Abstract
Hot-iron branding uses thermal injury to permanently identify cattle causing painful tissue damage. The primary objective was to examine the physiological and behavioral effects of oral meloxicam (MEL), compared to a control, administered at the time of hot-iron branding in Angus and Hereford steers and heifers. The secondary objectives were to investigate breed and sex effects on pain biomarkers. A total of 70 yearlings, consisting of 35 heifers and 35 steers (Angus, Hereford, or Angus × Hereford), were enrolled in the study. Animals were blocked by sex, randomized across weight, and assigned to receive MEL (1 mg/kg) or a placebo (CON). Biomarkers were assessed for 48 h after branding and included infrared thermography (IRT), mechanical nociceptive threshold (MNT), accelerometry and a visual analog scale (VAS), and serum cortisol and prostaglandin E2 metabolites (PGEM). Wound healing was assessed for 12 wk. Hair samples to quantify cortisol levels were taken prior to and 30 d post-branding. Responses were analyzed using repeated measures with calf nested in treatment as a random effect, and treatment, time, treatment by time interaction, breed, and sex as fixed effects. There was a treatment by time interaction for PGEM (P < 0.01) with MEL having lower values than CON at 6, 24, and 48 h (MEL: 18.34 ± 3.52, 19.61 ± 3.48, and 22.24 ± 3.48 pg/mL, respectively; CON: 32.57 ± 3.58, 37.00 ± 3.52, and 33.07 ± 3.48 pg/mL; P < 0.01). MEL showed less of a difference in maximum IRT values between the branded (2.27 ± 0.29 °C) and control site (3.15 ± 0.29 °C; P < 0.01). MEL took fewer lying bouts at 0–12 h (4.91 bouts ± 0.56) compared with CON (6.87 bouts ± 0.55; P < 0.01). Compared with Hereford calves, Angus calves exhibited greater serum but lower hair cortisol, greater PGEM, more lying bouts, and less healed wound scores at 3, 4, and 5 wk. Compared with heifers, steers exhibited lower PGEM, lower branding site and ocular IRT, higher MNT, and lower plasma meloxicam levels. Steers spent more time lying, took more lying bouts and had greater VAS pain, and more healed wound scores at 5 wk than heifers. Meloxicam administration at branding reduced branding and control site temperature differences and reduced lying bouts for the first 12 h. Breed and sex effects were observed across many biomarkers. Changes from baseline values for IRT, MNT, lying time, step count, VAS pain, and wound scoring all support that branding cattle is painful.
Keywords: branding, breed, cattle, gender, NSAID, pain
Lay Summary
Hot-iron branding uses thermal injury to permanently identify cattle causing painful tissue damage. The primary objective was to examine the effects of oral meloxicam (MEL), compared with a control, administered at the time of hot-iron branding in Angus and Hereford steers and heifers. The secondary objectives were to investigate breed and sex effects on pain biomarkers. A total of 70 yearlings, consisting of 35 heifers and 35 steers (Angus, Hereford, or Angus × Hereford), were enrolled. Animals were assigned to receive MEL or a placebo. Changes from baseline values for infrared thermography (IRT), mechanical nociceptive threshold, lying time, step count, visual analog scale score, and wound scoring all support that hot-iron branding cattle is painful and investigation into analgesic strategies is needed. MEL administration reduced IRT differences from the branding and control site and reduced lying bouts. Breed and sex effects were observed across a wide range of biomarkers and should be considered in future pain studies. The practicality of administering a nonsteroidal anti-inflammatory drug once at the time of branding is attractive. However, a multimodal approach using a combination of analgesics or longer acting analgesic option warrants further investigation to alleviate pain and discomfort caused by hot-iron branding.
Our results supported that hot-iron branding cattle is painful and oral meloxicam administration reduced infrared thermography differences from the branding and control site and reduced lying bouts. Breed and sex effects were observed across a wide range of biomarkers and should be considered in future pain studies.
Introduction
Hot-iron branding permanently identifies cattle via thermal injury causing painful tissue damage. In the most recent USDA survey, 75% of large cattle operations (200+ head) used hot-iron brands (USDA, 2019). Branding is used for identifying imported cattle, disease control, theft prevention, and permanent identification on open range. Hot-iron branding results in second or third degree burn injuries causing cell death and inflammation leading to a local edema (Laycock et al., 2013). Tucker et al. (2014b) showed that only 67% of brand wounds were fully healed after 10 wk.
Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit the cyclooxygenase enzymes, reduce inflammation, and decrease prostaglandin production which attenuates the response of the nervous system to noxious stimuli, which reduces the response to pain (Ochroch et al., 2003). Meloxicam has been shown to attenuate physiologic biomarkers associated with pain, stress, and inflammation across multiple food animal species (Van Engen et al., 2014; Pairis-Garcia et al., 2015; Colditz et al., 2019). Meloxicam reduced indicators of acute pain associated with knife castration along with branding (Meléndez et al., 2018). Pain biomarkers quantified in previous branding studies include cortisol, substance P, mechanical nociceptive threshold (MNT), infrared thermography (IRT), acute phase proteins, activity and pain behaviors, and wound healing (Tucker et al., 2014b; Meléndez et al., 2018). However, the effect of meloxicam on pain biomarkers has not been solely investigated at the time of hot-iron branding.
Meloxicam is a practical analgesic option for producers due to its long half-life of 27 h (Coetzee et al., 2009). The primary objective was to examine the physiological and behavioral effects of oral meloxicam (MEL) administered at the time of hot-iron branding compared with a control. Stafford and Mellor (2005) state that rigorously designed pain studies must make an allowance for animal breed and sex. Based on previous research (Heimbürge et al., 2020; Vesel et al., 2020), the authors hypothesized that differences in hair coat color due to breed would have an effect on hair cortisol concentrations. The secondary objectives were to further characterize the pain associated with branding and investigate the effects of breed and sex on pain biomarkers.
Materials and Methods
This experiment was conducted at the Colorado State University Agricultural Research, Development and Education Center and was reviewed and approved by the Colorado State University Institutional Animal Care and Use Committee (#19-9609A). Branding without any form of pain control is a standard procedure at this facility. Calves were monitored daily throughout the study for signs of swelling, bleeding, or lethargy. If any of these signs had been observed, the attending veterinarian would have been contacted immediately.
Animals and treatments
A total of 70 yearling calves consisting of 35 heifers (mean ± SE BW = 353 ± 6.32 kg) and 35 steers (mean ± SE BW = 373 ± 6.34 kg; Angus, Hereford, and Angus × Hereford) were enrolled onto the study (Table 1). Animals were blocked by sex, randomized across weight from lightest to heaviest, and assigned using the RAND function in Microsoft Excel (Microsoft Excel 2016, Microsoft Corporation, Redmond, WA) to one of two treatment groups, control (CON) or meloxicam (MEL; 1 mg/kg BW per os). Calves were weighed the day prior to branding and meloxicam dosage was determined based upon that measured BW. Meloxicam (Zydus Pharmaceuticals Inc., Pennington, NJ) was administered via 1 or 2 gelatin capsules (Torpac Inc., Fairfield, NJ) containing 15 mg tablets. Meloxicam was administered per os at a dosage of 1 mg/kg via 15 mg tablets, dosages were rounded to the nearest whole tablet (actual calculated dosage 0.999 mg/kg). Calves in the control group received 1 or 2 gelatin capsules (Torpac Inc.) containing the placebo—lactose monohydrate powder (Thermo Fisher Scientific, Waltham, MA) that is the binder in meloxicam tablets. Treatments were administered via a bolus gun at the time of branding. Calves were weighed again 30 d post-branding and individual weights were recorded.
Table 1.
Number of observations by treatment allocation, breed type, and sex calves undergoing hot-iron branding and receiving meloxicam at 1 mg/kg orally (MEL; n = 35) or placebo (CON; n = 35)
| Treatment assignment | Breed type | Sex | n |
|---|---|---|---|
| MEL | Angus | Heifer | 9 |
| MEL | Hereford | Heifer | 8 |
| MEL | Angus | Steer | 10 |
| MEL | Hereford | Steer | 6 |
| MEL | Angus × Hereford | Steer | 2 |
| CON | Angus | Heifer | 7 |
| CON | Hereford | Heifer | 10 |
| CON | Angus × Hereford | Heifer | 1 |
| CON | Angus | Steer | 6 |
| CON | Hereford | Steer | 10 |
| CON | Angus × Hereford | Steer | 1 |
Branding procedure
All animals were restrained in a hydraulic chute for approximately 5 min during branding. The branding site of approximately 400 cm2 was clipped prior to branding. Animals were branded on the left hip with an electric branding iron (L & H Branding Irons, Mandan, ND) by the same trained individual who conducts the branding procedure in calves at this facility annually. The hot branding iron (621 ± 8.49 °C) was placed on the left hip area of each calf for approximately 10–15 s. The branding iron symbol was the letters CSU. A control site was clipped on the opposite hip for IRT and MNT analysis.
