Abstract
Background
We evaluated whether integration of novel diets for donors and patients, in addition to faecal transplantation [FT], could increase FT remission rate in refractory ulcerative colitis [UC].
Methods
This was a blinded, randomised, controlled trial in adults with active UC, defined by a simple clinical colitis activity index [SCCAI] of ≥5 and ≤11 and endoscopic Mayo score 2–3, refractory to medication. Group 1 received free diet and single donor standard FT by colonoscopy on Day 1and rectal enemas on Days 2 and 14 without dietary conditioning of the donor. Group 2 received FT as above but with dietary pre-conditioning of the donor for 14 days and a UC Exclusion Diet [UCED] for the patients. Group 3 received the UCED alone. The primary endpoint was Week 8 clinical steroid-free remission, defined as SCCAI <3.
Results
Of 96 planned patients, 62 were enrolled. Remission Week 8 Group 1 was 2/17 [11.8%], Group 2 was 4/19 [21.1%], Group 3 was 6/15 [40%] [non-significant]. Endoscopic remission Group 1 was 2/17 [12%], Group 2 was 3/19 [16%], Group 3 was 4/15 [27%] [Group 1 vs 3 p = 0.38]. Mucosal healing [Mayo 0] was achieved only in Group 3 [3/15, 20%] vs 0/36 FT patients [p = 0.022]. Exacerbation of disease occurred in 3/17 [17.6%] of Group 1, 4/19 [21.1%] of Group 2, and 1/15 [6.7%] of Group 3 [Group 2 vs 3, p = 0.35].
Conclusions
UCED alone appeared to achieve higher clinical remission and mucosal healing than single donor FT with or without diet. The study was stopped for futility by a safety monitoring board.
Keywords: Ulcerative colitis, faecal transplantation, diet, microbiome, fibre
1. Introduction
The pathogenesis of ulcerative colitis [UC] is unknown. Recent studies have suggested that UC is associated with alterations of the gut microbiota1,2 and impaired mucous layer and epithelial barrier.3,4 The dysbiosis in UC is characterised by a reduction in short chain fatty acids [SCFAs]-producing taxa,5–7 and in some studies by an increase in potential pathobionts such as Escherichia or Ruminococcus gnavus,8 or hydrogen sulphide-reducing bacteria.6 Manipulation of the microbiota has become one of the most intriguing targets for intervention in inflammatory bowel diseases [IBD].
Dietary therapy has been used successfully in mild to moderate Crohn’s disease9 and may be effective in mild to moderate UC in children as well, though dietary interventions in UC are just getting under way [Sarbagili Shabat & Levine /in press] and data regarding dietary interventions in inflamed adults with UC are lacking. Faecal microbial transfer [FT] is another option for altering the microbiota, and is postulated to do so by replenishing or repopulating the microbiota with beneficial taxa from a healthy donor. FT has been shown to be effective in the short term in about 30% of cases.10–15 Success of FT appears to depend upon the choice of donor and its microbiota composition,10,14 and use of multi-donor high-frequency FTs.12 High-frequency FT appears to be associated with better outcomes but requires multiple weekly or daily transplants to achieve and maintain remission.12 Pooled multi-donor FTs may also offer an advantage,13 suggesting that colonisation of transplanted microbial communities does not occur or is lost and is dependent on frequent FTs to achieve outcomes. An additional approach that improved colonisation was use of antibiotics before FT.16 Although clinical remission rates are superior in FT groups compared with placebo groups,17 each regimen may have advantages or disadvantages.18 Use of multiple FTs or multiple donors may increase the risk of transmissible traits and diseases, and requires a large screened donor pool as well as frequent administration usually with enemas.
David et al. demonstrated that diet can rapidly change the microbiota and bacterial metabolism, such that consuming a high animal- and low plant-based diet significantly altered the composition of the microbiome, decreasing SCFAs-producing taxa.19
We hypothesised that a strategy incorporating a novel UC dietary intervention and donor diet might improve the effectiveness of FT as a treatment for active refractory UC patients.
2. Methods
2.1. Patient population
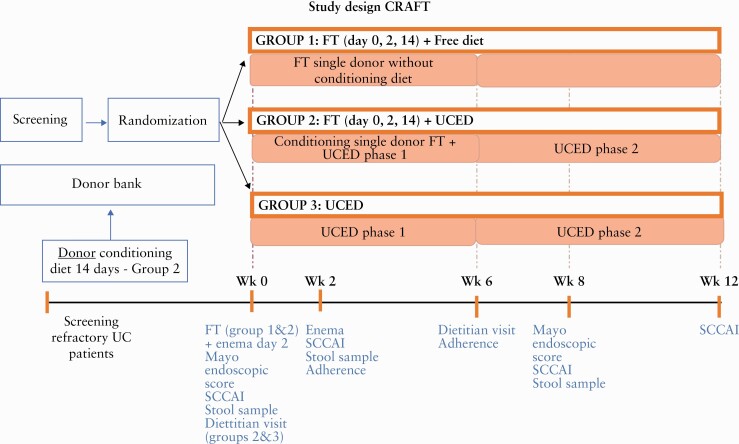
The Conditioning of Recipient And donor with Faecal Transplantation in Ulcerative Colitis: [CRAFT UC] trial was a single, blinded, randomised, controlled trial, involving three arms, in adults with refractory established UC. Patients with active disease and a colonoscopy demonstrating Mayo endoscopic score of ≥2 were randomised 1:1:1 to one of three groups; Group 1 were allowed to consume a free diet and received a standard FT by colonoscopy on Day 1 and rectal enemas from the same donor on Days 2 and 14, without dietary conditioning of the donor. Group 2 received FT by colonoscopy on Day 1 and rectal enemas from the same donor on Days 2 and 14, with dietary pre-conditioning of the donor for 14 days, and dietary treatment with an ulcerative colitis exclusion diet [UCED] after transplantation and for the following 12 weeks. Group 3 received the UCED alone without FT for 12 weeks [Figure 1]. The control arm with the diet was specifically introduced to enable us to separate the effect of the donor diet from the recipient diet. Group 3 with diet alone was designed to enable us to evaluate the independent effect of diet. Using single donors who provided samples before and after diet allowed us to evaluate the independent effect of donors. Group 1 allowed us to evaluate the independent effect of FT. The study was registered in five sites, but only four sites enrolled patients: the ‘Fondazione Policlinico Universitario A. Gemelli IRCCS’ Hospital in Rome, the Inflammatory Bowel Diseases units of the Tel Aviv Medical Center, the Wolfson Medical Center in Holon, and the Emek Medical Center in Afula, from Israel. Physicians enrolling patients were blinded to randomisation and to performance of colonoscopy, and for patients undergoing transplantation, blinded to the donor [pre-conditioned with diet or not] and to whether patients received the diet after transplantation.
