Abstract
Background:
Atypical sensory responsivity and sensory interests are now included in the DSM 5 diagnostic criteria for autism spectrum disorder (ASD) under the broad domain of restricted and repetitive behavior (RRB). However, relatively little is known about the emergence of sensory-related features and their relation to conventionally defined RRB in the first years of life.
Methods:
Prospective, longitudinal parent-report data using the Sensory Experiences Questionnaire (SEQ) were collected for 331 high-risk toddlers (74 of whom met diagnostic criteria for ASD at age 2) and 135 low-risk controls. Longitudinal profiles for SEQ scores were compared between groups across ages 12 to 24 months. Associations between SEQ measures and measures of RRB subtypes (based on Repetitive Behavior Scale, Revised) were also examined.
Results:
Longitudinal profiles for all SEQ scores significantly differed between groups. SEQ scores were elevated for the ASD group from age 12 months, with differences becoming more pronounced across the 12–24 month interval. At both 12 and 24 months, most measures derived from the SEQ were significantly associated with all subtypes of RRB.
Conclusions:
These findings suggest that differences in sensory responsivity may be evident in high-risk infants later diagnosed with ASD in early toddlerhood, and that the magnitude of these differences increase over the second year of life. The high degree of association between SEQ scores and RRB supports the conceptual alignment of these features but also raises questions as to explanatory mechanisms.
Keywords: sensory, longitudinal, development, repetitive behavior
Atypical behavioral responses to external stimuli are a common feature of autism spectrum disorder (ASD) (Baranek, David, Poe, Stone, & Watson, 2006; Ben-Sasson et al., 2008). Associated behaviors may be observed in response to a wide range of stimuli that are not specific to any one modality, and may take the form of approach or avoidance suggesting over- or under-sensitivity to a given stimulus. Reports of such behaviors date as far back as the first case descriptions of ASD by both Leo Kanner and Hans Asperger, each of whom independently noted extreme responses to external stimulation (Asperger, 1944/1991; Kanner, 1943). Despite a long-standing association with autism, however, it was not until recently that atypical sensory responsiveness was included as part of ASD diagnostic criteria.
The latest edition of the DSM addresses sensory responsiveness under the heading of restricted and repetitive behaviors (RRB), specifying or requiring “hyper- or hypo-responsivity to sensory input or unusual interests in sensory aspects of the environment” (APA, 2013). As reflected by DSM 5 criteria, atypical sensory responsiveness may be grossly disaggregated into subtypes or patterns. While this commonly includes responses indicating hyperresponsivity, hyporesponsivity, and sensory seeking behavior, there is no single conventional framework for describing sensory response patterns (Ausderau et al., 2014; Dunn, 2001; Lane, Dennis, & Geraghty, 2011). Irrespective of nomenclature or conceptual framework, patterns or clusters of sensory responses are not mutually exclusive, and individuals may exhibit behaviors indicative of one or more response patterns that may vary on the basis of modality, stimulus, or context (Ausderau et al., 2014; Ben-Sasson et al., 2008; Boyd et al., 2010; Lane, Molloy, & Bishop, 2014; Watson et al., 2011). For example, a child may exhibit distress associated with the din of a busy classroom but show no reaction to a loud vacuum; another may be insensitive to a range of visual and auditory stimuli but show marked reactivity to light touch. In the present study, we follow the empirically supported conceptual framework reflected by the DSM 5 (e.g., Ben-Sasson et al. 2009). This includes hyper-responsivity, which is defined by over-reactivity to or avoidance of sensory stimuli; hypo-responsivity, which is defined by absent, delayed, or attenuated responses to sensory stimuli; and sensory seeking, which is defined as volitional behavior associated with access to specific sensory stimulation (Watson et al. 2011).
While theoretical links between sensory responsiveness and RRB have not been well-developed, there is empirical support for their alignment overall (Boyd et al., 2010; Chen, Rodgers, & McConachie, 2009; Rogers, Hepburn, & Wehner, 2003; Wigham, Rodgers, South, McConachie, & Freeston, 2015). How these associations hold up at a more fine grained level is less clear, and may vary across children as well as across development (Ausderau et al., 2014; Boyd et al., 2010; Kirby, Boyd, Williams, Faldowski, & Baranek, 2017). Reports on the co-development of sensory responsiveness and RRB during the first years of life are particularly limited. In a qualitative study based on retrospective analysis of home videos, Freuler and colleagues (2012) found that sensory hypo-responsiveness and stereotypies were evident and relatively stable in six infants later meeting criteria for ASD. More recently, we reported that sensory responsiveness and RRB may share common underlying neural circuitry (Wolff et al., 2017).
There is limited evidence that unusual sensory reactivity may be evident in infancy in children who later receive a diagnosis of ASD (Freuler et al. 2012; Karmel et al., 2010). Overall, however, very little is known about the early development of sensory responsiveness in children with or without ASD. In a landmark study using retrospective video coding, Baranek and colleagues (1999) identified differences in object and motor stereotypies, but not sensory responsiveness per se, in infants later diagnosed with ASD. Multiple research groups have since studied the emergence of ASD by prospectively following infant siblings of children with the disorder, who are themselves at increased risk for diagnosis. To date, only a handful of empirical reports based on this design have focused on sensory responsiveness. Guiraud and colleagues (2011) identified reduced habituation and hyposensitivity to auditory stimuli in high-risk infants relative to controls. Mulligan and White (2012) found a relative lack of sensory differences between high- and low-risk 12-month-olds using video coding of motor and sensory behaviors. However, both Mulligan and White (2012) and Guiraud et al. (2011) focused on familial risk only and did not differentiate between infants on the basis of diagnostic status. In a study of 2-year-olds, Germani and colleagues (2014) found that high risk children with ASD showed differences in responses to auditory stimuli and sensory hyporesponsivity. However, groups did not significantly differ on multiple sensory response scores, though this may have been related to statistical power given a relatively small sample of children with ASD (n = 14).
