Abstract
Condensed tannins (CT), one of the most ubiquitous compounds in the plant kingdom, can modulate ruminal nutrient metabolism. Objectives were to study potential interactions of CT and polyunsaturated fatty acids (PUFA) on ruminal fermentation, biohydrogenation (BH), and methane production. Ruminal fluid obtained from lactating Holstein Friesian cows was used. All experiments were carried out as a completely randomized design with the same mixed diet: control (60:40 forage:concentrate) without supplement (CON), 2.5% soybean oil (SBO), and SBO + grape seed tannin extract (GSTE) at 0.2%, 0.4%, 0.6%, or 0.8% dietary DM (ST0.2, ST0.4, ST0.6, and ST0.8, respectively). Compared with CON (84.7 mM), total VFA concentration was not affected by SBO, but decreased (P < 0.001) with ST0.8 vs. ST0.6 (75.3 vs. 78.3 mM). Relative to CON, methane production was depressed (P < 0.001) by 17.7% and 28.0% in ST0.4 and ST0.8. The highest (P < 0.001) mean concentrations of c9,t11 CLA and C18:1 t11 were observed with ST0.4 compared with CON, but there was no difference between SBO and CT-containing diets. Disappearance of C18:2 c9,c12 was 49.1% vs. 50.3% in CON vs. SBO, whereas it ranged from 39.9% to 46.3% in CT-containing diets after 2 h incubation (P < 0.001). Concentrations of c9,t11 CLA with supplemental SBO and ST0.8 nearly peaked (P < 0.001) at 2 h incubation, but this fatty acid peaked (P > 0.05) at 6 h incubation and remained higher (P < 0.001; 15.9–17.0 µg/mL) at 24 h incubation with ST0.2, ST0.4, and ST0.6 compared with other diets (13.5–14.5 µg/mL). Compared with CON (50.6 µg/mL), concentration of C18:1 t11 with SBO and CT-containing diets reached a peak (P < 0.001; 241–265 µg/mL) at 12 h incubation. Concentration of C18:0 was 171%–231% higher (P < 0.001) with SBO and CT relative to CON at 24 h incubation. Overall, these results demonstrated that PUFA in SBO are more effective in modulating ruminal BH and CH4 production when combined with CT. However, high doses of added CT can reduce ruminal VFA concentration. Thus, a level of 0.4% GSTE added to diets containing 2.5% PUFA from plant origin might be suitable for optimizing ruminal fermentation and BH of C18:2 c9,c12 to fatty acid intermediates that could have beneficial effects to human health.
Keywords: condensed tannins, fatty acid biohydrogenation, methane production, ruminal fermentation, soybean oil
Lay Summary
Condensed tannins can modulate methane emissions and ruminal biohydrogenation, but effects depend on type and dose. We used an in vitro fermentation system to investigate the effect of increasing doses (0%, 0.2%, 0.4%, 0.6%, and 0.8% dry matter) of grape tannin seed extract (GSTE) in a diet supplemented at 2.5% dry matter with soybean oil on methane production and biohydrogenation. Feeding soybean oil and GSTE at 0.6% and 0.8% reduced content of ruminal volatile fatty acids. Methane production (mL/g dry matter) was lower in the diet containing GSTE at 0.4%. Inclusion of GSTE at 0.2% and 0.4% increased concentration of C18:2 c9,c112, C18:3n3, c9,t11 conjugated linoleic acid and total polyunsaturated fatty acids after 24 h of incubation. The present findings contribute to a better understanding of the effect of condensed tannins from grape seed extract on ruminal fermentation and biohydrogenation.
Feeding 0.4% grape seed tannin extract plus 2.5% soybean oil is suitable for optimizing in vitro ruminal fermentation, methane production, and formation of fatty acid intermediates.
Introduction
The main isomer of conjugated linoleic acid (CLA) in ruminant derived-foods is c9,t11 CLA (rumenic acid, RA), which can be formed directly during biohydrogenation (BH) of C18:2 c9,c12 (linoleic acid, LA) in the rumen or can be synthesized in the animals’ tissues using C18 t11 (vaccenic acid, VA) from the rumen as a precursor. The direct RA formation in the rumen contributes only a minimal amount of the total CLA in the tissues of ruminants (Griinari and Bauman, 1999). Dietary supplementation of linoleic-rich oils is effective for increasing RA and VA in the rumen, but these fatty acids (FA) are converted quickly to C18:0 (stearic acid, SA). Thus, the enrichment of RA in ruminant products via nutrition of the animal requires ideal approaches for enhancing the amount of RA and VA that bypass further metabolism in the rumen.
Tannins are a group of polyphenolic compounds present in forages and other feeds commonly used for feeding ruminants. Condensed tannins (CT) are one of two large groups of tannins, designated as proanthocyanidins, and are one of the most ubiquitous compounds in the plant kingdom (Yoshida et al., 2005). Feed sources containing CT are receiving increased attention among ruminant nutritionists due to the ability of these compounds to interact with ruminal microbiota, which can then affect protein, fiber, and lipid metabolism (Vasta et al., 2019). For instance, feeding high CT-containing feeds inhibited the terminal steps of BH (Khiaosa-Ard et al., 2009; Frutos et al., 2020). Furthermore, CT can also mitigate enteric methane (CH4) emissions from ruminants (Min et al., 2020). Several factors are known to affect activity and effectiveness of dietary tannins on CH4 production and ruminal BH, with type and dose of CT being most-often reported (Carreño et al., 2015; Min et al., 2020; Menci et al., 2021). Inclusion of plants such as Lotus corniculatus, Acacia mearnsii, and quebracho are the most common ways to enhance dietary CT intake by ruminants (Vasta et al., 2019).
Few studies used grape by-products as feed supplements for ruminants. For instance, feeding a combination of 300 g/d grape seed and 220 g/d extruded linseed (as feed) increased C18:1 t11 and c9,t11 CLA proportions in milk of dairy sheep (Correddu et al., 2016). Moate et al. (2014) reported that feeding grape marc at 27% of dietary DM decreased methane production in dairy cows. Recently, Zhang et al. (2020) reported that supplementation of 0.2% DM grape seed tannin extract (GSTE) reduced in vitro methane production. To the best of our knowledge, no study has evaluated potential dose–response effects of grape seed extract rich in CT (95% proanthocyanidins) on methane production and ruminal biohydrogenation both in vitro and in vivo. Thus, the present study aimed to determine in vitro the effect of increasing CT from grape seed tannin extract (GSTE) to a mixed diet containing soybean oil (SBO) on fermentation, CH4 production, and BH. We hypothesized that supplementing a proper dose of GSTE in mixed diets containing added SBO would moderate terminal steps of LA BH and reduce CH4 production with minimal effects on ruminal fermentation.