Figure 1.
Trial design.
2.2. Inclusion/exclusion criteria
Patients could be included if they had an established diagnosis of UC, disease confined to the large intestine, involving the rectosigmoid for at least 3 months, were 18–70 years of age, had mild to moderate active disease defined as SCCAI of ≥5 and ≤11 with Mayo endoscopic sub-score ≥2 despite therapy at the baseline colonoscopy, and had been refractory to at least one of the following drug regimens: mesalamine > 6 weeks, steroids >14 days, thiopurines >12 weeks, or biologics >12 weeks, and signed informed consent. Patients were excluded if: they had started a new biologic in the previous 12 weeks, had Clostridiodes difficile or any stool infection, had extra-intestinal manifestations, had an episode of acute severe colitis in the previous 3 months, were on calcineurin inhibitors, were pregnant, or had a Mayo endoscopic score 0–1 at the baseline colonoscopy. Patients were also excluded who had previously received an FT or the UCED, or had suffered from autoimmune disorders, renal failure, fever > 38oC or current infection, or neoplasia, or who had a colectomy.
2.3. Endpoints
The primary endpoint was intention to treat clinical remission determined by SCCAI score <3 at Week 8 between Group 1 and Group 2. Secondary endpoints were SCCAI and improvement in the SCCAI score at Weeks 8 and 12 for each of the groups, endoscopic remission score at Week 8 for each of the groups, and change in calprotectin at Week 8, as well as the need for additional therapy by Week 12 for all groups. Clinical response was defined as a decrease in SCCAI ≥3. Endoscopic remission was defined as Mayo score 0–1 after therapy, mucosal healing as Mayo score 0. Patients who failed to achieve remission by Week 8 or required additional therapy were considered as treatment failures on an intention to treat basis.
2.4. Study visits
Patients were seen at screening and randomised before colonoscopy. They were seen at a baseline visit Week 0 and at Weeks 2, 8, 12. Patients met with a coordinator/dietitian after colonoscopy, patients in Group 1 did not receive any dietary instruction, and Group 2 and Group 3 received detailed instructions regarding the UCED to be used over 12 weeks. A telephone conversation to assess SCCAI was made at Week 1 and Week 7. At each visit, adverse events and medications were recorded and SCCAI, physician global assessment [PGA], a complete blood count, C-reactive protein [CRP], albumin, and complete chemistry panel were performed. All patients without change in therapy had a repeat sigmoidoscopy performed at Week 8 with Mayo scoring of the most inflamed segment. A modified MARS (medication adherence rating scale) diet adherence questionnaire9 was completed on Weeks 2 and 8. High diet adherence was defined by finding high adherence on the questionnaire and by dietitian’s assessment based on direct questioning of compliance over the previous period. Poor compliance was defined by having low compliance in either assessment at any time point.
2.5. Donors
In order to evaluate the added benefit of the donor diet and to avoid bias by donor selection, the same pool of donors was used for Group 1 and Group 2. After a detailed screening process [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online] for the donors and after consent was obtained, the donors were asked to provide at least four stool samples during the first week prior to the diet [pre-conditioned samples], and then consumed an especially designed donor diet for 2 weeks, after which they provided at least another four stool samples [post-conditioning]. Thus, patients in Group 1 received FTs from the baseline samples before the diet, and those in Group 2 received FTs from donors after the 2-week diet [Table 2; see 3.3.]. All samples from donors were placed in a sterile container with an anaerobic generator bag [Anaerogen P, Oxoid, Basingstoke, UK] brought fresh to the central institution where the samples were prepared immediately as faecal effluents, frozen, and stored at -80oC at each site. All donor samples came from one of two central donor stool sites: in Israel at the Tel Aviv Medical Center and in Italy at the Gemelli Hospital in Rome. This ensured homogeneous screening and sample storage. Briefly, fresh donor stool [100 g] was immediately homogenised with 260 ml of 0.9% normal saline, using a household hand blender with minimal exposure to air throughout the process + 40ml glycerol [glycerol 1:10 of final volume]. The blend was filtered through thin gauze twice. The resulting liquid was divided into 50-ml tubes, that were immediately frozen [-80°C] and thawed before using at 37°C.
Table 2.