We previously reported that RRBs are elevated at 12 months of age in high-risk infants who later receive a diagnosis of ASD (Elison et al., 2014; Wolff et al., 2014). Herein, we turn our focus to sensory responsiveness. Our primary goal was to characterize early patterns of sensory responsiveness in high-risk toddlers who did and did not go on to meet diagnostic criteria for ASD. Given that atypical responses to sensory stimuli are now subsumed by the repetitive behavior domain in DSM 5, as well as evidence suggesting that these behaviors may emerge concurrently in toddlers (Freuler et al., 2012; Wolff et al., 2017), we were also interested in examining relations of sensory response and repetitive behavior subtypes. The aim of the present study was thus two-fold: 1) characterize patterns of sensory responsiveness across ages 12 to 24 months in high-risk children with and without ASD, and 2) examine the relations of sensory response measures to subtypes of RRB and select cognitive and adaptive outcomes.
Methods
Participants
Participants were part of the ongoing Autism Center of Excellence Network Infant Brain Imaging Study. Children were recruited, screened, and assessed at one of four clinical sites: University of North Carolina, Children’s Hospital of Philadelphia, University of Washington, and Washington University in St. Louis. Exclusion criteria were: (1) evidence of a genetic condition; (2) significant medical condition affecting development; (3) significant hearing or vision impairment; (4) birth weight < 2000g or gestational age < 36 weeks; (5) significant perinatal adversity or exposure to neurotoxins, (6) contraindication for MRI, (7) predominant home language other than English, (8) adopted or half siblings, (9) first degree relative with bipolar disorder, psychosis, or schizophrenia, and (10) twins.
The primary focus of this study was on prospective, longitudinal data from infants at high risk for ASD. High-risk siblings were defined as such if they had an older sibling with an existing diagnosis of ASD confirmed by the Autism Diagnostic Interview-Revised (Lord, Rutter, & Couteur, 1994) and Social Communication Questionnaire (SCQ; Rutter, Bailey, Lord, & Berument, 2003). To provide consistency with our previous work (e.g. Wolff et al., 2014), we also included data for infants with typically developing older siblings. These low-risk controls had siblings who screened negative for ASD on the SCQ and had no first degree relatives with a developmental disability. Study procedures were approved by institutional review and written, informed consent obtained for all participants.
Participants were grouped according to risk and diagnostic status at age 2 years based on clinical best-estimate diagnosis made by experienced clinicians using DSM-IV-TR criteria for autistic disorder or PDD-NOS. Diagnoses were informed by multiple assessments including the Autism Diagnostic Observation Scale (ADOS), ADI-R, and Mullen Scales of Early Learning (MSEL), which were administered to all participants by study personnel at age 2 years. Diagnoses were independently verified by a senior clinician naïve to classification. This approach yielded the following groups: high-risk with ASD (HR-ASD; n = 74), high-risk not meeting diagnostic criteria for ASD (HR-Neg; n = 257), and low-risk controls without ASD (LR-control; n = 135).
Measures
Sensory Experiences Questionnaire, version 2.1 (SEQ; Baranek, David, Poe, Stone, & Watson, 2006).
The SEQ is a parent-report measure of behavioral responses to a range of common sensory stimuli. It is comprised of 33 quantitative items using a 5-point rating scale to measure frequency ranging from “Almost Never” to “Almost Always”. The SEQ provides a total score as well as scores for specific sensory modalities, contexts, and response patterns. The present study focused on the SEQ total score; response pattern scores for hyperresponsiveness, hyporesponsiveness, and sensory seeking; and subscale scores for auditory, tactile, and visual domains. We did not examine modality scores for gustatory/olfactory or vestibular/proprioceptive as we reasoned a priori that these would be less informative given the age range of the present sample (e.g., scores based on items related to food refusal, mouthing of objects, and other age-appropriate behaviors). The SEQ has been shown to have good internal consistency (.80) and excellent test-retest reliability for children with ASD or developmental delay ages 5–72 months (Little et al., 2011) as well as evidence of convergent and concurrent validity (Baranek et al., 2006; Boyd et al. 2010; Watson et al. 2011).
Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000).
The ADOS is a semi-structured diagnostic assessment designed to elicit symptoms associated with ASD. Participants were assessed at age 2 using either module 1 or 2 (90% received module 1). Standardized symptom severity scores were calculated based on the ADOS (Gotham et al., 2009).
Mullen Scales of Early Learning (Mullen, 1995).
The MSEL is a standardized measure of cognitive and motor development appropriate for children from birth to age 68 months. It provides an Early Learning Composite (ELC) score, which indexes overall cognitive and motor development.
Repetitive Behavior Scale – Revised (RBS-R; Bodfish, Symons, Parker, & Lewis, 2000).
The RBS-R is a caregiver rated measure of RRB of 43 items across 6 subscales. Each item represents a discrete and observable behavioral topography. The RBS-R is comprised of six subscales that include 1) stereotyped motor, 2) self-injurious behavior, 3) compulsive behavior, 4) ritualistic behavior, 5) sameness behavior, and 6) restricted behaviors. The present study focused on subscale inventory scores with the exception of the compulsive behavior subscale as we deemed its content as not developmentally relevant to toddlers based on previous work (Wolff et al. 2014). Following previous factor analytic studies supporting a five-factor model, the ritualistic and sameness behavior subscales were combined (Lam & Aman, 2007; Mirenda et al., 2010).
Vineland Adaptive Behavior Scales-II (Vineland II; Sparrow, Balla, & Cicchetti, 2005).
The Vineland-II is a standardized, semi-structured assessment of adaptive behavior. The Adaptive Behavior Composite score (ABC) socialization, communication, and motor scores were derived from the Vineland II. Daily living scores were not examined for this study given limited utility and variability for these scores in toddlers.
