery of additional effector pathways (Th17, Th9) and the increasingly recognized role of various populations of regulatory T-cells (Tregs). The complexity is further intensified by the contribution of innate immunity during acute flares of the disease and the continuously expanding universe of mucosal cells, mostly represented currently by innate lymphoid cells (ILCs). These cell subsets and their respective cytokines not only organize the inflammatory network within the mucosa but also affect the long-term development of intestinal fibrosis. Myofibroblasts actively participate in this process and are indicated by the fact that these cells bear several cytokines receptors55 and toll-like receptors (TLRs),56 thus being capable of scavenging their surroundings and adopting their function, accordingly. The role of such IBD-related, inflammation-associated molecular factors will be examined in the context of their effects in regulating the expansion, activation, and removal of intestinal myofibroblasts, as well as controlling the MMP/TIMP balance and ECM remodeling.
TGFβ/Smad: the “Core” Fibrotic Pathway
The pleomorphic cytokine, TGFβ, especially its TGFβ1 isoform, is the most recognized regulator of fibrosis in both intestinal and extraintestinal organs. When TGFβ1 binds to its receptors, TGFβR1 and 2, it initiates intracellular signals that are further propagated by phosphorylated proteins Smad 2, 3, and 4 and counter-regulated by Smad 7.57 All isoforms of TGFβ and their receptors are increased in the lamina propria, lymphocytes, epithelial cells, and fibroblasts of patients with CD.58 The TGFβ1 mRNA localizes to lamina propria cells, especially those that lay closest to the luminal surface.59 More importantly, this upregulation appears to be more exaggerated in biopsy specimens and myofibroblasts that are obtained from strictured compared with nonstrictured areas of the intestines.22 This includes higher baseline and/or TGFβ-induced levels for TGFβ, phosphorylated Smad2/3, and TIMP-1 proteins and collagen, along with reduced expression of Smad7, and MMP-3 and -12.
Transforming growth factor β1 exerts its profibrotic function via several mechanisms. Activated TGFβ1 is the most potent inductor of α-SMA, facilitating the differentiation of human fibroblasts to myofibroblasts. It also contributes to the expansion of the myofibroblast pool by promoting EMT and Endo-MT and by augmenting the proliferation of myofibroblasts and rendering them resistant to apoptosis. In addition, TGFβ1 affects remodeling of the ECM by enhancing tissue expression of TIMP, thus decreasing the MMP:TIMP ratio, which diminishes local degradation of ECM and sustains fibrosis. Transforming growth factor β also was shown to augment the migration of intestinal myofibroblasts and their collagen-producing capacity.20,60,61 The profibrotic effects of TGFβ were also demonstrated in a human intestinal organoid fibrosis model, whereby stimulation with TGFβ resulted in upregulation of collagen type 1, fibronectin, α-SMA, actin contractile gene Myosin Light Chain Kinase (MYLK), and fibrogenic transcription factor megakaryoblastic leukemia 1 (MLK1).62 Proof-of-concept for a profibrotic role of TG-β is also provided by observations in animal models, reporting amelioration of fibrosis by TGFβ blockade63 and intestinal stricture formation in mice with forced overexpression of this cytokine.42
Overall, the TGFβ/Smad pathway has been viewed as a “core” pathway upon which IL-13 is added as an important upstream mediator (see later on) and connective tissue growth factor (CTGF) is considered a principal downstream module. This central core can be modified via the subsequent action of several pro-and antifibrotic factors. Connective tissue growth factor is uniformly co-expressed with TGFβ, including at intestinal strictures of CD patients.64 It then acts downstream by enhancing myofibroblast proliferation and ECM synthesis, thus critically contributing to tissue fibrosis.65 By acting downstream, CTGF offers the opportunity for therapeutic manipulation of the profibrotic effects of TGFβ without interfering with its regulatory homeostatic function.
IL-13: Profibrotic Effects of Th2 Immunity
Interleukin-13, the central effector cytokine of Th2-type immunity, has demonstrated profibrotic activity in several chronic extraintestinal organs, such as the liver and lung.66,67 These effects are mediated by the ability of IL-13 to induce upregulation of downstream TGFβ, promote myofibroblast differentiation, and augment collagen production. Of note, elevated mucosal IL-13 expression has been most reported in patients with UC and not CD, which is in opposition to the fact that fibrosis is mainly a characteristic of the latter. Data from animal models of inflammation, however, provide insight into this discrepancy by underscoring the importance of temporal associations in disease progression. This was explicitly observed in the SAMP mouse model with chronic ileitis, which recapitulates ileal-specific CD for several characteristics, including the development of fibrostenosis with prestenotic dilatation.50 The initial development of ileitis in SAMP mice is dominated by Th1/interferon (IFN)-γ responses. This is, however, followed by a second stage that occurs later on and is characterized immunologically by prominent increases of IL-13 (and IL-5).49 This second phase also coincides with the development of overt ileal strictures with prestenotic dilatation, typified by local upregulation of collagen 1 and 3 and downstream fibrotic factors, including insulin-like grown factor-1 and CTGF. A similar inflammation-to-fibrosis transition has also been reported in the TNBS model of colitis in mice.36,68,69 Colitis starts as a typical Th1 response, dependent on IL-12 expression, only to be reverted in later stages to an IL-13-dominant mucosal phenotype, which again coincides with the development of fibrosis that follows repetitive instillation of TNBS. Fibrosis can be abrogated by TGFβ blockade, confirming the presence of upstream IL-13 in the “core” fibrosis pathway. This effect is mediated via binding to the IL-13Rα2, as its deletion or neutralization ameliorated TGFβ-mediated fibrosis.
