Supplemental Digital Content is available in the text.
Keywords: COVID-19, MIS-C, Kawasaki disease, TSS
Background:
Distinguishing multisystem inflammatory syndrome in children (MIS-C) from coronavirus disease 2019 (COVID-19), Kawasaki disease (KD), and toxic shock syndrome (TSS) can be challenging. Because clinical management of these conditions can vary, timely and accurate diagnosis is essential.
Methods:
Data were collected from patients <21 years of age hospitalized with MIS-C, COVID-19, KD, and TSS in 4 major health care institutions. Patient demographics and clinical and laboratory data were compared among the 4 conditions, and a diagnostic scoring tool was developed to assist in clinical diagnosis.
Results:
A total of 233 patients with MIS-C, 102 with COVID-19, 101 with KD, and 76 with TSS were included in the analysis. Patients with MIS-C had the highest prevalence of decreased cardiac function (38.6%), myocarditis (34.3%), pericardial effusion (38.2%), mitral regurgitation (31.8%) and pleural effusion (34.8%) compared with patients with the other conditions. Patients with MIS-C had increased peak levels of C-reactive protein and decreased platelets and lymphocyte nadir counts compared with patients with COVID-19 and KD and elevated levels of troponin, brain natriuretic peptide and pro-brain natriuretic peptide compared with COVID-19. Diagnostic scores utilizing clinical findings effectively distinguished MIS-C from COVID-19, KD, and TSS, with internal validation showing area under the curve ranging from 0.87 to 0.97.
Conclusions:
Compared with COVID-19, KD, and TSS, patients with MIS-C had significantly higher prevalence of cardiac complications, elevated markers of inflammation and cardiac damage, thrombocytopenia, and lymphopenia. Diagnostic scores can be a useful tool for distinguishing MIS-C from COVID-19, KD, and TSS.
Multisystem inflammatory syndrome in children (MIS-C) occurring after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was first described in April 2020 in the United Kingdom and was quickly followed by descriptions of similar patients in other European countries and the United States.1–3 In the United Kingdom, this condition was termed pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2.4 Many patients with MIS-C had evidence of SARS-CoV-2 infection 2–6 weeks before illness onset.1,3,5 In the United States, as of August 27, 2021, 4661 MIS-C patients had been reported to the Centers for Disease Control and Prevention (CDC).6 Many patients diagnosed with MIS-C were reported to have asymptomatic infection or mildly symptomatic coronavirus disease 2019 (COVID-19) before their diagnosis of MIS-C. Most cases of COVID-19 in children reported in the literature are mild or asymptomatic, but severe disease and death have occurred.7,8 Risk factors for severe COVID-19 include chronic conditions like asthma, diabetes, immunosuppression and obesity.8,9
Distinguishing MIS-C from COVID-19 and other hyperinflammatory conditions can be challenging for health care providers. The clinical features of MIS-C can substantially overlap with those of COVID-19, Kawasaki disease (KD), and toxic shock syndrome (TSS).2,8,10–14 While the presence of anti–SARS-CoV-2 IgM or IgG has been a useful marker to distinguish MIS-C early in the pandemic, the specificity of seropositivity as a diagnostic test for MIS-C decreases as population immunity grows from ongoing exposure, COVID-19 illness, or following vaccination. Prompt recognition and identification of patients with MIS-C is important for appropriate and timely treatment.15
CDC initiated a multicenter collaborative project with the objective of collecting and analyzing demographic, clinical and laboratory data to better define the characteristics that distinguish MIS-C from COVID-19, KD and TSS. In addition, diagnostic scoring profiles were developed to provide simplified tools to help clinicians better distinguish MIS-C from COVID-19, KD and TSS.
METHODS
The study was conducted in collaboration with 4 institutions and approved by their retrospective Institutional Review Boards: Children’s Healthcare of Atlanta and Emory University, Atlanta, Georgia; Phoenix Children’s Hospital, Phoenix, Arizona; Arnold Palmer Hospital for Children/Orlando Health, Orlando, Florida and Washington University, St. Louis, Missouri. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.16 For MIS-C and COVID-19, medical records were reviewed for patients hospitalized from March 16, 2020, through February 21, 2021. For KD and TSS, inpatient medical records were reviewed during the pre–COVID-19 pandemic period of January 2019 through December 2019 to collect 100 cases of KD and October 2015 through December 2019 to collect 75 cases of TSS. To identify sufficient numbers of KD and TSS cases, patients diagnosed during the pandemic (starting from January 2020) were accepted if they tested negative for SARS-CoV-2.