Statistical Analysis
Longitudinal profiles for dependent variables derived from the SEQ were analyzed using a linear mixed model framework. As visual inspection of SEQ data indicated a right-skewed distribution, a natural log transformation was applied to the data. Model fit statistics confirmed better fit for log transformed SEQ variables relative to raw scores. Fixed effects included Group, Time, and the Group by Time interaction. Sex was included as a covariate in all models given the disproportionate number of males in the ASD group. Mullen ELC score at age 24 months was included to control for potential effects related to general cognitive ability. ELC scores from age 12 months were carried forward for three participants with incomplete 24 month MSEL data. Maternal education was assessed as a potential covariate but ultimately excluded given that it did not improve model fit based on fit statistics, and that maternal education had a negligible impact on other model effects. Omnibus tests were two-tailed with α = 0.05. Bonferroni corrected post-hoc comparisons were conducted following significant omnibus tests. Spearman rank-order correlations were used to examine the strength of association between SEQ scores and subscales from the RBS-R at the 12 and 24 months for HR-ASD and HR-Neg as RBS-R data. Spearman correlations were also used to examine associations of SEQ scores with ADOS severity scores for the HR-ASD. Spearman correlations were used for the RBS-R and ADOS as these data are ordinal. Pearson correlations were used to examine associations between SEQ scores and Mullen ELC and Vineland II scores at age 24 months for HR-ASD and HR-Neg.
Results
Descriptive data for study participants are presented in Table 1. Demographic data on race, ethnicity, or maternal education was missing or not reported for approximately 14% of the total sample. For the proportion of the sample for whom demographic data was available, groups did not differ by age at assessment or by race or ethnicity. Groups differed in level of maternal education, with LR mothers having disproportionately higher education.
Table 1.
Descriptive and demographic data
| HR-ASD | HR-Neg | LR control | p | |
|---|---|---|---|---|
| Total N1 | 74 | 257 | 135 | |
| Age 12 months | ||||
| n | 62 | 222 | 110 | |
| Age (months) | 12.7 (0.8) | 12.6 (0.6) | 12.6 (0.7) | .16 |
| Age 24 months | ||||
| n | 63 | 200 | 115 | |
| Age (months) | 24.7 (1.0) | 24.7 (1.0) | 24.7 (0.9) | .97 |
| Sex (% male) | 79.7a | 56.8b | 58.5b | .001 |
| Race (%) | .91 | |||
| More than one | 8.2 | 8.3 | 6.1 | |
| Asian/Black/Other1 | 3.3 | 3.7 | 5.2 | |
| White | 88.5 | 88.0 | 88.7 | |
| Hispanic (%) | 6.5 | 7.4 | 4.3 | .60 |
| Maternal ed (%) | <.001 | |||
| Some college or less | 43.5 | 30.1 | 13.0 | |
| College degree | 30.6 | 44.9 | 42.6 | |
| Graduate degree | 25.8 | 25.0 | 44.3 | |
| Mullen ELC | 81.1a (17.5) | 102.5b (16.1) | 111.0c (15.5) | <.001 |
| Vineland II | ||||
| ABC | 87.7a (14.5) | 101.3b (9.1) | 104.9b (7.7) | <.001 |
| Communication | 88.3a (11.6) | 102.0b (9.4) | 105.6b (7.7) | <.001 |
| Motor | 93.0a (14.8) | 100.3b (10.0) | 102.4b (8.5) | <.001 |
| Socialization | 89.1a (9.4) | 101.1b (10.0) | 103.1b (9.6) | <.001 |
| ADOS severity | 5.9a (1.9) | 1.6b (1.0) | 1.5b (1.0) | <.001 |
‘Other’ includes Alaskan native, American Indian, and Pacific Islander; Mean and SD provided for Mullen ELC, Vineland II, ADOS severity, and Age; p-values given based on Fisher’s exact test for sex, race, ethnicity, and maternal education, and ANOVA for Mullen ELC, ADOS severity score, and age. ADOS = Autism Diagnostic Observation Schedule; ELC = Early Learning Composite; ABC = Adaptive Behavior Composite
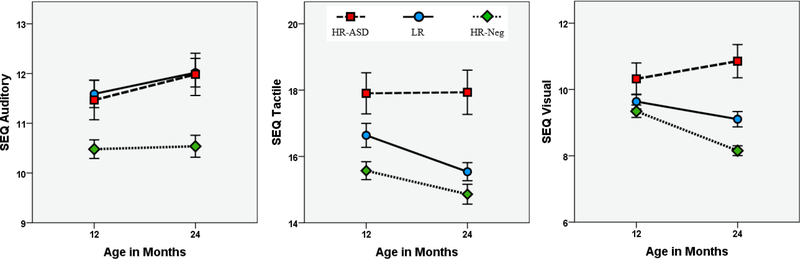
Results from the longitudinal analysis of SEQ data are presented in Table 2. The main effect of group was statistically significant for all measures at F(2,465) > 11.00, p < .001. At age 12 months, Bonferroni-corrected post-hoc results indicated that HR-ASD children scored higher in sensory hyper-responsivity relative to both HR-Neg and LR controls and higher in tactile modality scores than HR-Neg. There were several scales for which HR-Neg scored significantly lower than LR controls, including total SEQ, sensory seeking, and auditory and tactile scores. At age 24 months, posthoc tests indicated that the HR-ASD group scored uniformly higher than HR-Neg. Low risk controls scored significantly higher than HR-Neg on all scales except hyporesponsivity.
Table 2.