Materials and Methods
The study was performed at Laboratory of Ruminant Production Techniques, Department of Animal Sciences, College of Agriculture, Can Tho University, Viet Nam. All procedures were performed according to the ethical standards in the Helsinki Declaration of 1975, as revised in 2000, as well as the national law.
Experimental design and diets
Three in vitro experiments were performed in 1) 50-mL bottles to determine ruminal fermentation patterns, 2) 100-mL syringes to determine CH4 production, and 3) 500-mL flasks to determine FA BH. All experiments were carried out as a completely randomized design with the same treatment diets: control diet (60:40 forage to concentrate) without supplement (CON), 2.5% SBO, 2.5% SBO + GSTE at 0.2, 0.4, 0.6 or 0.8% (ST0.2, ST0.4, ST0.6, and ST0.8, respectively). Diets (Table 1) were formulated to meet nutrient requirements of lactating cows (NRC, 2001). Soybean oil was supplemented as a pure product. A commercial GSTE (IBPharco Co., Ltd., Ha Noi, Viet Nam) was added as a source of CT, which contained 95% proanthocyanidins.
Table 1.
Ingredients, chemical composition, and fatty acid profile of diets
| Item1 | Diet2 | |||||
|---|---|---|---|---|---|---|
| CON | SBO | ST0.2 | ST0.4 | ST0.6 | ST0.8 | |
| Ingredient, % DM | ||||||
| Elephant grass | 60.0 | 58.5 | 58.4 | 58.3 | 58.1 | 58.0 |
| Ground corn | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Rice bran | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Soybean meal | 23.6 | 24.7 | 24.8 | 24.9 | 25.0 | 25.0 |
| Copra meal | 4.20 | 2.11 | 1.94 | 1.78 | 1.61 | 1.44 |
| Limestone | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 |
| Dicalcium phosphate | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Salt (NaCl) | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Premix3 | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 |
| Soybean oil | – | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Grape seed tannin extract | – | – | 0.20 | 0.40 | 0.60 | 0.80 |
| Chemical composition, % DM unless otherwise noted | ||||||
| DM | 45.4 | 46.8 | 46.9 | 47.0 | 47.2 | 47.3 |
| OM | 89.0 | 89.2 | 89.0 | 88.8 | 88.6 | 88.4 |
| Ash | 9.88 | 9.72 | 9.71 | 9.69 | 9.68 | 9.67 |
| CP | 16.6 | 16.6 | 16.6 | 16.6 | 16.6 | 16.6 |
| EE | 2.62 | 4.85 | 4.83 | 4.81 | 4.79 | 4.77 |
| NDF | 47.3 | 45.6 | 45.5 | 45.3 | 45.2 | 45.0 |
| ADF | 32.7 | 31.4 | 31.3 | 31.2 | 31.1 | 31.0 |
| ADL | 6.02 | 5.76 | 5.74 | 5.72 | 5.70 | 5.68 |
| NFC | 23.6 | 23.2 | 23.4 | 23.6 | 23.7 | 23.9 |
| ME, Mcal/kg DM | 1.34 | 1.41 | 1.41 | 1.41 | 1.41 | 1.40 |
| Fatty acid profile, g/kg diet DM | ||||||
| C10:0 | 0.52 | 0.40 | 0.39 | 0.38 | 0.37 | 0.36 |
| C12:0 | 2.69 | 1.56 | 1.47 | 1.38 | 1.28 | 1.18 |
| C14:0 | 1.77 | 1.21 | 1.16 | 1.11 | 1.06 | 1.01 |
| C16:0 | 4.46 | 6.56 | 6.54 | 6.52 | 6.50 | 6.48 |
| C18:0 | 1.15 | 1.76 | 1.75 | 1.75 | 1.74 | 1.74 |
| C18:1 c9 | 3.28 | 10.7 | 10.7 | 10.7 | 10.7 | 10.7 |
| C18:2 c9,c12 | 6.50 | 19.3 | 19.3 | 19.3 | 19.2 | 19.2 |
| C18:3n3 | 4.19 | 5.04 | 5.03 | 5.02 | 5.01 | 5.01 |
| SFA | 11.9 | 13.1 | 12.9 | 12.7 | 12.5 | 12.3 |
| UFA | 14.3 | 35.5 | 35.5 | 35.4 | 35.4 | 35.4 |
| MUFA | 3.58 | 11.1 | 11.1 | 11.1 | 11.0 | 11.0 |
| PUFA | 10.8 | 24.4 | 24.4 | 24.4 | 24.3 | 24.3 |
DM, dry matter; OM, organic matter; Ash, total minerals; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; NFC, nonfiber carbohydrate; ME, metabolizable energy; SFA, saturated fatty acids; UFA, unsaturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
CON, control diet without added oil and grape seed tannin extract; SBO, 2,5% soybean oil; ST0.2, ST0.4, ST0.6, and ST0.8, SBO plus either 0.2%, 0.4%, 0.6%, or 0.8% grape seed tannin extract.
Provided per kg of premix including 450,000 IU vitamin A, 70,000 IU vitamin D3, 1,800 mg vitamin E, 320 g Ca, 57 g P, 32.5 g Mg, 11 g Na, 4.5 g Zn, 4.9 g Mn, 1 g Fe, 1 g Cu, 55 mg Se, and 40 mg biotin.
Inoculum and substrates
Ruminal contents were obtained from four lactating Holstein Friesian cows to conduct Exp. 1 and 2, whereas three lactating cows were donors for Exp. 3. Cows were fed fresh elephant grass and a 20% CP-containing concentrate (F:C 60:40, wt/wt on DM basis) twice a day at 8:00 and 17:00 h for 1 wk prior to sampling. Approximately 0.5 liter of ruminal digesta was collected from each cow just before the morning feeding using a stomach tube passed through the mouth. Ruminal fluid was filtered through a metal sieve with a pore size of 1 mm under a stream of CO2 at 39 °C. Donor fluid from each cow was kept separately and served as statistical replicates during in vitro experiments. Ruminal inocula was diluted (1:4, vol/vol) in medium solution prepared according to Menke and Steingass (1988) with minor adjustments. The KH2PO4 in the micromineral solution was reduced from 6.2 to 6.0 g, and the FeCl2.6H2O was increased from 0.8 to 8.0 g. Tryptone, l-cysteine hydrochloride, and sodium sulfide were added at 2.5, 0.313, and 0.313 g, respectively, into 1 liter of medium solution.
