Abstract
Background
Maternal biologic factors can affect fetal fraction in cell-free DNA-based prenatal screening assays, thereby limiting effectiveness. Higher rates of indeterminate results from low fetal fraction have been described in cases of autoimmune disease in pregnancy. Existing studies are confounded by the concomitant maternal use of anticoagulants, which may independently influence test characteristics.
Objective(s)
To evaluate differences in fetal fraction, indeterminate results, and total cell-free DNA concentration for women with autoimmune disease compared to controls using our in-house developed non-invasive prenatal screening platform in the absence of maternal anticoagulation use.
Study Design
A retrospective, single institution cohort study of a previously validated cell-free DNA-based non-invasive prenatal screening assay using a low-pass whole genome sequencing platform between 2017 and 2019. A diagnosis of autoimmune disease included systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and others. Immunomodulator therapies included biologics, corticosteroids, hydroxychloroquine, azathioprine, and intravenous immunoglobulin. Women on anticoagulation were excluded. We evaluated the association between autoimmune disease and fetal fraction, indeterminate results, and total cell-free DNA concentration using univariate and multivariate analyses, stratifying for immunomodulator therapy and adjusting for body mass index, fetal sex, and gestational age at sample collection.
Results
1,445 patients met inclusion criteria. Forty-three women had a confirmed autoimmune disease, with 25 not on immunomodulator therapy and 18 on immunomodulator therapy. The mean fetal fraction for women with autoimmune disease was significantly lower compared to controls (9.7% vs. 11.9%, p=0.004). The rate of indeterminate results was significantly higher for women with autoimmune disease compared to controls (16.3% vs. 3.5%; p<0.001) The total cell-free DNA concentration was not statistically different between groups (94.8 pg/uL for autoimmune disease vs. 83.9 pg/uL for controls, p=0.06). In logistic regression, women with autoimmune disease had a significantly higher odds of an indeterminate result compared to controls, (aOR 5.3, 95%CI 2.0, 14.2). Linear regression showed a significant negative association between autoimmune disease and fetal fraction (aβ −2.1, 95%CI −3.4, −0.6).
Stratifying by treatment status, mean fetal fraction was 9.8%, 9.6%, and 11.9% for women with autoimmune disease not on immunomodulator therapy, autoimmune disease on immunomodulator therapy, and controls, respectively (p=0.02). The rate of indeterminate results increased in a stepwise fashion from 3.5% to 11.1% to 20.0% for controls, autoimmune disease on immunomodulator therapy, and autoimmune disease not on immunomodulator therapy, respectively (p<0.001).
Logistic regression demonstrated higher odds of an indeterminate result for women with autoimmune disease not on immunomodulator therapy compared to controls, (aOR 7.3, 95%CI 2.3, 22.5). Autoimmune disease not on immunomodulator therapy was negatively associated with fetal fraction compared to controls (aβ −2.2, 95%CI −4.2, −0.3).
Conclusion(s)
Women with autoimmune disease have lower fetal fraction and higher rates of indeterminate results compared to women without autoimmune disease. There was no difference in total cell-free DNA concentration. Treatment of maternal autoimmune disease with immunomodulator therapy may decrease the indeterminate result rate.
Keywords: cell-free DNA concentration, fetal fraction, immunomodulator therapy, indeterminate result, non-invasive prenatal screening
CONDENSATION
Autoimmune disease in pregnancy is associated with higher rates of indeterminate cell-free DNA results due to low fetal fraction, independent of anticoagulation use.
INTRODUCTION:
Before its use for reliable non-invasive prenatal screening (NIPS) for aneuploidy, cell-free DNA (cfDNA) was investigated as a biomarker for several acute and chronic disease states including sepsis, trauma, transplant rejection, cancer, and autoimmune disease (AID).1–6 Cell-free DNA is present at very low levels in healthy individuals, resulting from physiologic cellular breakdown and active DNA release from living cells.7 In certain pathologic conditions like AID, cfDNA levels are significantly elevated compared to healthy controls. Although the underlying mechanisms are not completely understood, proposed etiologies include ineffective clearance of necrotic or apoptotic cellular material and formation of proinflammatory immune complexes.8 Additionally, studies have shown that cfDNA levels further increase with clinical exacerbations and decrease with immunomodulator (IM) treatments targeted at improving systemic inflammation.6
Fetal cfDNA, originating largely from placental trophoblast cells during pregnancy,9 is detected in maternal circulation as early as the first trimester, accounting for approximately 10–15% of the total cfDNA concentration.10–12 Evaluation of cfDNA from maternal plasma has emerged as the most reliable NIPS tool for detecting fetal aneuploidy with a failure rate of 0.8–12.2%.13 The most common reason for a test failure, or an indeterminate result, is a low fetal fraction (FF).14,15 Obesity, anticoagulation use, maternal neoplasms, organ transplantation, and fetal aneuploidy are all proposed maternal and fetal factors that may contribute to low FF by either increasing the maternal contribution or decreasing the fetal contribution to total cfDNA.9,12,16–19
One small study and two case reports have found an association between maternal AID and higher rates of indeterminate results due to low FF.20–22 However, the majority of these patients were on anticoagulation, which alone is associated with an increased rate of indeterminate results.16,17,23 We sought to evaluate differences in FF, indeterminate result rates, and total cfDNA concentration in pregnant women with AID, as well as the potential effect of IM therapies, in the absence of concomitant anticoagulation use. We hypothesized that women with AID have lower FF and a higher rate of indeterminate results compared to controls, owing to an overall increase in maternal cfDNA concentration, and that treatment with IM therapies leads to less indeterminate results from low FF.