Therefore, IL-13Rα2 offers a novel therapeutic opportunity in human diseases that are associated with fibrosis and induced by the IL-13/TGF-β axis. On the one hand, IL-13 signaling may be the precipitating factor for fibrostenotic complications in long-standing ulcerative colitis. On the other hand, the apparent low expression of IL-13 in patients with CD may be due to selection of patients with active inflammation but not complicated fibrosis-related phenotypes. In a recent study, muscle extracts from fibrotic areas of CD patients highly expressed IL-13, whereas local fibroblasts expressed the receptor IL-13Rα1 and responded to IL-13 by downregulating MMP-2 and TNF-induced MMP-1 and MMP-9 synthesis.70 The responsible profibrotic, IL-13-secreting cell, was identified as a population of IL-13Rα1, which are killer-cell immunoglobulin-like receptors (KIR)+ innate lymphoid cells (ILCs). Similar to IL-13, the inductive Th2 cytokine IL-4 has also been associated with fibrosis in several experimental and clinical models. Reports of such effects in IBD are currently lacking, wherein its expression is not elevated in either CD or UC. Furthermore, when comparisons were performed, it appears that IL-13 has a more pronounced antifibrotic role than IL-4.
Interleukin-33 is another cytokine that has been associated with Th2 immune responses71 and has also demonstrated profibrotic function.72 Sponheim et al examined biopsy specimens from IBD patients and found significant upregulation of IL-33 mRNA levels in untreated ulcerative colitis, along with localization in ulceration-associated myofibroblasts.73 Interestingly, IL-33-positive myofibroblasts were almost absent near the deep fissures seen in Crohn’s disease. Interleukin-33 may also drive intestinal fibrosis via its association to local eosinophils, which are known to also promote fibrogenesis. In a recent study, IL-33 was shown to augment eosinophil peroxidase and IL-13 secretion.74 Furthermore, epithelial IL-33 was increased in pediatric Crohn’s ileitis and strongly associated with ileal eosinophilia and complicated fibrostenotic disease. Fibroblasts that were exposed to eosinophils that were prestimulated with IL-33 increased their production of pro-inflammatory and profibrotic factors, whereas eosinophil-targeted treatment resulted in significant improvements in inflammation and tissue remodeling in a chronic ileitis model.74 Finally, IL-33 may also mediate profibrotic signals that originate from microbial factors. This was shown for the CD-associated pathobiont adherent-invasive Escherichia coli (AIEC), which persistently colonizes the inflamed gut; it also potentiated the mucosal production of the IL-33 receptor ST2 in the intestinal epithelium and led to substantial fibrosis.75 This effect was mediated by flagellin, as bacteria deficient for flagellin failed to induce ST2.
IFN-γ: Antifibrotic Effects of Th1 Immunity
Several lines of evidence support an antifibrotic role for the hallmark Th1 cytokine, interferon-γ, and also IL-12. In particular, it was shown that IFN-γ inhibits the expression of TGFβ and also blocks its downstream effects, such as phosphorylation of Smad3, the association of Smad3 with Smad4, and the induction of TGFβ-responsive genes, including lower expression of CTGF. It further increases the expression of the counter-regulatory Smad 7 protein.76 In accordance, mice were protected from experimental interstitial nephritis by treatment with a pegylated IFN-γ preparation that significantly reduced collagen 1, fibronectin, and α-SMA mRNA and protein expression.77 Similarly, the antifibrotic effects of IL-12 were shown in mice with either schistosome infection or with bleomycin-induced lung fibrosis. Although these results clearly demonstrate an antifibrotic role for Th1 responses, and despite the fact that both IL-12 and IFN-γ are upregulated at the mucosa of patients with CD, there is a paucity of studies regarding the role of Th1 immunity in intestinal fibrosis. Furthermore, therapeutic administration of IFN-γ in extraintestinal fibrotic diseases has failed to generate substantial clinical benefit.
IL-17: The Evolving Role of Th17 Responses in Intestinal Fibrosis
The Th17 lineage is the third major effector population of T-lymphocytes and the latest to be described. Its hallmark cytokine is IL-17A, although several other products of Th17 cells have also been described, which include IL-17F, IL-21, and IL-22; however, significant input is delivered by IL-23, IL-6, and TGFβ. Although a pro-inflammatory role for Th17 cells has been well-recognized, these cells also play important roles in the preservation of tissue homeostasis. In addition, their involvement in tissue repair and fibrosis has been elucidated. In fact, a role for IL-17 in extraintestinal fibrosis has been described,78,79 with recent studies also supporting its role in the intestine. In expression studies, increase in IL-17A was detected in areas of stenosis from patients with CD.80 Furthermore, intestinal myofibroblasts from strictured regions responded to IL-17A stimulation by upregulating the production of collagen, MMP-3, MMP-12, and TIMP, showing reduced migratory ability compared with controls.80 Further studies showed that colonic myofibroblasts express IL-17R and respond to stimulation from IL-17A (alone or in combination with IL-1β, TNF, or IL-4). This leads to elevated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and mitogen-activated protein (MAP) kinase-dependent production of IL-6, IL-8, MCP-1, leukemia inhibitory factor (LIF), and MMP-1, and MMP-3.81-83 In another study, the stimulatory effects of IL-17 on human intestinal myofibroblasts was shown to depend on an intermediate step of elevated expression of heat shock protein (HSP) 47.84 The potential role of IL-17 in fibrosis was recently explored by observing the effects of anti-IL-17 neutralization in the TNBS model of colitis.85 Following anti-IL-17 treatment, both inflammation and fibrosis were ameliorated, accompanied by reductions in collagen 3, IL-17, tumor necrosis factor, TIMP-1, and MMP-2. In addition, in another study using the same model, it was proposed that the mechanism for colitis amelioration via anti-IL-17A treatment involved inhibition of EMT.86 Overall, these studies support a profibrotic role of IL-17 in the intestine. It should be noted, however, that Th17 cells are characterized by high plasticity and frequently acquire alternative phenotypes reminiscent of Th1 or even Treg lineages. Moreover, IL-17A also exerts protective roles at the intestinal mucosa, as exemplified by the worsening of CD patients who were treated with antibodies against IL-17A87 or its receptor88 in recent clinical trials. Thus, their net effect in intestinal immunity towards tissue repair or inflammation and fibrosis should be further investigated.