Biomarker collection
Baseline sample collection was completed 24 h prior to branding. Biomarkers were then collected 6, 24, and 48 h post-branding.
Blood sampling
Blood samples were collected at the following timepoints: −24, 6, 24, and 48 h post-branding. Blood samples for serum cortisol and prostaglandin E2 metabolite (PGEM) determination were collected from the jugular vein via venipuncture. The whole blood samples were immediately transferred to tubes (Vacutainer, BD Diagnostics, Franklin Lakes, NJ) containing either no additive for cortisol determination or ethylenediaminetetraacetic acid anticoagulant for PGEM determination. Samples were immediately placed on ice after collection, centrifuged within 30 min of collection for 10 min at 1,500 × g, and serum and plasma were placed in cryovials via transfer pipette and stored at −80 °C.
Serum cortisol
Serum cortisol concentrations were determined using a commercially available radioimmunoassay kit (MP Biomedicals, Irvine, CA) following manufacturer specifications with minor modifications; the standard curve was extended to include 1 and 3 ng/mL by diluting the 10 and 30 ng/mL manufacturer-supplied standards, 1:10 respectively. The standard curve ranged from 1 to 300 ng/mL. A low (25 ng/mL) and high (150 ng/mL) quality control (QC) were run at the beginning and end of each set to determine inter-assay variability. Plain 12 × 75 mm polypropylene tubes were used as blank tubes to calculate nonspecific binding. Input for standards, QCs, and samples was adjusted to 50 µL. Samples were incubated at room temperature for 30 min prior to the addition of I-125. Manufacturer instructions were then followed. Tubes were counted on a gamma counter (Wizard2, PerkinElmer, Waltham, MA) for 1 min. The raw data file was then uploaded onto MyAssays Desktop software (version 7.0.211.1238, 21 Hampton Place, Brighton, UK) for concentration determination. Standard curves were plotted as a four parameter logistic curve. Samples with a coefficient of variation (CV) ˃18% were reanalyzed. The CV for the intra-assay variability was 16.7%, and the inter-assay variability was calculated to be 16.0%.
Hair cortisol
Cortisol concentrations were determined from hair samples collected at baseline and 30 d post-branding. Approximately 250 mg of hair was weighed out and cleaned by soaking overnight in ultrapure water and dried at room temperature for 1 d. Hair was then washed 3 times with 5 mL isopropanol with vigorous shaking and allowed to air dry at room temperature for 5 d. Hair samples were then ground with a mortar and pestle and stored for later analysis. Cortisol was extracted by adding 2 mL methanol to 40 mg of ground hair, which was sonicated for 30 min and then incubated for 18 h in a 50 °C hot water shaker running at 200 rpm. The supernatant was transferred to a clean 2 mL microcentrifuge tube and centrifuged at 13,000 × g for 5 min. Then 1.6 mL of the supernatant was transferred to a 13 × 100 mm glass tubes and evaporated using a CentriVap Concentrator (Labconco, Kansas City, MO). Samples were then reconstituted with 200 µL enzyme-linked immunosorbent assay (ELISA) buffer from the Cortisol ELISA kit (Cayman Chemical, Ann Arbor, MI) and ran in triplicate according to manufacturer instructions. Intra-and inter-assay variation were 12.9% and 6.35%, respectively.
Prostaglandin E2 metabolites
PGEM were analyzed using a commercially available ELISA kit (Cayman Chemical) following manufacturer specifications with minor modifications. Sample input was adjusted to 375 µL with 1.5 mL ice-cold acetone added for sample purification. Samples were incubated at −20 °C for 30 min., then centrifuged at 3,000 × g for 5 min. The supernatant was transferred to clean 13 × 100 mm glass tubes and evaporated using a CentriVap Concentrator (Labconco) overnight (approx. 18h). Samples were reconstituted with 375 µL of appropriate kit buffer. A 300 µL aliquot of the reconstituted sample was derivatized with proportionally adjusted kit components. Manufacturer protocol was then followed. Samples were diluted at 1:2 and ran in duplicate. Absorbance was measured at 405 nm after 60 min of development (SpectraMax i3, Molecular Devices, San Jose, CA). Sample results were excluded if the raw read exceeded the raw read of the highest standard (Standard 1; 50 pg/mL) or was below the lowest acceptable standard. The lowest acceptable standard was defined for each individual plate and was identified by excluding standards that had a ratio of absorbance of that standard to the maximum binding of any well (%B/B0) of ≥80% or ≤20%. Any individual sample outside the standard curve, with a %B/B0 outside the 20%–80% range, or a CV ˃15% were reanalyzed. The project average for PGEM intra-assay CV was 17.40% and inter-assay CV was 10.89%.
Infrared thermography
IRT images of the branding site, a control site on the opposite hip, which was also clipped, as well as the left eye capturing the medial canthus were taken at the following timepoints: −24, 6, 24, and 48 h post-branding using a research-grade infrared camera (Fluke TiX580, Fluke Corp, Everett, WA). Images were obtained at a 45 ° angle, 0.5 – 0.75 m from the branding site, control site, and eye. Infrared images were analyzed using research-specific computer software (SmartView v. 4.3, Fluke Thermography, Plymouth, MN) to determine maximum and minimum temperatures. The difference between the temperature of the medial canthus baseline and timepoints following branding were determined and difference between the temperature of the branding and control site were determined for each time point. These differences were used for statistical analysis. The ambient temperature was similar for all study timepoints, thus the authors did not apply an adjustment to temperature recordings. The images were taken in a shaded facility where sunlight did not impact the temperature recordings. No rain events occurred during the study.
Mechanical nociception threshold
The MNT was determined using a handheld algometer (FPX 100, Wagner Instruments, Greenwich, CT) with a 1 cm2 rubber tip at the following timepoints: −24, 6, 24, and 48 h post-branding. Animals were restrained in a hydraulic chute and allowed to stand for all MNT measurements. The MNT was measured at four locations surrounding the brand site and one control location on the opposite hip by applying slow, steadily increasing pressure until the animal responded. The average of three readings for each location at each time point was taken to eliminate bias and the mean used for analysis. The MNT was determined at the four locations in the same order each time, in a clockwise rotation, starting at the top location. The difference between the control site and each location around the brand site were determined for each time point. These differences were used for statistical analysis. The investigator determining the MNT was blinded to treatment and the reading of the algometer, to prevent testing bias. A second investigator recorded algometer readings, to prevent testing bias. A single investigator used the algometer throughout the entire study to alleviate bias, a CV of 0.13% was calculated.
Accelerometer activity
IceTag (IceRobotics Ltd., South Queensferry, Edinburgh, Scotland, UK) accelerometers were placed on the left rear leg of a subset of 30 animals, 12 h prior to branding. Fifteen accelerometers were placed on heifers and were equally and randomly assigned to the two treatment groups. Fifteen accelerometers were placed on steers and were equally and randomly assigned to the two treatment groups. In total, 15 accelerometers were randomly assigned to each of the two treatment groups and 15 accelerometers were assigned to each sex. Animals were chosen using the RAND function in Microsoft Excel (Microsoft Excel 2016). Accelerometers were removed after the 48-h post-branding timepoint and data were downloaded for analysis using IceManager2014 v 3.0 (IceRobotics Ltd.). Accelerometers recorded motion index, standing and lying time, step count, and lying bouts. One accelerometer did not record data throughout the study. Raw data was captured in 15 min increments and summed over 12 h time periods for statistical analysis. Data collection began at the −12 h timepoint at 7:00 p.m., thus 12 h increments were from 7:00 p.m. to 7:00 a.m. and 7:00 a.m. to 7:00 p.m.
Behavior scoring
Behavior scoring was performed by two trained evaluators blinded to treatment prior to calves being moved from their home pen into the working facility at each of the following timepoints: −24, 6, 24, and 48 h post-branding. Animals who were chosen using the RAND function in Microsoft Excel (Microsoft Excel 2016) to have accelerometers placed on their left rear leg were also used for behavior scoring. Calves were observed for a minute and then a visual analog scale (VAS) behavior score was assigned. The VAS used was a 100 mm (10 cm) line anchored at each end by descriptors of “No Pain” or “Severe Pain”. Six parameters were used to assess pain: depression, tail swishing or flicking, stance, head carriage, foot stomping or kicking, and wound licking. No pain was defined on the scale by being alert and quick to show interest, no tail swishing, a normal stance, head held above spine level, absence of foot stomping, and absence of wound licking. Severe pain was defined on the scale by being dull and showing no interest, more than three tail swishes per minute, legs abducted, head held below spine level, numerous stomps, and any wound licking. The evaluator marked the line between the two descriptors to indicate the pain intensity. A millimeter scale was used to measure the score from the zero anchor point to the evaluator’s mark. The mean VAS measures of the two evaluators were combined into one score for statistical analysis. Inter-observer reliability was not calculated.