MATERIALS AND METHODS
This is a single institution retrospective cohort study of cfDNA-based NIPS tests performed between May 2017 and December 2019. Since May 2017, the University of Washington has clinically used an internally validated NIPS assay that employs low-pass whole genome sequencing of cfDNA extracted from maternal plasma to provide a high-quality screen for autosomal and sex chromosome aneuploidies. Validation studies prior to clinical use confirmed that our test performance is in line with other commercially available platforms.
Study Population
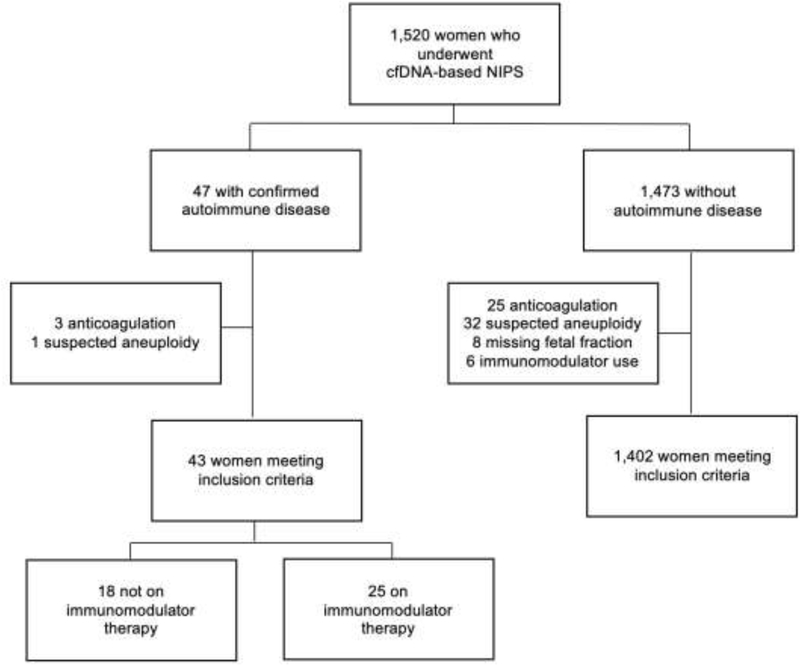
All patients with singleton gestations who completed first trimester NIPS through the University of Washington within the study period were eligible for inclusion. We sought to compare samples from women with and without AID. Additionally, we stratified women with AID by use of IM therapy at the time of NIPS, creating four study groups: all AID, AID on IM therapy, AID not on IM therapy, and controls without AID. For all groups we excluded women on anticoagulation, all NIPS results consistent with suspected aneuploidy, those with a missing FF, and those taking IM medications for non-autoimmune conditions (Figure 1). Patients on low-dose aspirin were included in the analysis.
Figure 1: Flowchart of study population.
Flow chart of the study population. cfDNA, cell-free DNA; NIPS, non-invasive prenatal screening.
Detailed maternal demographic, medical, and obstetric data was abstracted from the medical record. AIDs included systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease and other AID (immune thrombocytopenic purpura, Sjogren’s syndrome, Ankylosing spondylitis, Psoriatic arthritis, Guttate psoriasis, Behcet’s disease, and AID not otherwise specified). IM therapies included biologics, corticosteroids, hydroxychloroquine, azathioprine, and intravenous immunoglobulin (IVIG). Given the in-house nature of our platform, we have access to detailed test characteristics and medical records, allowing for review of any notable cases. Any such cases, particularly related to abnormal test results, were reconciled by authors, CML and RS. Fetal sex was confirmed via obstetric and neonatal records. Institutional Review Board approval was obtained from the University of Washington (STUDY00005540). Study data was collected and managed using REDCap electronic data capture tools hosted at the University of Washington.24,25
Sample collection and quantification of cfDNA
Whole blood from Streck (BCT1) tubes was centrifuged and plasma was isolated as per the package insert. cfDNA was extracted from plasma using the QIAsymphony Circulating DNA Kit. Total cfDNA concentration was measured by Qubit fluorometry, a highly sensitive method for quantification of intact double-stranded DNA (pg/uL). The Agilent TapeStation workflow, an automated electrophoresis system, was used to assess the size and integrity of the DNA and functions as a critical quality control step throughout next-generation library preparation, hybridization capture, and sample pooling before sequencing.
Sequencing and quantification of fetal fraction
Following library preparation with KAPA HyperPrep for adapter and index ligation followed by Agencourt AMPureXP purification and amplification, libraries were pooled using an equimolar strategy. Libraries were sequenced using an Illumina NextSeq 500 High Output 75 cycle kit with a 37bp paired-end read configuration. On average, each cfDNA sample was sequenced to a depth of approximately 20 million paired-end reads, corresponding to an average genome depth of 0.5X. Reads were aligned to the human reference genome (hg19) with Bowtie (version 1.1.2), and run metrics were calculated with Picard (version 1.141). The placental-derived fraction for each sample was calculated either by the percent of reads that align to the Y chromosome, or a custom bioinformatic algorithm based on the aggregate length distribution in sequencing reads for samples in which the Y chromosome was not present (i.e. female fetuses).26 An indeterminate result was defined as fetal fraction <4%. Failures due to technical issues, including samples with insufficient quantities of input DNA, were purposely excluded from the indeterminate group as these were related to collection or processing errors.
Statistical Analysis
Our primary outcome was to compare cfDNA FF among women with AID compared to controls. Secondary outcomes included differences in the rate of indeterminate results due to low FF and total cfDNA concentration among the groups. Primary and secondary outcomes were additionally analyzed after stratifying for IM use in AID subjects. Demographic and clinical characteristics for the groups were compared with ꭓ2 or Fisher’s exact test as appropriate. Continuous variables were tested for normality and compared using the t-test or Kruskal-Wallis test as appropriate. Multivariable logistic regression was performed to determine the odds of an indeterminate result based on the presence of AID and IM treatment status. Linear regression was performed to determine the association between FF and total cfDNA concentrations with AID and IM treatment status. Models were adjusted for fetal sex, gestational age, and BMI at the time of sample collection. All data were analyzed using STATA/IC 16.1 (Statacorp, College Station, TX) and SPSS version 26.0 (IBM Corp).