Novel Fibrogenic Cytokines: TL1A, IL-34, IL-36
The TL1A (TNFSF15) and its functional receptor DR3 (TNFRSF25) are members of the TNF/TNFR superfamilies with well-established and significant roles in chronic intestinal inflammation as universal costimulators of effector responses and regulators of Treg function.89 Recently, their involvement in intestinal fibrosis has also been suggested.90 The TL1A/DR3 amplify multiple immunological pathways that, when sustained, may lead to fibrosis, including Th2/IL-13- and Th17/IL-17A-dependent mechanisms. Moreover, expression of TL1A is detected in human intestinal myofibroblasts; it is upregulated in patients with CD and receives stimulatory signals from CD-associated pro-inflammatory cytokines (IFN-γ, TNF, and IL-1α), supernatants of intestinal tissue cultures from IBD patients, or supernatants of prestimulated epithelial cells.21 Interestingly, the latter express the receptor DR3, raising the possibility for epithelial-myofibroblast interactions via TL1A-DR3 binding. Thus, a mucosal amplification loop may be initiated by the local pro-inflammatory milieu, which then perpetuates itself through reciprocal stimulation of epithelial cells and intestinal myofibroblasts. Whether such interaction leads to increased collagen accumulation and fibrosis remains to be seen. Notably, significant elevations of both TL1A and DR3 expression have been reported in the ileitis- and fibrosis-prone SAMP mouse strain.91 Further support for a profibrogenic role of TL1A is derived by studies with TL1A transgenic mice that develop IL-13-dependent inflammation of the small bowel, which is associated with wall thickening and hypertrophy of the muscularis propria.92,93 Other studies reported increased intestinal accumulation of collagen in TL1A-Tg mice, along with the development of overt intestinal strictures at the small and proximal large bowel and increased local expression of TGFβ and IGF under colitogenic conditions within the context of DSS administration or T-cell adoptive transfer.47,94 Interestingly, treatment with antibodies against TL1A resulted in amelioration of inflammation and reversal of fibrosis in both colitogenic models, even when treatment was administrated late in the course of disease.47 Intestinal myofibroblasts were shown to express DR3 (albeit only a fraction of them) and respond to TL1A with increased expression of COL1A2 and IL-31Ra, whereas neutralization of TL1A reduced expression of α-SMA and vimentin and colonic expression of TGFβ1 and SMAD3 in the T-cell transfer model.95 These effects were further explored by Jacob et al who showed that the profibrotic effects of TL1A were regulated by the gut microbiome.96 In particular, germ-free TL1A-Tg mice were protected from spontaneous ileitis and showed diminished cecal collagen deposition. Furthermore, myofibroblasts from germ-free mice demonstrated decreased migration/proliferation and collagen production than those derived from specific pathogen-free mice. The authors were also able to identify possible bacterial genera and species that differentially promote fibrosis in the respective localizations in the context of TL1A overexpression.96 Although these results are intriguing, a true separation between anti-inflammatory and antifibrotic effects of TL1A over expression has not been clearly demonstrated. Nevertheless, these studies demonstrated for the first time that anti-inflammatory therapy, in this case TL1A neutralization, can not only prevent but also potentially reverse established intestinal fibrosis.
Interleukin-34 is a novel cytokine that signals through the colony-stimulating factor 1 receptor (CSF-1R). This cytokine acts on monocytes/macrophages and regulates their differentiation, survival, and function.97 Interleukin-34 and CSF-1R are upregulated in patients with Crohn’s disease and ulcerative colitis, specifically localizing to areas with active inflammation; they respond to pro-inflammatory stimuli and are decreased following successful treatment with anti-TNF antibodies.98 The potential involvement of IL-34 in IBD-associated fibrosis was recently demonstrated. Biopsies from fibrostenotic areas of patients with CD showed elevated expression of both IL-34 and CSFR-1 mRNA and protein.99 Furthermore, immunolocalization of both markers was detected in stromal cells and enhanced expression in fibroblasts isolated from patients with CD. Interleukin-34 showed profibrotic properties in vitro, as it induced increased production of collagens COL1A1 and COL3A1 by healthy fibroblasts via p38 MAP kinase-dependent mechanism and augmented wound healing. These effects were abrogated in fibroblasts that were rendered deficient for IL-34.
Interleukin-36 represents a group of cytokines that belong to the IL-1 family. Although they have been associated mostly with inflammatory effects, it was recently shown that signaling via the IL-36 receptor (IL-36R) has antifibrotic effects in murine models of chronic intestinal inflammation.100 Specifically, mice that were either deficient in IL-1Rrp2 (IL-36R) or were treated with neutralizing anti-36R mAbs were protected from developing fibrosis that was induced by either DSS-administration of TNBS treatment. More importantly, treatment with anti-IL-36R antibodies reversed established fibrosis in mice. The translational value of these findings was further highlighted by the finding that patients with fibrostenotic CD had significantly higher levels of intestinal IL-36A; this correlated to elevated numbers of activatedα-SMA positive fibroblasts. Recent studies have also implicated IL-36:IL-36R signaling in fibrogenesis in various organs, including the pancreas, lung, kidney, and heart, making this cytokine an attractive novel target for antifibrotic therapies.101
Therapeutic Implications of Pathogenetic Mechanisms
Do Anti-inflammatory Therapies Prevent Fibrotic Complications in Patients with IBD?
