Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are widespread and persistent pollutants that have been associated with elevated cholesterol levels. However, data on incident cardiovascular disease (CVD) is lacking.
Objectives:
We investigated the association of exposure to PFAS with risk of myocardial infarction and stroke and, subsidiary, with baseline blood lipids.
Methods:
This population-based nested case–control study included first incident myocardial infarction and stroke cases with matched controls from two Swedish cohorts: the Swedish Mammography Cohort-Clinical (SMC-C) and the Cohort of 60-year-olds (60YO). Baseline blood sampling occurred during 2003–2009 and 1997–1999 with follow-up through 2017 and 2014 for the SMC-C and the 60YO, respectively. Eight plasma PFAS concentrations were measured using targeted liquid chromatography–triple quadrupole mass spectrometry. Five of these were quantifiable in both cohorts; individual values and their standardized sum were categorized into tertiles based on the controls. First incident myocardial infarction () and ischemic stroke () cases were ascertained via linkage to the National Inpatient Register and the Cause of Death Register. Controls were randomly selected from each cohort after matching for age, sex, and sample date. Baseline blood lipids were measured in plasma or serum after overnight fasting.
Results:
Among the 1,528 case–control subjects, the mean (standard deviation) age was 66 (7.7) y and 67% of them were women. In multivariable-adjusted analyses, the third tertile of the standardized sum of five PFAS associated with higher cholesterol and lower triglyceride levels among controls at baseline (). The corresponding results were [95% confidence interval (CI): 0.53, 0.93] for CVD, 0.60 (95% CI: 0.39, 0.92) for myocardial infarction, and 0.83 (95% CI: 0.46, 1.50) for stroke.
Discussion:
This study indicated that exposure to PFAS, although associated with increased cholesterol levels, did not associate with an increased risk of myocardial infarction, stroke, or their composite end point. The findings improve our knowledge on potential health effects of environmental contaminants in the CVD context. https://doi.org/10.1289/EHP9791
Introduction
Cardiovascular health may be damaged by certain classes of environmental pollutants (GBD 2015 Risk Factors Collaborators 2016; O’Toole et al. 2008), one such group of interest is the fluorinated synthetic chemicals widely used for their water-, oil-, and stain-repelling properties. Per- and polyfluoroalkyl substances (PFAS) accumulate globally in the environment and, subsequently, also in humans (Lau et al. 2007) via contaminated food, food-contact materials, drinking water, dust, and contact with PFAS-containing products (Sunderland et al. 2019). There is consistent evidence for an association between PFAS and elevated total cholesterol in humans (EFSA CONTAM et al. 2018; Steenland et al. 2020; Sunderland et al. 2019). Underlying mechanisms may involve the disruption of fatty acid metabolism and lipid synthesis in the liver given that PFAS activate transcription factors for genes involved in lipid metabolism, including peroxisome proliferator-activated receptor () (U.S. EPA 2016; Bijland et al. 2011).
PFAS have been shown to be related to atherosclerosis development (Lind et al. 2017, 2018; Osorio-Yáñez et al. 2021) and could impact cardiovascular disease (CVD) risk via elevated cholesterol (Prospective Studies Collaboration et al. 2007), as well as via endocrine disruption (Kahn et al. 2020), oxidative stress (Liu et al. 2007), reduced immune response (DeWitt et al. 2019), and endothelial dysfunction (Lin et al. 2016). However, studies to date on PFAS and CVD are scarce, inconsistent, and with considerable methodological limitations (lack of temporality criterion, small sample sizes, or self-reported end points), as reviewed by the European Food Safety Agency (EFSA CONTAM et al. 2018). Therefore, high-quality epidemiological studies on PFAS and CVD are needed to provide a stronger basis for regulatory decisions. Thus, the present study investigated whether the observed association between PFAS and cholesterol translated into increased risk of CVD, that is, myocardial infarction and stroke. We assessed associations of seven different PFAS with different chain lengths with CVD risk using a nested case–control design, using bio-banked plasma and data from two population-based cohorts. We also assessed baseline associations with blood lipid fractions among the controls.
Methods
Study Population
The study used data from the Swedish Mammography Cohort-Clinical (SMC-C) (SIMPLER; https://www.simpler4health.se/) and the Cohort of 60-year-olds (60YO) (Karolinska Institutet; https://ki.se/en/imm/the-cohort-of-60-year-olds). The SMC, established between 1987 and 1990, included women born during 1914–1948 residing in Central Sweden (74% response rate, ) (Harris 2013). Between 2003 and 2009, all SMC-women of age living in Uppsala town and surrounding areas were invited for health examination (baseline in this study); 5,022 responders (61%) constituted the SMC-C. The 60YO cohort, established to assess CVD etiology, identified residents in Stockholm County turning 60 y old between July 1997 and June 1998 and randomly invited every third man and woman for a health examination between August 1997 and March 1999 (78% response rate, ). Both cohorts donated blood samples and completed a questionnaire (Wändell et al. 2007). Written or oral informed consent was obtained from all participants and the studies were approved by the regional ethical review board in Stockholm.
Ascertainment of Myocardial Infarction and Ischemic Stroke
From baseline blood sampling through 2017 for the SMC-C and 2014 for the 60YO, a total of 135 and 214 first incident cases of primary myocardial infarction and 173 and 183 first incident cases of ischemic stroke, respectively, were ascertained via linkage of the cohort to the National Inpatient Register [International Classification of Diseases (ICD), 10th Revision (WHO 2016): I21 and I63], among participants free of prevalent coronary heart disease or cerebrovascular disease. Outside hospital deaths from myocardial infarction from the Cause of Death Register were verified by autopsy reports. Register validation revealed that diagnosis was correct in 98% for myocardial infarction (validation study of men and women 45–70 years of age between 1992 and 1994), 98.6% for stroke (validation study of men, private communication) and 68.5% for nonfatal stroke (men and women 25–74 years of age between 1985 and 1989) as reviewed by Ludvigsson et al. (2011).
Nested Case–Control Study
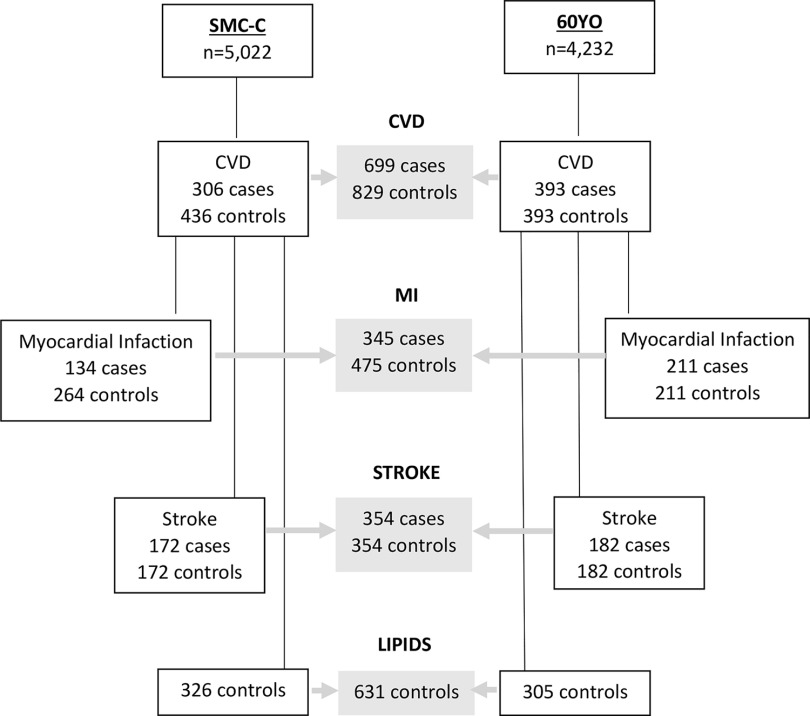
For each case, controls were randomly matched if alive and free from the case diagnosis at the time the case experienced the event (risk-set sampling). In the SMC-C, controls were matched (1:2 for myocardial infarction and 1:1 for stroke) based on age () and sample date (). In the 60YO, controls were matched (1:1) based on sex and sample date (). Plasma samples were missing for some subjects, leading to a final study population of 134 cases–264 controls (4 cases were matched 1:1) in the SMC-C and 211 case–control pairs in the 60YO for myocardial infarction and 172 pairs in the SMC-C and 182 pairs in the 60YO for ischemic stroke. Thus, 699 cases and 829 controls were available for total CVD assessment (Figure 1).
Figure 1.