Longitudinal results for SEQ scores from ages 12 to 24 months
| SEQ score | HR-ASD |
HR-Neg |
LR |
Group |
Group X Time |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | p | F | p | |
| Total | 23.54 | < .001 | 5.06 | .007 | ||||||
| 12 months | 57.92 | 12.53 | 52.99 | 11.02 | 57.69 | 11.27 | ||||
| 24 months | 60.27 | 14.78 | 49.88 | 10.45 | 56.04 | 10.26 | ||||
| Hyper | 13.89 | < .001 | 0.49 | .61 | ||||||
| 12 months | 22.27 | 6.18 | 19.57 | 4.56 | 20.16 | 4.19 | ||||
| 24 months | 24.02 | 5.92 | 20.73 | 4.74 | 21.90 | 4.01 | ||||
| Hypo | 12.96 | < .001 | 8.56 | < .001 | ||||||
| 12 months | 9.45 | 3.40 | 8.35 | 2.54 | 8.63 | 2.33 | ||||
| 24 months | 11.00 | 4.24 | 7.97 | 2.33 | 8.39 | 2.24 | ||||
| Seeking | 27.98 | < .001 | 3.79 | .023 | ||||||
| 12 months | 26.19 | 6.67 | 25.06 | 6.82 | 28.90 | 8.51 | ||||
| 24 months | 25.26 | 8.15 | 21.20 | 5.78 | 25.75 | 7.17 | ||||
| Auditory | 14.87 | < .001 | 0.58 | .56 | ||||||
| 12 months | 11.47 | 3.12 | 10.48 | 2.79 | 11.59 | 2.89 | ||||
| 24 months | 11.98 | 3.35 | 10.54 | 3.12 | 12.02 | 3.08 | ||||
| Tactile | 13.83 | < .001 | 0.63 | .53 | ||||||
| 12 months | 17.90 | 4.88 | 15.57 | 4.01 | 16.64 | 3.80 | ||||
| 24 months | 17.94 | 5.26 | 14.86 | 4.23 | 15.54 | 2.92 | ||||
| Visual | 11.21 | < .001 | 8.74 | < .001 | ||||||
| 12 months | 10.32 | 3.77 | 9.34 | 2.76 | 9.64 | 2.33 | ||||
| 24 months | 10.86 | 3.95 | 8.16 | 2.09 | 9.10 | 2.48 | ||||
SEQ = Sensory Experiences Questionnaire. Unadjusted descriptive statistics are presented; mean scores were log transformed for statistical testing.
Groups differed in slope from age 12 to 24 months for total SEQ score (F(2,465) = 5.06, p = .007), hyporesponsivity (F(2,465) = 8.56, p < .001), sensory seeking (F(2,465) = 3.79, p = .023), and visual modality score (F(2,465) = 8.74, p < .001). For total SEQ, hyporesponsivity, and visual modality, the interaction of Group by Time was characterized by increasing scores for HR-ASD and decreasing scores for HR-Neg and LR controls. Sensory seeking decreased for all groups, though this change was most pronounced for HR-Neg. To ensure results were not impacted by the 3 participants for whom 12 months MSEL data were used as a control variable, models were rerun excluding those participants. Exclusion of those participants had a negligible impact on the results. Unadjusted SEQ scores over the 12 to 24 month age interval are presented in Figures 1 and 2.
Figure 1. Total SEQ and sensory response pattern scores from ages 12 to 24 months.
Unadjusted scores from Sensory Experiences Questionnaire 2.1 (SEQ). Error bars are ±1 SE.
Figure 2. Sensory modality scores from ages 12 to 24 months.
Unadjusted scores from the Sensory Experiences Questionnaire 2.1 (SEQ). Error bars are ±1 SE.
Association of SEQ scores with RRB
HR-ASD.
Spearman correlation results for SEQ and RBS-R scores are presented in Table 3. At both 12 and 24 months, most subscale scores from the RBS-R were significantly positively correlated with most measures from the SEQ, with Time 2 characterized by stronger and more uniform correlations. As a follow-up, this analysis was rerun adjusting for MSEL ELC scores to control for general developmental level. Results did not appreciably differ from those obtained from the primary model (see Supplementary Information).
Table 3.
Rank-order correlations for SEQ and RBS-R scores among HR-ASD
| SEQ scores |
||||||
|---|---|---|---|---|---|---|
| RBS-R scores | Hyper | Hypo | Seeking | Auditory | Tactile | Visual |
| Age 12 months | ||||||
| Stereotypical | .31* | .38** | .28* | .10 | .36** | .36** |
| Self-injurious | .27* | .03 | .24 | .18 | .25* | .03 |
| Ritualistic/Sameness | .45*** | .41** | .17 | .15 | .42** | .46*** |
| Restricted | .30* | .30* | .25* | .18 | .28* | .37** |
| Age 24 months | ||||||
| Stereotypical | .27* | .33* | .55*** | .40** | .23 | .53*** |
| Self-injurious | .25* | .18 | .43** | .28* | .36** | .19 |
| Ritualistic/Sameness | .48*** | .46*** | .42** | .28* | .46*** | .60*** |
| Restricted | .53*** | .38** | .34** | .23 | .43** | .54*** |
RBS-R = Repetitive Behavior Scale, Revised; SEQ = Sensory Experiences Questionnaire
p < 0.001
p < 0.01
p < .05
HR-Neg.
At age 12 months, all RBS-R subscales were significantly correlated with all SEQ measures, p < .02. Spearman’s rho values ranged from a low of rs =.15 (SEQ auditory and RBS-R self-injury) to a high of rs =.38 (SEQ tactile and RBS-R ritual/sameness). At age 24 months, all RBS-R subscales were significantly correlated with all SEQ measures, p < .003. Spearman’s rho values ranged from a low of rs = .20 (SEQ auditory and RBS-R self-injury) to a high of rs =.42 (SEQ tactile and RBS-R stereotypy).
Association of SEQ scores with ASD symptom severity in HR-ASD
At age 24 months, ADOS calibrated severity scores and (total and social affect) were not significantly associated with any scores from the SEQ (all p > .07). Spearman’s rho values ranged from rs =.13 (with SEQ sensory seeking) to rs =.23 (with SEQ visual). To further verify this finding, we conducted a follow-up analysis using raw ADOS social affect scores. As with calibrated severity scores, no significant relations with SEQ measures was observed (all p > .08).