Given that the current pathogenetic model for IBD highlights an “inflammation to fibrosis” sequence, there is reasonable hope that effective anti-inflammatory therapies may prevent the development of fibrosis in patients with CD and UC. Optimism was further heightened after the development and clinical utilization of biological agents that have shown high efficacy in eliminating chronic IBD-related intestinal inflammation. Although more than 20 years has passed since the approval of infliximab for the therapy of CD, a clear answer is still awaiting. This may be due to several factors. There is currently lack of reliable biomarkers for fibrosis; thus, the initial development of fibrosis and its evolution over time cannot be definitively identified, including the effects of any therapy. This may be about to change, however, as international groups are currently working intensely to develop strict definitions for fibrosis-related outcomes for application in both clinical trials and everyday practice.102 At the moment, any antifibrotic outcome derived from anti-inflammatory therapies can only be judged indirectly by observing their effect on the rate of complications, especially surgical ones in patients with CD, as they are usually related to strictures. Although opposite views also occur, the overall trend points towards a measurable decrease in surgical rates following the introduction of biological therapies. Ma et al reported a 3.5% decrease in the overall rate of surgeries between 2002 and 2010, although this affected emergency cases only.103 Similarly, a significant difference in the 10-year risk of second surgery for CD was observed over time when studies conducted after 1980 were compared with those before this date.104 Finally, Dittrich et al found that surgical rates were decreased by 8.4% each year between 1993 and 2013, alongside a 36.2% annual increase in the use of anti-TNF therapy and a 2.2% annual decrease in the proportion of active smokers.105 As mentioned earlier, other studies contradict these results106; this is likely due to divergences in the penetration rates of biological therapies and the time of their introduction into individual therapeutic regimens. Indeed, anti-inflammatory therapies may be administered too late in the natural history of IBD, thus being unable to demonstrate a major impact on fibrosis. This is an important issue to be considered; early intervention may be the decisive factor, as preclinical studies and clinical observations have shown. In 2 murine models of intestinal fibrosis, namely the PG-PS rat107 and the Salmonella typhimurium mouse41 models, it was shown that neutralization of TNF was associated with prevention of fibrosis, but this benefit was only seen when intervention was administered at an early stage. In contrast, late administration of anti-TNF had no effect on fibrosis. These results are aligned with the well-established clinical observation that anti-TNF therapies are much more effective when administered in patients with a short, as opposed to long, duration of CD.108,109 Taken together, those data indicate that a “point of no return” does exist in CD and that biological therapy prevents fibrotic complications—but only if administered before this point is reached, especially in patients with factors indicative of a poor prognosis.110
Do Inflammation-independent Fibrotic Pathways Exist?
The studies mentioned previously clearly show that elimination of active inflammation is not sufficient to prevent or, more significantly, reverse intestinal fibrosis. It is therefore assumed that inflammation-independent mechanisms of fibrosis also occur. In other words, although postinjury tissue inflammation provides the signals that initiate fibrogenic responses, eventually the latter may progress independently of concomitant inflammation. There are several lines of evidence that support this scenario. First, this was shown in mice deficient in the transcription factor PU.1; this factor is required for the generation of myeloid and lymphoid progenitors from bone marrow stem cells. As such, PU.1-null mice are genetically incapable of mounting an adequate inflammatory response due to lack of functional macrophages and neutrophils; yet, they demonstrate an efficient and rapid repair of skin wounds.111 Second, embryos and neonatal organisms also show inflammation-independent would healing.112,113 Third, myofibroblasts in culture can effectively close wounds in migrations assays, again without the need for inflammatory input. Fourth, when fibrosis was induced in IL-10-/- mice via either DSS administration or heterotopic transplantation, no association was observed between expression of inflammatory vs fibrogenic factors.114 Finally, in a chronic TNBS model of inflammation-induced colonic fibrosis, it was reported that inflammation-related genes showed an early rise that was followed by a rapid decline.115 In contrast, profibrogenic extracellular matrix genes appeared early but remained overexpressed, even after the disappearance of inflammation.
The exact nature of noninflammatory fibrotic pathways in IBD remains largely unknown, but several mechanisms may act in isolation or synergistically. One such mechanism is the evasion of apoptosis by myofibroblasts.116 During normal would healing, when tissue repair is completed, myofibroblasts go through an elimination process that may take the form of deactivation (ie, becoming senescent cells or apoptotic death). In fibrotic states, however, by integrating signals from the local environment, myofibroblasts acquire a survival advantage and continue to remodel tissue, eventually leading to pathological scarring. Mechanical properties of the surrounding tissue provide additional noninflammatory triggers for the activated myofibroblast. In other organs, it has been demonstrated that increasing tissue stiffness is an early stage in fibrosis progression and precedes ECM deposition.117 In relation to this, Stewart et al recently showed that inflamed tissue from patients with CD had increased stiffness in combination with elevated expression of type 1 collagen compared with unaffected areas.118 Furthermore, myofibroblasts that were isolated from intestinal areas with stenosis demonstrated a “paradoxical” response in culture under high pressure conditions with downregulation of MMP3 expression, indicating further amplification of their profibrotic properties, which is in sharp contrast to the behavior of control myofibroblasts.25 The importance of tissue stiffness is also indicated by the postoperative natural history of CD, following right hemicolectomy for stenotic disease. It was shown that often fibrosis does not progress proximal to the anastomotic site, indicating that the decrease in intramural pressure permanently disrupts the disease process.119,120 A final factor to be considered is the effect of tissue- and cell-specific epigenetic alterations that may be integrated within fibrotic myofibroblasts and permanently alter their status.121 In fact, distinct methylation patterns of fibrotic CD-derived myofibroblasts have been reported.122 Recently, it was shown that subepithelial myofibroblasts derived from fibrostenotic areas of patients with Crohn’s disease displayed increased endoplasmic reticulum stress as a result of miR-199a-5p silencing, which resulted in augmented expression of TGFβ1 and collagen 1α1.123 Such epigenetic alterations may transform myofibroblasts from “healing-type” to “scar-type” phenotypes and perpetuate profibrotic pathways within the intestinal tissue of patients with IBD.
Can Established Fibrosis Be Reversed?