Flow chart of the prospective nested case–control design and the cross-sectional assessment of lipids using two pooled Swedish cohorts, SMC-C and 60YO. For MI in the SMC-C, there is a 1:1 match in four cases, due to missing missing/broken samples. For lipid analyses, controls without a matched case (due to missing/broken samples) that were excluded from the CVD/MI/stroke analyses were included in the lipid analyses, whereas controls used in both MI and stroke data sets were used only once. Controls on lipid-lowering medication at baseline and missing lipid concentrations were excluded ( additional for LDL analyses). Note: 60YO, Cohort of 60-year-olds; CVD, cardiovascular disease; LDL, low-density lipoprotein; MI, myocardial infarction; SMC-C, Swedish Mammography Cohort-Clinical cohort.
Cross-Sectional Assessment of Blood Lipid Levels
For the baseline cross-sectional evaluation of PFAS and blood lipids, we included data from all available controls, plus three controls with a missing case, and removed the duplicated controls (matched for both myocardial infarction and stroke; ). Those reporting high baseline cholesterol (either self-reported or ascertained via the prescribed drug register in the SMC-C and as self-reported in the 60YO; ) and those with missing lipid measurements () were excluded, leaving 631 controls (326 from the SMC-C and 305 from the 60YO; Figure 1). Blood lipids [i.e., total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides] and apolipoproteins (apoB and apoA1, in 60YO) were measured in blood plasma (SMC-C) or serum (60YO) after overnight fasting using routine hospital laboratories in the SMC-C and automated measurement systems in the 60YO (Halldin et al. 2007).
Baseline PFAS Measurements
Serum PFAS were measured at the Division of Occupational and Environmental Medicine at Lund University, applying a modified method previously described (Norén et al. 2021). In short, the proteins were precipitated using acetonitrile by vigorous shaking for 30 min of thawed samples. After centrifugation, an aliquot of the supernatant was analyzed using liquid chromatography–triple quadrupole linear ion trap mass spectrometry (QTRAP 5500, AB Sciex), using selected reaction monitoring in negative ion mode. For quality control (QC), five QC reference samples, four chemical blanks (water), and calibration standards were analyzed for each sample batch. The limit of detection (LOD) was three times the standard deviation of responses in chemical blanks (Table S1). QC samples results were used to calculate the between-run precision as the coefficient of variation (2-14%; Tables S1–S2).
All samples were analyzed within 5 wk. The laboratory participates in a quality control program from the University of Erlangen-Nuremberg, Germany, for perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) and in Interlaboratory Comparison Investigations/External Quality Assurance Schemes exercises for the analysis of perfluorohexane sulfonate (PFHxS), perfluoroheptanoic acid (PFHpA), PFOS, PFOA, perfluorononanoic acid (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA) and is approved by quality controls performed by the European Human Biomonitoring Initiative (HBM4EU) project (see “Appendix S1. Certificates quality control PFAS measurements (University of Erlangen-Nuremberg, Germany)” and “Appendix S2. Certificate quality control PFAS measurements (HBM4EU).” in the Supplemental Material).
The LOD ranged from for PFHpA to for PFOA. Of the eight PFAS that had measurable levels, concentrations of PFHxS, PFOS, PFOA, PFNA, and PFDA were for all participants. Concentrations of PFHpA and PFUnDA were for all participants in the SMC-C, but for 2.2% and 0.25% of participants in the 60YO, respectively, which were substituted with the LOD divided by the square root of 2. However, concentrations of PFDoDA were in 50.1% and 60.7% in the SMC-C and the 60YO cohorts, respectively, and because tertiles could not be accurately assessed, they were therefore excluded from the analysis. Furthermore, concentrations of PFOA and PFHpA were remarkably high in the SMC-C (50% of participants had values between and for PFOA and PFHpA, respectively), likely owing to contamination of the samples during sampling/storage, and they were therefore not considered in that cohort.
Thus, eventually, five long-chain PFAS—three carboxylated (PFNA, PFDA, and PFUnDA) and two sulfonated (PFHxS and PFOS)—were available in both the SMC-C and the 60YO cohorts, whereas two carboxylated PFAS—one short-chain (PFHpA) and one long-chain (PFOA)—were additionally available in the 60YO cohort.
Baseline Assessment of Covariates
Questionnaire information included age, sex, attained education, body mass index (BMI), comorbidities (i.e., diabetes, hypertension, and high cholesterol), family history of CVD (i.e., heart attack in a relative before 60 years of age in the SMC-C cohort and in any siblings, father, or mother in the 60YO cohort), smoking habits and physical activity (i.e., active when reported walking/biking was and exercise for the SMC-C and when reported activity was moderate or heavy for the 60YO). Covariates were selected based on a priori knowledge of CVD risk factors and lifestyle factors that could impact PFAS levels (Lindbohm et al. 2021; Rosengren et al. 2019).
We obtained information on food consumption (from a semiquantitative 124-item food frequency questionnaire in the SMC-C and a questionnaire with 17 food-related questions in the 60YO). For the SMC-C, we created a healthy diet score based on the eight-point scoring system (low to high adherence) of the modified Mediterranean diet score, reflecting consumption of fruits and vegetables, fermented dairy foods, whole grain/fiber-rich foods, legumes and nuts, fish, olive/rapeseed oil, and alcohol (in moderation) as positive components and with red and processed meat (as a negative component) (Tektonidis et al. 2015), which was collapsed into three categories. For the 60YO, the healthy diet score was constructed from a six-point scoring system based on the intake of fruits, vegetables, fish, alcohol (in moderation) as positive components and with meat and snacks as negative components, and also collapsed into three categories. Missing information on covariates (, with the exception of 16% for physical activity in the SMC-C) were replaced by a missing indicator category.
Statistical Analyses
Spearman’s rank correlation was used to assess pairwise relationships between different PFAS. Individual PFAS plasma concentrations were natural log-transformed and assessed as a continuous variable per 1 standard deviation (SD) increment. To create a sum of the PFAS, individual PFAS were standardized (rescaled with and ) and summed (). Individual PFAS and were also categorized into tertiles according to the cohort-specific distribution among the controls.
The baseline cross-sectional associations among the controls between PFAS and blood lipids were assessed using multivariable-adjusted linear regression analysis. Pooled results from both cohorts, using linear mixed effects models, are presented as with corresponding 95% confidence intervals (CIs).
The prospective associations between baseline PFAS and risk of CVD, myocardial infarction, and stroke were assessed using conditional logistic regression. Pooled results from the two cohorts are presented as odds ratios (ORs) with corresponding 95% CIs. To maximize statistical power, simple pooling was used in assessing total CVD because of low between-cohort heterogeneity ( statistic range: 0–34%, lowest ). Random-effects meta-analysis was used for assessing separate risk of myocardial infarction and stroke because the heterogeneity between the cohorts was larger ( statistic range: 0–86%, ) for separate outcomes.
Both assessments of PFAS with lipids and with CVD were adjusted in Model 1 for matching factors [i.e., age (in SMC-C), sex (in 60YO) and sample year] and in Model 2 were additionally adjusted for attained education (), BMI (as continuous), diabetes (yes/no), hypertension (yes/no), family history of CVD (yes/no), smoking habits (never/former/current/missing), physical activity (active/inactive/missing), and healthy diet score (4 categories, including “missing” category). To explore potential mediation by lipids on the PFAS and CVD risk association, lipids were included in additional models (Model 3 included LDL, whereas Model 4 included HDL and triglycerides). Furthermore, the potential effect modification by BMI (normal vs. overweight and obese ) was investigated using interaction terms for continuous PFAS. Adjusted (Model 2) coefficients (95% CIs) from linear mixed effects models stratified by BMI were visualized for a 1-SD increase in PFAS.
All statistical analyses were performed using the statistical software STATA (version 15.1; Stata Corp LP) and using the metan package for the meta-analysis. -Values were calculated based on two-sided tests, and the level of statistical significance was set at 0.05.
Results
Study population characteristics by case–control status in each cohort are summarized in Table 1 (see Tables S3 and S4 for summaries of myocardial infarction/stroke). The SMC-C compassed an older, female population with a later sampling date than the 60YO, which had mostly male cases and matched controls. The SMC-C cohort showed a lower prevalence of diabetes but had a higher prevalence of high cholesterol and fewer smokers compared with the 60YO cohort. Furthermore, except for PFOS, PFAS levels were higher in the SMC-C. Overall, the controls were more educated and less often smokers and had a lower prevalence of diabetes and hypertension and lower triglycerides levels than the cases. High correlations were observed between PFNA, PFDA, and PFUnDA (), whereas the lowest correlation was between PFHpA and PFUnDA () (Figure S1).
Table 1.