Donors and outcomes by group.
| Donor | Total remission | Group 1 pre-diet remission | Group 2 post-diet remission | Donor diet compliance |
|---|---|---|---|---|
| A [Israel] | 0/6 | 0/3 | 0/3 | High |
| B [Italy] | 0/10 | 0/5 | 0/5 | High |
| C [Italy] | 3/5 | 1/2 | 2/3 | High |
| D [Israel] | 0/5 | 0/3 | 0/2 | High |
| E [Israel] | 1/3 | 0/1 | 1/2 | High |
| F [Israel] | 0/4 | 0/2 | 0/2 | High |
| G [Israel] | 1/2 | - | 1/2 | High |
| H [Israel] | 1/1 | 1/1 | - | High |
| Total | 6/36 [16.6%] | 2/17 [11.8%] | 4/19 [21%] | High [100%] |
2.6. Dietary intervention
Patients in Group 2 [FT + diet] and Group 3 [diet alone] received detailed instructions for the UCED by a dietitian, with a uniform handout according to each stage. The UCED diet was designed to alter dietary components that may adversely affect goblet cells, mucus permeability, and microbiome composition, which were previously linked to UC. The following principles guiding food exclusion and addition included: decreased exposure to sulphated amino acids, total protein, haeme, animal fat, saturated fat, and food additives, and increased exposure to tryptophan and natural sources of pectin and resistant starch [Supplementary Figure 2, available as Supplementary data at ECCO-JCC online].6,20–23 The UCED comprises foods that are mandatory, such as certain fruits and vegetables, prescribed amounts of chicken, eggs, and yoghurt, others that are allowed with quantitative restriction, foods that can be consumed without limitation, and disallowed foods; it is rich in fruits and vegetables. A sample meal plan appears in Supplementary Table 1, available as Supplementary data at ECCO-JCC online. It has two stages, Weeks 0–6, and Weeks 7–12: the latter stage is more permissive. The donor diet instructions appear in Supplementary Table 2, available as Supplementary data at ECCO-JCC online.
2.7. Faecal transplantation recipients [Groups 1 and 2]
Patients were prepared for the FT with bowel lavage [2–3 L macrogol solution and clear fluids] the evening or morning before treatment. On the day of FT [Day 0], while fasting, they received a dose of loperamide and a single faecal transplantation by colonoscopy of 200 ml of faecal effluent. During the colonoscopy the effluent was infused as proximally as possible, with the full 200 ml introduced into the right side [caecum and ascending colon]. On Days 2 and 14, patients underwent an enema of 100 ml effluent. Patients were instructed to hold the effluent for least 15 min after the procedure. We did not use antibiotics before FT as, at the time this trial was designed [February 2016], there was no consensus regarding the need of using antibiotics before FT. There were two randomised controlled trials [RCTs] published during the time that this trial was conceived10,11 and both did not use antibiotics. Six h after the first transplantation, patients in Group 2 started the UCED diet. Patients in Group 1 or 2, who achieved remission but lost response between Weeks 2 and 8, could receive up to two rescue enemas during the 8 weeks of the study from their original donor, using the same type of sample [Group 1 no pre-conditioned sample, Group 2 pre-conditioned sample]. Patients who did not achieve remission or required additional therapy were considered failures in the intention to treat analysis.
2.8. Adverse events
All adverse events, serious adverse events, or worsening of disease were recorded. Due to safety concerns, whether from futility as previously shown in some studies or from FT-induced adverse events, an interim safety analysis took place after the first 34 FT patients, by an independent data safety monitoring board (DSMB) from non- participating institutions. A repeat DSMB meeting after further queries was held in December 2020 after queries were obtained from the Italian cohort, which had been delayed due to SARS COV-2.
2.9. Randomisation and blinding
Randomisation was started 1:1:1 for the first 60 patients until the first 20 patients in Group 3 were enrolled, and then was to continue as a two-arm study with Group 1 and Group 2 alone randomised 1:1 to complete at least 76 patients in the FT arms [effectively 2:2:1 by the end of the study]; the dietary arm was to have fewer patients [as this arm was designed to evaluate the independent role of diet on recipients’ clinical state and their microbiome] in blocks of 6, provided by opaque randomisation envelopes handed to the patient during enrolment after consent. In order to ensure physician blinding, a coordinator in each institution set up the study visits, met with the dietitians and patients, and ensured that patients received the appropriate donor sample and diet according their allocated group without the physicians’ knowledge.
2.10. Power analysis
Previous studies have shown roughly a 25–30% remission rate using multiple time point enemas over several weeks, while we assumed that less frequent time pointsfor FT in Group 1 would lead to lower remission rates [20% or less] from FT. We previously found that diet induced remission in 40% of patients in a pilot trial. We estimated that the study arm which combined diet and FT would lead to remission by 8 weeks in over 50% of patients. Assuming 50% remission in the experimental group [Group 2] and 20% in the control group [Group 1], 76 patients receiving FT were required to have an 80% chance of detecting, as significant at the 5% level, an increase in the primary outcome measure from 20% in the control group to 50% in the experimental group. In addition, we enrolled 20 patients into the diet arm through randomisation as a control arm, in order to prevent selection bias and to enable us to estimate the independent effect of diet on remission, and thereby try to assess the impact of donor conditioning and FT as independent variables.
2.11. Statistical analysis
Continuous variables were evaluated for distribution normality and reported as median [interquartile range, IQR] or mean [standard deviation, SD] as appropriate. Group differences in continuous variables were assessed by the Krushal‐Wallis non-parametric one-way analysis of variamce [ANOVA] test, using Bonferroni correction after the normality of distribution was rejected. Associations between nominal variables were performed with the Pearson chi square test or Fisher’s exact test. The primary endpoint of the proportion of patients in remission at Week 8 was analysed by the Pearson chi square test or Fisher’s exact test to the intention-to-treat [ITT] paradigm. Pairwise comparison of SCCAI at Week 0 versus Week 8 was analysed by the Wilcoxon signed rank test and was performed only in subjects with parameters at both time points. All statistical analyses were performed using the SPSS version 27 statistical analysis software [IBM, USA]; p-values <0.05 were considered to be significant.