Patients with MIS-C were <21 years of age, hospitalized with fever, multisystem involvement, laboratory evidence of inflammation, and positive SARS-CoV-2 test by reverse transcription polymerase chain reaction, serology, or antigen test. Patients with COVID-19 were <21 years of age, hospitalized with positive SARS-CoV-2 testing and with respiratory illness or at least two of the following symptoms (fever, chills, rigors, myalgia, headache, sore throat and new olfactory or taste disorder). Full descriptions of the MIS-C and COVID-19 case definitions, as well as the case definitions for KD and TSS, are provided in the Table, Supplemental Digital Content 1, http://links.lww.com/INF/E630. For MIS-C and COVID-19, the diagnoses were reviewed by a multidisciplinary team of clinicians at the hospitals. For a small number of patients in whom distinguishing between MIS-C and COVID-19 was challenging, CDC team members assisted in adjudicating the diagnosis that most clearly matched the patient’s clinical manifestation. Patients with KD and TSS were deliberately obtained from the prepandemic period to avoid diagnostic confusion. For a subanalysis, patients with KD shock syndrome (KDSS) were compared with MIS-C and COVID-19 patients with shock (definition in the Table, Supplemental Digital Content 1, http://links.lww.com/INF/E630).17
Medical record abstraction for all 4 diseases was completed using the same case report form that was developed for MIS-C national surveillance with slight revisions to reflect the case definitions used for each disease (Form, Supplemental Digital Content 2, http://links.lww.com/INF/E631).18 Information collected included patient demographics, clinical and laboratory characteristics, and illness outcomes. The peak and/or nadir laboratory values were collected but not the timing of laboratory results. Abstracted data were entered into a Research Electronic Data Capture database,19,20 and statistical analyses were performed using R, version 4.0.2,21 including the packages CatPredi,22 mice,23 glmnet,24 WeightedROC,25 and caret.26 Patient demographics, clinical manifestations, outcomes and laboratory findings of patients with MIS-C were compared with those of COVID-19, KD and TSS. Mann-Whitney U tests were used to evaluate differences between diseases for continuous variables, and the Fisher exact test was used to assess differences for categorical variables.
Diagnostic scores were created to provide a simplified tool to help clinicians distinguish patients with clinical features that overlap with MIS-C and either one or multiple of the other three clinical conditions. The outcomes of these diagnostic scores are not intended to be a definitive diagnosis of MIS-C. The purpose of these scores is to use key clinical characteristics to quickly assess a patient’s relative likelihood of having MIS-C in comparison to the other three conditions. Variables considered for these scores included comorbidities, clinical findings, and laboratory results. For easier clinical interpretation, continuous laboratory values were dichotomized using cutoff points that maximized discriminatory power between MIS-C and the other disease categories.27 Multiple imputation by chained equations using fully conditional specification was used to account for patients missing dichotomized normal/abnormal values for select laboratory markers (Table, Supplemental Digital Content 3, http://links.lww.com/INF/E632).23 Using least absolute shrinkage and selection operator (LASSO) for variable selection, models with a priori limit of 7 or fewer variables were identified for each diagnostic score.24 Patient data in the least absolute shrinkage and selection operator models were weighted such that the number and age distribution of MIS-C patients effectively matched that of the non–MIS-C patients in each comparison. Patient totals were balanced so that the scores could be applied in a cohort with equal numbers of MIS-C and non–MIS-C patients. Generalized linear models were used to generate regression coefficients, which were converted to diagnostic score points via rounding with an adjustment factor to minimize error. The end product was a set of clinical criteria with assigned point totals. Weighted area under the curve (AUC) values were calculated to quantify model fit of the diagnostic scores.25 In addition, the estimated probability of a patient having MIS-C based on the weighted regression results was calculated at each possible point total. To assess the generalizability of diagnostic scores, 10-fold internal cross-validation28 was used to evaluate the performance of the scores in patient data sets not used for model estimation, and corresponding kappa statistics were computed for each diagnostic score.
RESULTS
Overall, 512 patients were identified from the 4 institutions: 233 (45.5%) with MIS-C, 102 (19.9%) with COVID-19, 101 (19.7%) with KD (92 with complete and 9 with incomplete KD), and 76 (14.8%) with TSS. MIS-C patients had illness onset from March 16, 2020, through March 8, 2021, and COVID-19 patients from March 24, 2020, through February 21, 2021. The majority of patients with KD (94%) and TSS (83%) were identified through retrospective chart review from the pre–COVID-19 pandemic period (October 2015 to December 2019).
Table 1 provides an overview of demographics and clinical outcomes of the patients. Among patients with MIS-C, 147 (63.1%) were male—a proportion similar to that of patients with KD (66; 65.3%) but greater than that of COVID-19 (40; 39.2%) and TSS (23; 30.3%; both P < 0.001). MIS-C patient ages [median, 9 years; interquartile range (IQR), 5–13] were generally lower than those of COVID-19 patients (median, 15 years; IQR, 3–17) and TSS patients (median, 13 years; IQR, 9–16) but greater than KD patients (median, 3 years; IQR, 2–5). Among patients with MIS-C, 44.1% were non-Hispanic Black, compared with 34.7% of patients with COVID-19 (P = 0.139) and 14.5% of patients with TSS (P < 0.001). Patients with MIS-C were less likely to be non-Hispanic Asian (2.3%) compared with those with KD (13.1%; P < 0.001). Patients with MIS-C generally had longer median hospital days (5 days) compared with patients with COVID-19 (4 days; P = 0.009) and patients with KD (4 days; P < 0.001). Over three-fifths (62.2%) of patients with MIS-C were admitted to an intensive care unit, which was higher than for COVID-19 (49.0%; P = 0.030) and KD (10.9%; P < 0.001) but lower than for patients with TSS (81.6%; P = 0.002). Two patients died, one with COVID-19 and one with TSS. There were no deaths among patients with MIS-C or KD.
TABLE 1.