Baseline (2003–2009 and 1997–1999, respectively) characteristics by cardiovascular disease (CVD) case–control status of 742 women from the Swedish Mammography Cohort-Clinical and of 786 men and women from the Swedish Cohort of 60-year-olds.
| Characteristics | SMC-C cohort | 60YO cohort | ||
|---|---|---|---|---|
| CVD cases () | Controls () | CVD cases () | Controls () | |
| Sex [% ()] | ||||
| Female | 100 (306) | 100 (436) | 36 (141) | 36 (141) |
| Male | 0 (0) | 0 (0) | 64 (252) | 64 (252) |
| Age (y) | 72 (7.3) | 72 (7.3) | 61 (0.1) | 61 (0.1) |
| Sample year | 2006 (1.5) | 2006 (1.5) | 1998 (0.4) | 1998 (0.3) |
| Education (y) [% ()] | ||||
| 70 (212) | 68 (296) | 79 (289) | 67 (254) | |
| 30 (93) | 32 (138) | 21 (79) | 33 (125) | |
| Missing () | 1 | 2 | 25 | 14 |
| BMI () | 27 (4.6) | 26 (4.4) | 27 (4.3) | 27 (4.3) |
| History of diabetes [% ()] | ||||
| No | 95 (292) | 97 (424) | 91 (356) | 94 (370) |
| Yes | 4.6 (14) | 2.8 (12) | 9.4 (37) | 5.9 (23) |
| History of hypertension [% ()] | ||||
| No | 48 (146) | 59 (257) | 50 (198) | 62 (243) |
| Yes | 52 (160) | 41 (179) | 50 (195) | 38 (150) |
| History of high cholesterol [% ()] | ||||
| No | 72 (221) | 77 (336) | 93 (365) | 90 (353) |
| Yes | 28 (85) | 23 (100) | 7.1 (28) | 10 (40) |
| Family history of CVD [% ()] | ||||
| No | 62 (191) | 64 (280) | 56 (222) | 56 (220) |
| Yes | 38 (115) | 36 (156) | 44 (171) | 44 (173) |
| Smoking status [% ()] | ||||
| Never | 52 (152) | 59 (249) | 29 (108) | 46 (172) |
| Former | 32 (92) | 32 (133) | 37 (135) | 38 (144) |
| Current | 16 (47) | 8.8 (37) | 34 (123) | 16 (61) |
| Missing () | 15 | 17 | 27 | 16 |
| Physical activity [% ()] | ||||
| Active | 31 (77) | 33 (120) | 28 (100) | 33 (127) |
| Inactive | 70 (175) | 67 (249) | 72 (257) | 67 (255) |
| Missing () | 54 | 67 | 36 | 11 |
| Healthy diet score [% ()] | ||||
| Unhealthy | 21 (62) | 13 (56) | 40 (146) | 33 (127) |
| Moderately healthy | 61 (180) | 61 (256) | 35 (130) | 32 (124) |
| Healthy | 18 (53) | 25 (106) | 25 (92) | 34 (132) |
| Missing () | 11 | 18 | 25 | 10 |
| Total cholesterol (mmol/L) | 5.8 (1.1) | 5.8 (1.0) | 6.0 (1.0) | 5.9 (1.0) |
| LDL (mmol/L) | 3.5 (1.0) | 3.5 (0.9) | 4.0 (0.9) | 3.9 (0.9) |
| HDL (mmol/L) | 1.5 (0.4) | 1.6 (0.4) | 1.3 (0.4) | 1.4 (0.4) |
| Triglycerides (mmol/L) | 1.5 (0.7) | 1.3 (0.6) | 1.6 (1.0) | 1.4 (0.9) |
| ApoB (mmol/L)a | — | — | 1.2 (0.2) | 1.0 (0.2) |
| ApoA1 (mmol/L)a | — | — | 1.4 (0.3) | 1.5 (0.3) |
| PFHxS (ng/mL) | 5.28 (7.81) | 5.96 (7.64) | 3.21 (4.60) | 3.46 (6.45) |
| Median (IQR) | 2.69 (2.07–4.62) | 2.73 (2.01–5.59) | 2.34 (1.85–2.93) | 2.33 (1.84–2.91) |
| PFHpA (ng/mL)a | — | — | 0.08 (0.10) | 0.08 (0.08) |
| Median (IQR) | — | — | 0.05 (0.03–0.09) | 0.06 (0.03–0.1) |
| PFOS (ng/mL) | 18.4 (10.5) | 19.2 (17.6) | 26.5 (13.2) | 27.8 (14.9) |
| Median (IQR) | 16.8 (11.8–22.1) | 16.9 (12.5–21.8) | 24.8 (19.1–31.2) | 25.2 (19.0–34.4) |
| PFOA (ng/mL)a | — | — | 5.59 ( 3.44) | 5.91 (3.91) |
| Median (IQR) | — | — | 5.05 (3.85–6.63) | 5.31 (3.99–6.93) |
| PFNA (ng/mL) | 1.01 (0.52) | 1.02 (0.48) | 0.74 (0.39) | 0.81 (0.42) |
| Median (IQR) | 0.95 (0.67–1.19) | 0.92 (0.72–1.21) | 0.69 (0.48–0.89) | 0.71 (0.51–0.99) |
| PFDA (ng/mL) | 0.40 (0.21) | 0.43 (0.23) | 0.28 (0.15) | 0.30 (0.16) |
| Median (IQR) | 0.35 (0.27–0.48) | 0.38 (0.29–0.52) | 0.25 (0.18–0.34) | 0.26 (0.19–0.39) |
| PFUnDA (ng/mL) | 0.32 (0.19) | 0.36 (0.21) | 0.25 (0.14) | 0.29 (0.17) |
| Median (IQR) | 0.27 (0.19–0.4) | 0.31 (0.21–0.44) | 0.22 (0.16–0.31) | 0.24 (0.17–0.36) |
Note: Continuous variables are shown as mean (SD) if not otherwise stated. PFAS concentrations are presented as mean (SD) followed by median (IQR). —, not applicable; 60YO, Cohort of 60-year-olds; apo, apolipoprotein; BMI, body mass index; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; PFDA, perfluorodecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid; SD, standard deviation; SMC-C, Swedish Mammography Cohort-Clinical cohort.
Available for the 60YO cohort alone.
Overall, the vast majority of PFAS showed statistically significant associations, with higher total and LDL cholesterol, whereas associations in a favorable direction were observed with higher HDL cholesterol and apoA1 and lower triglycerides. Results for apoB showed mainly null associations (Figure 2; Table S5). An interaction between PFAS and BMI () was observed, with stronger associations of most PFAS with higher LDL and apoB among the overweight and obese, whereas no associations were found among the lean participants (Figure S2). The same effect modification by BMI was observed for total cholesterol, whereas there was no significant interaction for HDL (for most PFAS), triglycerides, or apoA1 (; Table S6).
Figure 2.