Currently, there are no approved antifibrotic therapies for patients with CD and established intestinal strictures. Consequently, research in this area follows the progress in other fibrotic conditions that are better studied, including liver cirrhosis and idiopathic pulmonary fibrosis (IPF). Nevertheless, there is a pivotal difference between intestinal and liver fibrosis, which relates to the fact that in the latter the triggering factor is almost always known (viral hepatitis, alcohol, or nonalcoholic fatty liver disease [NAFLD]). Thus, therapeutic efforts are mostly directed towards removing the precipitating cause, which has led to impressive successful results in the case of HCV and HBV liver disease. Idiopathic pulmonary fibrosis (IPF), on the other hand, is a chronic progressive condition that leads to lung fibrosis and gradual respiratory compromise. Recently, the first 2 purely antifibrotic therapies were approved for use in patients with IPF. Pirfenidone is a drug with both anti-inflammatory and antifibrotic properties. Its use in IPF was associated with slowing down the decline of forced vital capacity and improvement in progression free survival.124 Nintedanib is a tyrosine kinase inhibitor, and its administration to IPF patients led to a significant drop in the rate of annual decline of forced vital capacity.125,126 Although these drugs have not been tested in patients with IBD, their efficacy in IPF provides evidence for the feasibility of halting the progression of, and even reversing, established organ fibrosis. At the same time, novel antifibrotic therapies are currently under development based on their preclinical success. In one such effort, Truffi et al studied the effects of treatment with an antibody against fibrosis-associated protein (FAP) of cultured intestinal biopsies from stenotic and nonstenotic ileal segments of patients with CD.127 They demonstrated that treatment of stenotic tissues with the anti-FAP antibody significantly decreased the production of type 1 collagen and inhibited TIMP-1 expression, indicating that anti-FAP treatments may reestablish ECM homeostasis in CD. In another study, it was shown that inhibition of Rho kinases (ROCK) was effective in reversing established fibrosis in 2 experimental models. Interestingly the severity of underlying inflammation was not affected, indicating a fibrosis-specific effect.
Glossary
Abbreviations
- AIEC
adherent-invasive Escherichia coli
- CD
Crohn’s disease
- COL
collagen
- CSF-1R
colony stimulating factor 1 receptor
- CTGF
connective tissue growth factor
- DAMP
damage-associated molecular patterns
- DcR3
decoy receptor 3
- DR3
death-domain receptor 3
- DSS
dextran sodium sulfate
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- Endo-MT
endothelial-to-mesenchymal transition
- FAP
fibrosis associated protein
- HBV
hepatitis B virus
- HSP
heat shock protein
- IBD
inflammatory bowel disease
- IFN
interferon
- ILCs
innate lymphoid cells
- IPF
idiopathic pulmonary fibrosis
- KIR
killer-cell immunoglobulin-like receptors
- MAP
mitogen-activated protein kinase
- MCP-1
macrophage chemoattractant protein
- MLK1
megakaryoblastic leukemia 1
- MMPs
matrix metalloproteinases
- MYLK
myosin light chain kinase
- NAFLD
nonalcoholic fatty liver disease
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PAMP
pathogen-associated molecular pattern
- PG-PS
peptidoglycan-polysaccharide
- PRRs
pattern recognition receptors
- RAG
recombination-activating gene
- ROCK
Rho kinases
- SEMF
subepithelial myofibroblast
- SMA
smooth muscle actin
- TGF
transforming growth factor
- TIMPs
tissue inhibitors of metalloproteinases
- TL1A
TNF-like cytokine1A
- TLRs
toll-like receptors
- TNBS
trinitrobenzene sulfonic acid
- TNF
tumor necrosis factor
- UC
ulcerative colitis
Author Contributions
G.B., F.C.: study design. G.B.: literature review. G.B., T.T.P., F.C.: manuscript writing. G.B., T.T.P., F.C.: review, comments, and editing of final manuscript. All authors contributed to the article and approved the submitted version.
Funding
Research reported in this publication was supported by National Institutes of Health grants DK042191 and DK091222 (to F.C. and T.T.P.) and DK055812 (to F.C.). This publication was supported by the National Institutes of Health grant DK42991 (to F.C. and T.T.P.), DK055812, DK091222, and DK097948 (to F.C.), and the NIH Cleveland Digestive Diseases Research Core Center (DDRCC) Administrative Core (to F.C.).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Duffield JS, Lupher M, Thannickal VJ, et al. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silverstein MD, Loftus EV, Sandborn WJ, et al. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57. [DOI] [PubMed] [Google Scholar]
- 3. Gordon IO, Agrawal N, Willis E, et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47:922–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wynn TA, Ramalingam TR.. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henderson NC, Rieder F, Wynn TA.. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao S, Dejanovic D, Yao P, et al. Selective deletion of MyD88 signaling in α-SMA positive cells ameliorates experimental intestinal fibrosis via post-transcriptional regulation. Mucosal Immunol. 2020;13:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dovrolis N, Drygiannakis I, Filidou E, et al. Gut microbial signatures underline complicated Crohn’s disease but vary between cohorts; an in Silico approach. Inflamm Bowel Dis. 2019;25:217–225. [DOI] [PubMed] [Google Scholar]
- 8. Valatas V, Filidou E, Drygiannakis I, et al. Stromal and immune cells in gut fibrosis: the myofibroblast and the scarface. Ann Gastroenterol. 2017;30:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roulis M, Flavell RA.. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation. 2016;92:116–131. [DOI] [PubMed] [Google Scholar]
- 10. Rieder F, Fiocchi C.. Intestinal fibrosis in IBD–a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–235. [DOI] [PubMed] [Google Scholar]
- 11. Kuroda N, Masuya M, Tawara I, et al. Infiltrating CCR2+ monocytes and their progenies, fibrocytes, contribute to colon fibrosis by inhibiting collagen degradation through the production of TIMP-1. Sci Rep. 2019;9:8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flier SN, Tanjore H, Kokkotou EG, et al. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimshoni E, Yablecovitch D, Baram L, Dotan I, Sagi I.. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut 2015;64:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Bruyn M, Vandooren J, Ugarte-Berzal E, et al. The molecular biology of matrix metalloproteinases and tissue inhibitors of metalloproteinases in inflammatory bowel diseases. Crit Rev Biochem Mol Biol. 2016;51:295–358. [DOI] [PubMed] [Google Scholar]