Demographic and Clinical Overview of MIS-C, COVID-19, KD, and TSS Patients, United States
| Variable | MIS-C (n = 233) | COVID-19 (n = 102) | Kawasaki Disease (n = 101) | Toxic Shock Syndrome (n = 76) | |||
|---|---|---|---|---|---|---|---|
| n (%) | P Value* | n (%) | P Value* | n (%) | P Value* | ||
| Sex | |||||||
| Male | 147 (63%) | 40 (39%) | <0.001 | 66 (65%) | 0.712 | 23 (30%) | <0.001 |
| Age, y; median (IQR) | 9 (5–13) | 15 (3–17) | <0.001 | 3 (2–5) | <0.001 | 13 (9–16) | <0.001 |
| Race/ethnicity† | |||||||
| Hispanic | 60 (27%) | 32 (33%) | 0.349 | 14 (14%) | 0.014 | 5 (7%) | <0.001 |
| Black, non-Hispanic | 98 (44%) | 34 (35%) | 0.139 | 57 (58%) | 0.030 | 10 (14%) | <0.001 |
| White, non-Hispanic | 52 (23%) | 30 (31%) | 0.211 | 14 (14%) | 0.072 | 47 (68%) | <0.001 |
| Asian, non-Hispanic | 5 (2%) | 1 (1%) | 0.671 | 13 (13%) | <0.001 | 2 (3%) | 0.671 |
| AI/AN, non-Hispanic | 2 (1%) | 0 (0%) | 1.000 | 0 (0%) | 1.000 | 1 (1%) | 0.557 |
| Multiple | 3 (1%) | 1 (1%) | 1.000 | 1 (1%) | 1.000 | 0 (0%) | 1.000 |
| Other | 2 (1%) | 0 (0%) | 1.000 | 0 (0%) | 1.000 | 4 (6%) | 0.030 |
| Missing | 11 (-) | 4 (-) | 2 (-) | 7 (-) | |||
| Days in hospital, median (IQR)‡ | 5 (4–8) | 4 (2–8) | 0.009 | 4 (3–5) | <0.001 | 4 (3–6) | 0.080 |
| 1 | 1 (0%) | 11 (12%) | 0 (0%) | 2 (3%) | |||
| 2–7 | 169 (74%) | 58 (61%) | 88 (87%) | 57 (75%) | |||
| 8–14 | 50 (22%) | 15 (16%) | 9 (9%) | 8 (11%) | |||
| ≥15 | 9 (4%) | 11 (12%) | 4 (4%) | 9 (12%) | |||
| ICU admission | 145 (62%) | 50 (49%) | 0.030 | 11 (11%) | <0.001 | 62 (82%) | 0.002 |
| Days in ICU, median (IQR)§ | 3 (0–5) | 7 (2–13) | 0.001 | 5 (3–7) | 0.073 | 1 (0–2) | 0.174 |
| Underlying medical conditions | |||||||
| Obesity | 84 (36%) | 51 (50%) | 0.021 | 13 (13%) | <0.001 | 19 (25%) | 0.093 |
| Chronic lung disease | 24 (10%) | 25 (25%) | 0.001 | 5 (5%) | 0.139 | 6 (8%) | 0.658 |
| Days with fever¶ | |||||||
| Total, median (IQR) | 5 (4–7) | 4 (2–6) | <0.001 | 7 (5–8) | <0.001 | 3 (2–5) | <0.001 |
| At admission, median (IQR) | 4 (3–5) | 2 (0–4) | <0.001 | 4 (3–6) | 0.002 | 1 (0–3) | <0.001 |
| Outcome | |||||||
| Died | 0 (0%) | 1 (1%) | 0.304 | 0 (0%) | - | 1 (1.3%) | 0.246 |
Compared with patients with MIS-C.
Twenty-four with missing race/ethnicity.
Eleven patients had unknown length of hospital stay.
One hundred forty-eight patients admitted to the ICU had unknown length of ICU stay.
Forty-four patients had unknown length of fever.
AI indicates American Indian; AN, Alaska Native; ICU, intensive care unit.