RESULTS
A total of 1,520 women with singleton pregnancies completed cfDNA-based NIPS during the study period. Of the 1,445 women who met inclusion criteria, 3% (n=43) had a confirmed AID with 58% (n=25) not on IM therapy and 42% (n=18) on IM therapy (Figure 1). Of those on IM therapy, 13 were on single agent therapy and 5 were on more than one agent. There were higher rates of pregestational hypertension and low-dose aspirin use in women with AID on IM therapy. Other characteristics including maternal age at delivery, gravidity, body mass index (BMI), gestational age at sample collection, fetal sex, use of assisted reproductive technologies, and indication for testing were similar across groups (Table 1). The most common indication for screening was advanced maternal age (≥35 years) while other less common indications included first-line screening, ultrasound abnormalities, and abnormal serum screening results.
Table 1:
Patient Demographics
| AID (−) Immunomodulator Therapy (n=25) |
AID (+) Immunomodulator Therapy (n=18) |
No AID (n=1402) |
p-value | |
|---|---|---|---|---|
| Age (years) | 36.9 ± 4.5 | 34.7 ± 3.7 | 34.7 ± 5.0 | 0.1 |
| BMI (kg/m2) at sample collection | 27.9 ± 8.0 | 26.8 ± 4.8 | 27.3 ± 6.6 | 0.8 |
| Gravidity | 3 (1–5) | 3 (1–4) | 2 (1–4) | 0.2 |
| Pregestational hypertension | 2 (8.0) | 3 (16.7) | 86 (6.1) | 0.03 |
| ART pregnancy | 3 (12.0) | 1 (5.6) | 92 (6.6) | 0.41 |
| Gestational age at sample collection | 13.6 ± 3.9 | 14.0 ± 3.3 | 14.2 ± 4.4 | 0.8 |
| Female fetal sex | 13 (52.0) | 8 (44.4) | 537 (38.3) | 0.9 |
| Aspirin use | 4 (16.0) | 4 (22.2) | 53 (3.8) | <0.001 |
| Indication for testing | 0.8 | |||
| Advanced maternal age | 19 | 12 | 833 | |
| First-line screen | 3 | 4 | 303 | |
| Ultrasound abnormality | 1 | 1 | 163 | |
| Abnormal serum screen | 2 | 1 | 78 | |
| Other | 0 | 0 | 16 | |
| Type of AID | ||||
| Systemic lupus erythematous | 3 (12.0) | 5 (27.8) | 0 (0) | |
| Rheumatoid arthritis | 5 (20.0) | 1 (5.6) | 0 (0) | |
| Multiple sclerosis | 3 (12.0) | 0 (0) | 0 (0) | |
| Inflammatory bowel disease | 4 (16.0) | 7 (38.9) | 0 (0) | |
| Other* | 11 (44.0) | 5 (27.8) | 0 (0) | |
Data presented as mean ± SD, N (%), or median (interquartile range) AID, autoimmune disease; BMI, body mass index; ART, assisted reproductive technologies
Includes immune thrombocytopenic purpura, Sjogren’s syndrome, Ankylosing spondylitis, Psoriatic arthritis, Guttate psoriasis, Behcet’s disease, autoimmune disease not otherwise specified
The mean FF for women with AID was significantly lower compared to controls (9.7% vs. 11.9%, p=0.004) and, correspondingly, the indeterminate rate was significantly higher compared to controls (16.3% vs. 3.5%; p<0.001) (Table 2). Although the median total cfDNA concentration was higher for women with AID compared to controls, this did not reach statistical significance (94.8 pg/uL [IQR 68.7–138.0] vs. 83.9 pg/uL [IQR 59.0–117.0]; p=0.06).
Table 2:
Cell-free DNA test characteristics by maternal disease status
| AID (n=43) | No AID (n=1402) | p-value | |
|---|---|---|---|
| Fetal fraction (%) | 9.7 ± 5.6 | 11.9 ± 5.0 | 0.004 |
| Indeterminate rate | 7 (16.3) | 49 (3.5) | <0.001 |
| Total cfDNA (pg/uL) | 94.8 (68.7–138.0) | 83.9 (59.0–117.0) | 0.06 |
Data are mean ± SD, N (%), or median (interquartile range)
AID, autoimmune disease; cfDNA, cell-free DNA
Logistic regression analyses demonstrated that for women with AID the odds of an indeterminate result was approximately 5-fold higher compared to controls (OR 5.4, 95% CI 2.3,12.7). This remained significant after controlling for the gestational age at sample collection, BMI, and fetal sex (aOR 5.3, 95% CI 2.0, 14.2). In linear regression analysis, FF was negatively associated with maternal AID, controlling for previously listed confounders (aβ −2.1, 95% CI −3.4, −0.6). We found no significant association between total cfDNA concentration and AID (Table 3).