Wound scoring
Branding site wounds were assessed weekly for 12 wk following branding. Animals were walked through the working facility weekly and a photo was taken of the branding site. Wound healing was then scored by a trained evaluator, blinded to treatment, using a six-point scale adapted from Tucker et al. (2014b). A score of 1 represented all of the initial scab being present, 2: a majority of the brand covered by a scab, 3: minority of the initial scab present, 4: initial scab gone and tissue becoming re-pigmented, 5: secondary scabbing present and majority of tissue re-pigmented, a score of 6 represented no presence of scabbing and 100% re-pigmentation. The same evaluator scored all of the wound photos, comparing each photo to the photos on the six-point scale from Tucker et al. (2014b), intra-observer reliability was not calculated.
Plasma meloxicam
A 1 mg/mL stock solution of MEL (Toronto Research Chemical, North York, ON, Canada) and internal standard (IS) piroxicam (PIR; Toronto Research Chemical, North York, ON, Canada) was prepared in dimethyl sulfoxide and stored at −80 °C. Working solutions of MEL and PIR were prepared fresh daily in 4% phosphoric acid. Standards (ranging from 1 to 100 ng/mL), QCs, and a 100 ng/mL PIR solution were subsequently prepared in 4% phosphoric acid from the working solutions. Plasma collected in lithium heparin tubes was used for MEL determination. Standards, QCs, and samples were prepared in an untreated 96-well plate. A 100 µL aliquot of previously prepared standards and QCs in 4% phosphoric acid was mixed with 100 µL negative control (NEG CTRL) plasma and 100 µL of 100 ng/mL PIR IS. Samples were appropriately diluted in 4% phosphoric acid and a 100 µL aliquot was mixed with 100 µL 4% phosphoric acid and 100 µL of 100 ng/mL PIR IS. An IS control was prepared by mixing 100 µL NEG CTRL plasma, 100 µL 4% phosphoric acid, and 100 µL of 100 ng/mL PIR IS. A 100 µL aliquot of NEG CTRL plasma was mixed with 200 µL 4% phosphoric acid for a NEG CTRL. Samples, QCs, and standards were purified using Oasis HLB PRiME µElution (Waters Corp, Milford, MA) solid-phase extraction (SPE) cartridge and a positive pressure manifold. The mixtures from the 96-well plate were transferred to the SPE cartridge, were washed with 300 µL of 5% methanol, and analytes eluted with 50 µL acetonitrile–methanol (90:10, v/v) into a collection plate. An additional 50 µL of 0.2% formic acid in water was added to each well.
Collection plates were loaded onto an Acquity H Class ultra-performance liquid chromatography (UPLC) system coupled with a Xevo TQ-S tandem mass spectrometer set in ESI positive mode (MS/MS; Waters Corp) and a Vici DBS Mistral EVO-40 nitrogen generator. A Waters C18 HSS T3 50mm column held at 40 °C was used for chromatographic separation. Mobile phase A consisted of 0.1% formic acid in 18.2 MΩ.cm water, while mobile phase B consisted of 0.1% formic acid in acetonitrile with a flow rate of 0.4 mL/min. Run time was 3 min with the following gradient: 90% A from 0 to 0.5 min; 10% A from 0.5 to 2.0 min; and 90% A from 2.01 to 3 min. The quantifying transition for MEL was m/z 352.17→114.93 and the qualifying transition was m/z 352.17→140.98. The quantifying transition for PIR was m/z 332.22→94.94. Data acquisition and analysis were performed using MassLynx and TargetLynx software, respectively (Waters Corp). The standard curve was linear from 1 to 100 ng/mL and the correlation coefficient was accepted if it was at least 0.99. Samples with concentrations above the standard curve linear range were diluted 1:100 with 4% phosphoric acid and reanalyzed. The accuracy and precision of this assay was 101.93% and 1.96%, respectively, in QCs with a CV of 2.00%. Three heifers and one steer in the MEL treatment group did not have sufficient plasma meloxicam concentrations to likely have an analgesic effect (maximum concentration <1,000 ng/mL).
Statistical analyses
Concentrations of serum cortisol and PGEM were log-transformed for normality before statistical analysis. Continuous responses (i.e., IRT, MNT, accelerometer activity, VAS, serum and hair cortisol, and PGEM) were analyzed using a mixed effects model with repeated measures with calf as the experimental unit. Degrees of freedom were estimated using the Satterthwaite approximation. Unstructured repeated measures covariance and correlation matrices were used. Model assumptions were checked using residual by predicted plots. Calves nested in a treatment group were designated as a random effect, with treatment, time, treatment by time interaction, breed, and sex designated as fixed effects. F-tests were utilized for testing significance of main effects and interactions. If significant overall differences were identified, pairwise comparisons were performed using the Tukey honestly significant difference (HSD) test. Categorical responses (wound healing) were analyzed using contingency tables and Fisher’s exact test. Sample sizes of purebred Angus and Hereford calves were large enough to compare breed effects, crossbred Angus × Hereford sample size was not. Statistics were performed using statistical software (JMP Pro 15.1.0, SAS Institute, Inc., Cary, NC). Statistical significance was set a priori at P ≤ 0.05. Data were presented as least squares means.
Results
Outcome measure means are outlined by treatment in Table 2, by breed in Table 3, and by sex in Table 4.
Table 2.
Least squares means (upper and lower 95% confidence interval) of outcome variables by treatment for calves undergoing hot-iron branding and receiving meloxicam at 1 mg/kg orally (MEL; n = 35) or placebo (CON; n = 35)
| Variable | MEL | CON | TRT | TIME | TRT∗TIME |
|---|---|---|---|---|---|
| Mean serum cortisol, ng/mL | 14.27 | 14.37 | 0.73 | <0.01 | 0.86 |
| CI | 11.67 to 16.88 | 11.77 to 16.97 | |||
| Mean hair cortisol, pg/mL | 214.91 | 209.96 | 0.78 | <0.01 | 0.25 |
| CI | 182.19 to 247.62 | 175.58 to 244.35 | |||
| Mean PGEM, pg/mL | 24.73 | 34.54 | <0.01 | <0.01 | <0.01 |
| CI | 18.61 to 30.84 | 28.42 to 40.67 | |||
| Mean max IRT hip difference, °C | 2.28a | 3.15b | <0.01 | <0.01 | 0.39 |
| CI | 1.70 to 2.85 | 2.58 to 3.73 | |||
| Mean max IRT left hip, °C | 34.86 | 34.96 | 0.53 | <0.01 | 0.71 |
| CI | 34.56 to 35.15 | 34.66 to 35.26 | |||
| Mean max ocular IRT, °C | 36.61 | 36.53 | 0.60 | <0.01 | 0.57 |
| CI | 36.33 to 36.89 | 36.25 to 36.81 | |||
| Mean brand site MNT, kg F | 0.66 | 0.65 | 0.92 | <0.01 | 0.63 |
| CI | 0.62 to 0.69 | 0.62 to 0.69 | |||
| Mean control site MNT, kg F | 1.13 | 1.21 | 0.16 | <0.01 | 0.32 |
| CI | 1.03 to 1.24 | 1.11 to 1.31 | |||
| Mean motion index | 5,259 | 5,161 | 0.80 | <0.01 | 0.96 |
| CI | 4,557 to 5,962 | 4,469 to 5,852 | |||
| Mean lying time (proportion) | 0.30 | 0.30 | 0.83 | <0.01 | 0.42 |
| CI | 0.29 to 0.31 | 0.29 to 0.31 | |||
| Mean step count | 1,097 | 1,074 | 0.77 | <0.01 | 0.94 |
| CI | 951 to 1,243 | 930 to 1,217 | |||
| Mean lying bouts | 5.27 | 5.63 | 0.42 | 0.37 | 0.03 |
| CI | 4.46 to 6.07 | 4.84 to 6.42 | |||
| Mean VAS (1–100 mm) | 8.60 | 8.51 | 0.75 | <0.01 | 0.29 |
| CI | 8.10 to 9.09 | 8.00 to 9.01 | |||
| Mean ADG, kg/d | 0.91 | 0.86 | 0.61 | ||
| CI | 0.74 to 1.07 | 1.02 to 0.88 |
PGEM, prostaglandin E2 metabolite concentration; IRT, infrared thermography; MNT, mechanical nociceptive threshold; VAS, visual analog scale; ADG, average daily gain.