2.12. Donor microbiome analysis
Metagenomic DNA was purified using DNeasy PowerMag Soil DNA extraction kit [Qiagen] optimised for Tecan automated platform. Next-generation sequencing [NGS] libraries were prepared using Nextera DNA library prep [Illumina] and sequenced on a NovaSeq sequencing platform [Illumina]. Sequencing was performed with 75-bp single-end reads with the depth of 10 million reads per sample. We filtered metagenomic reads containing Illumina adapters, filtered low-quality reads, and trimmed low-quality read edges. We detected host DNA by mapping with Bowtie to the human genome with inclusive parameters, and removed those reads. Bacterial relative abundance [RA] estimation was performed by mapping bacterial reads to species-level genome bins [SGB] representative genomes.24 We selected all SGB representatives with at least 5 genomes in a group, and for these representatives’ genomes kept only unique regions as a reference dataset. Mapping was performed using Bowtie,25 and abundance was estimated by calculating the mean coverage of unique genomic regions across the 50% most densely covered areas, as previously described.26 Featured names include the lowest taxonomy level identified.
2.13. Ethical consideration
The CRAFT study [NCT 02734589] was approved by the ethics committee in all the hospitals where it was conducted: Wolfson, Tel Aviv Sourasky, and Haemek Medical Centers in Israel and in A. Gemelli IRCCS in Italy.
3. Results
3.1. Study population
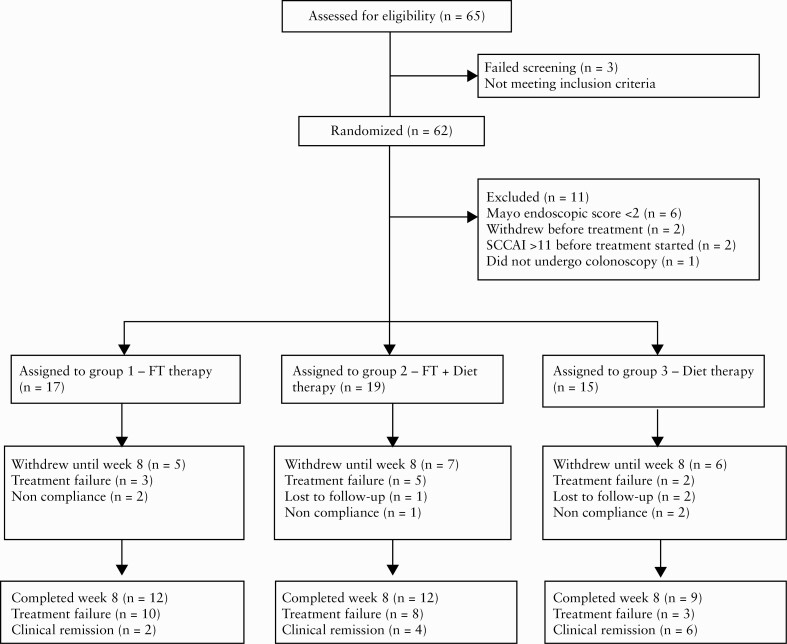
We enrolled eight donors [six from Israel, two from Rome] and 62 patients: 22/62 [35.4%] from Italy and 40 [64.6%] from Israel, from May 2017 until December 2020. Eleven patients were excluded after randomisation due to lack of active inflammation during colonoscopy, withdrawal before receiving any treatment, or violation of exclusion criteria. The flow of patients is depicted in Figure 2.
Figure 2.
Participant flow diagram.
Approximately 55% of patients [28/51] were failing a biologic or corticosteroid at enrolment. Over 50% of patients had failed at least one biologic before enrolment [Table 1]. An independent DSMB reviewed queried data on December 2020, and recommended suspension of the trial due to futility after 53% of the intended patients had been enrolled.
Table 1.
Characteristics of the study patients at baseline.
| Characteristic | Total [n = 51] | Group 1: FT [n = 17] | Group 2: FT + UCED [n = 19] | Group 3: UCED [n = 15] |
|---|---|---|---|---|
| Female gender, n [%] | 14 [27.5] | 5 [29.4] | 5 [26.3] | 4 [26.7] |
| Age [years], mean [SD] | 40.4 [12.5] | 43.1 [14.7] | 43.5 [10.5]a | 33.3 [9.8]a |
| Disease duration [years], median [IQR] | 7.9 [3.2–13.4] | 7.1 [3.3–18.0] | 11.2 [4.7–16.9] | 7.9 [1.9–9.4] |
| CRP, mg/dL, median [IQR] | 0.7 [0.2–1.6] | 0.8 [0.2–1.7] | 0.9 [0.3–1.8] | 0.4 [0–1.1] |
| SCCAI | ||||
| Median [IQR] | 7 [5–12] | 7 [6–9] | 8 [6–10] | 6 [5–8] |
| Range | 5–11 | 5–11 | 5–11 | 5–11 |
| Disease location | ||||
| Extensive | 18 [35.3] | 5 [29.4] | 9 [47.4] | 4 [26.7] |
| Left-sided | 23 [45.1] | 6 [35.3] | 7 [36.8] | 10 [66.7] |
| Proctitis | 10 [19.6] | 6 [35.3] | 3 [15.8] | 1 [6.7] |
| Mayo endoscopic score, median [IQR] | 2 [2–3] | 2 [2–3] | 2 [2–3] | 2 [2–3] |
| BMI, median [IQR] | 23.2 [20.3–26.0] | 22 [20.7–28.3] | 23.8 [21–26] | 22.4 [18.8–25.9] |
| Albumin, g/L, mean [SD] | 41.3 [3.7] | 40.4 [3.2] | 41.6 [4.2] | 42.1 [3.1] |
| Current or last treatment enrolment, n [%] | ||||
| 5-ASA [oral or oral and topical] | 38 [74.5] | 11 [64.7] | 14 [73.7] | 13 [86.7] |
| Steroids | 15 [29.4] | 7 [41.2] | 5 [26.3] | 3 [20] |
| Biologics | 13 [25.5] | 5 [29.4] | 5 [26.3] | 3 [20] |
| None | 8 [15.7] | 3 [17.6] | 3 [15.8] | 2 [13.3] |
| Immunomodulators | 1 [2] | - | - | 1 [6.7] |
| Antibiotic | 1 [2] | 1 [5.9] | - | - |
| Refractory to biologic treatment, n [%] | 28 [54.9] | 12 [70.6] | 11 [57.9] | 5 [33.3] |
FT, faecal transplanation; UCED, ulcerative colitis exclusion diet; SD, standard deviation; IQR, interquartile range; CRP, C-reactive protein; SCCAI, Simple Clinical Colitis Activity Index; BMI, body mass index; 5-ASA, 5-aminosalicylic acid.