- 15. Rieder F, Karrasch T, Ben-Horin S, et al. Results of the 2nd scientific workshop of the ECCO (3): basic mechanisms of intestinal healing. J Crohns Colitis. 2012;6:373–385. [DOI] [PubMed] [Google Scholar]
- 16. Burke JP, Mulsow JJ, O’Keane C, et al. Fibrogenesis in Crohn’s disease. Am J Gastroenterol 2007;102:439–448. [DOI] [PubMed] [Google Scholar]
- 17. Allocca M, Fiorino G, Bonifacio C, et al. Noninvasive multimodal methods to differentiate inflamed vs fibrotic strictures in patients with Crohn’s Disease. Clin Gastroenterol Hepatol. 2019;17:2397–415. [DOI] [PubMed] [Google Scholar]
- 18. Lingala S, Ghany MG.. Natural history of hepatitis C. Gastroenterol Clin North Am. 2015;44:717–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55. [DOI] [PubMed] [Google Scholar]
- 20. Drygiannakis I, Valatas V, Sfakianaki O, et al. Proinflammatory cytokines induce cross-talk between colonic epithelial cells and subepithelial myofibroblasts: implication in intestinal fibrosis. J Crohns Colitis. 2013;7:286–300. [DOI] [PubMed] [Google Scholar]
- 21. Bamias G, Filidou E, Goukos D, et al. Crohn’s disease-associated mucosal factors regulate the expression of TNF-like cytokine 1A and its receptors in primary subepithelial intestinal myofibroblasts and intestinal epithelial cells. Transl Res. 2017;180:118–130.e2. [DOI] [PubMed] [Google Scholar]
- 22. Di Sabatino A, Jackson CL, Pickard KM, et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut. 2009;58:777–789. [DOI] [PubMed] [Google Scholar]
- 23. Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. [DOI] [PubMed] [Google Scholar]
- 24. Johnson LA, Rodansky ES, Sauder KL, et al. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis. 2013;19:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Bruyn JR, van den Brink GR, Steenkamer J, et al. Fibrostenotic phenotype of myofibroblasts in Crohn’s Disease is dependent on tissue stiffness and reversed by LOX inhibition. J Crohns Colitis. 2018;12:849–859. [DOI] [PubMed] [Google Scholar]
- 26. Lampugnani MG. Cell migration into a wounded area in vitro. Methods Mol Biol. 1999;96:177–182. [DOI] [PubMed] [Google Scholar]
- 27. Stelzner M, Helmrath M, Dunn JC, et al. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shim KY, Lee D, Han J, et al. Microfluidic gut-on-a-chip with 3D villi structure. Biomed Microdevices 2017;19:37. [DOI] [PubMed] [Google Scholar]
- 30. Workman MJ, Gleeson JP, Troisi EJ, et al. Enhanced utilization of induced pluripotent stem cell-derived human intestinal organoids using microengineered chips. Cell Mol Gastroenterol Hepatol. 2018;5:669–677.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giuffrida P, Curti M, Al-Akkad W, et al. Decellularized human gut as a natural 3D platform for research in intestinal fibrosis. Inflamm Bowel Dis. 2019;25:1740–1750. [DOI] [PubMed] [Google Scholar]
- 32. De Salvo C, Ray S, Pizarro TT.. Mechanisms and models for intestinal fibrosis in IBD. Dig Dis. 2014;32Suppl 1:26–34. [DOI] [PubMed] [Google Scholar]
- 33. Neurath M, Fuss I, Strober W.. TNBS-colitis. Int Rev Immunol. 2000;19:51–62. [DOI] [PubMed] [Google Scholar]
- 34. Wirtz S, Popp V, Kindermann M, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–1309. [DOI] [PubMed] [Google Scholar]
- 35. Lund PK, Zuniga CC.. Intestinal fibrosis in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol. 2001;17:318–323. [DOI] [PubMed] [Google Scholar]
- 36. Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. [DOI] [PubMed] [Google Scholar]
- 37. Sartor RB, Cromartie WJ, Powell DW, et al. Granulomatous enterocolitis induced in rats by purified bacterial cell wall fragments. Gastroenterology. 1985;89:587–595. [DOI] [PubMed] [Google Scholar]
- 38. van Tol EA, Holt L, Li FL, et al. Bacterial cell wall polymers promote intestinal fibrosis by direct stimulation of myofibroblasts. Am J Physiol. 1999;277:G245–G255. [DOI] [PubMed] [Google Scholar]
- 39. Mourelle M, Salas A, Guarner F, et al. Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology. 1998;114:519–526. [DOI] [PubMed] [Google Scholar]
- 40. Grassl GA, Valdez Y, Bergstrom KS, et al. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology. 2008;134:768–780. [DOI] [PubMed] [Google Scholar]
- 41. Johnson LA, Luke A, Sauder K, et al. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a “Top-Down” approach to intestinal fibrosis in mice. Inflamm Bowel Dis 2012;18:460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vallance BA, Gunawan MI, Hewlett B, et al. TGF-beta1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G116–G128. [DOI] [PubMed] [Google Scholar]
- 43. Motomura Y, Khan WI, El-Sharkawy RT, et al. Induction of a fibrogenic response in mouse colon by overexpression of monocyte chemoattractant protein 1. Gut. 2006;55:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bamias G, Martin C 3rd, Marini M, et al. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868–4874. [DOI] [PubMed] [Google Scholar]
- 45. Siakavellas SI, Sfikakis PP, Bamias G.. The TL1A/DR3/DcR3 pathway in autoimmune rheumatic diseases. Semin Arthritis Rheum. 2015;45:1–8. [DOI] [PubMed] [Google Scholar]
- 46. Shih DQ, Barrett R, Zhang X, et al. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. Plos One. 2011;6:e16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shih DQ, Zheng L, Zhang X, et al. Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis. Mucosal Immunol. 2014;7:1492–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pizarro TT, Pastorelli L, Bamias G, et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bamias G, Martin C, Mishina M, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–666. [DOI] [PubMed] [Google Scholar]
- 50. Rivera-Nieves J, Bamias G, Vidrich A, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–982. [DOI] [PubMed] [Google Scholar]