Different superscripts indicate significant differences between treatment groups (P ≤ 0.05).
Table 3.
Least squares means (upper and lower 95% confidence interval) of outcome variables by breed (Angus or Hereford) for calves undergoing hot-iron branding and receiving meloxicam at 1 mg/kg orally (MEL; n = 35) or placebo (CON; n = 35)
| Variable | Angus | Hereford | Breed |
|---|---|---|---|
| Mean serum cortisol, ng/mL | 14.81a | 10.69b | <0.01 |
| CI | 12.73 to 16.89 | 8.66 to 12.72 | |
| Mean hair cortisol, pg/mL | 199.75a | 260.23b | <0.01 |
| CI | 173.29 to 226.21 | 234.73 to 285.73 | |
| Mean PGEM, pg/mL | 33.52a | 23.77b | <0.01 |
| CI | 28.61 to 38.43 | 19.01 to 28.53 | |
| Mean max IRT hip difference, °C | 2.46 | 2.59 | 0.63 |
| CI | 2.00 to 2.91 | 2.14 to 3.03 | |
| Mean max IRT left hip, °C | 35.11 | 34.77 | 0.13 |
| CI | 34.87 to 35.35 | 34.54 to 35.00 | |
| Mean max ocular IRT, °C | 36.44 | 36.63 | 0.49 |
| CI | 36.22 to 36.67 | 36.41 to 36.85 | |
| Mean brand site MNT, kg F | 0.67 | 0.69 | 0.13 |
| CI | 0.64 to 0.69 | 0.66 to 0.71 | |
| Mean control site MNT, kg F | 1.16 | 1.21 | 0.72 |
| CI | 1.08 to 1.24 | 1.13 to 1.29 | |
| Mean motion index | 5,381 | 4,870 | 0.43 |
| CI | 4,763 to 5,999 | 4,319 to 5,421 | |
| Mean lying time, Proportion | 0.31 | 0.31 | 0.61 |
| CI | 0.30 to 0.31 | 0.30 to 0.31 | |
| Mean step count | 1,127 | 1,017 | 0.42 |
| CI | 999 to 1,256 | 903 to 1,132 | |
| Mean lying bouts | 6.42a | 5.43b | 0.04 |
| CI | 5.71 to 7.13 | 4.80 to 6.06 | |
| Mean VAS (1–100 mm) | 8.54 | 8.62 | 0.95 |
| CI | 8.09 to 8.98 | 8.22 to 9.02 | |
| Mean ADG (kg/d) | 0.94 | 0.88 | 0.72 |
| CI | 0.81 to 1.08 | 0.75 to 1.01 |
PGEM, prostaglandin E2 metabolite concentration; IRT, infrared thermography; MNT, mechanical nociceptive threshold; VAS, visual analog scale; ADG, average daily gain.
Different superscripts indicate significant differences between treatment groups (P ≤ 0.05).
Table 4.
Least squares means (upper and lower 95% confidence interval) of outcome variables by sex (heifer or steer) for calves undergoing hot-iron branding and receiving meloxicam at 1 mg/kg orally (MEL; n = 35) or placebo (CON; n = 35)
| Variable | Heifer | Steer | Sex |
|---|---|---|---|
| Mean serum cortisol, ng/mL | 14.25 | 14.40 | 0.87 |
| CI | 11.53 to 16.96 | 11.92 to 16.87 | |
| Mean hair cortisol, pg/mL | 209.11 | 215.76 | 0.72 |
| CI | 170.26 to 247.96 | 187.43 to 244.09 | |
| Mean PGEM, pg/mL | 36.14a | 23.13b | 0.02 |
| CI | 29.77 to 42.52 | 17.30 to 28.95 | |
| Mean max IRT hip difference, °C | 2.53 | 2.90 | 0.24 |
| CI | 1.93 to 3.13 | 2.35 to 3.45 | |
| Mean max IRT left hip, °C | 35.44a | 34.38b | <0.01 |
| CI | 35.13 to 35.75 | 34.09 to 34.66 | |
| Mean max ocular IRT, °C | 36.85a | 36.28b | <0.01 |
| CI | 36.56 to 37.15 | 36.02 to 36.55 | |
| Mean brand site MNT, kg F | 0.64 | 0.66 | 0.24 |
| CI | 0.61 to 0.68 | 0.63 to 0.70 | |
| Mean control site MNT, kg F | 1.11a | 1.23b | 0.04 |
| CI | 1.01 to 1.22 | 1.13 to 1.33 | |
| Mean motion index | 5,536 | 4,884 | 0.11 |
| CI | 4,829 to 6,243 | 4,194 to 5,575 | |
| Mean lying time, Proportion | 0.29a | 0.32b | <0.01 |
| CI | 0.28 to 0.30 | 0.31 to 0.33 | |
| Mean step count | 1,161 | 1,009 | 0.07 |
| CI | 1,014 to 1,308 | 865 to 1,153 | |
| Mean lying bouts | 4.68a | 6.22b | <0.01 |
| CI | 3.87 to 5.49 | 5.43 to 7.01 | |
| Mean VAS (1–100 mm) | 8.24a | 8.87b | 0.03 |
| CI | 7.75 to 8.73 | 8.36 to 9.37 | |
| Mean ADG (kg/d) | 0.30a | 1.46b | <0.01 |
| CI | 0.13 to 0.48 | 1.30 to 1.62 |
PGEM, prostaglandin E2 metabolite concentration; IRT, infrared thermography; MNT, mechanical nociceptive threshold; VAS, visual analog scale; ADG, average daily gain.
Different superscripts indicate significant differences between treatment groups (P ≤ 0.05).
Serum cortisol
There was no evidence of a significant treatment effect (P = 0.73) or treatment by time interaction (P = 0.86) for serum cortisol concentrations, but there was evidence of a time effect (P < 0.01). Serum cortisol concentrations were greater at −24 h (18.93 ng/mL, 95% CI: 16.28 to 21.58) compared with 24 and 48 h (12.57 ng/mL, 95% CI: 9.90 to 15.24 ng/mL and 11.97 ng/mL, 95% CI: 9.31 to 14.63 ng/mL, respectively; P < 0.01). Serum cortisol concentrations were greatest at −24 h (18.93 ng/mL, 95% CI: 16.28 to 21.58 ng/mL) and declined from 6 to 48 h following branding, however there were not significant differences between the 6, 24, and 48 h time points (13.82 ng/mL, 95% CI 11.13 to 16.50 ng/mL; 12.57 ng/mL, 95% CI: 9.90 to 15.24 ng/mL; and 11.97 ng/mL, 95% CI: 9.31 to 14.63 ng/mL, respectively; P > 0.06).
There was a significant breed effect (P < 0.01) with Angus calves having greater serum cortisol concentrations (14.81 ng/mL, 95% CI: 12.73 to 16.89 ng/mL) compared with Hereford calves (10.69 ng/mL, 95% CI: 8.66 to 12.72 ng/mL; P = 0.02). There was no evidence of a sex effect on serum cortisol concentrations (P = 0.87).
Hair cortisol
There was no evidence of a significant treatment effect (P = 0.78) or treatment by time interaction (P = 0.25) for hair cortisol concentrations, but there was evidence of a time effect (P < 0.01). Hair cortisol concentrations were greater at baseline (250 pg/mL, 95% CI: 219 to 281 pg/mL) compared with 30 d post-branding (175 pg/mL, 95% CI: 143 to 207 pg/mL; P < 0.01).
There was a significant breed effect (P < 0.01) with Hereford calves having greater hair cortisol concentrations (260 pg/mL, 95% CI: 235 to 286 pg/mL) compared with Angus calves (200 pg/mL, 95% CI: 173 to 226 pg/mL; P < 0.01). There was no evidence of a sex effect on hair cortisol concentrations (P = 0.72).