Table 3:
Association of maternal disease status with fetal fraction, rate of indeterminate result and total cell-free DNA concentration
| AID (n=43) | No AID (n=1402) | |
|---|---|---|
|
Fetal fraction %
β (95% CI) | ||
| Unadjusted | −2.2 (−3.7, −0.7) | 0 (Reference) |
| Adjusted * | −2.1 (−3.4, −0.6) | 0 (Reference) |
|
Indeterminate rate
OR (95% CI) | ||
| Unadjusted | 5.4 (2.3, 12.7) | 1.0 (Reference) |
| Adjusted * | 5.3 (2.0–14.2) | 1.0 (Reference) |
|
Total cfDNA
β (95% CI) | ||
| Unadjusted | 27.8 (−15.1, 70.6) | 0 (Reference) |
| Adjusted * | 24.7 (−19.2, 68.7) | 0 (Reference) |
AID, autoimmune disease; CI, confidence interval; cfDNA, cell-free DNA
Adjusted for BMI, fetal sex, and gestational age at sample collection
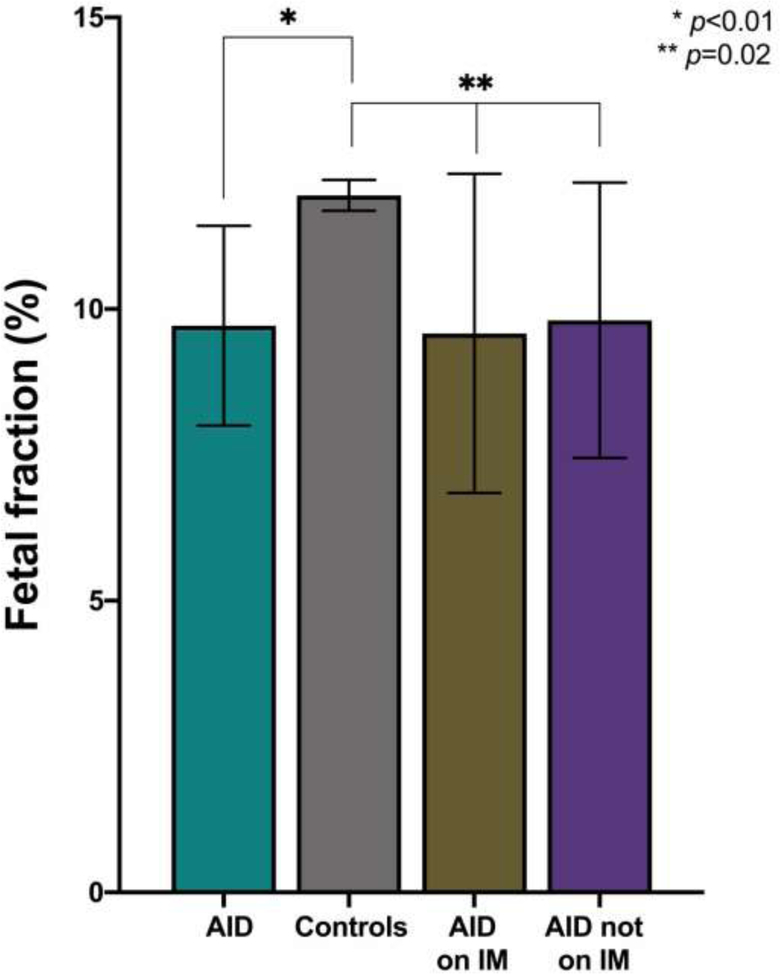
After stratifying women with AID based on IM treatment status, we found that the mean FF remained significantly lower for women with AID compared to controls (9.8% AID not on IM, 9.6% AID on IM, and 11.9% controls, p=0.02) (Table 4, Figure 2).
Table 4:
Cell-free DNA test characteristics by maternal autoimmune disease and immunomodulator therapy status
| AID (−) Immunomodulator Therapy (n=25) |
AID (+) Immunomodulator Therapy (n=18) |
No AID (n=1402) |
p-value | |
|---|---|---|---|---|
| Fetal fraction (%) | 9.8 ± 5.7 | 9.6 ± 5.5 | 11.9 ± 5.0 | 0.02 |
| Indeterminate rate | 5 (20.0) | 2 (11.1) | 49 (3.5) | 0.001 |
| Total cfDNA (pg/uL) | 96.4 (73.5–134.0) | 94.8 (63.1–163.0) | 83.9 (59.0–117.0) | 0.08 |
Data are mean ±SD, N (%), median (interquartile range)
AID, autoimmune disease; cfDNA, cell-free DNA
Figure 2: Fetal fraction in maternal autoimmune disease.
Mean fetal fraction, demonstrating comparisons between controls and study groups. AID, autoimmune disease; IM, immunomodulator.