Association of SEQ scores with cognitive and adaptive behavior
HR-ASD.
At age 24 months, MSEL ELC and Vineland II ABC and motor scores were not significantly associated with any SEQ measures (all p > .08). However, Vineland II socialization score was significantly associated with total SEQ (r = −.51, p < .001), hyperresponsivity (r = −.48, p < .001), hyporesponsivity (r = −.42, p = .002), and sensory seeking (r = −.33, p = .015), as well as auditory (r = −.29, p = .04), tactile (r = −.53, p < .001), and visual (r = −.45, p = .001) modality scores. Vineland II communication scores were also significantly associated with total SEQ (r = −.46, p = .001), hyporesponsivity (r = −.42, p = .002), and sensory seeking (r = −.41, p = .002), as well as auditory (r = −.40, p = .003), and visual (r = −.55, p <.001) modality scores. Vineland II communication scores were not significantly associated with hyperresponsivity (r = −.21, p = .13) or tactile modality score (r = −.25, p = .07).
HR-Neg.
At age 24 months, MSEL ELC were significantly associated with SEQ sensory seeking (r = −.18, p = .012) and visual modality (r = −.22, p = .002) scores. All Vineland II scores were significantly associated with hyporesponsivity and tactile modality scores, ranging from a low of r = −.14 (hyporesponsivity and Vineland II motor) to a high of r = −.24 (hyporesponsivity and Vineland II ABC). Total SEQ and visual modality scores were significantly associated with all Vineland II measures except motor score, ranging from a low of r = −.17 (total SEQ and Vineland II communication) to a high of r = −.25 (visual modality and Vineland II ABC). Of Vineland II scores, hyperresponsivity was significantly negatively associated with ABC, motor, and socialization scores (all r > −.19, p < .007), while sensory seeking was only associated with socialization, r = −.17, p = .02. SEQ auditory scores were not significantly associated with any Vineland II scores (p > .45).
Discussion
We found that parent-reported sensory responsiveness differed as early as age 12 months between high-familial risk toddlers who did and did not later meet diagnostic criteria for ASD. Differences were characterized at both 12 and 24 months of age by higher reported frequency of responses to sensory stimuli among children who developed ASD relative to high risk children who did not. At age 12 months, differences were particularly pronounced on items reflecting general hyperresponsivity and for items involving tactile stimulation. Results from our longitudinal analyses indicate that frequency of atypical sensory responses increased overall for children with ASD over the second year of life, while generally decreasing for children who did not develop the disorder.
The finding that low risk controls were intermediate to high risk children with and without ASD for most SEQ scores was unexpected. The most parsimonious interpretation of this result pertains to the potential effect of rater bias. By definition, parent raters from the HR group have an older child with ASD; the parents of LR controls do not. It is likely that results for the LR control group reflect differences in experience with ASD and knowledge of concepts related to it; this consideration is key given that the validity of the SEQ in typically developing populations has not been established. We would caution readers to interpret data for the low risk control group with this important potential bias in mind. While comparison of HR-Neg to HR-ASD likely controls for bias related to parent knowledge and experience with ASD, it is possible that scores for HR-Neg reflect a ‘normalizing’ effect whereby parents under-rate sensory behaviors for their typically developing children. However, we have not observed such an effect with parent-rated measures of other ASD-related behaviors (e.g, Rescorla et al. 2017; Wolff et al. 2014).
Findings from studies of older children indicate that hyporesponsiveness to sensory stimuli best differentiates those with ASD from typically developing and developmental delayed peers (Ausderau et al., 2016; Ben-Sasson et al., 2009). We had anticipated that a similar pattern might be observed in HR infants, following longstanding theory, in addition to empirical data, suggesting that under-registration of the external environment could be linked to the pathogenesis of ASD (Rogers & Ozonoff, 2005). Although we did see evidence that HR-ASD infants exhibited significantly higher scores for hyporesponsivity, the most robust differences at age 12 months were on scores related to hyperresponsivity. This may indicate a particular target for further study, as it is possible that hyperresponsivity to stimuli (somatosensory in particular) may be an early manifestation of atypical nervous system development (Mikkelsen, Wodka, Mostofsky, & Puts, 2016). Exploring this matter further could yield insights as to developmental mechanisms underlying the emergence of ASD as well as provide new targets for screening and intervention. For example, several theoretical models posit that altered processing of sensory stimuli in infancy might contribute directly to the accumulation of later-emerging social communication deficits in children with ASD (Thye et al. 2018). Alternatively, it is plausible that our results reflect a more complex truth, wherein response patterns within and between children are characterized by high levels of variability and heterogeneity, reflecting the interplay of multiple aspects of development during a period of rapid maturation. Data driven subgrouping of infants on the basis of age related response patterns for sensory responsiveness and RRB might delineate phenotypes that map onto specific aspects of neurobiology and inform ASD pathogenesis (Robertson & Baron-Cohen, 2017; Uljarević et al., 2017).
Adaptive behavior composite scores and Mullen ELC scores were not associated with any measures from the SEQ. These null results are not entirely surprising as studies of older children have been inconsistent with regard to whether sensory symptoms covary as a function of either adaptive behavior or IQ (Baranek et al., 2006; Liss et al., 2006; Rogers et al., 2003; Sanz-Cervera et al., 2015). Together with a lack of relation to symptom severity as measured by the ADOS, it would appear that frequency of atypical sensory responsivity is relatively independent of impairment among toddlers with ASD. It is possible, however, that a link between sensory measures and ASD severity or adaptive function and IQ emerge later in development. We did find that all SEQ scores were significantly negatively associated with social skills as measured by the Vineland II. This is consistent with our previous work showing a similar effect between socialization and RRB, and suggests that behaviors comprising the RRB domain in general may interfere specifically with adaptive social opportunities (Wolff et al., 2014). We also found that most SEQ measures were significantly negatively correlated with communication, but not motor scores, from the Vineland II. This likely reflects the high degree of correspondence between social and communication skills in young children. It is worth noting that, in general, SEQ scores were more strongly associated with other parent-report measures than with direct measures, such as the ADOS and MSEL.