Prostaglandin E2 metabolites
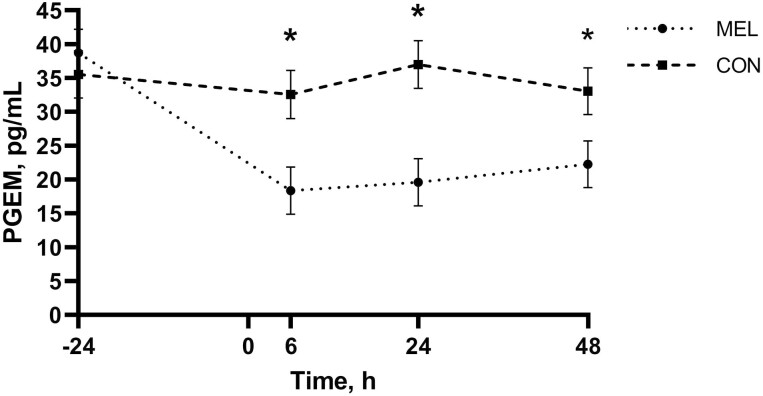
There was evidence of a treatment by time interaction for PGEM concentrations (P < 0.01; Figure 1) with calves in the MEL group having lower concentrations than CON at 6, 24, and 48 h (MEL: 18.34, 19.61, and 22.24 pg/mL, respectively; CON: 32.57, 37.00, and 33.07 pg/mL, respectively; P < 0.01). The MEL group specifically had lower PGEM concentrations at 6, 24, and 48 h (18.34 pg/mL, 95% CI: 11.37 to 25.31 pg/mL; 19.61 pg/mL, 95% CI: 12.71 to 26.50 pg/mL; and 22.24 pg/mL, 95% CI: 15.34 to 29.14 pg/mL, respectively) relative to −24 h (38.72 pg/mL, 95% CI: 31.82 to 45.61 pg/mL; P < 0.01).
Figure 1.
Mean PGEM concentrations measured in pg/mL over the duration of the study for each of the two treatment groups receiving oral meloxicam at 1 m/kg (MEL; n = 35) or placebo (CON; n = 35) following hot-iron branding. Error bars indicate SEM. Asterisk denotes timepoints where a statistically significant difference (P ≤ 0.05) was observed between treatment groups.
There was a significant breed effect with Angus calves exhibiting higher serum PGEM concentrations (33.52 pg/mL, 95% CI: 28.61 to 38.43 pg/mL) relative to Hereford calves (23.77 pg/mL, 95% CI: 19.01 to 28.53 pg/mL; P = 0.02). There was a significant sex effect with heifers exhibiting greater PGEM concentrations (36.14 pg/mL, 95% CI: 29.77 to 42.52 pg/mL) than steers (23.13 pg/mL; 95% CI: 17.30 to 28.95 pg/mL; P < 0.01).
Infrared thermography
There was evidence of a treatment effect (P < 0.01) and time effect (P < 0.01) for maximum IRT values on the left hip (branded side of the calf) minus the right hip (control), but the treatment by time interaction was not significant (P = 0.39). Calves in the MEL group had a smaller difference in maximum temperature between the branded and control site (2.27 °C, 95% CI: 1.70 to 2.85 °C) relative to CON (3.15 °C, 95% CI: 2.58 to 3.73 °C; P < 0.01). Calves showed larger differences at 24 and 48 h (3.87 °C, 95% CI: 3.31 to 4.43 °C and 3.30 °C, 95% CI: 2.74 to 3.85 °C, respectively) compared with −24 and 6 h (1.64 °C, 95% CI: 1.08 to 2.19 °C and 2.06, 95% CI: 1.50 to 2.61 °C, respectively; P < 0.01). There was no evidence of breed or sex effects for the difference in maximum IRT hip readings (P > 0.24).
There was evidence of a time effect for left hip (branded side of the calf) maximum IRT values (P < 0.01) with values at −24 and 6 h (35.33 °C, 95% CI: 34.91 to 35.76 °C and 35.79 °C, 95% CI: 35.37 to 36.22 °C, respectively) being greater than at 24 and 48 h (34.25 °C, 95% CI: 33.83 to 34.68 °C and 34.25 °C, 95% CI: 33.82 to 34.68 °C; P < 0.01). There was no evidence of a treatment (P = 0.53), treatment by time interaction (P = 0.71), or breed effect (P = 0.13) for left hip maximum IRT values. There was evidence of sex effect (P < 0.01) with heifers having greater left hip maximum IRT values (35.44 °C, 95% CI: 35.13 to 35.57 °C) compared with steers (34.38 °C, 95% CI: 34.09 to 34.66 °C).
There was evidence of a time effect for maximum ocular temperatures (P < 0.01) with values at −24 and 6 h (37.44 °C, 95% CI: 37.12 to 37.76 °C and 37.08 °C, 95% CI: 36.77 to 37.40 °C, respectively) being greater than at 24 and 48 h (35.74 °C, 95% CI: 35.42 to 36.06 °C and 36.01 °C, 95% CI: 35.69 to 36.33 °C, respectively; P < 0.01). There was no evidence of a treatment (P = 0.60), treatment by time interaction (P = 0.57), or breed effect (P = 0.49) for maximum ocular IRT values. There was evidence of sex effect (P < 0.01) with heifers having maximum ocular IRT values (36.85 °C, 95% CI: 36.56 to 37.15 °C) compared with steers (36.28 °C, 95% CI: 36.02 to 36.55 °C).
Mechanical nociception threshold
There was evidence of a time effect for mean brand site MNT values (P < 0.01) with thresholds at 6 and 24 h (0.56 kg F, 95% CI: 0.52 to 0.59 kg F and 0.55 kg F, 95% CI: 0.51 to 0.59 kg F, respectively) being lower than at −24 and 48 h (0.80 kg F, 95% CI: 0.76 to 0.83 kg F and 0.71 kg F, 95% CI: 0.68 to 0.75 kg F, respectively; P < 0.01). There was no evidence of a treatment (P = 0.92), treatment by time interaction (P = 0.63), breed (P = 0.13), or sex effect (P = 0.24) for mean brand site MNT values.
There was evidence of a time effect for mean control site MNT values (P < 0.01) with the threshold at 48 h (1.33 kg F, 95% CI: 1.21 to 1.46 kg F) being greater than at all other timepoints (−24, 6, and 24 h; 1.03 kg F, 95% CI: 0.91 to 1.1.6; 1.20 kg F, 95% CI: 1.08 to 1.32 kg F; and 1.12 kg F, 95% CI: 1.00 to 1.25 kg F, respectively; P < 0.03). There was no evidence of a treatment (P = 0.16), treatment by time interaction (P = 0.32), or breed effect (P = 0.72) for mean control site MNT values. There was a sex effect (P = 0.04) with steers having a greater mean control site threshold (1.23 kg F, 95% CI: 1.13 to 1.33 kg F) compared with heifers (1.11 kg F, 95% CI: 1.01 to 1.22 kg F).
Accelerometer
There was evidence of a time effect for motion index (P < 0.01). Motion index at 0 to 12 h (13,063, 95% CI: 12,373 to 13,754) was greater than at all other time points (P < 0.01) and at 24 to 36 h (7,692, 95% CI: 7,002 to 8,383) was greater than at baseline (−12 to 0 h), 12 to 24, and 36 to 48 h (2,229, 95% CI: 1,539 to 2,920; 1,489, 95% CI: 799 to 2,180; and 1,578, 95% CI: 888 to 2,269, respectively; P < 0.01). There was no evidence of a treatment (P = 0.80), treatment by time interaction (P = 0.96), breed (P = 0.43), or sex effect (P = 0.11) for motion index.
There was evidence of a time effect for standing and lying time (P < 0.01). A greater proportion of time was spent lying than standing at 12 to 24 and 36 to 48 h (0.40 and 0.40, 95% CI: 0.39 to 0.42) relative to −12 to 0, 0 to 12, and 24 to 36 h (0.35, 95% CI: 0.34 to 0.37; 0.12, 95% CI: 0.13 to 0.16; and 0.21, 95%: 0.20 to 0.23, respectively; P < 0.01). There was no evidence of a treatment (P = 0.83), treatment by time interaction (P = 0.42), or breed effect (P = 0.61) for standing and lying time. There was a sex effect (P < 0.01) with steers spending a greater proportion of time lying (0.32, 95% CI: 0.31 to 0.33) relative to heifers (0.29, 95% CI: 0.28 to 0.30).
There was evidence of a time effect for step count (P < 0.01) with calves taking less steps at 12 to 24 and 36 to 48 h (347 steps, 95% CI: 206 to 488 steps and 360 steps, 95% CI: 219 to 500 steps) compared with −12 to 0, 0 to 12, and 24 to 36 h (551 steps, 95% CI: 410 to 691 steps; 2,569, 95% CI: 2,428 to 2,710 steps; and 1,600 steps, 1,459 to 1,741 steps, respectively; P < 0.01). There was no evidence of a treatment (P = 0.77), treatment by time interaction (P = 0.94), breed (P = 0.42), or sex effect (P = 0.07) for step count.