- 51. Corridoni D, Chapman T, Antanaviciute A, et al. Inflammatory bowel disease through the lens of single-cell RNA-seq technologies. Inflamm Bowel Dis 2020;26:1658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Higuchi Y, Kojima M, Ishii G, et al. Gastrointestinal fibroblasts have specialized, diverse transcriptional phenotypes: a comprehensive gene expression analysis of human fibroblasts. PLoS One. 2015;10:e0129241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kinchen J, Chen HH, Parikh K, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175:372–386.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ferrer-Mayorga G, Niell N, Cantero R, et al. Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts. Sci Rep. 2019;9:8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Filidou E, Valatas V, Drygiannakis I, et al. Cytokine receptor profiling in human colonic subepithelial myofibroblasts: a differential effect of Th polarization-associated cytokines in intestinal fibrosis. Inflamm Bowel Dis. 2018;24:2224–2241. [DOI] [PubMed] [Google Scholar]
- 56. Otte JM, Rosenberg IM, Podolsky DK.. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. [DOI] [PubMed] [Google Scholar]
- 57. Letterio JJ, Roberts AB.. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. [DOI] [PubMed] [Google Scholar]
- 58. di Mola FF, Friess H, Scheuren A, et al. Transforming growth factor-betas and their signaling receptors are coexpressed in Crohn’s disease. Ann Surg. 1999;229:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Babyatsky MW, Rossiter G, Podolsky DK.. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–984. [DOI] [PubMed] [Google Scholar]
- 60. Leeb SN, Vogl D, Falk W, et al. Regulation of migration of human colonic myofibroblasts. Growth Factors 2002;20:81–91. [DOI] [PubMed] [Google Scholar]
- 61. McKaig BC, McWilliams D, Watson SA, et al. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol. 2003;162:1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rodansky ES, Johnson LA, Huang S, et al. Intestinal organoids: a model of intestinal fibrosis for evaluating antifibrotic drugs. Exp Mol Pathol 2015;98:346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ma Y, Guan Q, Bai A, et al. Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm Bowel Dis. 2010;16:1040–1050. [DOI] [PubMed] [Google Scholar]
- 64. Li C, Iness A, Yoon J, et al. Noncanonical STAT3 activation regulates excess TGF-β1 and collagen 1 expression in muscle of stricturing Crohn’s disease. J Immunol. 2015;194:3422–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. [DOI] [PubMed] [Google Scholar]
- 66. Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA.. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fichtner-Feigl S, Strober W, Kawakami K, et al. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med 2006;12:99–106. [DOI] [PubMed] [Google Scholar]
- 69. Fichtner-Feigl S, Young CA, Kitani A, et al. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology 2008;135:2003–13, 13 e1-7. [DOI] [PubMed] [Google Scholar]
- 70. Bailey JR, Bland PW, Tarlton JF, et al. IL-13 promotes collagen accumulation in Crohn’s disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? Plos One. 2012;7:e52332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. [DOI] [PubMed] [Google Scholar]
- 72. Lopetuso LR, Scaldaferri F, Pizarro TT.. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sponheim J, Pollheimer J, Olsen T, et al. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177:2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Masterson JC, Capocelli KE, Hosford L, et al. Eosinophils and IL-33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing Crohn’s ileitis. Inflamm Bowel Dis. 2015;21:2429–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Imai J, Kitamoto S, Sugihara K, et al. Flagellin-mediated activation of IL-33-ST2 signaling by a pathobiont promotes intestinal fibrosis. Mucosal Immunol. 2019;12:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ulloa L, Doody J, Massagué J.. Inhibition of transforming growth factor-beta/Smad signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. [DOI] [PubMed] [Google Scholar]
- 77. Poosti F, Bansal R, Yazdani S, et al. Selective delivery of IFN-γ to renal interstitial myofibroblasts: a novel strategy for the treatment of renal fibrosis. Faseb J. 2015;29:1029–1042. [DOI] [PubMed] [Google Scholar]
- 78. Yoshizaki A, Yanaba K, Iwata Y, et al. Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J Immunol. 2010;185:2502–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baldeviano GC, Barin JG, Talor MV, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–1655. [DOI] [PubMed] [Google Scholar]
- 80. Biancheri P, Pender SL, Ammoscato F, et al. The role of interleukin 17 in Crohn’s disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair. 2013;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Andoh A, Hata K, Araki Y, Fujiyama Y, Bamba T.. Interleukin (IL)-4 and IL-17 synergistically stimulate IL-6 secretion in human colonic myofibroblasts. Int J Mol Med 2002;10:631–4. [PubMed] [Google Scholar]
- 82. Hata K, Andoh A, Shimada M, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–G1044. [DOI] [PubMed] [Google Scholar]
- 83. Yagi Y, Andoh A, Inatomi O, et al. Inflammatory responses induced by interleukin-17 family members in human colonic subepithelial myofibroblasts. J Gastroenterol. 2007;42:746–53. [DOI] [PubMed] [Google Scholar]
- 84. Honzawa Y, Nakase H, Shiokawa M, et al. Involvement of interleukin-17A-induced expression of heat shock protein 47 in intestinal fibrosis in Crohn’s disease. Gut. 2014;63:1902–1912. [DOI] [PubMed] [Google Scholar]
- 85. Li J, Liu L, Zhao Q, Chen M.. Role of interleukin-17 in pathogenesis of intestinal fibrosis in mice. Dig Dis Sci. 2020;65:1971–1979. [DOI] [PubMed] [Google Scholar]
- 86. Zhang HJ, Zhang YN, Zhou H, et al. IL-17A promotes initiation and development of intestinal fibrosis through EMT. Dig Dis Sci. 2018;63:2898–2909. [DOI] [PubMed] [Google Scholar]
- 87. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s Disease. Am J Gastroenterol. 2016;111:1599–1607. [DOI] [PubMed] [Google Scholar]
- 89. Siakavellas SI, Bamias G.. Tumor necrosis factor-like cytokine TL1A and its receptors DR3 and DcR3: important new factors in mucosal homeostasis and inflammation. Inflamm Bowel Dis. 2015;21:2441–2452. [DOI] [PubMed] [Google Scholar]