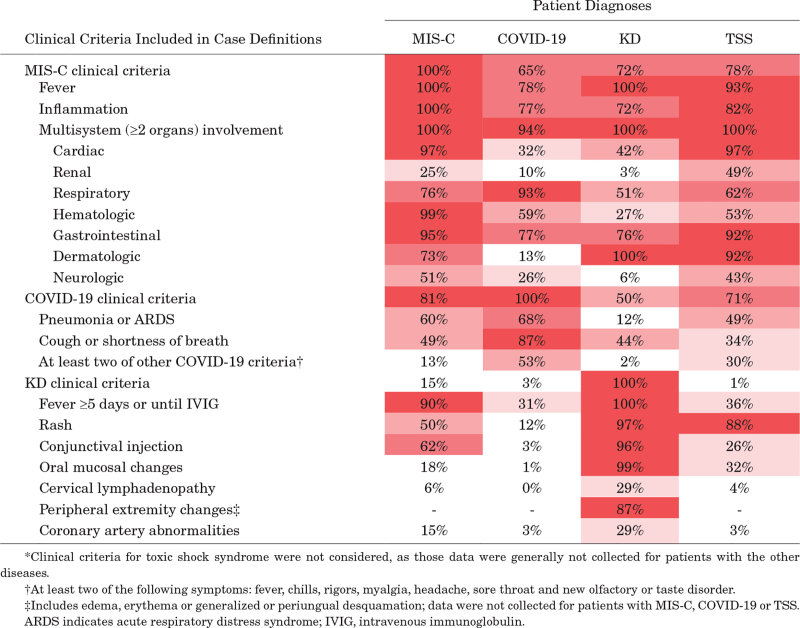
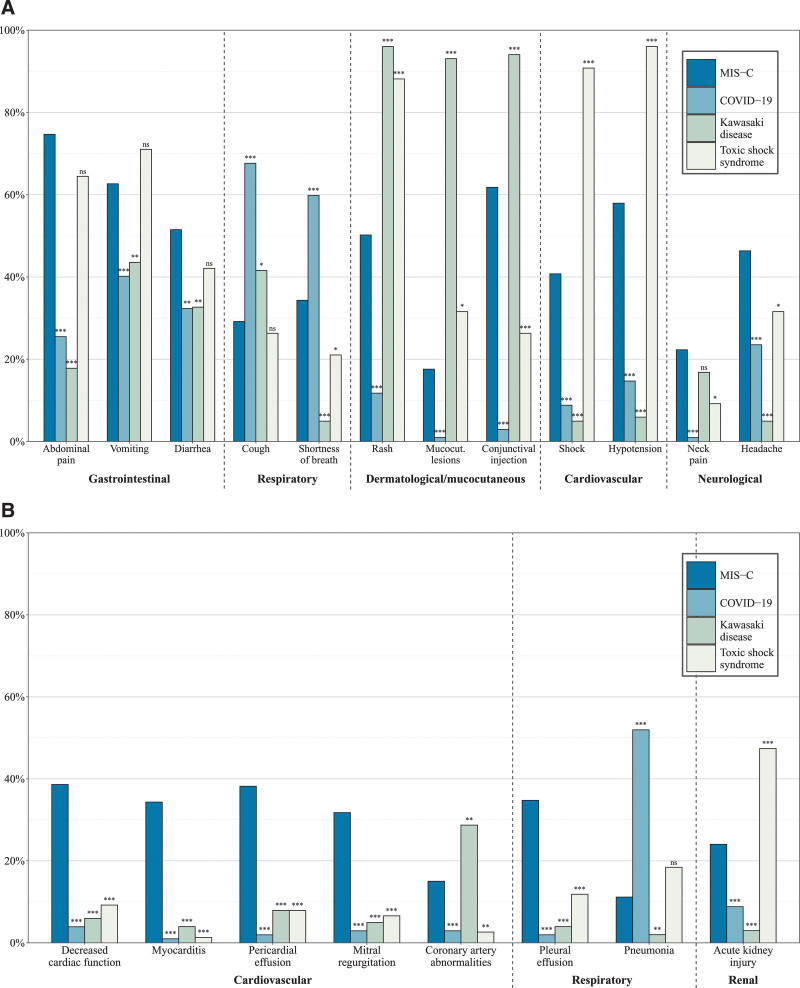
As shown in Table 2, the clinical manifestations of MIS-C, COVID-19, KD, and TSS had substantial overlap. The clinical features of MIS-C included in the case definition [fever, inflammation, and multisystem (≥2 organs) involvement] were reported in 65% of COVID-19, 72% of KD, and 78% of TSS patients (Table 2). However, compared with patients with COVID-19, patients with MIS-C were more likely to have gastrointestinal symptoms such as abdominal pain (74.7% vs. 25.5%; P < 0.001), vomiting (62.7% vs. 40.2%; P < 0.001) and diarrhea (51.5% vs. 32.4%; P = 0.001), rash (50.2% vs. 11.8%; P < 0.001), conjunctival injection (61.5% vs. 3.0%; P < 0.001) and neck pain (22.3% vs. 1.0%; P < 0.001; Figure 1A). They were also more likely to have pleural effusion (34.8% vs. 2.0%; P < 0.001) and cardiovascular involvement, including shock (40.1% vs. 8.8%; P < 0.001), hypotension (57.9% vs. 14.7%; P < 0.001), decreased cardiac function (38.6% vs. 3.9%; P < 0.001), myocarditis (34.3% vs. 1.0%; P < 0.001), pericardial effusion (38.2% vs. 2.0%; P < 0.001), mitral regurgitation (31.8% vs. 2.9%; P < 0.001), and coronary artery abnormalities (15.0% vs. 2.9%; P < 0.001; Figure 1B). Compared with patients with MIS-C, patients with COVID-19 had a higher prevalence of respiratory manifestations, including cough (67.6% vs. 29.2%; P < 0.001), shortness of breath (59.8% vs. 34.3%; P < 0.001), and pneumonia (52.0% vs. 11.1%; P < 0.001).
Table 2.
Percentage of Patients With MIS-C, COVID-19, KD, and TSS Who Met Clinical Criteria for Each Disease*
FIGURE 1.
Proportions with signs and symptoms and clinical findings of interest for patients with MIS-C, COVID-19, KD, and TSS. Proportions with signs and symptoms (A) and clinical findings (B). P values from Fisher exact tests for difference in proportions compared with MIS-C patients denoted as ns (not significant),* (P < 0.05), ** (P < 0.01), and *** (P < 0.001).