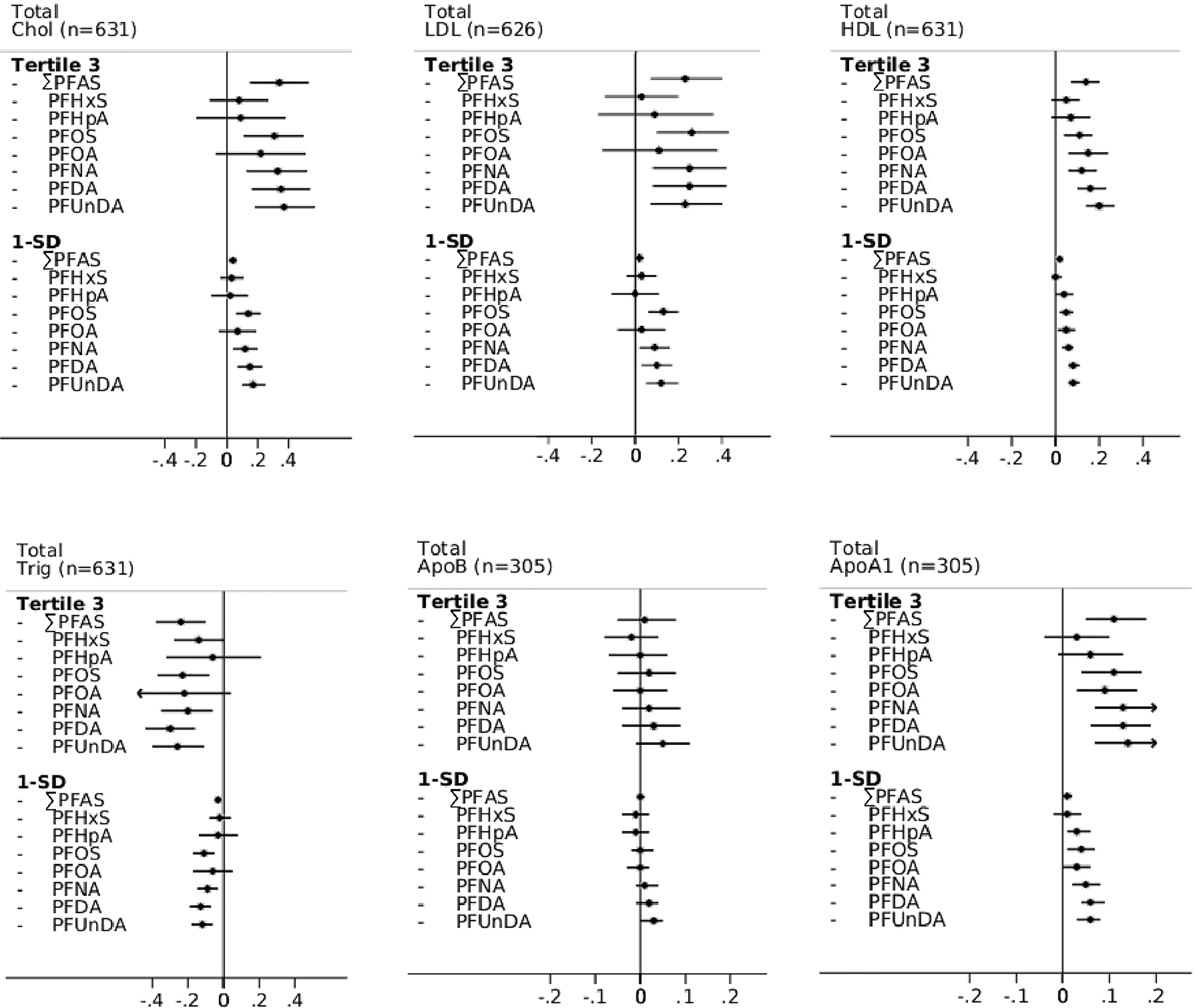
Multivariable-adjusted cross-sectional associations in controls between baseline PFAS plasma concentrations and total cholesterol, LDL, HDL, triglycerides, apoB, and apoA1 of two Swedish pooled cohorts (SMC-C baseline: 2003–2009 and 60YO baseline: 1997–1999), estimated using linear mixed effects models—apoB, apoA1, PFHpA, and PFOA results are from the 60YO cohort alone. Adjusted (95% CIs) are presented according to PFAS tertiles (using Tertile 1 as reference), as well as by 1-SD increment in natural log-transformed plasma PFAS concentrations (ng/mL). Models were adjusted for age, sex, sampling date, education, BMI, diabetes, hypertension, family history of CVD, smoking habits, physical activity, and healthy diet score. Individual PFAS were standardized (rescaled with and ) and summed (). Note: 60YO, Cohort of 60-year-olds; BMI, body mass index; apo, apolipoprotein; Chol, cholesterol; CI, confidence interval; CVD, cardiovascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PFDA, perfluorodecanoic acid; PFAS, per- and polyfluoroalkyl substances; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid; SD, standard deviation; SMC-C, Swedish Mammography Cohort-Clinical cohort; Trig, triglycerides.
PFAS levels were overall inversely associated [: (95% CI: 0.53, 0.93)] with risk of CVD after pooling the cohorts (Table 2). Further adjusting for baseline lipid levels (LDL or HDL and triglycerides) had only marginal impact (Models 3 and 4, Table 2). Similar associations were found in individual cohorts (Table S7). Specific assessment of myocardial infarction and ischemic stroke in random-effects meta-analyses showed an overall similar pattern of nonsignificant inverse associations, although slightly more inconsistencies between cohorts were found (Figures 3 and 4; Tables S8 and S9). There was no indication of interactions with BMI (, Table S6).
Table 2.
Multivariable-adjusted prospective associations between baseline PFAS plasma concentrations and subsequent risk of cardiovascular disease (CVD) in 1,528 men and women from two pooled Swedish cohorts, estimated using conditional logistic regression—PFHpA and PFOA results are from the 60YO cohort alone.
| Exposure categories | Pooled cohorts () | |||||
|---|---|---|---|---|---|---|
| OR of incident CVD (95% CI) | ||||||
| Case/control () | Median (IQR) (mmol/L)a | Model 1 | Model 2 | Model 3 | Model 4 | |
| Tertile 1 | 270/278 | (, ) | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 253/275 | (, 0.10) | 0.94 (0.74, 1.20) | 1.03 (0.79, 1.34) | 1.01 (0.77, 1.31) | 1.05 (0.81, 1.37) |
| Tertile 3 | 176/276 | 3.46 (2.09, 5.70) | 0.64 (0.49, 0.82) | 0.70 (0.53, 0.93) | 0.68 (0.51, 0.90) | 0.73 (0.55, 0.97) |
| 1-SD log | — | — | 0.95 (0.92, 0.98) | 0.97 (0.93, 1.00) | 0.96 (0.93, 0.99) | 0.97 (0.94, 1.00) |
| PFHxS | ||||||
| Tertile 1 | 244/277 | 1.73 (1.44, 1.95) | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 237/276 | 2.52 (2.32, 2.74) | 0.98 (0.76, 1.25) | 0.95 (0.72, 1.24) | 0.92 (0.70, 1.20) | 0.96 (0.73, 1.26) |
| Tertile 3 | 218/276 | 4.97 (3.20, 11.1) | 0.87 (0.67, 1.13) | 0.96 (0.72, 1.28) | 0.94 (0.70, 1.25) | 0.99 (0.74, 1.32) |
| 1-SD log | — | — | 0.93 (0.84, 1.04) | 0.95 (0.85, 1.06) | 0.94 (0.84, 1.06) | 0.96 (0.85, 1.07) |
| PFHpA b | ||||||
| Tertile 1 | 153/132 | 0.03 (0.02, 0.03) | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 120/130 | 0.06 (0.05, 0.07) | 0.79 (0.56, 1.12) | 0.69 (0.46, 1.03) | 0.64 (0.42, 0.97) | 0.68 (0.45, 1.02) |
| Tertile 3 | 120/131 | 0.13 (0.10, 0.18) | 0.78 (0.55, 1.11) | 0.75 (0.50, 1.11) | 0.68 (0.45, 1.03) | 0.72 (0.48, 1.08) |
| 1-SD log | — | — | 0.93 (0.81, 1.08) | 0.95 (0.81, 1.12) | 0.94 (0.79, 1.11) | 0.95 (0.80, 1.12) |
| PFOS | ||||||
| Tertile 1 | 250/278 | 12.9 (10.0, 17.3) | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 228/276 | 21.9 (17.1, 25.7) | 0.89 (0.70, 1.15) | 0.92 (0.70, 1.21) | 0.86 (0.65, 1.14) | 0.95 (0.73, 1.26) |
| Tertile 3 | 221/275 | 32.3 (24.8, 38.9) | 0.87 (0.67, 1.13) | 0.90 (0.68, 1.20) | 0.87 (0.65, 1.16) | 0.94 (0.71, 1.26) |
| 1-SD log | — | — | 0.89 (0.80, 0.99) | 0.91 (0.81, 1.03) | 0.89 (0.79, 1.01) | 0.93 (0.82, 1.05) |
| PFOA b | ||||||
| Tertile 1 | 135/131 | 3.41 (2.66, 3.94) | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 142/131 | 5.25 (4.82, 5.70) | 1.05 (0.76, 1.45) | 1.14 (0.78, 1.65) | 1.03 (0.70, 1.50) | 1.17 (0.80, 1.70) |
| Tertile 3 | 116/131 | 7.63 (6.88, 9.18) | 0.84 (0.58, 1.20) | 0.90 (0.60, 1.37) | 0.81 (0.52, 1.24) | 0.93 (0.61, 1.42) |
| 1-SD log | — | — | 0.90 (0.77, 1.04) | 0.91 (0.77, 1.08) | 0.87 (0.73, 1.04) | 0.92 (0.77, 1.09) |
| PFNA | ||||||
| Tertile 1 | 260/279 | 0.51 (0.40, 0.60) | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 234/276 | 0.81 (0.71, 0.93) | 0.89 (0.71, 1.13) | 0.88 (0.68, 1.14) | 0.83 (0.64, 1.08) | 0.88 (0.68, 1.14) |
| Tertile 3 | 205/274 | 1.28 (1.11, 1.55) | 0.80 (0.62, 1.02) | 0.91 (0.69, 1.20) | 0.87 (0.66, 1.14) | 0.93 (0.71, 1.23) |
| 1-SD log | — | — | 0.87 (0.78, 0.96) | 0.91 (0.81, 1.02) | 0.89 (0.79, 1.00) | 0.92 (0.82, 1.03) |
| PFDA | ||||||
| Tertile 1 | 256/285 | 0.20 (0.15, 0.26) | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 254/269 | 0.31 (0.25, 0.38) | 1.02 (0.80, 1.29) | 1.17 (0.90, 1.52) | 1.13 (0.87, 1.47) | 1.19 (0.92, 1.55) |
| Tertile 3 | 189/275 | 0.52 (0.45, 0.66) | 0.73 (0.56, 0.95) | 0.81 (0.61, 1.08) | 0.78 (0.58, 1.04) | 0.86 (0.64, 1.15) |
| 1-SD log | — | — | 0.83 (0.75, 0.93) | 0.89 (0.79, 1.00) | 0.87 (0.77, 0.98) | 0.90 (0.80, 1.02) |
| PFUnDA | ||||||
| Tertile 1 | 288/294 | 0.16 (0.13, 0.19) | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 236/260 | 0.27 (0.24, 0.31) | 0.89 (0.70, 1.13) | 0.99 (0.76, 1.29) | 0.99 (0.76, 1.28) | 1.01 (0.78, 1.32) |
| Tertile 3 | 175/275 | 0.48 (0.40, 0.60) | 0.61 (0.47, 0.79) | 0.76 (0.57, 1.02) | 0.73 (0.54, 0.97) | 0.80 (0.59, 1.07) |
| 1-SD log | — | — | 0.79 (0.71, 0.88) | 0.86 (0.76, 0.97) | 0.84 (0.74, 0.95) | 0.87 (0.77, 0.99) |
Note: Adjusted ORs (95% CIs) of incident CVD (myocardial infarction or stroke) are presented according to the PFAS tertiles as well as by 1-SD increment in natural log-transformed plasma PFAS concentrations (ng/mL). Model 1: adjusted for matching factors (sex, age, sampling date). Model 2: additionally adjusted for education, BMI, diabetes, hypertension, family history of CVD, smoking habits, physical activity, and healthy diet score. Model 3: additionally adjusted for LDL (19 observations deleted due to missing LDL). Model 4: additionally adjusted for HDL and triglycerides. Individual PFAS were standardized (rescaled with and ) and summed (). —, not applicable; 60YO, Cohort of 60-year-olds; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; OR, odds ratio; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid; SD, standard deviation; SMC-C, Swedish Mammography Cohort-Clinical cohort.