Elephant grass was ground throughout a Retsch mill (Cutting Mill SM 100 model, Retsch, Haan, Germany) with a mesh size of 1 mm before in vitro experiments. Feed ingredients were mixed as described in Table 1 and stored at −20 °C until incubations were conducted. Soybean oil was dissolved in absolute ethanol (99.99%) at 1:15 (wt/vol) and used for Exp. 1 and 2, whereas this oil was emulsified with tween 20 solution (P9416, Sigma–Aldrich, USA) for Exp. 3. Chemical composition of the diets is presented in Table 1.
In vitro incubation
In Exp. 1, substrates at 312.5 mg DM were fed into 50-mL bottles and then 125 μL of oil-ethanol solution added, which provided an estimated 7.82 mg of added oil. A 25-mL mixture of ruminal fluid from each donor cow and medium solution were added into each bottle. Bottles were then incubated in a shaking incubator (ISS-4075R, Jeiotech, Korea) at 120 rpm, 39 °C for 24 h. The fermentation reaction was stopped by placing bottles in cold ice, and pH value of the fermentation solution was measured immediately (HI-5522, Hanna Instruments, Inc., USA). The fermented liquor was then filtered through four layers of cheesecloth. Samples used for analyses of NH3-N and VFA concentrations were acidified with 1 M H2SO4 (10:1, vol:vol), centrifuged at 10,000 × g for 15 min. The supernatant was collected and stored at −20 °C. Four replicates in Exp. 1 were run at once.
In Exp. 2, 625 mg DM of substrate was weighed into 100-mL glass syringes and then 250 μL of oil-ethanol solution added, which provided an estimated 15.63 mg of added oil. Two hundred fifty microliters of absolute ethanol without substrates or oil was added to four blank syringes. A 50-mL mixture of ruminal fluid and medium solution was introduced into 100-mL gastight glass syringes. The lower end of the syringes was closed afterwards, and they were incubated in a slow-shaking water bath (WNB 45, Memmert, Germany) at 39 °C for 48 h. Gas volume produced was recorded at 2, 4, 6, 8, 10, 12, 18, 24, 30, 36, 42, and 48 h of incubation. Total gas production was collected into multi-layer foil gas sampling bags (Cat. No: 22952, Restek, USA) and stored until analysis of CH4 concentration. Four replicates in Exp. 2 were run at once.
In Exp. 3, strained ruminal fluid (40 mL) from each cow was added into 500-mL flasks containing warm (39 °C) medium (160 mL) and substrates (2.5 g). Emulsified oil solution was then directly added into the flasks. Cultures were continuously mixed in a slow-shaking water bath (WNB 45, Memmert, Germany) at 39 °C under continuous flushing with CO2 gas for 24 h. Samples for FA analysis (2.5 mL) were taken at 0, 2, 4, 6, 12, and 24 h into glass culture tubes fitted with a teflon-lined screw cap. Reactions were immediately stopped by cooling samples in an ice bath (A24B, Thermo Scientific, USA) and then stored at −20 °C until FA analysis. The experiment was repeated three times on separate days, where there was one bottle used per treatment/cow/day.
Chemical analysis
Feed samples were analyzed for dry matter (DM), organic matter (OM), crude protein (CP), ether extract (EE), and ash using standard methods (AOAC, 1990). Crude protein (N×6.25) was determined by the Kjeldahl method. Ether extract was determined using ether in a Soxtec extraction system. Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were analyzed following the methods of Van Soest et al. (1991). Concentration of ruminal NH3-N was analyzed by the Kjeldahl method (AOAC, 1990). Methane concentration in gas samples was measured via a gas analyzer (GeoTech GA5000, Queensway, UK).
Concentrations of individual volatile fatty acids (VFA) were determined using a gas chromatograph (Thermo Scientific Trace 1310 GC system, Waltham, MA) equipped with a flame ionization detector. The inlet and detector temperatures were maintained at 220 °C. Aliquots (1 μL) were injected with a split ratio of 10:1 into a 30 m × 0.25 mm × 0.25 μm Nukol fused-silica capillary column (Cat. No: 24107, Supelco, Sigma-Aldrich, St. Louis, MO) with helium carrier gas set to a flow rate of 1 mL/min and initial oven temperature at 80 °C. The oven temperature was held constant at the initial temperature for 1 min and thereafter increased at 20 °C/min to a temperature of 180 °C and held for 1 min, and increased at 10 °C/min to a final temperature of 200 °C, and a final run time of 14 min. Individual VFA peaks were identified based on their retention times compared with external standards including acetic, propionic, butyric, valeric, iso-butyric, and iso-valeric acids (Sigma–Aldrich, Louis, MO).
Lipids in feed samples (1 g) were extracted based on the traditional Folch procedure (Folch et al., 1957), with minor modifications as described by Li and Watkins (2001). Incubated samples (2.5 mL) were freeze-dried (Cat. No: 7752040, Labconco, Kansas, MO) prior to FA composition analysis. One milliliter of internal standard (2 mg C19:0/mL hexane) was added to all extracted lipids in feed samples and evaporated to complete dryness under a N2 stream. Dried incubated samples were mixed with 1 mL of internal standard (0.05 mg C21:0/mL toluene). Dried lipids of feed samples were then methylated with 3 mL of NaOH in methanol (0.5 M) followed by 2 mL of acetyl chloride in methanol (1:5 vol/vol), whereas dried samples from incubations were methylated with 1 mL of NaOH in methanol (0.5 M) followed by 1.5 mL of acetyl chloride in methanol (1:5 vol/vol). The FA methyl esters (FAME) were extracted twice with 2 mL of hexane and pooled extracts were evaporated under N2 stream until dryness. The residue was dissolved in 1 mL of hexane and analyzed using a gas chromatograph (Thermo Scientific Trace 1310 GC system, Waltham, MA) equipped with a flame ionization detector. Aliquots (1 μL) were injected at a split ratio of 50:1 into a 100 m × 0.25 mm × 0.25 μm high polar fused silica capillary column (Cat. No: 24056, Supelco Inc., Bellefonte, PA) with helium carrier gas set to a flow rate of 1 mL/min. The initial oven temperature was programmed at 50 °C and held for 4 min, then increased to 120 °C at 10 °C/min, held for 1 min, then increased up to 180 °C at 5 °C/min, held for 18 min, then increased up to 200 °C at 2 °C/min, held for 15 min, and then increased up to 230 °C at 2 °C/min, held for 19 min. The injector and detector temperatures were at 270 °C and 300 °C, respectively. Individual FAME were identified by comparison of retention times with 37 component FAME mix of standard (Cat. No: CRM47885, Supelco Inc, Bellefonte, PA), CLA mix of standard (Cat. No: O5632, Sigma–Aldrich, Louis, MO), and C18:1 t11 standard (Cat. No: CRM46905, Supelco Inc, Bellefonte, PA). Fatty acids in diets are expressed as g/100 g of total FA while FA in the incubated samples are expressed as μg/mL. Concentrations of FA at 0, 2, 4, 6, 12, and 24 h of incubation for each treatment are included in Supplementary Tables 1–6.