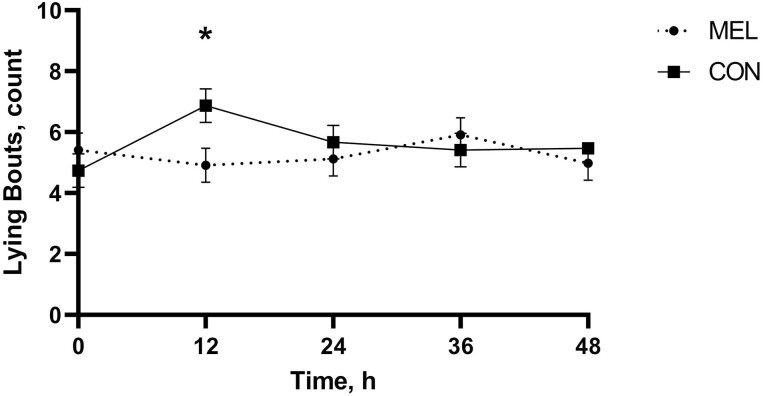
There was evidence of a treatment by time interaction for lying bouts (P = 0.03; Figure 2). CON calves took more lying bouts at 0 to 12 h (6.87 bouts, 95% CI: 5.78 to 796 bouts) relative to −12 to 0 h (4.74 bouts, 95% CI: 3.65 to 5.83 bouts; P = 0.03). Calves in the MEL group took fewer lying bouts at 0 to12 h (4.91 bouts, 95% CI: 3.79 to 6.03 bouts) compared with CON (6.87 bouts, 95% CI: 5.78 to 7.96; P < 0.01). There was a breed effect (P = 0.04) with Angus calves taking more lying bouts (6.42 bouts, 95% CI: 5.71 to 7.13 bouts) relative to Hereford calves (5.43 bouts, 95% CI: 4.80 to 6.06 bouts). There was evidence of sex effect (P < 0.01) with steers taking more lying bouts (6.22 bouts, 95% CI: 5.43 to 7.01 bouts) compared with heifers (4.70 bouts, 95% CI: 3.87 to 5.49 bouts).
Figure 2.
Mean lying bouts over the duration of the study for each of the two treatment groups receiving MEL at 1 m/kg (n = 35) or placebo (CON; n = 35) following hot-iron branding. Error bars indicate SEM. Asterisk denotes timepoints where a statistically significant difference (P ≤ 0.05) was observed between treatment groups.
Visual analog scale
There was a time effect for VAS score (P < 0.01) with pain scores being lowest at −24 h (4.64 mm, 95% CI: 3.97 to 5.32 mm) compared with 6, 24, and 48 h (7.48 mm, 95% CI: 6.80 to 8.17 mm; 11.13 mm, 95% CI: 10.44 to 11.81 mm; and 10.95 mm, 95% CI: 10.27 to 11.64 mm, respectively; P < 0.01). Scores at 6 h (7.48 mm, 95% CI: 6.80 to 8.17 mm) were also lower than at 24 and 48 h (11.13 mm, 95% CI: 10.44 to 11.81 mm and 10.95 mm, 95% CI: 10.27 to 11.64 mm, respectively; P < 0.01). There was no evidence of a treatment (P = 0.75), treatment by time interaction (P = 0.28), or breed effect (P = 0.95) on VAS score. There was a sex effect (P = 0.03) with steers having higher mean VAS scores (8.87 mm, 95% CI: 8.36 to 9.37 mm) compared with heifers (8.23 mm, 95% CI: 7.75 to 8.73 mm).
Wound healing
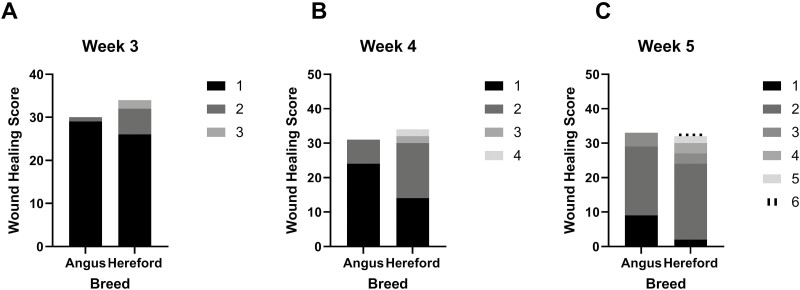
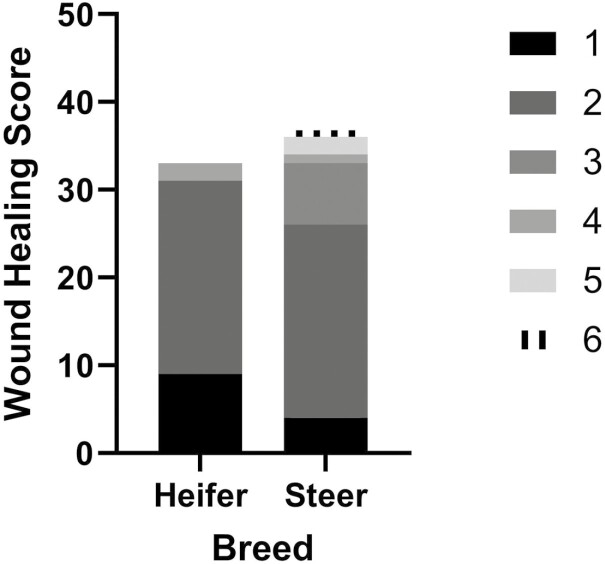
There was no evidence of a treatment effect for wound scoring (P = 0.69). There was evidence of a breed effect on wound scoring at 3, 4, and 5 wk (P < 0.05; Figure 3) with Hereford calves having greater wound scores (more healed) at 3, 4, and 5 wk (3 wk—score 2: 6, score 3: 2; 4 wk—score 2: 16, score 3: 2, score 4: 2; 5 wk—score 2: 22, score 3: 3, score 4: 3, score 5: 2, score 6: 1) compared with Angus calves (3 wk—score 2: 1, score 3: 0; 4 wk—score 2: 7, score 3: 0, score 4: 0; 5 wk—score 2: 20, score 3: 4, score 4: 0, score 5: 0, score 6: 0). There was a sex effect on wound scoring at 5 wk (P = 0.01; Figure 4) with steers having greater wound scores (more healed—score 2: 22, score 3: 7, score 4: 1, score 5: 2, score 6: 1) than heifers (score 2: 22, score 3: 0, score 4: 2, score 5: 0, score 6: 0).
Figure 3.
Individual wound healing scores for each of the two breed types following hot-iron branding for week 3 (3.A), 4 (3.B), and 5 (3.C) when significant differences (P < 0.05) between breeds were observed.
Figure 4.
Individual wound healing scores by sex following hot-iron branding for week 5 when a significant sex effect (P = 0.01) was observed.
Average daily gain
There was no evidence of a treatment (P = 0.61) or breed effect (P = 0.72) for average daily gain (ADG) for the 30-d following branding. There was evidence of a sex effect (P < 0.01) with steers having a greater ADG (1.46 kg/d, 95% CI: 1.30 to 1.62 kg/d) compared with heifers (0.30 kg/d, 95% CI: 0.13 to 0.48 kg/d).
Plasma meloxicam
Plasma meloxicam level means at 6 h post-branding were 2,029 ng/mL (95% CI: 1,517 to 2,541 ng/mL), at 24 h were 3,420 ng/mL (95% CI: 2,908 to 3,932 ng/mL), and at 48 h were 1,798 ng/mL (95% CI: 1,285 to 2,310 ng/mL). There was no evidence of a breed effect on plasma meloxicam levels (P = 0.98). There was evidence of a sex effect (P = 0.05) with heifers having greater plasma meloxicam levels (2,748 ng/mL; 95% CI: 2,102 to 3,393 ng/mL) compared with steers (2,083 ng/mL; 95% CI: 1,554 to 2,613 ng/mL; P = 0.05).
Discussion
Globally, branding ranges from being banned or limited to freeze branding, to still being a very common industry practice in many parts of the world (Spoolder et al., 2016; Adcock et al., 2018; Müller et al., 2018). Branding is not mandatory in the majority of states in the United States but is required for cattle imported unless they are for immediate slaughter from Canada; two forms of identification are required for cattle from Mexico, with a brand typically being one form (AVMA, 2011; USDA-APHIS, 2018; USDA-APHIS-VS, 2018). Brand inspections are often required for cattle to be sold or cross state lines and branding is generally required for cattle grazing open range (Patent, 2018; Utah Department of Agriculture, 2020). Previous studies have quantified pain from hot-iron branding (Lay et al., 1992; Schwartzkopf-Genswein et al., 1997) and have begun to investigate analgesic strategies (Tucker et al., 2014b; Meléndez et al., 2018; Moreno Berggren, 2019). Meloxicam has shown promise in reducing physiologic pain biomarkers following a combination of castration and branding procedures (Meléndez et al., 2018), and has been evaluated in cattle branded on the jaw (Moreno Berggren, 2019) which is not a common practice in the United States.