The score are standardized values.
Estimated from the 60YO cohort alone.
Figure 3.
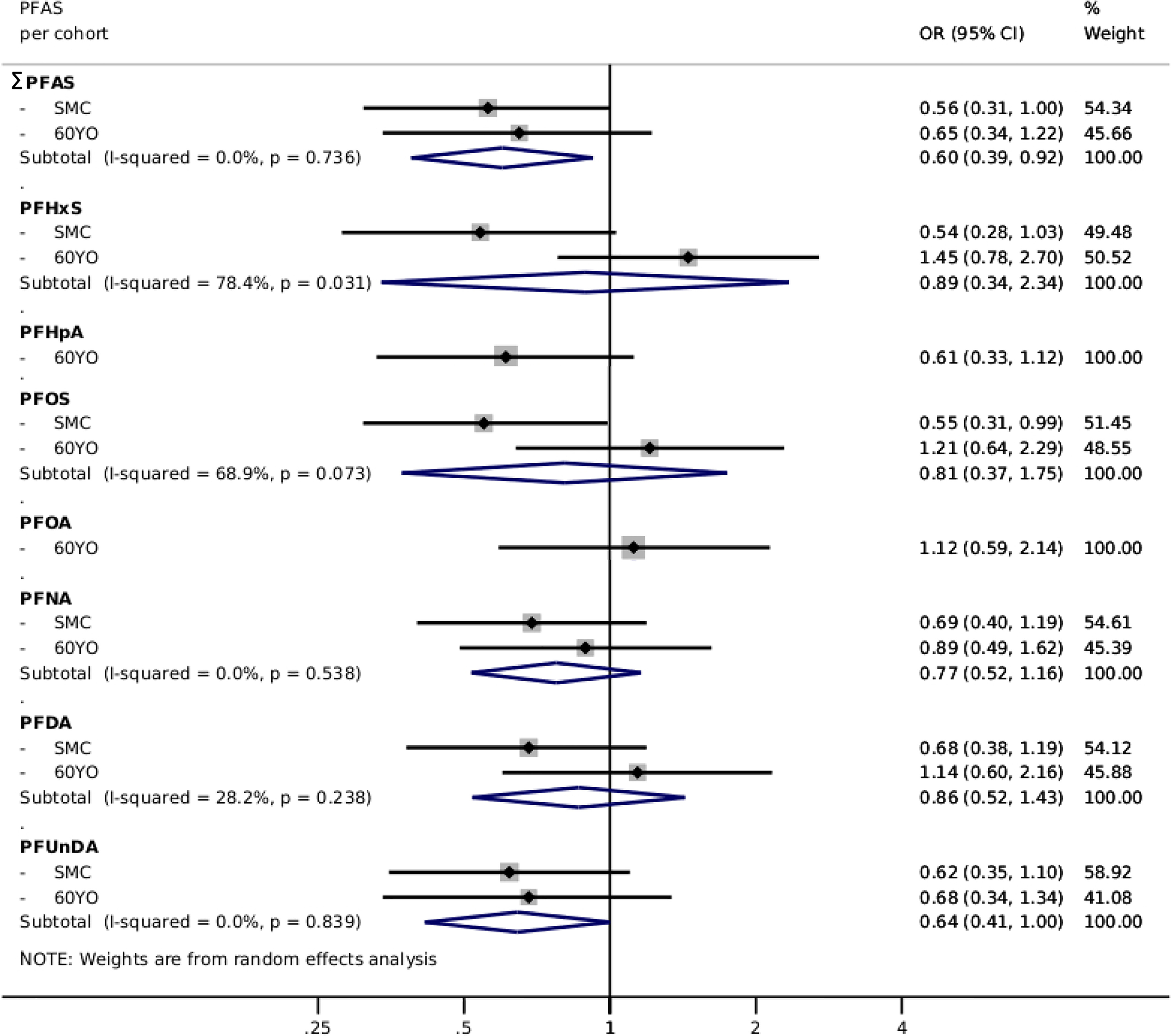
Multivariable-adjusted risk of myocardial infarction, presented as pooled ORs (95% CIs) from two Swedish cohorts (SMC-C: and 60YO: ) using random effects meta-analysis, comparing the third tertile of each PFAS with the first tertile—PFHpA and PFOA results are from the 60YO cohort alone. Estimations adjusted according to Model 2: sex, age, sampling date, education, BMI, diabetes, hypertension, family history of CVD, smoking habits, physical activity, and healthy diet score. Individual PFAS were standardized (rescaled with and ) and summed (). Note: 60YO, Cohort of 60-year-olds; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; OR, odds ratio; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid; SD, standard deviation; SMC-C, Swedish Mammography Cohort-Clinical cohort.
Figure 4.