Statistical analysis
Data in Exp. 1 and 2 were statistically analyzed with the GLM procedure for completely randomized designs. The statistical model was Yij = μ + Di + εij, where Yij is the dependent variable, μ is the overall mean, Di is the diet effect, and εij is the random residual error. Data in Exp. 3 were statistically analyzed with the MIXED procedure for completely randomized designs with repeated measures (hours). The statistical model used was Yijk= µ + Di + Tj + (D×T)ij + εijk, where Yijk is the dependent variable, µ is the overall mean, Di is the fixed effect of diet, Tj is the fixed effect of incubation time, (D×T)ij is the fixed effect of interaction between diet and incubation time, and εijk is the random residual error. Cow inoculum source was considered as a random factor. The most-suitable covariance structure was homogeneous autoregressive order 1. Significant differences among diet means were statistically compared using Tukey. Significant effect of diet was declared at P < 0.05. Statistical tests were performed using SAS OnDemand for Academics (SAS Institute, Inc., Cary, NC).
Results
Ruminal fermentation and methane production
Ammonia-N concentration ranged from 45.5 to 52.2 mg/dL (Table 2) and did not differ significantly, but SBO and CT-containing diets reduced (P < 0.001) pH after 24 h incubation. Supplementation of SBO alone or in combination with GSTE at 0.2% and 0.4% had no effect on total VFA concentration, but combining SBO and GSTE at 0.6% and 0.8% reduced VFA concentration relative to CON by 7.56% to 11.1% (P < 0.001). Compared with ST0.2 (61.3%), acetate proportion was lower (P < 0.05) with ST0.8 (59.7%). In contrast, compared with CON, increasing levels of GSTE in the diet increased (P < 0.01) propionate proportion, which led to a decrease (P < 0.01) in acetate/propionate with ST0.6 and ST0.8. Compared with CON, increasing supplementation of GSTE in the diet resulted in a linear decrease (P < 0.001) of isobutyrate proportion and a linear increase (P < 0.001) in isovalerate proportion.
Table 2.
Ruminal fermentation characteristics after 24 h incubation
| Item1 | Diet2 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | SBO | ST0.2 | ST0.4 | ST0.6 | ST0.8 | |||
| pH | 6.81c | 6.98bc | 7.04ab | 7.04ab | 7.10a | 7.21a | 0.08 | <0.001 |
| NH3-N, mg/dL | 45.5 | 50.8 | 45.9 | 52.2 | 50.4 | 52.2 | 5.62 | 0.367 |
| Total VFA, mM | 84.7a | 81.0ab | 82.4ab | 80.2abc | 78.3bc | 75.3c | 2.37 | <0.001 |
| Acetate, % | 60.4ab | 60.7ab | 61.3a | 61.0ab | 60.1ab | 59.7b | 0.66 | 0.029 |
| Propionate, % | 19.1c | 19.7bc | 19.6bc | 19.7b | 20.5ab | 21.0a | 0.44 | 0.001 |
| Butyrate, % | 11.5ab | 11.2ab | 11.0b | 11.2ab | 11.7ab | 11.8a | 0.33 | 0.025 |
| Valerate, % | 2.96 | 2.83 | 2.74 | 2.81 | 2.79 | 2.85 | 0.10 | 0.104 |
| Isobutyrate, % | 1.82a | 1.74a | 1.66ab | 1.67ab | 1.53ab | 1.44b | 0.13 | 0.009 |
| Isovalerate, % | 4.26a | 3.84ab | 3.64ab | 3.72ab | 3.41b | 3.22b | 0.31 | 0.003 |
| Acetate/propionate | 3.16a | 3.08ab | 3.13ab | 3.10ab | 2.93bc | 2.84c | 0.09 | 0.001 |
VFA, volatile fatty acids.
CON, control diet without added oil and grape seed tannin extract; SBO, 2.5% soybean oil; ST0.2, ST0.4, ST0.6, and ST0.8: SBO plus either 0.2%, 0.4%, 0.6%, or 0.8% grape seed tannin extract.
Means within a row with different superscripts are significantly different (P < 0.05).
Supplemental SBO did not change total gas production after 48 h incubation (Table 3), but compared with CON (171 mL), ST0.6 and ST0.8 led to lower (P < 0.05) values (157 mL). Total gas production with ST0.8 decreased (P < 0.05) from early stages of incubation (6 h) with this trend remaining until 48 h incubation (Figure 1). Interestingly, CH4 concentration was reduced (P < 0.01) when GSTE was supplemented at 0.8% in the diet. Production of CH4 (mL/g DM) with ST0.4 and ST0.8 was suppressed (P < 0.01) by 17.5% and 28.0% relative to CON (Table 3).
Table 3.
Methane production after 48 h incubation
| Item1 | Diet2 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | SBO | ST0.2 | ST0.4 | ST0.6 | ST0.8 | |||
| Total gas, mL | 171ab | 174a | 172ab | 165ab | 157b | 157b | 7.46 | 0.010 |
| CH4, % | 17.2a | 15.1ab | 14.9ab | 14.7ab | 15.5ab | 13.4b | 1.13 | 0.006 |
| CH4, mL | 29.3a | 26.1ab | 25.7ab | 24.2b | 24.3ab | 21.1b | 2.25 | 0.002 |
| CH4, mL/g DM | 46.8a | 41.8ab | 41.0ab | 38.6b | 38.8ab | 33.7b | 3.61 | 0.002 |
DM, dry matter.