We observed strong associations between most subtypes of RRB as measured by the RBS-R and measures from the SEQ, a matter which we will discuss in detail for the remainder of this section. The co-occurrence of atypical sensory responsiveness and RRB has long been appreciated (Ornitz, 1974), and clinical consensus has led to the domain of RRB subsuming sensory responsivity as part of diagnostic criteria. However, there has been relatively little progress made toward elucidating the nature of this relationship ( Uljarević et al., 2017; Wolff et al., 2017; for an illuminating exchange see Lewis, Baumeister, & Mailman, 1987, & Lovaas, Newsom, & Hickman, 1987). While there is some indication that sensory hyperresponsivity may be most closely tied to RRB (Boyd et al., 2010) or sensory seeking and interests (Ausderau et al., 2014), the literature overall is relatively lean and inconsistent (e.g., Foss-Feig, Heacock, & Cascio, 2012; Wigham et al., 2015). The present results overall suggest a strong correspondence between RRB and sensory responsivity insofar as nearly every measure of the former was significantly correlated with nearly every measure of the latter. This pattern of results, while less robust, was nonetheless also observed among the HR-Neg group, suggesting interplay between sensory responsivity and RRB across a typical-atypical continuum. It is also notable that the present findings, when considered alongside previous work, suggest that RRB and sensory symptoms are evident (albeit subtle) and co-occuring in infants who develop ASD before the average age of diagnosis.
An ongoing challenge to the field is that we have yet to develop a compelling nomological network describing the behavioral and biological correspondence between varieties of sensory responsivity and RRB (Cascio et al., 2016; Rogers & Ozonoff, 2005; Wolff et al., 2017). Repetitive behaviors and atypical sensory responses appear to hang together -- but why? The present work adds to existing literature but arguably also complicates larger conceptual questions given that we observed significant associations between nearly all RBS-R and SEQ measures. This lack of specificity precludes the winnowing of specific behavioral relations that might inform neurobiological mechanisms and help identify specific targets for intervention (Boyd et al., 2010). Finding that “everything correlates with everything” may represent what Paul Meehl referred to as the “crud factor” : the correlations may be real, but we lack a unifying theory to explain them (Meehl, 1990).
Constructing a theoretical framework that accounts for the varied, ubiquitous relations observed between these many forms of behavior is a daunting prospect. Progress in this regard is critical, however, if we are to reach more precise conclusions and vertically advance our knowledge of the RRB domain. One clear way forward long acknowledged in the literature is to improve our capacity to measure constructs related to RRB. This could be accomplished through developmentally informed, dimensional approaches to assessment (Uljarević et al., 2017; Wolff, Boyd, & Elison, 2016) as well as direct measures that yield results more proximal to underlying mechanisms (Barney, Tervo, Wilcox, & Symons, 2017; Cascio et al., 2012; Duerden et al., 2015). It is also worth considering whether we are measuring the right thing, particularly in regard to sensory-related constructs given the inferences involved in linking observable outward behavior to nervous system function. A little over a decade ago Rogers and Ozonoff (2005) concluded that: “...in reality construct validity has not been established in any of the sensory domains.” While progress has certainly been made since then, the recommendation that we leverage an interdisciplinary approach grounded in neurobiology remains essential (e.g., Cascio et al., 2016; Wodka et al., 2016).
Limitations
One clear limitation of the present work is our inability to draw strong inferences about LR control participants. Resolving this issue will require validation studies in typically developing samples or the application of direct measures that overcome potential issues related to rater bias and construct validity. While we considered presenting data only on HR siblings, we believed it was important to include LR data if only to ensure transparency about potential confounds associated with the measurement of key ASD-related constructs. There is also some potential for bias in parent-report measures of behavior with the HR group. For example, parent perspective or awareness related to their child’s overall development may impact the likelihood of endorsing behaviors measured by the RBS-R or SEQ. Likewise, we cannot discount the limitations of indirect measurement, particularly when it relies on proxy inferences related to the internal and unconfirmed states of others, for example in the rating of some items related to hypo-responsiveness (Cascio, Woynaroski, Baranek, & Wallace, 2016; Kirby et al., 2017). Items associated with the construct of hyporesponsivity pose a particular measurement challenge in that they entail rating the frequency or degree to which a behavioral response is absent. Another limitation relates to age of diagnosis. While diagnoses made at age 2 years are relatively stable over time (Lord et al., 2006; Shen et al., 2013), there is variability associated with ASD onset and we expect that diagnostic status will change for a small proportion our sample as they age (Davidovitch, Levit-Binnun, Golan, & Manning-Courtney, 2015; Ozonoff et al., 2015). Whether the pattern of results observed here will generalize across a wider age range is an important issue, and we are currently following-up participants from the present study at school-age to account for later childhood outcomes.
Conclusion
Atypical patterns of sensory responsivity are evident in high-risk infants who later meet diagnostic criteria for ASD as early as age 12 months. Most sensory response patterns, such as hyper- and hypo-responsivity, increased among HR-ASD infants over early toddlerhood, while in general decreasing for HR infants who did not meet diagnostic criteria. This pattern of results extended across sensory modalities, including auditory, tactile, and visual. While we included results for low risk infants, we believe comparisons between LR and HR infants may be subject to rater bias related to knowledge of, and experience with, ASD. An important goal for future research will be to develop measures that overcome this limitation, including direct measures of reactivity. While much remains uncertain about the correspondence between sensory responsivity and restricted and repetitive behaviors, it has become increasingly clear that these features emerge early and in tandem among infants who develop ASD.
Supplementary Material
Key Points.
Atypical sensory features were recently added to DSM 5 diagnostic criteria for ASD within the domain of repetitive behavior.