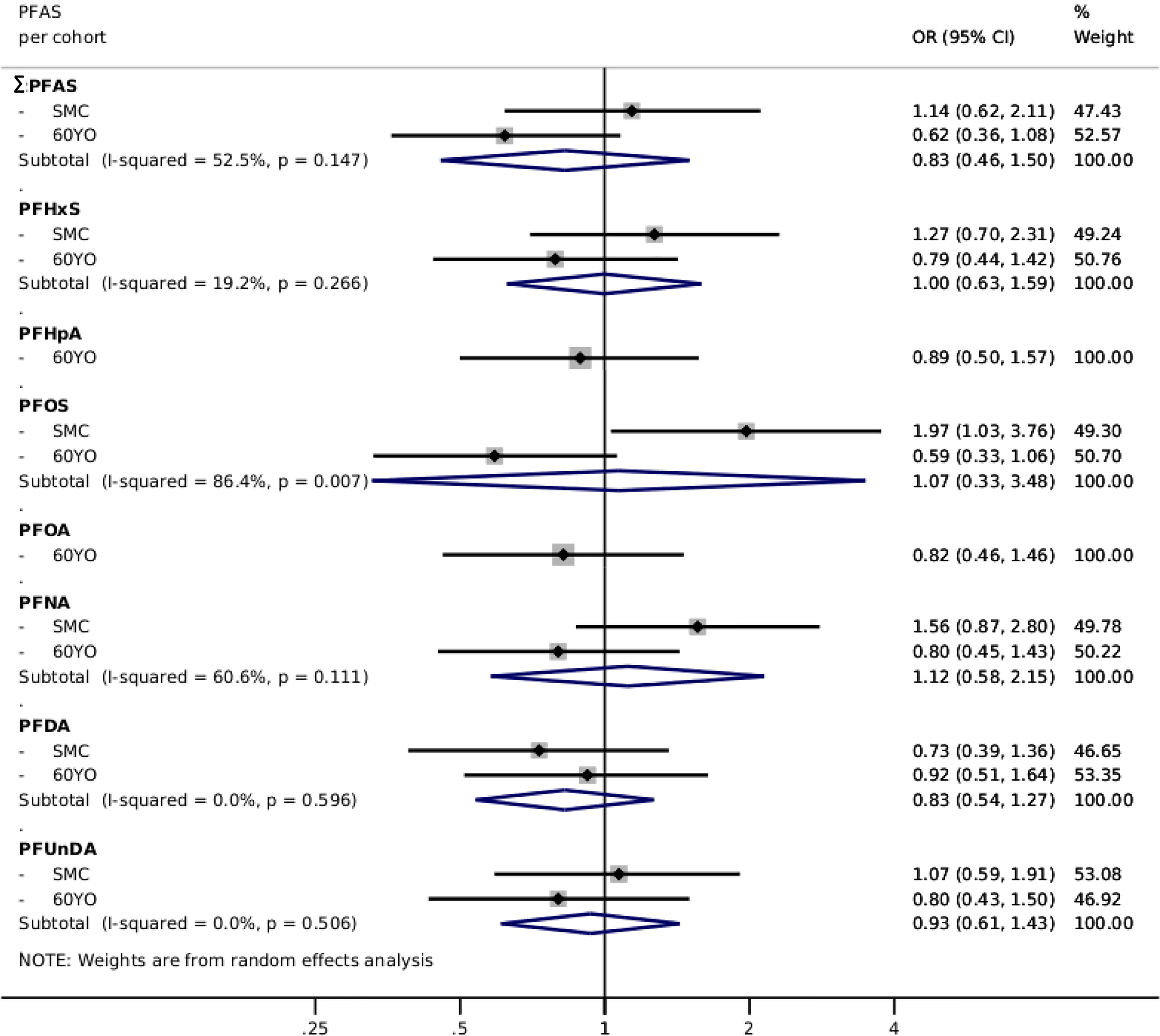
Multivariable-adjusted risk of stroke, presented as pooled ORs (95% CIs) from two Swedish cohorts (SMC-C: and 60YO: ) using random effects meta-analysis, comparing the third tertile of each PFAS with the first tertile—PFHpA and PFOA results are from the 60YO cohort alone. Estimations adjusted according to Model 2: sex, age, sampling date, education, BMI, diabetes, hypertension, family history of CVD, smoking habits, physical activity, and healthy diet score. Individual PFAS were standardized (rescaled with and ) and summed (). Note: 60YO, Cohort of 60-year-olds; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; OR, odds ratio; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid; SD, standard deviation; SMC-C, Swedish Mammography Cohort-Clinical cohort.
Discussion
In this large prospective nested case–control study, despite statistically significant cross-sectional associations between PFAS and increased total and LDL cholesterol among the controls, we observed overall null associations between PFAS and risk of CVD, myocardial infarction, and stroke. If anything, these associations displayed an inverse tendency.
Although in our study the median PFAS levels were not particularly high [e.g., PFOS levels approximated the previously established lower bound benchmark doses (Dong et al. 2019)], we still observed statistically significantly associations for , PFOS, PFNA, PFDA, and PFUnDA with higher total- and LDL cholesterol. There were no significant associations for PFHxS, PFHpA and PFOA, possibly due to lower potency of the shorter chain lengths (PFHxS and PFHpA) (Wolf et al. 2008) or due to lower power (PFOA) given that this was assessed only in the 60YO cohort. Our findings align with several risk assessments (ATSDR 2021; EFSA CONTAM et al. 2018; IARC 2018), reviews (Steenland et al. 2020; Sunderland et al. 2019), and other large studies showing positive associations (Fitz-Simon et al. 2013; Frisbee et al. 2010; Steenland et al. 2009). In contrast to these potential atherogenic associations observed, we found associations with lower triglycerides and higher HDL. Interestingly, stronger associations were found for the newer, less abundant and longer chain PFAS compounds (PFNA, PFDA, and PFUnDA), which may be related to differences in potency (Bijland et al. 2011; Buhrke et al. 2013).
PFAS-related molecular mechanisms underlying altered lipid metabolism are not yet clarified. Proposed pathways include increased cholesterol absorption or synthesis, impaired mobilization, reduced reverse transportation (Fletcher et al. 2013), reduced turnover to bile acids (Behr et al. 2020), and sterol imbalance (Monroe and Dobs 2013). These processes may be impacted through PFAS-activation of transcription factors, such as (Bijland et al. 2011), constitutive androstane receptor (CAR) (Abe et al. 2017), Pregnane X Receptor (PXR) (Bijland et al. 2011), and endocrine receptor () (Benninghoff et al. 2011). Evidence for these pathways mainly comes from in vitro or animal studies that have shown hypocholesteremia and reduced triglycerides upon PFAS exposure (Bijland et al. 2011). Apart from our observed lower triglyceride levels with increasing PFAS, an overall hypocholesteremia in animal data contrasts with the elevated cholesterol observed in many epidemiological studies, including this one. Discrepancies in doses or differences in physiology may explain interspecies differences (Bjork and Wallace 2009; Golforoush et al. 2020; Lau et al. 2007). Furthermore, diet may play a modifying role given that hypercholesteremia has been shown in animals maintained on a high-fat diet (Rebholz et al. 2016). In humans, obesity has been shown to modify the associations between PFAS and blood lipid levels (Jain and Ducatman 2019), and we also found stronger associations for LDL and apoB among overweight and obese participants. This suggests that obese individuals are more susceptible to alterations in lipid metabolism, potentially due to liver steatosis (Bagley et al. 2017; Jain and Ducatman 2019). However, cautious interpretation is recommended because stratification by baseline BMI could introduce (collider stratification) bias (Inoue et al. 2020).
Evidence for a causal relationship between LDL cholesterol and CVD is strong (Prospective Studies Collaboration et al. 2007; Cholesterol Treatment Trialists’ Collaborators et al. 2012), particularly for myocardial infarction (Yusuf et al. 2020), mainly owing to the role of blood lipids in atherosclerosis (Geovanini and Libby 2018). In addition, HDL cholesterol and triglycerides may be relevant, although the evidence for causality is less conclusive (Emerging Risk Factors Collaboration et al. 2009; Farnier et al. 2021; Musunuru and Kathiresan 2016; Schwartz et al. 2012). Thus, the impact of PFAS on cholesterol levels may translate into increased CVD risk. However, this study showed null associations with an inverse tendency for PFAS and CVD. Our findings align with another study with retrospectively modeled PFOA and prospective coronary artery disease (retrospective self-reported diagnosis verified by medical records) performed on workers and residents in the Mid-Ohio Valley C8 cohort (Winquist and Steenland 2014), with a smaller prospective nested case–control study (Mattsson et al. 2015), and with a large cross-sectional study on self-reported diagnosis of stroke using data from the C8 cohort (Hutcheson et al. 2020). In contrast, a cross-sectional study using the National Health and Nutrition Examination Surveys cohort based on self-reported diagnosis of five different CVD outcomes found that several PFAS exposures (among which were PFOS and PFNA) associated with increased CVD (Huang et al. 2018), another cross-sectional study found a positive association between PFOA and self-reported CVD (Shankar et al. 2012), and an ecological study in the Veneto Region in Italy found increased rate ratios for myocardial infarction (Mastrantonio et al. 2018).
The present study found a tendency for inverse associations of PFAS with CVD risk, which was unexpected because of the consistent evidence for an association with PFAS and elevated cholesterol, as reviewed by the European Food Safety Agency (EFSA CONTAM et al. 2018). It is possible that the LDL increase associated with PFAS exposure was not enough to increase the risk for CVD. On the other hand, we hypothesize that the PFAS-associated higher HDL and apoA1 as well as lower triglyceride levels may negate the detrimental effect of the PFAS-associated higher LDL levels on CVD risk. In addition, PFAS associations with apoB, a better marker for atherosclerosis risk than LDL (Sniderman et al. 2003), were weaker. However, inclusion of lipids (LDL or HDL and triglycerides) in the CVD analysis models did not change PFAS associations. Furthermore, diet or environmental factors can covary with PFAS and alter blood lipid levels, for example, fish is the main source of PFAS among regular consumers of fish (Bjorke-Monsen et al. 2020; Domingo and Nadal 2017; Vestergren et al. 2012) and fish consumption associates with higher HDL cholesterol levels in the blood (Alhassan et al. 2017) and thus could confound PFAS–HDL and PFAS–CVD relationships (Leung Yinko et al. 2014). However, associations remained similar after adjustment for diet. Another possible explanation for the slightly reduced CVD risk is the potential of PFAS to diminish the immune response (Grandjean et al. 2017; Salihovic et al. 2020), possibly through activation (DeWitt et al. 2009), given that inflammation aggravates atherosclerotic plaque formation and rupture (Geovanini and Libby 2018).