CON, control diet without added oil and grape seed tannin extract; SBO, 2.5% soybean oil; ST0.2, ST0.4, ST0.6, and ST0.8: SBO plus either 0.2%, 0.4%, 0.6%, or 0.8% grape seed tannin extract.
Means within a row with different superscripts are significantly different (P < 0.05).
Figure 1.
Cumulative gas production during 48 h incubation.
Fatty acid profile and biohydrogenation
In general, treatment diets affected (P < 0.05) mean concentrations of some individual FA, but no significant difference was detected between SBO and CT-containing diets (Table 4). Interestingly, there were interactions (P < 0.01; Table 4) between diet and incubation time for the main FA involved in the BH process of LA including RA, VA, and SA. Compared with 0 h, LA concentration in CON, SBO, ST0.2, ST0.4, ST0.6, and ST0.8 after 2 h incubation decreased by 49.1%, 50.3%, 44.9%, 46.3%, 43.7%, and 39.9%, respectively (P < 0.001; Figure 2). At the end of incubation, LA concentration was 10.1 vs. 18.7 µg/mL in CON vs. SBO, whereas in the CT-containing diets concentrations averaged 18.9 vs. 27.0 µg/mL in CON vs. SBO (P < 0.001). Formation of RA increased rapidly at 2 h of incubation (P < 0.001; Figure 3). Concentrations of this FA with SBO and ST0.8 nearly reached a peak at a very early stage of incubation (2 h) and then remained unchanged until 12 h incubation. In contrast, RA concentrations with ST0.2, ST0.4, and ST0.6 peaked at mid stages of incubation (6 h). Furthermore, compared with other diets (13.5–14.5 µg/mL), ST0.2, ST0.4, and ST0.6 led to highest (P < 0.001) RA concentrations (15.9–17.0 µg/mL) at 24 h incubation.
Table 4.
The changes in mean amounts (μg/mL) of fatty acid during 24 h incubation
| Fatty acid1 | Diet2 | SEM | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON | SBO | ST0.2 | ST0.4 | ST0.6 | ST0.8 | D | T | D×T | ||
| C4:0 | 0.12 | 0.13 | 0.12 | 0.11 | 0.10 | 0.15 | 0.02 | 0.299 | 0.003 | 0.016 |
| C6:0 | 0.53b | 0.62b | 0.83a | 0.62b | 0.59b | 0.63b | 0.06 | 0.001 | <0.001 | 0.029 |
| C8:0 | 0.67 | 0.58 | 0.56 | 0.52 | 0.39 | 0.44 | 0.16 | 0.606 | 0.007 | 0.643 |
| C10:0 | 2.65a | 1.81b | 1.90b | 1.78b | 1.81b | 1.69b | 0.18 | <0.001 | <0.001 | 0.002 |
| C11:0 | 0.47ab | 0.39b | 0.46ab | 0.41ab | 0.45ab | 0.41ab | 0.02 | 0.007 | <0.001 | 0.111 |
| C12:0 | 31.8a | 23.9b | 25.3b | 23.4b | 23.9b | 21.8b | 1.41 | <0.001 | <0.001 | 0.101 |
| C13:0 | 1.22 | 1.15 | 1.16 | 1.19 | 1.14 | 1.15 | 0.08 | 0.929 | <0.001 | 0.265 |
| C14:0 | 17.4a | 13.4b | 12.8b | 13.1b | 12.2c | 11.2b | 0.78 | <0.001 | <0.001 | 0.055 |
| C14:1 | 0.04 | 0.03 | 0.04 | 0.04 | 0.04 | 0.03 | 0.01 | 0.766 | <0.001 | 0.205 |
| C15:0 | 8.32 | 7.86 | 11.0 | 9.45 | 8.42 | 9.07 | 1.37 | 0.302 | <0.001 | 0.124 |
| C15:1 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.01 | 0.992 | 0.009 | 0.906 |
| C16:0 | 57.2b | 80.7a | 82.3a | 82.9a | 80.3a | 78.4a | 4.19 | <0.001 | <0.001 | 0.629 |
| C16:1 | 1.34 | 1.34 | 1.39 | 1.38 | 1.40 | 1.34 | 0.14 | 0.994 | <0.001 | 0.003 |
| C17:0 | 1.32 | 1.60 | 1.58 | 1.54 | 1.49 | 1.49 | 0.10 | 0.124 | <0.001 | 0.147 |
| C17:1 | 0.02 | 0.04 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.281 | 0.212 | 0.600 |
| C18:0 | 41.1b | 77.5a | 79.9a | 76.6a | 75.1a | 72.1a | 8.67 | 0.002 | <0.001 | <0.001 |
| C18:1 t9 | 0.31b | 0.70a | 0.56ab | 0.58ab | 0.59ab | 0.56ab | 0.10 | 0.014 | <0.001 | 0.033 |
| C18:1 t11 | 39.6b | 134a | 143a | 147a | 141a | 134a | 19.1 | <0.001 | <0.001 | 0.005 |
| C18:1 c9 | 26.3b | 66.8a | 68.3a | 68.1a | 66.9a | 65.4a | 2.72 | <0.001 | <0.001 | <0.001 |
| C18:2 t9,t12 | 0.13 | 0.18 | 0.21 | 0.23 | 0.18 | 0.19 | 0.03 | 0.074 | <0.001 | 0.067 |
| C18:2 c9,c12 | 33.2b | 108a | 116a | 115a | 115a | 111a | 8.00 | <0.001 | <0.001 | <0.001 |
| c9,t11 CLA | 13.4b | 14.8ab | 14.5ab | 15.2a | 14.2ab | 13.6ab | 0.59 | 0.040 | <0.001 | <0.001 |
| c10,c12 CLA | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.051 | <0.001 | 0.028 |