All p > .05 except where indicated.
a p = 0.03
3.2. Response and remission
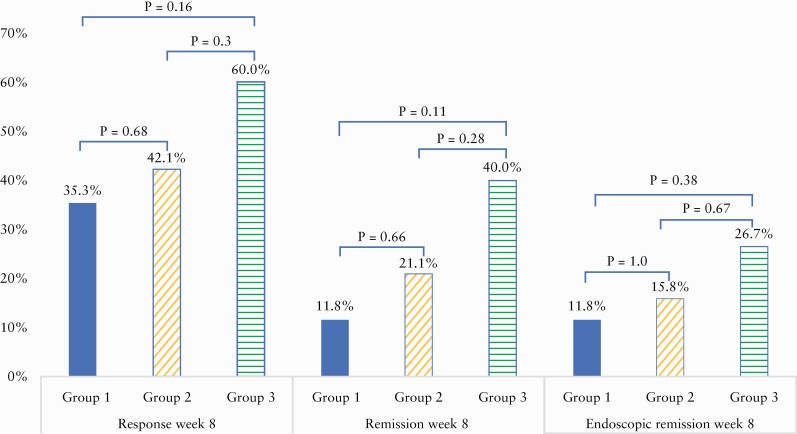
Response and remission by group at Week 8 are portrayed in Figure 3. Intention to treat response and remission rates were 35.3% and 11.8% for Group 1, 42.1% and 21.1% for Group 2, and 60% and 40% for Group 3, respectively [NS], using a less rigorous cut-off for remission as SCCAI <5 did not change the outcome [23.5%, 26.3%, 40% for Groups 1, 2, 3, respectively]. Endoscopic remission at Week 8 was highest for patients in Group 3 [Figure 3], of whom 4/15 [26.6%] achieved endoscopic remission, and lowest in Group 1 [2/17, 11.7%], p = 0.38. Mayo endoscopic score of 0 was achieved only in patients in Group 3 [3/15, 20%] and in 0/36 patients receiving FT, p = 0.022.
Figure 3.
Clinical response, clinical remission, and endoscopic remission at Week 8 by intention to treat.
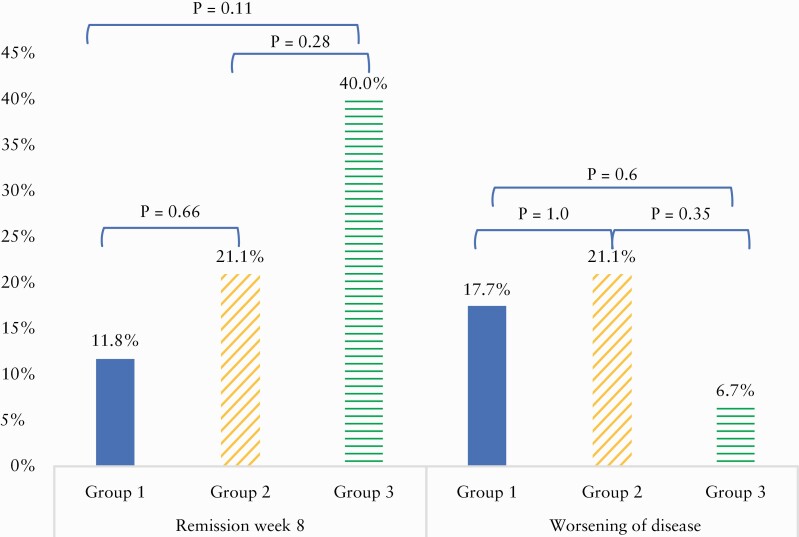
The median SCCAI declined from 7 [IQR 6–9] to 6 [IQR 3.25–9] in Group 1 [p = 0.14], from 8 [IQR 6–10] to 6 [IQR 1.25–10] in Group 2 [p = 0.08], and from 6 [IQR 5–8] to 2 [IQR 0–6] in Group 3 [p = 0.02]. However, a significant proportion of patients also worsened after FT by Week 8 compared with baseline. Comparison of remission vs worsening for each group is portrayed in Figure 4.
Figure 4.
Remission versus deterioration at Week 8 by group [intention to treat].
At Week 12, 9/12 [75%] patients in remission had sustained good response [SCCAI <5], [Group 1 n = 1, Group 2 n = 3, Group 3 n = 5]. One patient from Group 3 was not classified as remission due to arthritis, though there was no longer bleeding nor increased stool frequency. Sustained remission at Week 12 was present in 7/12 [58%] of patients who obtained clinical remission at Week 8 [SCCAI <3] [all from Group 2 or Group 3], without any additional therapy [Group 2 n = 3, Group 3 n = 4]. Two patients were lost to follow-up and one relapsed between Weeks 8 to 12. ITT sustained remission for Group 1, Group 2, Group 3 was 0/17 [0%], 3/19 [15.7%], and 4/15 [26.7%], respectively, by SCCAI remission, and 5/15 [33%] in Group 3 based on luminal remission [no bleeding or diarrhoea]. One patient in Group 1 had received a rescue enema at Week 8 after losing response between Weeks 2 to 8. Despite an improvement in condition, the patient did not enter clinical remission at Week 12.