Changes from baseline in IRT, MNT, lying time, step count, VAS pain score, and wound scoring from the current study all support that hot-iron branding cattle is painful. The greatest serum cortisol levels observed were at baseline and 6 h after branding with calves likely becoming more acclimated to moving through the chute at subsequent time points and thus exhibiting a reduced stress response. The first collection time point following branding was at 6 h which did not allow for an acute cortisol response to be captured, if it did exist. Previous research has shown a serum cortisol spike 20 and 40 min following branding (Schwartzkopf-Genswein et al., 1997) which were timepoints not assessed in the current study. Greater hair cortisol values were observed at baseline which likely represented an additive effect of multiple potentially stressful and painful events over time, compared with 30 d post-branding hair cortisol values. A treatment effect was not observed at the 30 d timepoint indicating that meloxicam administration did not have a significant effect on hair cortisol values compared with controls. Calves who received meloxicam had lower PGEM concentrations than controls at 6, 24, and 48 h which is consistent with previous findings suggesting that NSAIDs reduce prostaglandin E2 concentrations over the duration of action of the drug (Stock et al., 2016; Martin et al., 2021).
Calves in the MEL group had less of a difference in IRT readings between the branding and control site on their opposite hip than CON. The branding site IRT readings were higher at −24 and 6 h relative to 24 and 48 h. Previous findings have shown that hot-iron branding caused elevated readings at the branding site 168 h post-branding (Schwartzkopf-Genswein and Stookey, 1997) which is beyond the duration of the present study. Differences in pressure sensitivity of the branding site have been shown to be most pronounced in the days immediately following branding but lasting out to 71 d (Tucker et al., 2014b). In the present study, the lowest nociceptive thresholds were observed at 6 and 24 h following branding around the branding site. The control site threshold was greatest at the end of the study indicating that calves had not become sensitized to the algometer.
There was evidence of a diurnal effect in accelerometer activity with calves being more active during the 0–12 h and 24–36 h daytime increments. The −12 to 0, 12 to 24, and 36 to 48 h increments corresponded with the same time of day. Calves spent more time lying and took less steps at 12–24 and 36–48 h compared with baseline and CON calves took more lying bouts than MEL from 0 to 12 h indicating that increased lying time and lying bouts may have been due to calves being uncomfortable or painful. Previous literature has shown that calves who received an NSAID (flunixin IV) spent less time lying on the day of branding (Tucker et al., 2014b). This was not observed in the present study which may be due to route of administration and thus different pharmacokinetics. VAS scores based on pain behavior were greatest at 24 and 48 h indicating that calves were still painful at the end of the study sampling period.
Steers and heifers were wound scored until 8 wk following branding, the heifers were then turned out to pasture and the steers continued to be scored out to 12 wk following branding. At 8 wk, wound scores ranged from 2 to 6 with the majority of calves being scored 3–6. At 11 wk, all the steers scored a 5 or 6 except one calf. At 12 wk, all the steers scored a 5 or 6 with 17 calves scoring a 6, and 2 calves scoring a 5, indicating that at 12 wk nearly all of the branding sites were completely healed. These results show consistencies in healing time and associated scores outlined in Tucker et al. (2014a, 2014b). However, in the previously mentioned studies, branding sites were beginning to be fully healed at 8 wk, whereas branding sites were identified as fully healed beginning at 6 wk in the current study. Differences in wound healing time may be due to variability in the branding procedure such as branding iron temperature and length of time the iron touched the animal.
Meloxicam has been shown to have a positive effect on weight gain 5 d immediately following branding (Moreno Berggren, 2019). A second weight was recorded for animals in the current study 30 d following branding and ADG was calculated. No statistical differences were observed between treatments, though the MEL group ADG was numerically larger. Further investigation via a study with power designed to detect performance differences following NSAID administration at branding is warranted.
Plasma meloxicam levels were greatest at 24 h following administration. The 24 h mean was greater than that reported by Van Engen et al. (2014) at 24 h following MEL administration at 1 mg/kg in similarly sized calves who were transported to the feedyard following dosing. The level of activity, stress, hydration, fed, and metabolic state may have differed between calves in the current study and those observed by Van Engen et al. (2014) which are all factors that could influence plasma meloxicam levels. Coetzee et al. (2009) investigated the bioavailability of MEL in Holstein calves relative to intravenous administration and found that MEL demonstrated excellent bioavailability when corrected for dose.
Stafford and Mellor (2005) make the point that rigorously designed pain studies must make an allowance for animal breed and sex. Breed differences were observed for serum and hair cortisol, PGEM, lying bouts, and wound healing scoring in the present study. Angus calves had greater serum cortisol but lower hair cortisol concentrations, higher PGEM concentrations, took more lying bouts, and had lower (less healed) wound healing scores at 3, 4, and 5 wk following branding compared with Hereford calves. Breed differences were not observed by Schwartzkopf-Genswein et al. (1997) but a smaller sample size and larger pool of breed types was used in that study. Past studies have consistently found differences in cortisol concentrations between black and white hair color (Heimbürge et al., 2020; Vesel et al., 2020). The impact of pigmentation differences on hair cortisol and wound healing warrants further investigation.
Sex differences were observed for PGEM, branding site and ocular IRT, MNT, lying time, lying bouts, VAS, wound healing scoring, ADG, and plasma meloxicam levels. Steers had lower PGEM concentrations, lower branding site and ocular temperatures, a higher nociceptive threshold, spent more time lying and took more lying bouts, had higher VAS scores, had higher wound scores (healed more quickly) at 5 wk, had higher ADG, and lower plasma meloxicam levels than heifers. Hair cortisol samples from 14 heifers were not able to be included in the analysis, negatively impacting sample size, thus if a sex difference in hair cortisol levels did exist, there was likely insufficient power to detect it. Three heifers and one steer in the MEL treatment group did not have sufficient plasma meloxicam concentrations to likely have an analgesic effect. It is probable that these calves regurgitated a portion of the meloxicam tablets contained in the bolus. Human research has shown that male subjects have higher pain thresholds and tolerance, are less discriminative between painful sensations, and the NSAID ibuprofen has been shown to be less effective in women (Walker and Carmody, 1998; Vallerand and Polomano, 2000). The males in this study were castrated which may have influenced sex differences. The need for further investigation into whether these differences exist in cattle among intact males, castrated males, and females is apparent to better characterize and alleviate pain.
These data suggest that meloxicam administration at hot-iron branding reduced the difference in temperature between the branding and control site and reduced lying bouts for the first 12 h following branding. However, meloxicam administration alone did not have an effect on the majority of pain biomarkers collected in the present study. The practicality of administering an NSAID once at the time of branding is attractive. However, a multimodal approach using a combination of analgesics or longer acting analgesic option warrants further investigation to alleviate pain and discomfort caused by hot-iron branding.
Conclusions
These results show that IRT, MNT, lying time, step count, VAS score, and wound scoring all support that hot-iron branding cattle is painful and investigation into analgesic strategies is needed. MEL administration reduced IRT differences from the branding and control site and reduced lying bouts. Breed and sex effects were observed across a wide range of biomarkers and should be considered in future pain studies. The need for long-acting analgesic options that demonstrate pain alleviation across multiple biomarkers is apparent and would be beneficial to alleviating pain from routine husbandry procedures like branding.
Acknowledgments
This project was supported by the College of Veterinary Medicine at Kansas State University. Miriam Martin is a Foundation for Food and Agriculture Research (FFAR) fellow supported by grant number 548795. Drs. Kleinhenz and Coetzee are supported by the Agriculture and Food Research Initiative Competitive, grant numbers 2017-67015-27124, 2020-67030-31479, 2020-67015-31540, 2020-67015-31546, and 2021-67015-34084 from the USDA National Institute of Food and Agriculture.
Glossary
Abbreviations
- CON
control
- CV
coefficient of variation
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- IRT
infrared thermography
- MEL
oral meloxicam
- MNT
mechanical nociceptive threshold
- NEG CTRL
negative control
- NSAID
nonsteroidal anti-inflammatory drug
- PGEM
prostaglandin E2 metabolite
- QC
quality control
- RIA
radioimmunoassay
- UPLC
ultra-performance liquid chromatography
- VAS
visual analog scale
Conflict of interest statement
The authors do not have any conflicts of interest to disclose.