| t10,c12 CLA | 0.20 | 0.22 | 0.15 | 0.21 | 0.21 | 0.19 | 0.04 | 0.570 | 0.019 | 0.454 |
| C18:3n6 | 0.06 | 0.04 | 0.05 | 0.04 | 0.05 | 0.06 | 0.01 | 0.275 | <0.001 | 0.370 |
| C18:3n3 | 18.3b | 21.9a | 22.2a | 22.3a | 22.0a | 21.7a | 1.14 | 0.012 | <0.001 | 0.010 |
| C20:0 | 3.73ab | 3.40b | 4.13a | 4.05a | 3.94a | 3.87ab | 0.15 | <0.001 | <0.001 | <0.001 |
| C20:1n9 | 0.38b | 0.61ab | 0.56ab | 0.68a | 0.68a | 0.59ab | 0.09 | 0.038 | <0.001 | 0.176 |
| C20:2 | 0.16 | 0.17 | 0.14 | 0.15 | 0.15 | 0.15 | 0.01 | 0.613 | <0.001 | 0.051 |
| C20:3n6 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.01 | 0.576 | 0.004 | 0.108 |
| C20:3n3 | 0.09 | 0.09 | 0.08 | 0.07 | 0.07 | 0.08 | 0.01 | 0.852 | <0.001 | 0.771 |
| C20:4n6 | 0.04 | 0.06 | 0.05 | 0.06 | 0.07 | 0.06 | 0.02 | 0.532 | 0.679 | 0.733 |
| C20:5n3 | 0.13 | 0.13 | 0.14 | 0.10 | 0.08 | 0.09 | 0.03 | 0.134 | <0.001 | 0.110 |
| C22:0 | 1.81 | 1.73 | 2.37 | 2.32 | 2.27 | 1.93 | 0.39 | 0.398 | <0.001 | <0.001 |
| C22:1n9 | 0.07 | 0.05 | 0.07 | 0.07 | 0.06 | 0.06 | 0.01 | 0.574 | <0.001 | 0.343 |
| C22:2 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.01 | 0.222 | <0.001 | 0.284 |
| C22:6n3 | 0.10 | 0.10 | 0.11 | 0.11 | 0.10 | 0.10 | 0.01 | 0.846 | <0.001 | 0.905 |
| C23:0 | 0.57 | 0.39 | 0.45 | 0.58 | 0.58 | 0.46 | 0.08 | 0.115 | <0.001 | 0.023 |
| C24:0 | 2.68 | 2.47 | 2.46 | 2.92 | 2.34 | 2.33 | 0.34 | 0.489 | <0.001 | <0.001 |
| C24:1n9 | 0.32 | 0.28 | 0.24 | 0.23 | 0.26 | 0.28 | 0.04 | 0.326 | <0.001 | 0.013 |
| Total FA | 306b | 567a | 595a | 593a | 578a | 557a | 26.7 | <0.001 | <0.001 | 0.103 |
| SFA | 171b | 217a | 227a | 221a | 214a | 207a | 13.3 | 0.006 | <0.001 | <0.001 |
| UFA | 134b | 349a | 368a | 371a | 363a | 350a | 15.0 | <0.001 | <0.001 | 0.104 |
| MUFA | 68.4b | 204a | 214a | 218a | 211a | 203a | 18.1 | <0.001 | <0.001 | 0.030 |
| PUFA | 66.0b | 145a | 154a | 153a | 152a | 148a | 8.88 | <0.001 | <0.001 | <0.001 |
| RA/LA | 0.76a | 0.29b | 0.26b | 0.26b | 0.27b | 0.30b | 0.05 | <0.001 | <0.001 | 0.001 |
| VA/RA | 3.03b | 8.34a | 8.42a | 8.62a | 8.82a | 9.00a | 1.12 | <0.001 | <0.001 | 0.001 |
| SA/VA | 7.07 | 9.80 | 9.74 | 9.68 | 9.65 | 9.63 | 3.17 | 0.938 | <0.001 | 0.999 |
FA, fatty acids; SFA, saturated fatty acids; UFA, unsaturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; RA, rumenic acid; LA, linoleic acid; VA, vaccenic acid; SA, stearic acid.
CON, control diet without added oil and grape seed tannin extract; SBO, 2.5% soybean oil; ST0.2, ST0.4, ST0.6, and ST0.8, SBO plus either 0.2%, 0.4%, 0.6%, or 0.8% grape seed tannin extract.
Means within a row with different superscripts are significantly different (P < 0.05).
Figure 2.
Temporal change of C18:2 c9,c12 concentration during 24 h incubation.
Figure 3.
Temporal change of c9,t11 CLA concentration during 24 h incubation.
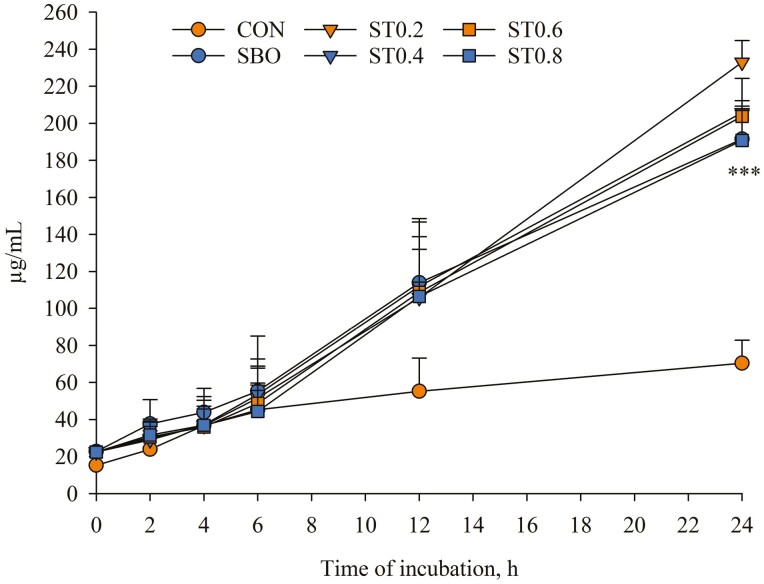
It was noteworthy that production of VA with CON peaked (57.2 µg/mL) at 4 h incubation and then decreased slowly until end of incubations (Figure 4); however, with SBO and CT-containing diets, this FA increased markedly and almost reached peak concentrations (241–265 µg/mL) at 12 h incubation. It was impressive that production of VA increased continuously from 0 to 24 h incubation with ST0.6, whereas it decreased (P < 0.001) from 12 to 24 h incubation with other diets. As a result, formation of SA was very slow and did not differ (P > 0.05) among diets during the first 6 h of incubation (Figure 5); however, relative to CON, SA concentration at 24 h incubation was 171%–231% higher (P < 0.001) with SBO and CT-containing diets. In the present study, mean amounts of MUFA and PUFA were higher (P < 0.001) with SBO and CT-containing diets (Table 4). Compared with CON, supplementation of SBO and GSTE in the diet reduced (P < 0.001) RA/VA, but increased (P < 0.001) VA/RA. However, inclusion of SBO alone or in combination with GSTE in the diet had no effect (P > 0.05) on SA/VA after 24 h incubation.