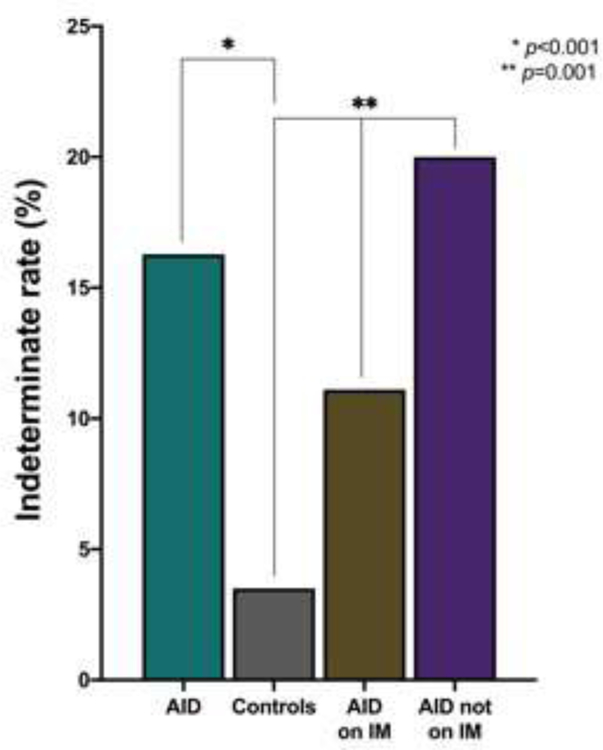
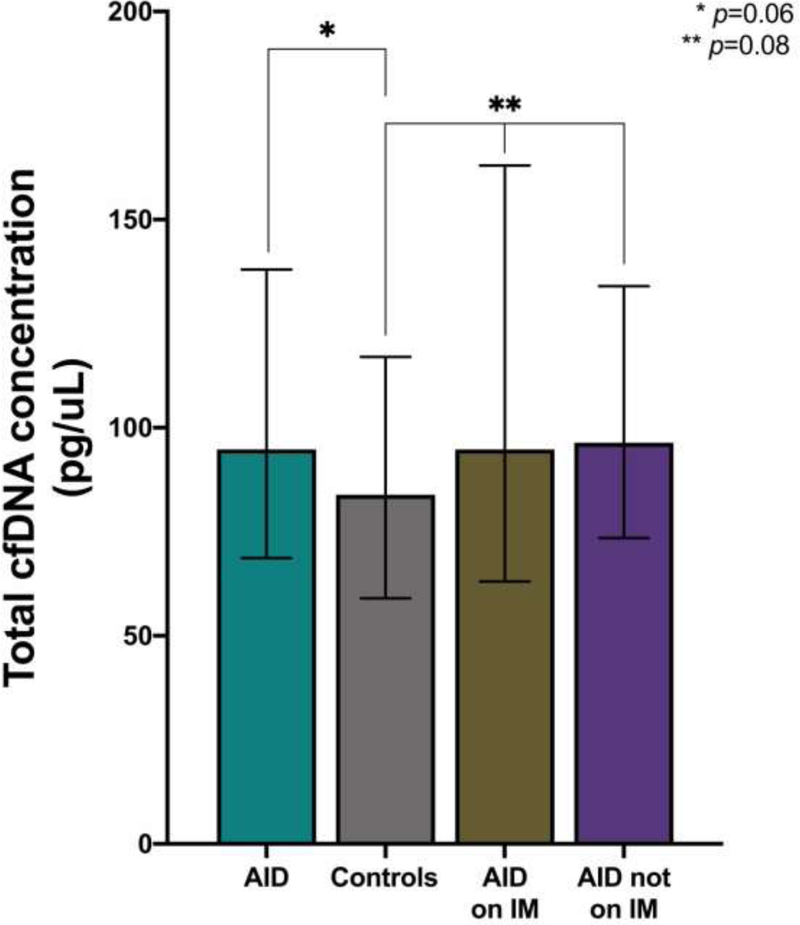
Correspondingly, there was a stepwise increase in the rate of indeterminate results from 3.5% to 11.1% to 20.0% for controls, women with AID on IM therapy and women with AID not on IM therapy, respectively (p=0.001) (Figure 3). Of those with indeterminate results, 36 in the control group and 4 in the AID group not on IM therapy underwent repeat testing with 31% and 75% having a second indeterminate result, respectively. The 2 women in the AID group on IM with indeterminate results did not have repeat testing. Although there was no significant difference in the total cfDNA concentration between groups, there was a trend towards increasing total cfDNA concentration from a median of 83.9 pg/uL (IQR 59.0–117.0) to 94.8 pg/uL (IQR 63.1–163.0) to 96.4 pg/uL (IQR 73.5–134.0) for controls, women with AID on IM therapy, and women with AID not on IM therapy, respectively (p=0.08) (Figure 4).
Figure 3: Indeterminate result rate in maternal autoimmune disease.
Indeterminate result rates, demonstrating comparisons between controls and study groups. AID, autoimmune disease; IM, immunomodulator.
Figure 4: Total cfDNA concentration in maternal autoimmune disease.
Total cfDNA, demonstrating comparisons between controls and study groups. AID, autoimmune disease; IM, immunomodulator.
Logistic regression analyses demonstrated that women with AID not on IM therapy had an approximately 7-fold higher odds of an indeterminate result compared to controls (OR 6.90, 95% CI 2.5–19.1). This remained significant in the adjusted analysis (aOR 7.3, 95% CI 2.3–22.5; p=0.001). For women with AID on IM therapy, there was no significant difference in the odds of an indeterminate result compared to controls in both unadjusted and adjusted analyses (OR 3.5, 95% CI 0.8, 15.4 and aOR 2.4, 95% CI 0.3, 19.4). In linear regression analysis, FF was negatively associated with AID not on IM therapy, controlling for the aforementioned confounders (aβ −2.2, 95% CI −4.2, −0.3). For women with AID on IM therapy, there was no significant association with FF compared to controls (aβ −1.8, 95% CI −4.1, 0.5). Additionally, we found no significant association between total cfDNA concentration and AID among the stratified groups (Table 5).