3.3. Disease severity and response
There appeared to be a correlation between disease severity and outcomes, as remission was obtained in 0/17 patients with an SCCAI ≥9 at enrolment compared with 12/34 [35.3%] patients with SCCAI <9, p = 0.004. Focusing just upon this milder subset of patients with SCCAI <9, remission was obtained in 6/12 [50%] with diet alone versus 6/22 [27.3%] in both FT groups, p = 0.25. Though not significant, doubling of remission rates with diet vs FT suggests that diet is as good or better than FT even for this segment. Patients failing biologic treatment were also less likely to achieve remission [p < 0.001], as only 1/28 biologic-refractory patients [Group 2] achieved clinical remission with the assigned intervention.
3.4. Donors and outcomes
The association between donor and patient outcomes is presented in Table 2. Response depended more upon the donor than upon dietary conditioning which did not have any effect on outcomes. One donor appeared to account for half of the success rate [donor C], used in only five FTs. We also examined the shift in microbiota after the donors’ diet among 7/8 donors, using shotgun metagenomics. We could not analyse the sample from donor H due to Covid-19 precautions. We did not detect any post-diet microbial shift, including in alpha diversity [Supplementary Figure 3a, available as Supplementary data at ECCO-JCC online], Firmicutes to Bacteroides ratio, or composition [not shown]. Richness declined in 5/7 donors examined [Supplementary Figure 3b]. This supports the notion that the donor diet did not have an impact upon outcomes.
3.5. Adverse events and compliance:
Adverse events are portrayed in Table 3: there were no serious adverse events associated with diet or FT. Adherence to the diet by the donors was available for 8/8 [100%] donors, all eight demonstrated high compliance. Adherence to the diet by patients was available for 15/19 [78.9%] in Group 2, of whom 80% [12/15] had high compliance at Week 2. Among 12 patients who reached Week 8 in this group, 9/12 [75%] had high compliance. In Group 3, data were available for 14/15 [93.3%] patients and all 14 [100%] had high compliance after 2 weeks. By Week 8, data regarding compliance were available for eight among nine patients who reached Week 8: all eight [100%] were highly compliant. Using non-compliance imputed for all missing data or withdrawals, 26/34 [76.4%] and 17/34 [50%] patients treated with diet were highly compliant at Week 2 and at Week 8, respectively, in an intention to treat analysis.
Table 3.
Adverse events.
| Total [n = 51] | FMT [n = 17] | FMT + UCED [n = 19] | UCED [n = 15] | |
|---|---|---|---|---|
| Disease exacerbation | 8 [15.7] | 3 [17.6] | 4 [21.0] | 1 [6.7] |
| Fever | 4 [7.8] | 2 [11.8] | 1 [5.3] | 1 [6.7] |
| Abdominal pain | 3 [5.9] | 1 [5.9] | 2 [10.5] | 0 |
| Diarrhoea | 2 [3.9] | 0 | 2 [10.5] | 0 |
| Chills | 2 [3.9] | 0 | 2 [10.5] | 0 |
| Weakness | 2 [3.9] | 2 [11.8] | 0 | 0 |
| Lack of appetite | 2 [3.9] | 1 [5.9] | 0 | 1 [6.7] |
| Flatulence | 1 [2.0] | 0 | 1 [5.3] | 0 |
| Upper respiratory | 1 [2.0] | 0 | 1 [5.3] | 0 |
| Nausea | 1 [2.0] | 0 | 0 | 1 [6.7] |
| Parageusia | 1 [2.0] | 1 [5.9] | 0 | 0 |
| Elevated ALT | 1 [2.0] | 1 [5.9] | 0 | 0 |
| Malignancy/death | 0 | 0 | 0 | 0 |
Data are presented as n or n [%] as numbers of individual events and not number of patients; some may have had more than one event.
FMT, faecal microbiome transfer; UCED, ulcerative colitis exclusion diet; ALT, alanine amino-transferase.
4. Discussion
Current therapy for 5-ASA unresponsive or severe patients with UC is based on immune suppression. The ability to target the microbiome by FT or diet would allow therapy based on modulation of the microbiome instead of immune suppression. In the current study, FT was largely unsuccessful in generating clinical remission in our patient population, which consisted of patients failing existing medications. Steroid-free clinical remission was achieved in 20% or less of patients in the two FT arms, whereas an equivalent proportion of patients in either arm became worse. Among patients receiving FT [Group 1 and Group 2], only patients in Group 2, receiving diet, demonstrated a non- significant trend in reduction of SCCAI at Week 8 compared with baseline [p = 0.08]. However, remission data were less optimistic, as FT [Group 1] obtained remission in only 11% of patients, whereas Group 2 obtained clinical remission in 21% of cases, both lower than the expected remission rates.10–13 Endoscopic remission at Week 8 did not differ between Group 1 and Group 2, occurring only in 12% and 16%, respectively,
It is perplexing to note that the very same diet, that appeared to be successful for inducing clinical remission and mucosal healing in Group 3, did not appear to have this effect when combined with FT in Group 2.
We can only speculate that diet alone may have succeeded better than FT with diet, because FT during inflammation may have actually destabilised the microbiome further in patients who flared or did not respond to FT. A second possibility is that patients in Group 2 might have had more severe disease even if this was not significant, as SCCAI at baseline was highest for Group 2. More severe inflammation could impair the utilisation of beneficial metabolites such as SCFAs. Ferrer-Pincon et al. have demonstrated that in the presence of tumour necrosis factor [TNF]+, metabolism and transcription of SLC16A1, ABCG2, and GPR43 decreased and resulted in reduced butyrate consumption by epithelial cells.27 Though FT failed to provide the anticipated outcomes when combined with diet as we hypothesised, we cannot extrapolate this finding to high-intensity multi-donor FTs. We chose single donor FT in order to be able to rigorously identify if it was the donor or the UCED that led to benefit, and as such the results might have been better had we been able to find a better donor or pooled donor samples.