Figure 4.
Temporal change of C18:1 t11 concentration during 24 h incubation.
Figure 5.
Temporal change of C18:0 concentration during 24 h incubation.
Discussion
Effect of oil and CT on ruminal fermentation and methane production
Tannins are useful phytochemicals for modulating ruminal microbial fermentation in a beneficial way (Vasta et al., 2019). However, it has proven challenging to identify doses that are effective, yet have minimal detrimental effects on ruminal fermentation. The fact that total VFA concentration was reduced with ST0.6 and ST0.8 suggested disturbances in carbohydrate metabolism in ruminal fluid. Menci et al. (2021) reported a linear reduction of in vitro total VFA concentration when tannin extracts were added from 0 to 30 g/kg of the diet in sheep. In a continuous culture using ruminal fluid from Holstein cows, Roca-Fernández et al. (2020) reported a decrease of 44.7% in total VFA concentration when CT was increased from 0.29% to 7.56% of diet DM. Thus, at least in sheep, CT at high concentrations can have an inhibitory effect on fibrolytic bacteria populating the rumen with consequential effects on fermentation pathways (Costa et al., 2018).
A noteworthy result in the current study was the linear decrease in acetate/propionate ratio with increasing levels of CT. In certain cases, a decrease in acetate/propionate ratio is associated with feeding high-grain diets, low pH, and reduction in fiber digestion. This result suggested that a combination of oil and CT in the diet was more effective than oil alone in impairing fiber digestion. Similar results were reported by Menci et al. (2021). Despite the lack of difference in ruminal NH3-N concentration in this study, a reduction in ruminal degradability of dietary protein with CT-containing diets as compared to CON was possible as indicated by the decrease of isobutyrate and isovalerate (Costa et al., 2018; Roca-Fernández et al., 2020; Menci et al., 2021). Thus, these data underscored the strong effect of CT on aspects of ruminal protein metabolism.
The reduction in CH4 production when oil and CT were fed in combination underscored a synergistic effect of these feed sources. Our findings were similar to previous reports on the effect of combinations of anti-methanogenic feed additives on CH4 production. For example, when cottonseed oil and tannin were fed to dairy cows (Williams et al., 2020) and a fish-soybean oil blend and CT extract were added in vitro to ruminal fluid from dairy cows (Szczechowiak et al., 2016), the effect on CH4 production of the combined treatments was greater than any treatment given individually.
Effect of oil and CT on FA biohydrogenation
Addition of SBO positively influenced the concentrations of VA, RA, MUFA, and PUFA. The reduced RA/LA, but increased VA/RA with oil-supplemented diets revealed that greater content of lipid in diets either delayed the BH of LA, or increased the formation of VA rapidly. The lack of difference of SA/VA among diets suggested that the formation of SA was not affected by dietary oil content and CT. Furthermore, the similar concentration of SA between SBO and CT-containing diets during the incubation indicated that CT did not inhibit the final step of LA BH. The greater concentrations of VA and RA in CT-containing diets at 24 h of incubation demonstrated that CT from grape seed extract had the ability to inhibit partially the BH of both VA and RA in the rumen. This result strengthens our hypothesis that CT can affect FA metabolism during the process of ruminal BH.
Condensed tannins have antibacterial properties and, thus, can modulate populations of ruminal bacteria involved in BH (Vasta et al., 2019). However, published studies examining the effects of tannins on FA metabolism in ruminants have reported contrasting responses. For instance, Carreño et al. (2015) reported that supplementation of grape extract at 80 g/kg DM in sheep diets reduced ruminal BH of LA after a 12 h in vitro incubation by acting both on the conversion of RA to VA and VA to SA. In contrast, no effect of CT on in vitro proportions of VA and RA was detected when quebracho tannin extract was added at 30 g/kg DM to the diet of sheep (Menci et al., 2021), but supplementing the same level of this tannin extract in the diet caused a reduction of both VA and RA in milk fat of dairy cows (Henke et al., 2017). Compared with chestnut, quebracho, and Cistus ladanifer, the study of Costa et al. (2017) revealed that supplementation of grape seed extract resulted in higher ruminal C18:1 t11 concentrations. We speculate that the lack of consistency among studies, in general, might be due to factors such as type of substrate incubated, amount of lipid in the diet, tannin type and concentration, and whether the study was in vitro or in vivo.
Conclusion
Soybean oil is more effective in modulating ruminal fermentation, CH4 production, and BH of LA when associated with CT. However, at high doses of added CT, ruminal VFA concentration can be reduced indicating negative impacts on fermentation. Thus, a level of 0.4% grape seed tannin extract added to diets containing 2.5% SBO would be suitable for optimizing ruminal fermentation, CH4 production, and BH of LA to fatty acid intermediates that could have beneficial effects to human health.
Supplementary Material
Acknowledgments
This study was financially supported by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under Grant No. 106.05-2017.310. We expressed the special thanks to facility support from Department of Animal Sciences, College of Agriculture, Can Tho University, Viet Nam. Dr. L. P. T. also offers the sincere thanks to the Fulbright Scholar Program (2020-2021), the U.S. Department of State by providing him an opportunity to work and prepare the manuscript of this paper at Mammalian NutriPhysioGenomics Laboratory under the supervision of Prof. J. J. L.