Table 5:
Association of immune modulator therapy with fetal fraction, indeterminate result rate and total cfDNA concentration
| AID (−) Immunomodulator Therapy (n=25) |
AID (+) Immunomodulator Therapy (n=18) |
No AID (n=1402) |
|
|---|---|---|---|
|
Fetal fraction %
β (95% CI) | |||
| Unadjusted | −2.1 (−4.1, 0.2) | −2.4 (−4.7, −0.02) | 0 (Reference) |
| Adjusted * | −2.2 (−4.2, −0.3) | −1.8 (−4.1, 0.5) | 0 (Reference) |
|
Indeterminate rate
OR (95% CI) | |||
| Unadjusted | 6.9 (2.5, 19.1) | 3.5 (0.8, 15.4) | 1.0 (Reference) |
| Adjusted * | 7.3 (2.3, 22.5) | 2.4 (0.3, 19.4) | 1.0 (Reference) |
|
Total cfDNA
β (95% CI) | |||
| Unadjusted | 38.5 (−19.2, 92.2) | 15.5 (−50.5, 81.5) | 0 (Reference) |
| Adjusted * | −44.0 (−12.6, 100.7) | −3.5 (−71.8, 64.8) | 0 (Reference) |
AID, autoimmune disease; CI, confidence interval; cfDNA, cell-free DNA
Adjusted for BMI, fetal sex, and gestational age at sample collection
COMMENT
Principal Findings
We found that women with AID had a significantly lower mean FF on cfDNA-based NIPS compared to controls, with correspondingly higher indeterminate result rates, without differences in total cfDNA concentration. These findings were obtained in the absence of concomitant anticoagulation. The indeterminate result rate progressively increased from the lowest rate in controls, to women with AID on IM therapy, followed by women with AID not on IM therapy. Stratified regression analysis demonstrated that maternal AID not on IM therapy negatively correlated with FF and that these women had a 7-fold increased odds of an indeterminate result compared to controls. Treatment of AID with IM therapy tempered these effects, as this group was statistically similar to controls. We found no statistical difference in total cfDNA concentration between groups, however, there may be a trend towards higher concentrations in women with AID with or without IM therapies.
Results in the context of what is known
Our findings add to a growing body of literature suggesting the complex role maternal medical conditions, specifically AID, may play in cfDNA-based NIPS testing. To our knowledge, no prior studies have specifically assessed the effect of maternal AID on such tests in the absence of anticoagulation. This is important as anticoagulation is independently associated with low FF and higher test failure.16,17,23 We elected to include patients on aspirin, as this has not been demonstrated to influence cfDNA prenatal screening test characteristics.27 Additionally, we utilized our robust medical records to clinically confirm AID, followed by stratification based on confirmation of IM use at the time of sample collection. As such, our data demonstrate that not only does the presence of maternal AID influence test characteristics, but so does IM treatment of these diseases.
Clinical Implications
Given its prolific use, understanding biologic factors influencing cfDNA-based NIPS is important for providers counseling patients and triaging failed tests for women with underlying conditions like AID. The initial clinical concern in the setting of an indeterminate result is the risk of fetal aneuploidy.28 However as mounting evidence reveals additional factors that increase the risk of a failed test from low FF, providers should be thoughtful when considering next steps of management given the additional cost, anxiety and potential diagnostic delay that could result from repeated indeterminate results. More information is needed to develop appropriate clinical algorithms for approaching failed tests.
We found differences in test results for all women with AID regardless of IM treatment status. Although heterogeneity of AID diagnoses in our cohort precludes precise assessment of individual disease severity, it is reasonable to posit that IM therapy was indicated in cases with more severe disease. Interestingly, our data suggest that differences in cfDNA test characteristics are more pronounced in women with AID not on IM therapy, who presumably have mild or quiescent disease. Thus, it seems that the presence of AID alone, regardless of its apparent clinical severity, may impact cfDNA test characteristics. The precise interaction between underlying AID, IM therapy and disease severity is unknown at this time. Despite the assumption that IM treatment may indicate increased underlying AID severity, this group was statistically similar to controls in stratified analysis. Although the regression analyses were non-significant for AID on IM compared to controls, the absolute value of indeterminate result rates fell between that of controls and AID not on IM therapy. Future studies with a larger sample sizes may provide additional power to further evaluate the potential modulating effect of IM therapy on cfDNA FF and indeterminate results for women with AID.
Research Implications
Limited data is currently available regarding the potential mechanisms contributing to altered cfDNA test characteristics in AID. Dabi et al. recently investigated the relationship between heparin treatment and cfDNA-based NIPS results and found that the presence of maternal AID was associated with an increased rate of an indeterminate result independent of anticoagulation use.20 Although this is consistent with our findings, all patients in this smaller cohort had concurrent heparin use, as such, this association was determined based on inclusion of AID as a confounder in regression analyses. Two additional case reports have described patients with AID and multiple failed cfDNA assays, although results in one case are confounded by anticoagulation use.21 In the second, a result was obtained on cfDNA-based NIPS on the third attempt, coinciding with the initiation of IM therapies.22 Although this may suggest a treatment-related effect, the known serial increase in FF with advancing gestation may have also played an important role.
Studies in both non-pregnant and pregnant women suggest an increased amount of circulating total cfDNA in the setting of various AIDs.29–35 This has led others to posit that this observed low FF results from excess maternal contribution to the total cfDNA concentration in the setting of systemic inflammation or impaired clearance mechanisms. Interestingly, we found no significant difference in total cfDNA concentration among women with AID compared to controls; however, a trend towards increasing cfDNA concentration in AID suggests possible limitations of our small sample size. Although the FF was not different in stratified analysis between women with AID on IM and women with AID on no IM in our cohort, the indeterminate rate was significantly lower for women with AID on IM therapy. This suggests that IM therapies may improve assay performance via currently unknown mechanisms. Comparisons across currently available studies are limited by differences in clinical cohorts, test methodology, and confounders, such as concomitant anticoagulation use.
Strengths and Limitations
Our study has multiple strengths. To specifically delineate relationships between AID and NIPS test characteristics, we carefully selected our clinical cohort to exclude all subjects on anticoagulation at the time of sample collection. Additionally, we utilized a single validated platform in current clinical use with a uniform FF cutoff for interpretation (<4%), removing any issues related to test performance of differing platforms. Due to the in-house nature of our testing platform, we have access to detailed laboratory and clinical data, allowing us to reconcile all cases with an indeterminate result to identify the underlying reason, such that all indeterminate results are truly due to a low FF and not other potential errors along the testing pipeline. Access to our robust and detailed medical records allowed us to clinically confirm every AID case and the presence of IM use at the time of sample collection, in turn allowing us to investigate these two clinical scenarios (AID on IM therapy and those not on IM therapy) separately. Importantly we did not exclude pregnancies based on fetal sex and included fetal sex as a biologic variable in all regression analyses, in line with contemporary research guidelines.36,37
Our study is limited by its retrospective design and small sample size of women with AID. Although we present novel findings, our study does not provide mechanisms to explain how AID or IM therapy affect cfDNA-based NIPS test characteristics. Finally, although we adjusted for known confounders, our results may be explained by additional unmeasured factors not gleaned from the medical records.