Compared with MIS-C, patients with KD were more likely to have rash (96.0% vs. 50.2%; P < 0.001), conjunctival injection (94.1% vs. 61.8%; P < 0.001), and coronary artery abnormalities (28.7% vs. 15.0%; P = 0.006) but were significantly less likely to have all other cardiovascular outcomes, as well as gastrointestinal, neurologic, or renal involvement (Figure 1A and B). Patients with TSS had lower rates of shortness of breath (21.1% for TSS patients vs. 34.3% for MIS-C patients; P = 0.032), conjunctival injection (26.3% vs. 61.8%; P < 0.001), neck pain (9.2% vs. 22.3%; P = 0.011), and pleural effusion (11.8% vs. 34.8%; P < 0.001) but were more likely to have rash (88.2% vs. 50.2%; P < 0.001) and acute kidney injury (47.4% vs. 24.0%; P < 0.001).
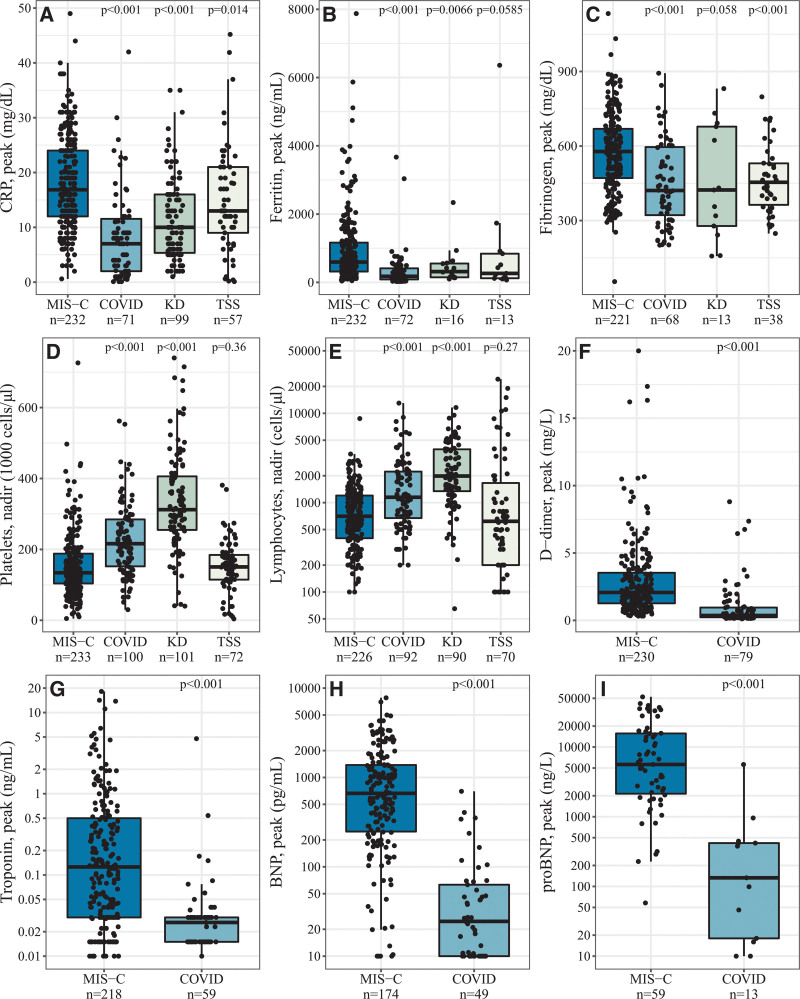
A summary of laboratory findings is shown in Figure 2 and Table, Supplemental Digital Content 4, http://links.lww.com/INF/E633. Compared with patients with COVID-19 and KD, patients with MIS-C had higher peak C-reactive protein (CRP), fibrinogen, and ferritin and lower platelet and lymphocyte count nadir. D-dimer and cardiac biomarkers (troponin, brain natriuretic peptide, and pro-brain natriuretic peptide) were not frequently collected among patients diagnosed with KD and TSS; peak levels of these four laboratory tests were significantly higher in patients with MIS-C compared with patients with COVID-19.
FIGURE 2.
Laboratory markers of interest in patients with MIS-C, COVID-19, KD, and TSS. D-dimer, troponin, BNP, and proBNP (panels F-I), do not include comparisons with KD and TSS due to insufficient sample size.
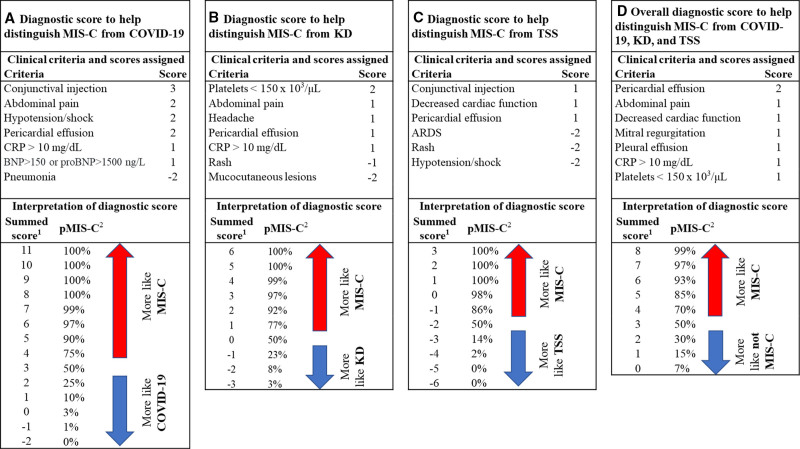
Four diagnostic scores were developed to distinguish MIS-C from COVID-19, KD and TSS independently or as a group for patients with multiple overlapping clinical features (Figure 3). The first score in Figure 3A assesses the likelihood of a patient having MIS-C compared with COVID-19. Seven criteria were included in this diagnostic score, each corresponding to a specific number of points. Conjunctival injection, for example, had 3 points, and abdominal pain, hypotension/shock and pericardial effusion each had 2 points. The points corresponding to the patients’ findings are then added to provide an overall summed score that correlates with the relative likelihood of MIS-C compared with COVID-19. Figure 3B–D outlines the diagnostic scores developed to distinguish MIS-C, respectively, from KD, TSS and patients with clinical manifestations overlapping with all 3 conditions. Assessment of the diagnostic scores using internal cross-validation indicated that the diagnostic scores in Figure 3A were effective at discriminating MIS-C from COVID-19 with a kappa value of 0.78 (AUC, 0.97). The diagnostic scores in Figure 3B and C also had good diagnostic accuracy with kappa values of 0.87 (AUC, 0.96) and 0.73 (AUC, 0.94), respectively. The score differentiating MIS-C from the 3 overlapping conditions had a kappa value of 0.57 (AUC, 0.87; Figure, Supplemental Digital Content 5, http://links.lww.com/INF/E634).