Glossary
Abbreviations
- ADF
acid detergent fiber
- ADL
acid detergent lignin
- BH
biohydrogenation
- CLA
conjugated linoleic acid
- CP
crude protein
- CT
condensed tannin
- DM
dry matter
- EE
ether extract
- FA
fatty acid
- FAME
fatty acid methyl ester
- GSTE
grape seed tannin extract
- LA
linoleic acid
- ME
metabolizable energy
- MUFA
monounsaturated fatty acid
- NDF
neutral detergent fiber
- NFC
non-fiber carbohydrate
- OM
organic matter
- PUFA
polyunsaturated fatty acid
- RA
rumenic acid
- SA
stearic acid
- SBO
soybean oil
- SFA
saturated fatty acid
- UFA
unsaturated fatty acid
- VA
vaccenic acid
- VFA
volatile fatty acid
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- AOAC . 1990. Official methods of analysis. 15th ed. Arlington (VA): Assoc. Off. Anal. Chem. [Google Scholar]
- Carreño, D., Hervás G., Toral P. G., Belenguer A., and Frutos P.. . 2015. Ability of different types and doses of tannin extracts to modulate in vitro ruminal biohydrogenation in sheep. Anim. Feed Sci. Technol. 202:42‐51. doi: 10.1016/j.anifeedsci.2015.02.003. [DOI] [Google Scholar]
- Correddu, F., Gaspa G., Pulina G., and Nudda A.. . 2016. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep. J. Dairy Sci. 99:1725–1735. doi: 10.3168/jds.2015-10108. [DOI] [PubMed] [Google Scholar]
- Costa, M., Alves S. P., Cabo Â., Guerreiro O., Stilwell G., Dentinho M. T., and Bessa R. J.. . 2017. Modulation of in vitro rumen biohydrogenation by Cistus ladanifer tannins compared with other tannin sources. J. Sci. Food Agric. 97:629–635. doi: 10.1002/jsfa.7777. [DOI] [PubMed] [Google Scholar]
- Costa, M., Alves S. P., Cappucci A., Cook S. R., Duarte A., Caldeira R. M., McAllister T. A., and Bessa R. J. B.. . 2018. Effects of condensed and hydrolyzable tannins on rumen metabolism with emphasis on the biohydrogenation of unsaturated fatty acids. J. Agric. Food Chem. 66(13):3367‐3377. doi: 10.1021/acs.jafc.7b04770. [DOI] [PubMed] [Google Scholar]
- Folch, J., Lees M., and Sloane Stanley G. H.. . 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- Frutos, P., Hervás G., Natalello A., Luciano G., Fondevila M., Priolo A., and Toral P. G.. . 2020. Ability of tannins to modulate ruminal lipid metabolism and milk and meat fatty acid profiles. Anim. Feed Sci. Technol. 269:114623. doi: 10.1016/j.anifeedsci.2020.114623. [DOI] [Google Scholar]
- Griinari, J. M., and Bauman D. E.. 1999. Biosynthesis of conjugated linoleic acid and its incorporation into meat and milk in ruminants. In: Yurawecz M. P., Mossoba M. M., Kramer J. K. G., Pariza M. W. and Nelson G. J., editors, Advances in conjugated linoleic acid research No. 1. Champaign (IL): AOCS Press. p. 180–200. [Google Scholar]
- Henke, A., Westreicher-Kristen E., Molkentin J., Dickhoefer U., Knappstein K., Hasler M., and Susenbeth A.. . 2017. Effect of dietary quebracho tannin extract on milk fatty acid composition in cows. J. Dairy Sci. 100:6229–6238. doi: 10.3168/jds.2016-12149. [DOI] [PubMed] [Google Scholar]
- Khiaosa-Ard, R., Bryner S. F., Scheeder M. R. L., Wettstein H. R., Leiber F., Kreuzer M., and Soliva C. R.. 2009. Evidence for the inhibition of the terminal step of ruminal α-linolenic acid biohydrogenation by condensed tannins. J. Dairy Sci. 92(1):177‐188. doi: 10.3168/jds.2008-1117. [DOI] [PubMed] [Google Scholar]
- Li, Y., and Watkins B. A.. 2001. Analysis of fatty acids in food lipids, Current protocols in food analytical chemistry No. 1. Hoboken (NJ): John Wiley & Sons, Inc. p. D1‐ 2.7. [Google Scholar]
- Menci, R., Coppa M., Torrent A., Natalello A., Valenti B., Luciano G., Priolo A., and Niderkorn V.. . 2021. Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Anim. Feed Sci. Technol. 278:114977. doi: 10.1016/j.anifeedsci.2021.114977. [DOI] [Google Scholar]
- Menke, K. H., and Steingass H.. 1988. Estimation of the energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim. Res. Dev. 28:7‐55. [Google Scholar]
- Min, B. R., Solaiman S., Waldrip H. M., Parker D., Todd R. W., and Brauer D.. . 2020. Dietary mitigation of enteric methane emissions from ruminants: a review of plant tannin mitigation options. Anim. Nutr. 6(3):231‐246. doi: 10.1016/j.aninu.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moate, P. J., Williams S. R., Torok V. A., Hannah M. C., Ribaux B. E., Tavendale M. H., Eckard R. J., Jacobs J. L., Auldist M. J., and Wales W. J.. . 2014. Grape marc reduces methane emissions when fed to dairy cows. J. Dairy Sci. 97:5073–5087. doi: 10.3168/jds.2013-7588. [DOI] [PubMed] [Google Scholar]
- NRC . 2001. National Research Council: nutrient requirements of dairy cattle. Washington, DC: National Academy Press. [Google Scholar]
- Roca-Fernández, A. I., Dillard S. L., and Soder K. J.. . 2020. Ruminal fermentation and enteric methane production of legumes containing condensed tannins fed in continuous culture. J. Dairy Sci. 103:7028–7038. doi: 10.3168/jds.2019-17627. [DOI] [PubMed] [Google Scholar]
- Szczechowiak, J., Szumacher-Strabel M., El-Sherbiny M., Pers-Kamczyc E., Pawlak P., and Cieslak A.. . 2016. Rumen fermentation, methane concentration and fatty acid proportion in the rumen and milk of dairy cows fed condensed tannin and/or fish-soybean oils blend. Anim. Feed Sci. Technol. 216:93‐107. doi: 10.1016/j.anifeedsci.2016.03.014. [DOI] [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Vasta, V., Daghio M., Cappucci A., Buccioni A., Serra A., Viti C., and Mele M.. . 2019. Invited review. Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: experimental evidence and methodological approaches. J. Dairy Sci. 102:3781–3804. doi: 10.3168/jds.2018-14985. [DOI] [PubMed] [Google Scholar]
- Williams, S. R. O., Hannah M. C., Eckard R. J., Wales W. J., and Moate P. J.. . 2020. Supplementing the diet of dairy cows with fat or tannin reduces methane yield, and additively when fed in combination. Animal 14(S3):s464–s472. doi: 10.1017/S1751731120001032. [DOI] [PubMed] [Google Scholar]
- Yoshida, T., Hatano T., and Ito H.. . 2005. Chapter seven—High molecular weight plant poplyphenols (tannins): prospective functions. In: J. T. Romeo, editor, Recent advances in phytochemistry No. 39. Oxford: Elsevier. p. 163‐190. [Google Scholar]
- Zhang, H., Tong J., Wang Z., Xiong B., and Jiang L.. . 2020. Illumina MiSeq sequencing reveals the effects of grape seed procyanidin on rumen archaeal communities in vitro. Asian-Australas. J. Anim. Sci. 33:61–68. doi: 10.5713/ajas.19.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.