Limitations and Strengths
Our analyses were limited by the PFOA and PFHpA contamination of blood samples in the SMC-C, and we cannot exclude that small effects on CVD risk were missed owing to limited power or low PFAS levels. However, our sample size was relatively large and lower PFAS levels allow for drawing inference for the general population. The generalizability of the study is limited to seniors, this is, nevertheless, the most critical group for CVD. There may be potential residual or unmeasured confounding. Confounding by PFAS binding to blood lipoproteins is unlikely because PFAS bind mainly to albumin with little affinity for lipoproteins (Forsthuber et al. 2020). However, given the cross-sectional nature of our lipid analyses, we could not establish the temporal relation or make causal inferences for the associations between PFAS and lipid fractions, and there may be reverse causality for PFAS and cholesterol through shared excretion in the bile (EFSA CONTAM et al. 2018; Zhao et al. 2015). Last, PFAS-related elevated cholesterol may result in increased lipid-lowering medication use during follow-up, subsequently lowering the risk of CVD. However, cholesterol levels did not differ strongly between cases and controls at baseline. Important strengths are the prospective design; long follow-up for risk of CVD; and that PFAS concentrations may well reflect the long-term exposure, given that high intra-class correlation coefficients have been shown over a 10-y period (Donat-Vargas et al. 2019); measurement of several commonly occurring PFAS in blood plasma; as well as robust case selection from register linkages, which limit the possibility for reverse causality and exposure and outcome misclassification. In addition, elaborate questionnaires and visit information allowed for adjustment of many potentially important covariates.
Conclusions
We confirmed PFAS cross-sectional associations with elevated cholesterol, which has been indicated as one of the main adverse outcomes of exposure to PFOS and PFOA. However, this did not translate into increased CVD risk in our study.
Supplementary Material
Acknowledgments
We thank A. Rönnholm, Å. Amilon, and M. Bengtsson for analyzing the samples. We acknowledge SIMPLER for provisioning of facilities and experimental support by the Swedish Research Council [2017-00644 (to A.W.). The computations were performed on resources provided by SNIC-SENS (the Swedish National Infrastructure for Computing that provides secure handling of sensitive data) through the Uppsala Multidisciplinary Center for Advanced Computational Science under Project SIMP2019015. This work was supported by the Swedish Research Council [2017-00822 (to A.Å.)].
References
- Abe T, Takahashi M, Kano M, Amaike Y, Ishii C, Maeda K, et al. 2017. Activation of nuclear receptor CAR by an environmental pollutant perfluorooctanoic acid. Arch Toxicol 91(6):2365–2374, PMID: , 10.1007/s00204-016-1888-3. [DOI] [PubMed] [Google Scholar]
- Alhassan A, Young J, Lean MEJ, Lara J. 2017. Consumption of fish and vascular risk factors: a systematic review and meta-analysis of intervention studies. Atherosclerosis 266:87–94, PMID: , 10.1016/j.atherosclerosis.2017.09.028. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2021. Toxicological profile for perfluoroalkyls. Atlanta, GA: ATSDR. https://www.atsdr.cdc.gov/ToxProfiles/tp200.pdf [accessed 10 March 2022]. [PubMed] [Google Scholar]
- Bagley BD, Chang SC, Ehresman DJ, Eveland A, Zitzow JD, Parker GA, et al. 2017. Perfluorooctane sulfonate-induced hepatic steatosis in male Sprague Dawley rats is not attenuated by dietary choline supplementation. Toxicol Sci 160(2):284–298, PMID: , 10.1093/toxsci/kfx185. [DOI] [PubMed] [Google Scholar]
- Behr AC, Kwiatkowski A, Ståhlman M, Schmidt FF, Luckert C, Braeuning A, et al. 2020. Impairment of bile acid metabolism by perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in human HepaRG hepatoma cells. Arch Toxicol 94(5):1673–1686, PMID: , 10.1007/s00204-020-02732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE. 2011. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol Sci 120(1):42–58, PMID: , 10.1093/toxsci/kfq379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijland S, Rensen PC, Pieterman EJ, Maas ACE, van der Hoorn JW, van Erk MJ, et al. 2011. Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-leiden CETP mice. Toxicol Sci 123(1):290–303, PMID: , 10.1093/toxsci/kfr142. [DOI] [PubMed] [Google Scholar]
- Bjork JA, Wallace KB. 2009. Structure-activity relationships and human relevance for perfluoroalkyl acid–induced transcriptional activation of peroxisome proliferation in liver cell cultures. Toxicol Sci 111(1):89–99, PMID: , 10.1093/toxsci/kfp093. [DOI] [PubMed] [Google Scholar]
- Bjorke-Monsen AL, Varsi K, Averina M, Brox J, Huber S. 2020. Perfluoroalkyl substances (PFASs) and mercury in never-pregnant women of fertile age: association with fish consumption and unfavorable lipid profile. BMJ Nutr Prev Health 3(2):277–284, PMID: , 10.1136/bmjnph-2020-000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrke T, Kibellus A, Lampen A. 2013. In vitro toxicological characterization of perfluorinated carboxylic acids with different carbon chain lengths. Toxicol Lett 218(2):97–104, PMID: , 10.1016/j.toxlet.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists’ Collaborators; Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. 2012. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 380(9841):581–590, PMID: , 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Blossom SJ, Schaider LA. 2019. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol 29(2):148–156, PMID: , 10.1038/s41370-018-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, et al. 2009. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol 39(1):76–94, PMID: , 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Nadal M. 2017. Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J Agric Food Chem 65(3):533–543, PMID: , 10.1021/acs.jafc.6b04683. [DOI] [PubMed] [Google Scholar]
- Donat-Vargas C, Bergdahl IA, Tornevi A, Wennberg M, Sommar J, Kiviranta H, et al. 2019. Perfluoroalkyl substances and risk of type II diabetes: a prospective nested case-control study. Environ Int 123:390–398, PMID: , 10.1016/j.envint.2018.12.026. [DOI] [PubMed] [Google Scholar]
- Dong Z, Wang H, Yu YY, Li YB, Naidu R, Liu Y. 2019. Using 2003–2014 U.S. NHANES data to determine the associations between per- and polyfluoroalkyl substances and cholesterol: trend and implications. Ecotoxicol Environ Saf 173:461–468, PMID: , 10.1016/j.ecoenv.2019.02.061. [DOI] [PubMed] [Google Scholar]
- EFSA CONTAM (EFSA Panel on Contaminants in the Food Chain); Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, et al. 2018. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J 16(12):e05194, PMID: , 10.2903/j.efsa.2018.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302(18):1993–2000, PMID: , 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnier M, Zeller M, Masson D, Cottin Y. 2021. Triglycerides and risk of atherosclerotic cardiovascular disease: an update. Arch Cardiovasc Dis 114(2):132–139, PMID: , 10.1016/j.acvd.2020.11.006. [DOI] [PubMed] [Google Scholar]
- Fitz-Simon N, Fletcher T, Luster MI, Steenland K, Calafat AM, Kato K, et al. 2013. Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology 24(4):569–576, PMID: , 10.1097/EDE.0b013e31829443ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, et al. 2013. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int 57–58:2–10, PMID: , 10.1016/j.envint.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Forsthuber M, Kaiser AM, Granitzer S, Hassl I, Hengstschläger M, Stangl H, et al. 2020. Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environ Int 137:105324, PMID: , 10.1016/j.envint.2019.105324. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, et al. 2010. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med 164(9):860–869, PMID: , 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Risk Factors Collaborators. 2016. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1659–1724, PMID: , 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geovanini GR, Libby P. 2018. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond) 132(12):1243–1252, PMID: , 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- Golforoush P, Yellon DM, Davidson SM. 2020. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res Cardiol 115(6):73, PMID: , 10.1007/s00395-020-00829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Timmermann A, et al. 2017. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J Immunotoxicol 14(1):188–195, PMID: , 10.1080/1547691X.2017.1360968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldin M, Rosell M, de Faire U, Hellénius ML. 2007. The metabolic syndrome: prevalence and association to leisure-time and work-related physical activity in 60-year-old men and women. Nutr Metab Cardiovasc Dis 17(5):349–357, PMID: , 10.1016/j.numecd.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Harris H, Håkansson N, Olofsson C, Stackelberg O, Julin B, Åkesson A, et al. 2013. The Swedish mammography cohort and the cohort of Swedish men: study design and characteristics of two population-based longitudinal cohorts. OA Epidemiology 1(2):16, 10.13172/2053-079X-1-2-943. [DOI] [Google Scholar]