We found differences at age 12 months in parent-reported sensory responsivity between children at high risk for autism who did versus did not receive a diagnosis at age 2 years.
Differences at age 12 months were strongest for sensory hyper-responsivity and responsivity to tactile stimuli.
Scores for high-risk children who developed ASD increased from age 12 to 24 months while decreasing for those who did not develop the disorder.
Measures of sensory-related behaviors were significantly associated with a wide range of restricted and repetitive behaviors.
Acknowledgements
This study was supported by funds from the National Institutes of Health under awards R01-HD055741 and P30-HD003110 to JP and K01-MH101653 to JW. Additional funding support has been provided from Autism Speaks and the Simons Foundation (SFARI Grant 140209). The funders had no role in study design, data collection, analysis, data interpretation, or the writing of the report. The authors would like to thank the IBIS families for their continued participation in this research.
Abbreviations:
- HR
high risk
- RBS-R
Repetitive Behavior Scale, Revised
- RRB
restricted and repetitive behaviors
- SEQ
Sensory Experiences Questionnaire
Footnotes
The Infant Brain Imaging Study (IBIS) Network is an NIH funded Autism Center of Excellence project and consists of a consortium of 8 universities in the U.S. and Canada. Clinical Sites: University of North Carolina: J. Piven (IBIS Network PI), H.C. Hazlett, C. Chappell; University of Washington: S. Dager, A. Estes, D. Shaw; Washington University: K. Botteron, R. McKinstry, J. Constantino, J. Pruett; Children’s Hospital of Philadelphia: R. Schultz, J. Pandey, S. Paterson; University of Alberta: L. Zwaigenbaum; University of Minnesota: J. Ellison, J. Wolff; Data Coordinating Center: Montreal Neurological Institute: A.C. Evans, D.L. Collins, G.B. Pike, V. Fonov, P. Kostopoulos, S. Das, L. MacIntyre; Image Processing Core: University of Utah: G. Gerig; University of North Carolina: M. Styner; Statistical Analysis Core: University of North Carolina: H. Gu
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Asperger H (1991). “Autistic psychopathy” in childhood. In Frith U (Ed.), Autism and Asperger Syndrome (pp. 37–92). Cambridge: Cambridge University Press. [Google Scholar]
- Ausderau KK, Furlong M, Sideris J, Bulluck J, Little LM, Watson LR, … Baranek GT (2014). Sensory subtypes in children with autism spectrum disorder: latent profile transition analysis using a national survey of sensory features. Journal of Child Psychology and Psychiatry, 55(8), 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausderau KK, Sideris J, Little LM, Furlong M, Bulluck JC, & Baranek GT (2016). Sensory subtypes and associated outcomes in children with autism spectrum disorders. Autism Research, 9(12), 1316–1327. [DOI] [PubMed] [Google Scholar]
- Ausderau K, Sideris J, Furlong M, Little LM, Bulluck J, & Baranek GT (2014). National survey of sensory features in children with ASD: factor structure of the sensory experience questionnaire (3.0). Journal of Autism and Developmental Disorders, 44(4), 915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT (1999). Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders, 29(3), 213–24. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, & Watson LR (2006). Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, 47(6), 591–601. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Watson LR, Boyd BA, Poe MD, David FJ, & McGuire L (2013). Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Development and Psychopathology, 25(2), 307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barney CC, Tervo R, Wilcox GL, & Symons FJ (2017). A case-controlled investigation of tactile reactivity in young children with and without global developmental delay. American Journal on Intellectual and Developmental Disabilities, 122(5), 409–421. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, & Carter AS (2008). Sensory clusters of toddlers with autism spectrum disorders: differences in affective symptoms. Journal of Child Psychology and Psychiatry, 49(8), 817–25. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, & Gal E (2009). A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, & Lewis MH (2000). Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders, 30(3), 237–43. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, & Miller H (2010). Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research, 3(2), 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Moana-Filho EJ, Guest S, Nebel MB, Weisner J, Baranek GT, & Essick GK (2012). Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Research, 5(4), 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Woynaroski T, Baranek GT, & Wallace MT (2016). Toward an interdisciplinary approach to understanding sensory function in autism spectrum disorder. Autism Research, 9(9), 920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, Rodgers J, & McConachie H (2009). Restricted and repetitive behaviours, sensory processing and cognitive style in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(4), 635–42. [DOI] [PubMed] [Google Scholar]
- Davidovitch M, Levit-Binnun N, Golan D, & Manning-Courtney P (2015). Late diagnosis of autism spectrum disorder after initial negative assessment by a multidisciplinary team. Journal of Developmental & Behavioral Pediatrics, 36(4), 227–234. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Taylor MJ, Lee M, McGrath PA, Davis KD, & Roberts SW (2015). Decreased sensitivity to thermal stimuli in adolescents with autism spectrum disorder: Relation to symptomatology and cognitive ability. Journal of Pain, 16(5), 463–471. [DOI] [PubMed] [Google Scholar]
- Dunn W (2001). The sensations of everyday life: empirical, theoretical, and pragmatic considerations. American Journal of Occupational Therapy, 55(6), 608–20. [DOI] [PubMed] [Google Scholar]
- Elison JT, Wolff JJ, Reznick JS, Botteron KN, Estes AM, Gu H, … Piven J (2014). Repetitive behavior in 12-month-olds later classified with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 53(11), 1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Heacock JL, & Cascio CJ (2012). Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Research in Autism Spectrum Disorders, 6(1), 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freuler A, Baranek GT, Watson LR, Boyd BA, & Bulluck JC (2012). Precursors and trajectories of sensory features: qualitative analysis of infant home videos. American Journal of Occupational Therapy, 66(5), e81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germani T, Zwaigenbaum L, Bryson S, Brian J, Smith I, Roberts W, … Vaillancourt T (2014). Brief report: assessment of early sensory processing in infants at high-risk of autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(12), 3264–70. [DOI] [PubMed] [Google Scholar]