If the results of FT were a disappointment in this trial, the effect of the diet was the unexpected silver lining. The UCED appeared to be quite effective in some of our patients, as 40% of patients failing medical therapy entered clinical remission and only one patient had worsening of disease after starting the diet. There was a significant decline in SCCAI [p = 0.02], and the endoscopic remission data were better with diet than with FT or FT with diet. Among non-severe patients with SCCAI 5–9, remission was obtained in 6/12 [50%] with diet alone versus 6/22 [27.3%] with both FT groups, though this was not significant given the small size of the cohort at the time the study was stopped. Complete mucosal healing [Mayo score 0] was significantly better and occurred only in patients from Group 3. There is a paucity of data regarding the successful use of diet as a prospective intervention in UC and to achieve mucosal healing. A recent study demonstrated that the addition of one week of exclusive enteral nutrition to intravenous steroids during acute severe colitis decreased steroid failure and improved 6-month composite outcomes.28 A case report in acute severe colitis setting, FT combining with diet, also demonstrated clinical and endoscopic improvement.29
The unsatisfactory outcomes with both FT arms coupled with better outcomes with diet led to our DSMB’s recommendation to stop the trial for futility.
Though the UCED provided to patients seems to show promise as an independent therapy that requires further study, the donor diet failed to have any detectable clinical or microbiological impact. One of the strengths of the study is that we used the same single donor with or without a donor diet to decrease heterogeneity induced by multiple donors; however, there was no discernable increase in success for FT with post-diet samples compared with pre-diet samples. We could not demonstrate any significant change in composition or diversity when comparing the stool sample from each donor before and after diet. This could be due to the short duration of the diet which might be insufficient to alter the microbiome in healthy individuals, as it did not appear to be related to compliance with the diet among the donors. We elected to use a 2-week diet, as we felt that normal healthy volunteers would be unlikely to keep a restrictive diet beyond 2 weeks, and obtaining donors is a difficult task with the high screening failure rate we and others have observed.30 In fact, the success rate for donors was dependent more upon the donor than the donor diet. One donor [donor C, from Italy] achieved 3/5 [60%] successful FTs leading to clinical remission, whereas no other donor was associated with more than one successful FT. This finding of a single successful donor is similar to the outcome in other trials.10 Due to SARS COV-2 circumstances, we could not analyse the pooled microbiome of patients at the time of writing this article.
There are a few strengths but many limitations to our study and interpretation. We employed a rigorous protocol that enrolled only inflamed patients with Mayo 2–3 inflammation, of whom 50% had failed at least one biologic therapy and 55% were failing steroids or a biologic at enrolment: this ensured rigor but might have been a high bar for any therapy and more rigorous entry criteria than some previous trials. The most significant limitation is the premature closure of the study and subsequent small sample size, but this was an important ethical consideration, as it was unlikely that we could have proven benefit for the primary endpoint with the remaining patients, and worsening of disease was as common as benefit in the FT arms. We suggest that one of the most important take-home messages from this trial is that diet may have an under-appreciated role in the treatment of UC and that further studies are required. The UECD is currently being investigated in a randomised controlled trial. Finally, portraying worsening of disease along with response rates with therapy might help clinicians to better interpret data from clinical trials with FT.
Supplementary Material
Contributor Information
Chen Sarbagili Shabat, Pediatric Gastroenterology Unit, PIBD Research Center, Wolfson Medical Center, Holon, Israel; Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Franco Scaldaferri, Dipartimento di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore – Fondazione Policlinico ‘A. Gemelli’ IRCCS, Rome, Italy; Cemad [CENTER for Digestive Disease], UOC Medicina Interna e Gastroenterologia, Fondazione Policlinico ‘A. Gemelli’ IRCCS, Rome, Italy.
Eran Zittan, Gastroenterology Institute, IBD Unit, Haemek Medical Center, Afula, Israel.
Ayal Hirsch, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Department of Gastroenterology and Hepatology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Maria Chiara Mentella, UOC di Nutrizione Clinica, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
Tania Musca, UOC di Nutrizione Clinica, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
Nathaniel Aviv Cohen, Department of Gastroenterology and Hepatology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Yulia Ron, Department of Gastroenterology and Hepatology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Naomi Fliss Isakov, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Department of Gastroenterology and Hepatology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Jorge Pfeffer, Department of Gastroenterology and Hepatology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Michal Yaakov, Pediatric Gastroenterology Unit, PIBD Research Center, Wolfson Medical Center, Holon, Israel.
Caterina Fanali, Cemad [CENTER for Digestive Disease], UOC Medicina Interna e Gastroenterologia, Fondazione Policlinico ‘A. Gemelli’ IRCCS, Rome, Italy.
Laura Turchini, Cemad [CENTER for Digestive Disease], UOC Medicina Interna e Gastroenterologia, Fondazione Policlinico ‘A. Gemelli’ IRCCS, Rome, Italy.
Luca Masucci, Istituto di Microbiologia, Università Cattolica del Sacro Cuore – Fondazione Policlinico ‘A. Gemelli’ IRCSS, Rome, Italy; Dipartimento Scienze di Laboratorio e Infettivologiche, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Gianluca Quaranta, Istituto di Microbiologia, Università Cattolica del Sacro Cuore – Fondazione Policlinico ‘A. Gemelli’ IRCSS, Rome, Italy; Dipartimento Scienze di Laboratorio e Infettivologiche, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Nitzan Kolonimos, Gastroenterology Institute, IBD Unit, Haemek Medical Center, Afula, Israel.
Anastasia Godneva, Department of Computer Science and Applied Mathematics, Weizmann Institute of Science, Rehovot, Israel.
Adina Weinberger, Department of Computer Science and Applied Mathematics, Weizmann Institute of Science, Rehovot, Israel.
Uri Kopylov, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel.
Arie Levine, Pediatric Gastroenterology Unit, PIBD Research Center, Wolfson Medical Center, Holon, Israel; Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Nitsan Maharshak, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Department of Gastroenterology and Hepatology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Funding
This study was supported by a ECCO Pioneer Prize, Litwin IBD Pioneer grant, and the Azrieli, Solomon and Beker foundations.