- Huang M, Jiao J, Zhuang P, Chen X, Wang J, Zhang Y. 2018. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environ Int 119:37–46, PMID: , 10.1016/j.envint.2018.05.051. [DOI] [PubMed] [Google Scholar]
- Hutcheson R, Innes K, Conway B. 2020. Perfluoroalkyl substances and likelihood of stroke in persons with and without diabetes. Diab Vasc Dis Res 17(1):1479164119892223, PMID: , 10.1177/1479164119892223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2018. Perfluororoctanoic acid. IARC Monogr Eval CArinog Risks Hum 110:37–110. [Google Scholar]
- Inoue K, Goto A, Sugiyama T, Ramlau-Hansen CH, Liew Z. 2020. The confounder-mediator dilemma: should we control for obesity to estimate the effect of perfluoroalkyl substances on health outcomes? Toxics 8(4):125, PMID: , 10.3390/toxics8040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB, Ducatman A. 2019. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Sci Total Environ 653:74–81, PMID: , 10.1016/j.scitotenv.2018.10.362. [DOI] [PubMed] [Google Scholar]
- Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. 2020. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol 8(8):703–718. ], PMID: , 10.1016/S2213-8587(20)30129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: , 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Leung Yinko SS, Stark KD, Thanassoulis G, Pilote L. 2014. Fish consumption and acute coronary syndrome: a meta-analysis. Am J Med 127(9):848–857.e2, PMID: , 10.1016/j.amjmed.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Lin CY, Chen PC, Lo SC, Torng PL, Sung FC, Su TC. 2016. The association of carotid intima-media thickness with serum level of perfluorinated chemicals and endothelium-platelet microparticles in adolescents and young adults. Env Int 94:292–299, PMID: , 10.1016/j.envint.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Lind PM, Salihovic S, Stubleski J, Kärrman A, Lind L. 2018. Changes in plasma levels of perfluoroalkyl substances (PFASs) are related to increase in carotid intima-media thickness over 10 years—a longitudinal study. Environ Health 17(1):59, PMID: , 10.1186/s12940-018-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Salihovic S, van Bavel B, Lind L. 2017. Circulating levels of perfluoroalkyl substances (PFASs) and carotid artery atherosclerosis. Environ Res 152:157–164, PMID: , 10.1016/j.envres.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Lindbohm JV, Sipilä PN, Mars N, Knüppel A, Pentti J, Nyberg ST, et al. 2021. Association between change in cardiovascular risk scores and future cardiovascular disease: analyses of data from the Whitehall II longitudinal, prospective cohort study. Lancet Digit Health 3(7):e434–e444, PMID: , 10.1016/S2589-7500(21)00079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yu K, Shi X, Wang J, Lam PKS, Wu RSS, et al. 2007. Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat Toxicol 82(2):135–143, PMID: , 10.1016/j.aquatox.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. 2011. External review and validation of the Swedish National Inpatient Register. BMC Public Health 11:450, PMID: , 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrantonio M, Bai E, Uccelli R, Cordiano V, Screpanti A, Crosignani P. 2018. Drinking water contamination from perfluoroalkyl substances (PFAS): an ecological mortality study in the Veneto Region, Italy. Eur J Public Health 28(1):180–185, PMID: , 10.1093/eurpub/ckx066. [DOI] [PubMed] [Google Scholar]
- Mattsson K, Rignell-Hydbom A, Holmberg S, Thelin A, Jönsson BAG, Lindh CH, et al. 2015. Levels of perfluoroalkyl substances and risk of coronary heart disease: findings from a population-based longitudinal study. Environ Res 142:148–154, PMID: , 10.1016/j.envres.2015.06.033. [DOI] [PubMed] [Google Scholar]
- Monroe AK, Dobs AS. 2013. The effect of androgens on lipids. Curr Opin Endocrinol Diabetes Obes 20(2):132–139, PMID: , 10.1097/MED.0b013e32835edb71. [DOI] [PubMed] [Google Scholar]
- Musunuru K, Kathiresan S. 2016. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ Res 118(4):579–585, PMID: , 10.1161/CIRCRESAHA.115.306398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norén E, Lindh C, Glynn A, Rylander L, Pineda D, Nielsen C. 2021. Temporal trends, 2000–2017, of perfluoroalkyl acid (PFAA) concentrations in serum of Swedish adolescents. Environ Int 155:106716, PMID: , 10.1016/j.envint.2021.106716. [DOI] [PubMed] [Google Scholar]
- O’Toole TE, Conklin DJ, Bhatnagar A. 2008. Environmental risk factors for heart disease. Rev Environ Health 23(3):167–202, PMID: , 10.1515/reveh.2008.23.3.167. [DOI] [PubMed] [Google Scholar]
- Osorio-Yáñez C, Sanchez-Guerra M, Cardenas A, Lin PD, Hauser R, Gold DR, et al. 2021. Per- and polyfluoroalkyl substances and calcifications of the coronary and aortic arteries in adults with prediabetes: results from the Diabetes Prevention Program outcomes study. Environ Int 151:106446, PMID: , 10.1016/j.envint.2021.106446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prospective Studies Collaboration, Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, et al. 2007. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370(9602):1829–1839, PMID: , 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- Rebholz SL, Jones T, Herrick RL, Xie C, Calafat AM, Pinney SM, et al. 2016. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol Rep 3:46–54, PMID: , 10.1016/j.toxrep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren A, Smyth A, Rangarajan S, Ramasundarahettige C, Bangdiwala SI, AlHabib KF, et al. 2019. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: the Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob Health 7(6):e748–e760, PMID: , 10.1016/S2214-109X(19)30045-2. [DOI] [PubMed] [Google Scholar]
- Salihovic S, Lind L, Larsson A, Lind PM. 2020. Plasma perfluoroalkyls are associated with decreased levels of proteomic inflammatory markers in a cross-sectional study of an elderly population. Environ Int 145:106099, PMID: , 10.1016/j.envint.2020.106099. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367(22):2089–2099, PMID: , 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A. 2012. Perfluorooctanoic acid and cardiovascular disease in US adults. Arch Intern Med 172(18):1397–1403, PMID: , 10.1001/archinternmed.2012.3393. [DOI] [PubMed] [Google Scholar]
- Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Jungner I, et al. 2003. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment. Lancet 361(9359):777–780, PMID: , 10.1016/S0140-6736(03)12663-3. [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Stein CR, Bartell SM, Darrow L, Lopez-Espinosa MJ, et al. 2020. Review: evolution of evidence on PFOA and health following the assessments of the C8 Science Panel. Environ Int 145:106125, PMID: , 10.1016/j.envint.2020.106125. [DOI] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. 2009. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol 170(10):1268–1278, PMID: , 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: , 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tektonidis TG, Åkesson A, Gigante B, Wolk A, Larsson SC. 2015. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: a population-based cohort study. Atherosclerosis 243(1):93–98, PMID: , 10.1016/j.atherosclerosis.2015.08.039. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2016. Health Effects Support Document for Perfluorooctanoic Acid (PFOA). EPA 822-R-16-003. https://www.epa.gov/sites/production/files/2016-05/documents/pfoa_hesd_final_508.pdf [accessed 10 March 2022].
- Vestergren R, Berger U, Glynn A, Cousins IT. 2012. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ Int 49:120–127, PMID: , 10.1016/j.envint.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Wändell PE, Wajngot A, de Faire U, Hellénius ML. 2007. Increased prevalence of diabetes among immigrants from non-European countries in 60-year-old men and women in Sweden. Diabetes Metab 33(1):30–36, PMID: , 10.1016/j.diabet.2006.07.001. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2016. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en [accessed 10 March 2022].
- Winquist A, Steenland K. 2014. Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ Health Perspect 122(12):1299–1305, PMID: , 10.1289/ehp.1307943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf CJ, Takacs ML, Schmid JE, Lau C, Abbott BD. 2008. Activation of mouse and human peroxisome proliferator−activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol Sci 106(1):162–171, PMID: , 10.1093/toxsci/kfn166. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. 2020. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 395(10226):795–808, PMID: , 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zitzow JD, Ehresman DJ, Chang SC, Butenhoff JL, Forster J, et al. 2015. Na+/taurocholate cotransporting polypeptide and apical sodium-dependent bile acid transporter are involved in the disposition of perfluoroalkyl sulfonates in humans and rats. Toxicol Sci 146(2):363–373, PMID: , 10.1093/toxsci/kfv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.