Conclusions
Maternal AID was associated with lower FF, higher indeterminate result rates and no difference in total cfDNA concentration compared to controls. Women with AID not on IM therapy had an increased odds of an indeterminate result due to low FF, while those on IM treatment did not, suggesting a mitigating role of IM therapy. We did not find significant differences in total cfDNA concentration between the groups, suggesting contribution of additional factors aside from dilution from maternal sources. Future studies directed at identifying biologic mechanisms that influence indeterminate cfDNA results in the setting of maternal AID and the effects of various IM therapies on NIPS test characteristics are needed.
AJOG AT A GLANCE.
A: Why was this study conducted?
Interpreting cell-free DNA (cfDNA) results in the context of biologic factors is important when counseling and managing indeterminate results.
The impact of maternal autoimmune disease, independent of anticoagulation use, on cfDNA screening is poorly understood.
B: What are the key findings?
Fetal fraction was lower in autoimmune disease versus controls.
Indeterminate result rates were higher in autoimmune disease, regardless of immunomodulator use, versus controls.
Total cfDNA concentration was not different in autoimmune disease versus controls.
C: What does this study add to what is already known?
In the absence of anticoagulation, maternal autoimmune disease alone contributes to lower fetal fraction and higher indeterminate result rates.
Low fetal fraction in the presence of autoimmune disease may not be solely explained by an excess of maternally-derived cell-free DNA.
Use of immunomodulatory therapy in maternal autoimmune disease may lower indeterminate result rates.
HIGHLIGHTS.
Maternal autoimmune disease contributes to low cell-free DNA fetal fraction and higher rates of indeterminate results in non-invasive prenatal screening.
Immunomodulator therapy in maternal autoimmune disease may mitigate the increased odds of indeterminate cell-free DNA prenatal screening results.
Total cell-free DNA concentration is not significantly different in pregnant women with and without autoimmune disease.
SOURCES OF FUNDING
The study was funded by the National Institutes of Health (K08HL150169), which had no involvement in study design, in the collection, analysis, or interpretation of data or in the writing of the report or decision to submit the article for publication. Additional funding for this study was supported by the National Center for Advancing Translational Sciences (UL1TR002319, KL2TR002317, TL1TR002318).
Footnotes
DISCLOSURE STATEMENT
The authors report no conflict of interest.
PAPER PRESENTATION
Preliminary findings of this study were presented as a poster presentation (#644) at the Society for Maternal Fetal Medicine’s 41st Annual Pregnancy Virtual Meeting; January 25–30, 2021.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saukkonen K, Lakkisto P, Pettilä V, et al. Cell-free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin Chem. 2008;54(6):1000–1007. doi: 10.1373/clinchem.2007.101030 [DOI] [PubMed] [Google Scholar]
- 2.Rainer TH. Plasma DNA, prediction and post-traumatic complications. Clin Chim Acta. 2001;313(1–2):81–85. doi: 10.1016/s0009-8981(01)00653-2 [DOI] [PubMed] [Google Scholar]
- 3.Rainer TH, Wong LKS, Lam W, et al. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin Chem. 2003;49(4):562–569. doi: 10.1373/49.4.562 [DOI] [PubMed] [Google Scholar]
- 4.Knight SR, Thorne A, Lo Faro ML. Donor-specific Cell-free DNA as a Biomarker in Solid Organ Transplantation. A Systematic Review. Transplantation. 2019;103(2):273–283. doi: 10.1097/TP.0000000000002482 [DOI] [PubMed] [Google Scholar]
- 5.Sun K, Jiang P, Chan KCA, et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci U S A. 2015;112(40):E5503–5512. doi: 10.1073/pnas.1508736112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duvvuri B, Lood C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front Immunol. 2019;10:502. doi: 10.3389/fimmu.2019.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandel P, Metais P. Nuclear Acids In Human Blood Plasma. C R Seances Soc Biol Fil. 1948;142(3–4):241–243. [PubMed] [Google Scholar]
- 8.Aucamp J, Bronkhorst AJ, Badenhorst CPS, Pretorius PJ. The diverse origins of circulating cell-free DNA in the human body: a critical re-evaluation of the literature. Biol Rev Camb Philos Soc. 2018;93(3):1649–1683. doi: 10.1111/brv.12413 [DOI] [PubMed] [Google Scholar]
- 9.Bianchi DW, Chudova D, Sehnert AJ, et al. Noninvasive Prenatal Testing and Incidental Detection of Occult Maternal Malignancies. JAMA. 2015;314(2):162–169. doi: 10.1001/jama.2015.7120 [DOI] [PubMed] [Google Scholar]
- 10.Lo YM, Tein MS, Lau TK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998;62(4):768–775. doi: 10.1086/301800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canick JA, Palomaki GE, Kloza EM, Lambert-Messerlian GM, Haddow JE. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn. 2013;33(7):667–674. doi: 10.1002/pd.4126 [DOI] [PubMed] [Google Scholar]
- 12.Ashoor G, Syngelaki A, Poon LCY, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell-free DNA at 11–13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. 2013;41(1):26–32. doi: 10.1002/uog.12331 [DOI] [PubMed] [Google Scholar]