Conflict of Interest
AL has received grants, honoraria, speaker or consulting fees, or IP licensing, from Janssen, Megapharm, Takeda, Ferring, and Nestle Health Science. FS has received speaker or consulting fees from Janssen, MSD, Takeda, Sanofi, Amgen, Ferring, Fresenius. UK has received consulting/lecture fees from Abbvie, Jannsen, Takeda, MSD, Medtronic, Pfizer; research grants from Janssen, Medtronic, Takeda. NM has received grants, speaking and/ or consulting fees from Pfizer, Takeda, Janssen, Ferring, BiomX, Abbott, BMS, and Nestle Health Science.
Author Contributions
AL, NM conceived of the study and with CSS designed the study. NM and FS were responsible for FMT protocols and national donor recruitment and sample storage in each country. AL secured grants for funding. CSS, NFI, and CF managed and monitored data. CSS, AL, FS, and NM analysed and reviewed clinical data, interpreted the results, and drafted the report. AW and AS performed donor microbial analyses and interpreted the results. NM, FS, EZ, MMC, MT, NAC, YR, NFI, JF, MY, FC, TL, LM, NK, GQ recruited patients and provided the data. NM and FS critically reviewed the manuscript. All authors read and approved the final version of the report.
References
- 1. Davenport M, Poles J, Leung JM, et al. Metabolic alterations to the mucosal microbiota in inflammatory bowel disease. Inflamm Bowel Dis 2014;20:723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishida A, Inoue R, Inatomi +O, Bamba S, Naito Y, Andoh A. Gut microbiota in the p athogenesis of inflammatory bowel disease. Clin J Gastroenterol 2018;11:1–10. [DOI] [PubMed] [Google Scholar]
- 3. Johansson ME, Gustafsson JK, Holmén-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014;63:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Post S, Jabbar KS, Birchenough G, et al. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019;68:2142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. James SL, Christophersen CT, Bird AR, et al. Abnormal fibre usage in UC in remission. Gut 2015;64:562–70. [DOI] [PubMed] [Google Scholar]
- 6. Khalil NA, Walton GE, Gibson GR, Tuohy KM, Andrews SC. In vitro batch cultures of gut microbiota from healthy and ulcerative colitis [UC] subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int J Food Sci Nutr 2014;65:79–88. [DOI] [PubMed] [Google Scholar]
- 7. Zhuang X, Liu C, Zhan S, et al. Gut microbiota profile in pediatric patients with inflammatory bowel disease: a systematic review. Front Pediatr 2021;9:626232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turner D, Bishai J, Reshef L, et al. Antibiotic cocktail for pediatric acute severe colitis and the microbiome: the PRASCO randomised controlled trial. Inflamm Bowel Dis 2020;26:1733–42. [DOI] [PubMed] [Google Scholar]
- 9. Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomised controlled trial. Gastroenterology 2019;157:440–50.e8. [DOI] [PubMed] [Google Scholar]
- 10. Moayyedi P, Surette MG, Kim PT, et al. Faecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomised controlled trial. Gastroenterology 2015;149:102–9.e6. [DOI] [PubMed] [Google Scholar]
- 11. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomised controlled trial of faecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149:110–8.e4. [DOI] [PubMed] [Google Scholar]
- 12. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017;389:1218–28. [DOI] [PubMed] [Google Scholar]
- 13. Costello SP, Hughes PA, Waters O, et al. Effect of faecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomised clinical trial. JAMA 2019;321:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuentes S, Rossen NG, van der Spek MJ, et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J 2017;11:1877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scaldaferri F, Pecere S, Petito V, et al. Efficacy and mechanisms of action of faecal microbiota transplantation in ulcerative colitis: pitfalls and promises from a first meta-analysis. Transplant Proc 2016;48:402–7. [DOI] [PubMed] [Google Scholar]
- 16. Ishikawa D, Sasaki T, Osada T, et al. Changes in intestinal microbiota following combination therapy with faecal microbial transplantation and antibiotics for ulcerative colitis. Inflamm Bowel Dis 2017;23:116–25. [DOI] [PubMed] [Google Scholar]
- 17. Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther 2017;46:213–24. [DOI] [PubMed] [Google Scholar]
- 18. Fang H, Fu L, Wang J. Protocol for faecal microbiota transplantation in inflammatory bowel disease: a systematic review and meta-analysis. Biomed Res Int 2018;2018:8941340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Llewellyn SR, Britton GJ, Contijoch EJ, et al. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 2018;154:1037–46.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vidal-Lletjós S, Beaumont M, Tomé D, Benamouzig R, Blachier F, Lan A. Dietary protein and amino acid supplementation in inflammatory bowel disease course: What impact on the colonic mucosa? Nutrients 2017;9:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh V, Yeoh BS, Walker RE, et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019;68:1801–12. [DOI] [PubMed] [Google Scholar]
- 23. Tomas J, Mulet C, Saffarian A, et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci U S A 2016;113:E5934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pasolli E, Asnicar F, Manara S, et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 2019;176:649–62.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012;9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Korem T, Zeevi D, Suez J, et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 2015;349:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrer-Picón E, Dotti I, Corraliza AM, et al. Intestinal inflammation modulates the epithelial response to butyrate in patients with inflammatory bowel disease. Inflamm Bowel Dis 2020;26:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahu P, Kedia S, Vuyyuru SK, et al. Randomised clinical trial: exclusive enteral nutrition versus standard of care for acute severe ulcerative colitis. Aliment Pharmacol Ther 2021;53:568–76. [DOI] [PubMed] [Google Scholar]
- 29. Costello SP, Day A, Yao CK, Bryant RV. Faecal microbiota transplantation [FMT] with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep 2020;13:e233135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cammarota G, Ianiro G, Kelly CR, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019;68:2111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.