FIGURE 3.
Four diagnostic scores using clinical criteria to help optimally distinguish between MIS-C, COVID-19, KD, and TSS. Panel A: MIS-C and COVID-19, panel B: MIS-C and KD, panel C: MIS-C and TSS, and panel D: MIS-C and COVID-19, KD, and TSS.
The probability calculation assumes a similar number of patients with MIS-C and the other conditions are compared. The following number (and percent) of patients were missing laboratory test results: CRP, 53 (10.3%); lymphocytes, 34 (6.6%); platelets, 6 (1.2%); fibrinogen, 46 (13.7%); troponin, 58 (17.3%); ferritin, 31 (9.3%) and BNP/proBNP, 43 (12.8%). For patients with missing values, multiple imputation was used to determine the likelihood of abnormal lab values. Criteria with negative coefficients indicate that the presence of the criterion decreases the probability that the patient has MIS-C (ie, the absence of the criterion increases the probability that the patient has MIS-C). For a given patient, their plausible diagnoses determine which diagnostic score would be most applicable. 1The summed score: all criteria that applies to the patient is added together for the total score. 2pMIS-C is the estimated probability that a patient with each given summed score has MIS-C. ARDS indicates acute respiratory distress syndrome; BNP, brain natriuretic peptide; pMIS-C, probability of MIS-C.
In a subanalysis, patients with MIS-C shock were compared with patients with COVID-19 shock and KDSS (Table, Supplemental Digital Content 6, http://links.lww.com/INF/E635). Compared with patients with KDSS, patients with MIS-C shock were more likely to report abdominal pain (77.0% vs. 27.3%; P = 0.003) and headache (49.2% vs. 0%; P = 0.001), whereas patients with KDSS were more likely to have coronary artery dilatation or aneurysm (63.6% vs. 17.7%; P = 0.002) and rash (90.9%–48.7%; P = 0.009). Patients with MIS-C shock had lower lymphocyte counts (median, 580/μL vs. 1350/μL) and shorter duration of fever (median, 5 vs. 7 days; P = 0.020). Differences between MIS-C shock and COVID-19 shock were generally similar to differences between all MIS-C patients and COVID-19 patients, including more frequent cardiac and gastrointestinal involvement in MIS-C shock patients and pneumonia in COVID-19 shock patients (Table, Supplemental Digital Content 6, http://links.lww.com/INF/E635).
DISCUSSION
MIS-C, COVID-19, KD and TSS are all conditions with the potential for multisystem involvement and systemic inflammation, yet they are distinct disorders with potentially different underlying pathophysiology that require different approaches for clinical management. The present study highlights demographic, clinical and laboratory differences among the 4 disorders that can assist in establishing a diagnosis and optimal management of affected children. MIS-C was more commonly associated with abdominal pain, diarrhea, decreased cardiac function, myocarditis, pericardial effusion, mitral regurgitation, pleural effusion and neck pain. Decreased cardiac function, myocarditis, pericardiac effusion, mitral regurgitation and pleural effusion occurred almost exclusively in patients with MIS-C.29 Patients with COVID-19 had the highest rates of cough, shortness of breath and pneumonia with the lowest rates of cardiovascular complications and dermatologic symptoms. Patients with KD had the highest rates of dermatologic symptoms and the lowest rates of shock, hypotension, respiratory complications, and renal injury. Patients with TSS had the highest rates of shock, hypotension and renal injury.