- 13.Yaron Y The implications of non-invasive prenatal testing failures: a review of an under-discussed phenomenon. Prenat Diagn. 2016;36(5):391–396. doi: 10.1002/pd.4804 [DOI] [PubMed] [Google Scholar]
- 14.Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–1597. doi: 10.1056/NEJMoa1407349 [DOI] [PubMed] [Google Scholar]
- 15.Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):302–314. doi: 10.1002/uog.17484 [DOI] [PubMed] [Google Scholar]
- 16.Grömminger S, Erkan S, Schöck U, et al. The influence of low molecular weight heparin medication on plasma DNA in pregnant women. Prenat Diagn. 2015;35(11):1155–1157. doi: 10.1002/pd.4668 [DOI] [PubMed] [Google Scholar]
- 17.Burns W, Koelper N, Barberio A, et al. The association between anticoagulation therapy, maternal characteristics, and a failed cfDNA test due to a low fetal fraction. Prenat Diagn. 2017;37(11):1125–1129. doi: 10.1002/pd.5152 [DOI] [PubMed] [Google Scholar]
- 18.Revello R, Sarno L, Ispas A, Akolekar R, Nicolaides KH. Screening for trisomies by cell-free DNA testing of maternal blood: consequences of a failed result. Ultrasound Obstet Gynecol. 2016;47(6):698–704. doi: 10.1002/uog.15851 [DOI] [PubMed] [Google Scholar]
- 19.Palomaki GE, Kloza EM. Prenatal cell-free DNA screening test failures: a systematic review of failure rates, risks of Down syndrome, and impact of repeat testing. Genet Med. 2018;20(11):1312–1323. doi: 10.1038/gim.2018.22 [DOI] [PubMed] [Google Scholar]
- 20.Dabi Y, Guterman S, Jani JC, et al. Autoimmune disorders but not heparin are associated with cell-free fetal DNA test failure. J Transl Med. 2018;16(1):335. doi: 10.1186/s12967-018-1705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui CYY, Tan WC, Tan EL, Tan LK. Repeated failed non-invasive prenatal testing in a woman with immune thrombocytopenia and antiphospholipid syndrome: lessons learnt. BMJ Case Rep. 2016;2016. doi: 10.1136/bcr-2016-216593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui L, Bethune M, Weeks A, Kelley J, Hayes L. Repeated failed non-invasive prenatal testing owing to low cell-free fetal DNA fraction and increased variance in a woman with severe autoimmune disease. Ultrasound Obstet Gynecol. 2014;44(2):242–243. doi: 10.1002/uog.13418 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura N, Sasaki A, Mikami M, et al. Nonreportable rates and cell-free DNA profiles in noninvasive prenatal testing among women with heparin treatment. Prenat Diagn. 2020;40(7):838–845. doi: 10.1002/pd.5695 [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu SCY, Chan KCA, Zheng YWL, et al. Size-based molecular diagnostics using plasma DNA for noninvasive prenatal testing. Proc Natl Acad Sci U S A. 2014;111(23):8583–8588. doi: 10.1073/pnas.1406103111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhlmann-Capek M, Chiossi G, Singh P, et al. Effects of medication intake in early pregnancy on the fetal fraction of cell-free DNA testing. Prenat Diagn. 2019;39(5):361–368. doi: 10.1002/pd.5436 [DOI] [PubMed] [Google Scholar]
- 28.Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. Circulating cell free DNA testing: are some test failures informative? Prenat Diagn. 2015;35(3):289–293. doi: 10.1002/pd.4541 [DOI] [PubMed] [Google Scholar]
- 29.Bartoloni E, Ludovini V, Alunno A, et al. Increased levels of circulating DNA in patients with systemic autoimmune diseases: A possible marker of disease activity in Sjögren’s syndrome. Lupus. 2011;20(9):928–935. doi: 10.1177/0961203311399606 [DOI] [PubMed] [Google Scholar]
- 30.Rykova E, Sizikov A, Roggenbuck D, et al. Circulating DNA in rheumatoid arthritis: pathological changes and association with clinically used serological markers. Arthritis Res Ther. 2017;19(1):85. doi: 10.1186/s13075-017-1295-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tug S, Helmig S, Menke J, et al. Correlation between cell free DNA levels and medical evaluation of disease progression in systemic lupus erythematosus patients. Cell Immunol. 2014;292(1–2):32–39. doi: 10.1016/j.cellimm.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Song Y, Chang J, et al. High levels of circulating cell-free DNA are a biomarker of active SLE. Eur J Clin Invest. 2018;48(11):e13015. doi: 10.1111/eci.13015 [DOI] [PubMed] [Google Scholar]
- 33.Yalavarthi S, Gould TJ, Rao AN, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67(11):2990–3003. doi: 10.1002/art.39247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, Dong Y, Zhang Y, et al. Antiphospholipid antibody-activated NETs exacerbate trophoblast and endothelial cell injury in obstetric antiphospholipid syndrome. J Cell Mol Med. 2020;24(12):6690–6703. doi: 10.1111/jcmm.15321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrablicova Z, Tomova K, Tothova L, et al. Nuclear and Mitochondrial Circulating Cell-Free DNA Is Increased in Patients With Inflammatory Bowel Disease in Clinical Remission. Front Med (Lausanne). 2020;7:593316. doi: 10.3389/fmed.2020.593316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller LR, Marks C, Becker JB, et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017;31(1):29–34. doi: 10.1096/fj.201600781R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnegard ME, Whitten LA, Hunter C, Clayton JA. Sex as a Biological Variable: A 5-Year Progress Report and Call to Action. J Womens Health (Larchmt). 2020;29(6):858–864. doi: 10.1089/jwh.2019.8247 [DOI] [PMC free article] [PubMed] [Google Scholar]