- 90. Valatas V, Kolios G, Bamias G.. TL1A (TNFSF15) and DR3 (TNFRSF25): a co-stimulatory system of cytokines with diverse functions in gut mucosal immunity. Front Immunol. 2019;10:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bamias G, Mishina M, Nyce M, et al. Role of TL1A and its receptor DR3 in 2 models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006;103:8441–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Meylan F, Song YJ, Fuss I, et al. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4:172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Taraban VY, Slebioda TJ, Willoughby JE, et al. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol. 2011;4:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barrett R, Zhang X, Koon HW, et al. Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am J Pathol. 2012;180:636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li H, Song J, Niu G, et al. TL1A blocking ameliorates intestinal fibrosis in the T cell transfer model of chronic colitis in mice. Pathol Res Pract. 2018;214:217–227. [DOI] [PubMed] [Google Scholar]
- 96. Jacob N, Jacobs JP, Kumagai K, et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol. 2018;11:1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lelios I, Cansever D, Utz SG, et al. Emerging roles of IL-34 in health and disease. J Exp Med. 2020;217: e20190290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Franzè E, Monteleone I, Cupi ML, et al. Interleukin-34 sustains inflammatory pathways in the gut. Clin Sci. 2015;129:271–280. [DOI] [PubMed] [Google Scholar]
- 99. Franzè E, Dinallo V, Laudisi F, et al. Interleukin-34 stimulates gut fibroblasts to produce collagen synthesis. J Crohns Colitis. 2020;14:1436–1445. [DOI] [PubMed] [Google Scholar]
- 100. Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156:1082–1097.e11. [DOI] [PubMed] [Google Scholar]
- 101. Melton E, Qiu H.. Interleukin-36 cytokine/receptor signaling: a new target for tissue fibrosis. Int J Mol Sci. 2020;21:6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Danese S, Bonovas S, Lopez A, et al. Identification of endpoints for development of antifibrosis drugs for treatment of Crohn’s Disease. Gastroenterology. 2018;155:76–87. [DOI] [PubMed] [Google Scholar]
- 103. Ma C, Moran GW, Benchimol EI, et al. Surgical rates for crohn’s disease are decreasing: a population-based time trend analysis and validation study. Am J Gastroenterol. 2017;112:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn’s disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol. 2014;109:1739–1748. [DOI] [PubMed] [Google Scholar]
- 105. Dittrich AE, Sutton RT, Haynes K, et al. Incidence rates for surgery in Crohn’s Disease have decreased: a population-based time-trend analysis. Inflamm Bowel Dis. 2020;26:1909–1916. [DOI] [PubMed] [Google Scholar]
- 106. Stokes AL, Kulaylat AN, Rocourt DV, et al. Rates and trends for inpatient surgeries in pediatric Crohn’s disease in the United States from 2003 to 2012. J Pediatr Surg. 2018;53:1334–1338. [DOI] [PubMed] [Google Scholar]
- 107. Schmiedlin-Ren P, Reingold LJ, Broxson CS, et al. Anti-TNFα alters the natural history of experimental Crohn’s disease in rats when begun early, but not late, in disease. Am J Physiol Gastrointest Liver Physiol. 2016;311:G688–G698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol. 2010;105:1574–1582. [DOI] [PubMed] [Google Scholar]
- 109. Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohns Colitis. 2013;7:213–221. [DOI] [PubMed] [Google Scholar]
- 110. Govani SM, Stidham RW, Higgins PD.. How early to take arms against a sea of troubles? The case for aggressive early therapy in Crohn’s disease to prevent fibrotic intestinal strictures. J Crohns Colitis. 2013;7:923–927. [DOI] [PubMed] [Google Scholar]
- 111. Martin P, D’Souza D, Martin J, et al. Wound healing in the PU.1 null mouse–tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. [DOI] [PubMed] [Google Scholar]
- 112. Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68-69:106–121. [DOI] [PubMed] [Google Scholar]
- 113. Redd MJ, Cooper L, Wood W, et al. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hünerwadel A, Fagagnini S, Rogler G, et al. Severity of local inflammation does not impact development of fibrosis in mouse models of intestinal fibrosis. Sci Rep. 2018;8:15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wu F, Chakravarti S.. Differential expression of inflammatory and fibrogenic genes and their regulation by NF-kappaB inhibition in a mouse model of chronic colitis. J Immunol. 2007;179:6988–7000. [DOI] [PubMed] [Google Scholar]
- 116. Hinz B, Lagares D.. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16:11–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Georges PC, Hui JJ, Gombos Z, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–G1154. [DOI] [PubMed] [Google Scholar]
- 118. Stewart DC, Berrie D, Li J, et al. Quantitative assessment of intestinal stiffness and associations with fibrosis in human inflammatory bowel disease. Plos One. 2018;13:e0200377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Maconi G, Sampietro GM, Cristaldi M, et al. Preoperative characteristics and postoperative behavior of bowel wall on risk of recurrence after conservative surgery in Crohn’s disease: a prospective study. Ann Surg. 2001;233:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yamamoto T, Fazio VW, Tekkis PP.. Safety and efficacy of strictureplasty for Crohn’s disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50:1968–1986. [DOI] [PubMed] [Google Scholar]
- 121. Li C, Kuemmerle JF.. The fate of myofibroblasts during the development of fibrosis in Crohn’s disease. J Dig Dis. 2020;21:326–331. [DOI] [PubMed] [Google Scholar]
- 122. Sadler T, Bhasin JM, Xu Y, et al. Genome-wide analysis of DNA methylation and gene expression defines molecular characteristics of Crohn’s disease-associated fibrosis. Clin Epigenetics. 2016;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li C, Grider JR, Murthy KS, et al. Endoplasmic reticulum stress in subepithelial myofibroblasts increases the TGF-β1 activity that regulates fibrosis in Crohn’s Disease. Inflamm Bowel Dis. 2020;26:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): 2 randomised trials. Lancet. 2011;377:1760–9. [DOI] [PubMed] [Google Scholar]
- 125. Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. [DOI] [PubMed] [Google Scholar]
- 126. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. [DOI] [PubMed] [Google Scholar]
- 127. Truffi M, Sorrentino L, Monieri M, et al. Inhibition of fibroblast activation protein restores a balanced extracellular matrix and reduces fibrosis in Crohn’s Disease strictures ex vivo. Inflamm Bowel Dis. 2018;24:332–345. [DOI] [PubMed] [Google Scholar]