Literature Cited
- Adcock, S. J., Tucker C. B., Weerasinghe G., and Rajapaksha E.. . 2018. Branding practices on four dairies in Kantale, Sri Lanka. Animals. 8:137. doi: 10.3390/ani8080137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVMA. 2011. Welfare implications of hot-iron branding and its alternatives. [accessed September 30, 2021]. https://www.avma.org/resources-tools/literature-reviews/welfare-implications-hot-iron-branding-and-its-alternatives#references.
- Coetzee, J. F., KuKanich B., Mosher R., and Allen P. S.. . 2009. Pharmacokinetics of intravenous and oral meloxicam in ruminant calves. Vet. Ther. 10:E1–E8. [PubMed] [Google Scholar]
- Colditz, I. G., Paull D. R., Lloyd J. B., Johnston L., and Small A. H.. . 2019. Efficacy of meloxicam in a pain model in sheep. Aust. Vet. J. 97:23–32. doi: 10.1111/avj.12779 [DOI] [PubMed] [Google Scholar]
- Heimbürge, S., Kanitz E., Tuchscherer A., and Otten W.. . 2020. Within a hair’s breadth–factors influencing hair cortisol levels in pigs and cattle. Gen. Comp. Endocrinol. 288:113359. doi: 10.1016/j.ygcen.2019.113359 [DOI] [PubMed] [Google Scholar]
- Keisha Patent. 2018. Branding in Nebraska: a fresh look at an old practice. [accessed February 28, 2022]. https://nebraskalegislature.gov/pdf/reports/research/snapshot_branding_2018.pdf
- Lay, D. C., Jr, Friend T. H., Randel R. D., Bowers C. L., Grissom K. K., and Jenkins O. C.. . 1992. Behavioral and physiological effects of freeze or hot-iron branding on crossbred cattle. J. Anim. Sci. 70:330–336. doi: 10.2527/1992.702330x [DOI] [PubMed] [Google Scholar]
- Laycock, H., Valente J., Bantel C., and Nagy I.. . 2013. Peripheral mechanisms of burn injury-associated pain. Eur. J. Pharmacol. 716:169–178. doi: 10.1016/j.ejphar.2013.01.071 [DOI] [PubMed] [Google Scholar]
- Martin, M. S., Kleinhenz M., Viscardi A., Curtis A., Johnson B., Montgomery S., and Coetzee J.. . 2021. A comparison of local anesthetic effectiveness in reducing pain associated with dehorning in dairy calves. Omaha (NE): American Society of Animal Science Midwest Meeting. [Google Scholar]
- Meléndez, D. M., Marti S., Pajor E. A., Moya D., Gellatly D., Janzen E. D., and Schwartzkopf-Genswein K. S.. . 2018. Effect of subcutaneous meloxicam on indicators of acute pain and distress after castration and branding in 2-mo-old beef calves. J. Anim. Sci. 96:3606–3621. doi: 10.1093/jas/sky245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Berggren, A. 2019. Impact of analgesia on health status and productivity of calves during hot-iron branding in Mato Grosso. Uppsala (Brazil): Swedish University of Agricultural Sciences. [Google Scholar]
- Müller, B. R., Soriano V. S., de Oliveira Sans E. C., Schnaider M. A., and Molento C. F. M.. . 2018. Perception of beef cattle producers in the state of Paraná regarding animal identification by hot iron branding. Rev. Acad. Ciênc. Anim. 16:1–10. doi: 10.7213/1981-4178.2018.161002 [DOI] [Google Scholar]
- Ochroch, E. A., Mardini I. A., and Gottschalk A.. . 2003. What is the role of NSAIDs in pre-emptive analgesia? Drugs 63:2709–2723. doi: 10.2165/00003495-200363240-00002 [DOI] [PubMed] [Google Scholar]
- Pairis-Garcia, M. D., Johnson A. K., Abell C. A., Coetzee J. F., Karriker L. A., Millman S. T., and Stalder K. J.. . 2015. Measuring the efficacy of flunixin meglumine and meloxicam for lame sows using a GAITFour pressure mat and an embedded microcomputer-based force plate system. J. Anim. Sci. 93:2100–2110. doi: 10.2527/jas.2014-8796 [DOI] [PubMed] [Google Scholar]
- Schwartzkopf-Genswein, K., and Stookey J.. . 1997. The use of infrared thermography to assess inflammation associated with hot-iron and freeze branding in cattle. Can. J. Anim. Sci. 77:577–583. doi: 10.4141/A97-019 [DOI] [Google Scholar]
- Schwartzkopf-Genswein, K., Stookey J., de Passillé A., and Rushen J.. . 1997. Comparison of hot-iron and freeze branding on cortisol levels and pain sensitivity in beef cattle. Can. J. Anim. Sci. 77:369–374. doi: 10.4141/A96-127 [DOI] [Google Scholar]
- Spoolder, H., Schone M., and Bracke M.. . 2016. Initiatives to reduce mutilations in EU livestock production. [accessed February 28, 2022]. https://edepot.wur.nl/374964.
- Stafford, K. J., and Mellor D. J.. . 2005. The welfare significance of the castration of cattle: a review. N. Z. Vet. J. 53:271–278. doi: 10.1080/00480169.2005.36560 [DOI] [PubMed] [Google Scholar]
- Stock, M. L., Barth L. A., Van Engen N. K., Millman S. T., Gehring R., Wang C., Voris E. A., Wulf L. W., Labeur L., Hsu W. H., . et al. 2016. Impact of carprofen administration on stress and nociception responses of calves to cautery dehorning. J. Anim. Sci. 94:542–555. doi: 10.2527/jas.2015-9510 [DOI] [PubMed] [Google Scholar]
- Tucker, C. B., Mintline E. M., Banuelos J., Walker K. A., Hoar B., Drake D., and Weary D. M.. . 2014a. Effect of a cooling gel on pain sensitivity and healing of hot-iron cattle brands. J. Anim. Sci. 92:5666–5673. doi: 10.2527/jas.2014-7860 [DOI] [PubMed] [Google Scholar]
- Tucker, C. B., Mintline E. M., Banuelos J., Walker K. A., Hoar B., Varga A., Drake D., and Weary D. M.. . 2014b. Pain sensitivity and healing of hot-iron cattle brands. J. Anim. Sci. 92:5674–5682. doi: 10.2527/jas.2014-7887 [DOI] [PubMed] [Google Scholar]
- USDA. 2019. Beef 2017, “Beef cow-calf management practices in the United States, 2017, report 1”. [accessed January 23, 2022]. https://www.aphis.usda.gov/animal_health/nahms/beefcowcalf/downloads/beef2017/Beef2017_dr_PartI.pdf.
- USDA-APHIS. 2018. Branding requirements for bovines imported into the United States from Mexico. In: Animal and plant health inspection service. [accessed January 23, 2022]. https://www.federalregister.gov/documents/2018/12/14/2018-27150/branding-requirements-for-bovines-imported-into-the-united-states-from-mexico.
- USDA-APHIS-VS. 2018. National import export services protocol for the importation of cattle or bison from Canada into the United States. In: Animal and plant health inspection service. [accessed January 23, 2022]. https://www.aphis.usda.gov/regulations/vs/iregs/animals/downloads/ca-protocol-imp-cattle-bison.pdf
- Utah Department of Agriculture. 2020. Utah requirements for the sale and movement of livestock. [accessed January 23, 2022]. https://ag.utah.gov/farmers/animal-industry/brand-inspection-and-registration/utah-requirements-for-the-sale-and-movement-of-livestock/
- Vallerand, A. H., and Polomano R. C.. . 2000. The relationship of gender to pain. Pain Manag. Nurs. 1(3 Suppl 1):8–15. doi: 10.1053/jpmn.2000.9759 [DOI] [PubMed] [Google Scholar]
- Van Engen, N. K., Stock M. L., Engelken T., Vann R. C., Wulf L. W., Karriker L. A., Busby W. D., Lakritz J., Carpenter A. J., Bradford B. J., . et al. 2014. Impact of oral meloxicam on circulating physiological biomarkers of stress and inflammation in beef steers after long-distance transportation. J. Anim. Sci. 92:498–510. doi: 10.2527/jas.2013-6857 [DOI] [PubMed] [Google Scholar]
- Vesel, U., Pavič T., Ježek J., Snoj T., and Starič J.. . 2020. Welfare assessment in dairy cows using hair cortisol as a part of monitoring protocols. J. Dairy Res. 87(S1):72–78. doi: 10.1017/S0022029920000588 [DOI] [PubMed] [Google Scholar]
- Walker, J. S., and Carmody J. J.. . 1998. Experimental pain in healthy human subjects: gender differences in nociception and in response to ibuprofen. Anesth. Analg. 86:1257–1262. doi: 10.1097/00000539-199806000-00023 [DOI] [PubMed] [Google Scholar]