Case definitions alone were not effective at differentiating MIS-C from COVID-19, KD and TSS; 65%–78% of patients with the latter 3 diseases met the most prominent clinical features included in MIS-C case definition. The utility of SARS-CoV-2 testing in distinguishing these conditions has declined over time as a larger proportion of the population can test positive as a result of natural infection or immunization. Although antinucleoprotein antibody assays can distinguish prior SARS-CoV-2 infection from COVID-19 vaccination, their utility and availability have limitations. Because treatment for each of the conditions can differ, ensuring the correct diagnosis is essential to the provision of rapid and appropriate clinical management. The diagnostic scores summarized in Figure 3 should assist with timeliness and accuracy of diagnosing MIS-C in patients who may have substantial clinical overlap with the other conditions. For example, if a patient was reported to have abdominal pain (2 points), CRP >10 mg/dL (1 point), brain natriuretic peptide >150 ng/L (1 point) and none of the other aforementioned criteria in Figure 3A, the patient is assigned a total score of 4. In a cohort with a roughly equal number of patients with MIS-C and COVID-19, a score of 4 suggests a 75% probability that this patient has MIS-C. If the same patient presents with pneumonia and CRP >10 mg/dL as the only clinical criteria, the total score would be −1, indicating a MIS-C probability of 1% and a likely diagnosis of COVID-19. The diagnostic scores are meant to be a guide for clinicians to assess the likelihood of MIS-C in patients with significant clinical overlap with the other conditions. The variables included in the model are not necessarily components of the case definitions. The diagnostic scores go beyond the case definition to help clinicians evaluate patients’ diagnosis based on the totality of available clinical and laboratory data in patients with overlapping features. The diagnostic scores are not intended to assist with determining whether the case meets the MIS-C case definition for reporting purposes.
The findings in this study present similar results to previously published studies comparing MIS-C with KD, KD shock and TSS.30,31 Patients with MIS-C were more likely to be male and younger in age compared with patients with COVID-19. The racial distribution mirrors that of other published data on MIS-C.3,5 Overall, patients with KD had the highest percentage of Asian persons and the longest median length of fever. Patients with TSS were more likely to be non-Hispanic White and female; they have the highest rate of intensive care unit admission. Patients with MIS-C had increased peak levels of CRP compared with patients with COVID-19 and KD and decreased platelets and lymphocyte nadir counts compared with patients with COVID-19 and KD. These laboratory values demonstrate that MIS-C is a highly inflammatory condition with significant cardiac involvement.30–32 The laboratory values for TSS were not statistically significant except for fibrinogen, which was lower than MIS-C but similar to COVID-19 and KD.
Strengths of the present study include involvement of multicenter sites and utilization of a standardized data collection instrument and chart abstraction for all 4 conditions. Clinical data were abstracted by clinicians from each of the 4 hospitals, and abstractors had direct access to medical records. Although the diagnostic scores were internally validated and were successful at distinguishing MIS-C from the other disease categories, future external validation using different sets of patients can improve their application. The diagnostic scores can potentially be integrated into electronic health records for ease of implementation.
The study has several limitations. First, the 4 institutions are in geographically distinct locations, but the majority of KD patients were identified from a single location, potentially decreasing the racial, ethnic and geographic diversity. However, data from patients with MIS-C, COVID-19 and TSS were provided by all 4 partnering hospitals, which improves the diversity of patient data. Second, the case report form did not include details for some of the variables, such as type of neck pain. Third, because of the retrospective data collection, clinical and laboratory data may not have been consistently collected or recorded in the medical records. For instance, troponin was not routinely tested in most patients with KD and TSS; it was often elevated in MIS-C but not typically in KD.4,33 Lastly, serial test results were unavailable; only the highest and lowest laboratory values were collected. In previous studies, the most abnormal values are generally recorded at or shortly after illness presentation, indicating that those values closely approximate laboratory findings early in the course of hospitalization when patient diagnosis is most critical.34,35
CONCLUSION
MIS-C can be challenging to differentiate from COVID-19 and other hyperinflammatory conditions. Patients with a history of SARS-CoV-2 infection or positive serology for SARS-CoV-2 who present with gastrointestinal and cardiac dysfunction, elevated markers of inflammation and significantly elevated cardiac markers are more likely to have MIS-C than patients with respiratory symptoms requiring noninvasive respiratory support. Patients with KD typically have a lower median age, more often have mucocutaneous lesions and coronary artery abnormalities, and less frequently have cardiac dysfunction and myocarditis. Patients with TSS were more likely to be non-Hispanic White and female with shock and hypotension and low rates of cardiovascular complications. In patients with substantial overlap in clinical manifestation, the diagnostic scores can be a valuable tool for distinguishing MIS-C from COVID-19, KD and TSS.
Supplementary Material
Footnotes
E.J.A. is involved in clinical trials for Pfizer, Sanofi Pasteur, MedImmune, Regeneron, PaxVax, GSK, Merck, Janssen, and Micron; consulting for Pfizer, Sanofi Pasteur, and Medscape; and DSMB for Kentucky Bioprocessing, Inc, and Sanofi Pasteur. K.C.E. is associated with the Arizona United Rheumatology Alliance as the Past Board Chair. C.M.K. received funding as the principal investigator for Washington University for this project from the Centers for Disease Control and Prevention (CDC). F.R.L. received funding as the principal investigator for Orlando Health for this project from the CDC. F.R.L. received an honoraria from Tyme, Inc, as a Medical Advisory Board member. R.B.R. received funding as the principal investigator for Phoenix Children’s Hospital for this project from the CDC. C.A.R. received funding as the principal investigator for Emory Healthcare for this project from the CDC; she has also received start-up funding and pilot funding for specimen collection for the Center for Childhood Infections and Vaccines of Children’s Healthcare of Atlanta and Emory University. Co-inventor of a pending patent (US20180333477A1) for respiratory syncytial virus vaccine technology, licensed to Meissa Vaccines, Inc, with royalties paid to Emory University. NIH R61 RADx-rad funding for predicting Viral-Associated Inflammatory Disease Severity in Children With Laboratory Diagnostics and Artificial Intelligence (PreVAIL)–Emory site PI; overall PI is Charles Chiu, MD, PhD, at UCSF; Genentech, Inc, funding as a coinvestigator; Emory PI is Ann Chahroudi, MD, PhD, VTEU, HHSN272201300018I role: as a coinvestigator; Emory PI is Nadine Rouphael, MD, MS, BioFire, Janssen, MedImmune, Micron, Moderna, PaxVax, Pfizer investigatory in clinical trials with support to Emory University. This study was supported by the CDC. The CDC provided funds for the principal investigators and abstractors and laboratory sample collection and testing. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
S.G.-C. and J.A. contributed equally as co-first authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dufort EM, Koumans EH, Chow EJ, et al. New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team . New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. Available at: https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf. Accessed December 16, 2020. [DOI] [PubMed]
- 5.Belay ED, Abrams J, Oster ME, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Multisystem Inflammatory Syndrome in Children, Cases in the U.S. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States | CDC. Available at: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance. Accessed April 3, 2021.
- 7.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. COVID-19 in Children and Teens. Available at: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/children/symptoms.html. Accessed February 14, 2021.
- 9.Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16:413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldstein LR, Tenforde MW, Friedman KG, et al. Overcoming COVID-19 Investigators . Overcoming COVID-19 Investigators Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corwin DJ, Sartori LF, Chiotos K, et al. Distinguishing multisystem inflammatory syndrome in children from Kawasaki disease and benign inflammatory illnesses in the SARS-CoV-2 pandemic. Pediatr Emerg Care. 2020;36:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabeerdoss J, Pilania RK, Karkhele R, et al. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noval Rivas M, Porritt RA, Cheng MH, et al. COVID-19-associated multisystem inflammatory syndrome in children (MIS-C): a novel disease that mimics toxic shock syndrome-the superantigen hypothesis. J Allergy Clin Immunol. 2021;147:57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Rheumatology. Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-1. Available at: https://www.rheumatology.org/Portals/0/Files/ACR-COVID-19-Clinical-Guidance-Summary-MIS-C-Hyperinflammation.pdf. Accessed February 16, 2021.
- 16.§ See e.g., 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
- 17.Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:783–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Multisystem Inflammatory Syndrome in Children, Healthcare Professionals. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C) | CDC. Available at: https://www.cdc.gov/mis/mis-c/hcp/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fmis%2Fhcp%2Findex.html. Accessed February 23, 2021.
- 19.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Minor BL, et al. REDCap Consortium . REDCap Consortium The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team (R: A language and environment for statistical computing. 2020). R Foundation for Statistical Computing, . URL Available at: https://www.R-project.org/. Accessed May 5, 2021. [Google Scholar]
- 22.Barrio I, Rodriguez-Alvarez MX. CatPredi: Optimal Categorisation of Continuous Variables in Prediction Models. 2017: R package version 1.1. Available at: https://CRAN.R-project.org/package=CatPredi. Accessed May 5, 2021.
- 23.Van Buuren S, Groothuis-Oudshoorn K. mice: multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. Available at: https://www.jstatsoft.org/v45/i03/. Accessed May 5, 2021 [Google Scholar]
- 24.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Hocking TD. WeightedROC: Fast, Weighted ROC Curves. 2020: R package version 2020.1.31. Available at: https://CRAN.R-project.org/package=WeightedROC. Accessed May 5, 2021.
- 26.Kuhn M. caret: Classification and Regression Training. 2020: R package version 6.0-86. Available at: https://CRAN.R-project.org/package=caret. Accessed May 5, 2021.
- 27.Barrio I, Arostegui I, Rodríguez-Álvarez MX, et al. A new approach to categorising continuous variables in prediction models: proposal and validation. Stat Methods Med Res. 2017;26:2586–2602. [DOI] [PubMed] [Google Scholar]
- 28.Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection. Vol. 14, No. In: International Joint Conference on Artificial Intelligence (IJCAI), August 20 1995 2, pp. 1137–45. [Google Scholar]
- 29.Rubens JH, Akindele NP, Tschudy MM, et al. Acute covid-19 and multisystem inflammatory syndrome in children. BMJ. 2021;372:n385. [DOI] [PubMed] [Google Scholar]
- 30.Kostik MM, Bregel LV, Avrusin IS, et al. Distinguishing between multisystem inflammatory syndrome, associated with COVID-19 in children and the Kawasaki disease: development of preliminary criteria based on the data of the retrospective Multicenter Cohort Study. Front Pediatr. 2021;109:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittaker E, Bamford A, Kenny J, et al. PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . PIMS-TS Study Group and EUCLIDS and PERFORM Consortia Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozsurekci Y, Gürlevik S, Kesici S, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic in Turkey: first report from the Eastern Mediterranean. Clin Rheumatol. 2021;12:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Checchia PA, Borensztajn J, Shulman ST. Circulating cardiac troponin I levels in Kawasaki disease. Pediatr Cardiol. 2001;22:102–106. [DOI] [PubMed] [Google Scholar]
- 34.Gruber CN, Patel RS, Trachtman R, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell. 2020;183:982–995.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riollano-Cruz M, Akkoyun E, Briceno-Brito E, et al. Multisystem inflammatory syndrome in children related to COVID-19: a New York City experience. J Med Virol. 2021;93:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]