- Guiraud JA, Kushnerenko E, Tomalski P, Davies K, Ribeiro H, & Johnson MH (2011). Differential habituation to repeated sounds in infants at high risk for autism. Neuroreport, 22(16), 845–9. [DOI] [PubMed] [Google Scholar]
- Kanner L (1943). Autistic disturbances of affective contact. Nervous Child, 2, 217–250. [PubMed] [Google Scholar]
- Karmel BZ, Gardner JM, Meade LS, Cohen IL, London E, Flory MJ, … Harin A (2010). Early medical and behavioral characteristics of NICU infants later classified with ASD. Pediatrics, 126(3), 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AV, Boyd BA, Williams KL, Faldowski RA, & Baranek GT (2017). Sensory and repetitive behaviors among children with autism spectrum disorder at home. Autism, 21(2), 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, & Aman MG (2007). The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(5), 855–866. [DOI] [PubMed] [Google Scholar]
- Lane AE, Dennis SJ, & Geraghty ME (2011). Brief report: Further evidence of sensory subtypes in autism. Journal of Autism and Developmental Disorders, 41(6), 826–831. [DOI] [PubMed] [Google Scholar]
- Lane AE, Molloy CA, & Bishop SL (2014). Classification of children with autism spectrum disorder by sensory subtype: A case for sensory-based phenotypes. Autism Research, 7(3), 322–333. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Baumeister AA, & Mailman RB (1987). A neurobiological alternative to the perceptual reinforcement hypothesis of stereotyped behavior: a commentary on “Self-stimulatory behavior and perceptual reinforcement”. Journal of Applied Behavior Analysis, 20(3), 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, & Kinsbourne M (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10(2), 155–172. [DOI] [PubMed] [Google Scholar]
- Little LM, Freuler AC, Houser MB, Guckian L, Carbine K, David FJ, & Baranek GT (2011). Psychometric validation of the Sensory Experiences Questionnaire. American Journal of Occupational Therapy, 65(2), 207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, & Pickles A (2006). Autism from 2 to 9 years of age. Archives of General Psychiatry, 63(6), 694–701. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Lovaas I, Newsom C, & Hickman C (1987). Self-stimulatory behavior and perceptual reinforcement. Journal of Applied Behavior Analysis, 20(1), 45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE (1990). Why summaries of research on psychological theories are often uninterpretable. Psychological Reports, 66(1), 195–244. [Google Scholar]
- Mikkelsen M, Wodka EL, Mostofsky SH, & Puts NAJ (2018). Autism spectrum disorder in the scope of tactile processing. Developmental Cognitive Neuroscience, 29, 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenda P, Smith IM, Vaillancourt T, Georgiades S, Duku E, Szatmari P, … Zwaigenbaum L (2010). Validating the Repetitive Behavior Scale-revised in young children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 40(12), 1521–30. [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen Scales of Early Learning. Circle Pines, MN: AGS Publishing. [Google Scholar]
- Mulligan S, & White BP (2012). Sensory and motor behaviors of infant siblings of children with and without autism. American Journal of Occupational Therapy, 66(5), 556–66. [DOI] [PubMed] [Google Scholar]
- Ornitz EM (1974). The modulation of sensory input and motor output in autistic children. Journal of Autism and Childhood Schizophrenia, 4(3), 197–215. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, … Iosif A-M (2015). Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. Journal of Child Psychology and Psychiatry, 56(9), 988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla LA, Winder-Patel BM, Paterson SJ, Pandey J, Wolff JJ, Schultz RT, & Piven J (2017). Autism spectrum disorder screening with the CBCL/1½−5: Findings for young children at high risk for autism spectrum disorder. Autism. Advance online publication. doi: 10.1177/1362361317718482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, & Baron-Cohen S (2017). Sensory perception in autism. Nature Reviews Neuroscience, 18(11), 671–684. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, & Wehner E (2003). Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders, 33(6), 631–42. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, & Ozonoff S (2005). Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry, 46(12), 1255–1268. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C, & Berument S (2003). Social Communication Questionnaire. Los Angeles: Western Psychological Services. [Google Scholar]
- Sanz-Cervera P, Pastor-Cerezuela G, Fernández-Andrés M-I, & Tárraga-Mínguez R (2015). Sensory processing in children with autism spectrum disorder: Relationship with non-verbal IQ, autism severity and attention deficit/hyperactivity disorder symptomatology. Research in Developmental Disabilities, 45–46, 188–201. [DOI] [PubMed] [Google Scholar]
- Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, … Amaral DG (2013). Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain, 136(9), 2825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Balla D, & Cicchetti D (2005). Vineland adaptive behavior scales: second edition. Shoreview, MN: AGS Publishing. [Google Scholar]
- Thye MD, Bednarz HM, Herringshaw AJ, Sartin E, & Kana RK (2018). The impact of atypical sensory processing on social impairments in autism spectrum disorder. Developmental Cognitive Neuroscience, 29, 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarević M, Baranek G, Vivanti G, Hedley D, Hudry K, & Lane A (2017). Heterogeneity of sensory features in autism spectrum disorder: Challenges and perspectives for future research. Autism Research, 10(5), 703–710. [DOI] [PubMed] [Google Scholar]
- Watson LR, Patten E, Baranek GT, Poe M, Boyd BA, Freuler A, & Lorenzi J (2011). Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. Journal of Speech, Language, and Hearing Research, 54(6), 1562–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigham S, Rodgers J, South M, McConachie H, & Freeston M (2015). The interplay between sensory processing abnormalities, intolerance of uncertainty, anxiety and restricted and repetitive behaviours in autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(4), 943–52. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Puts NAJ, Mahone EM, Edden RAE, Tommerdahl M, & Mostofsky SH (2016). The role of attention in somatosensory processing: A multi-trait, multi-method analysis. Journal of Autism and Developmental Disorders, 46(10), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Botteron KN, Dager SR, Elison JT, Estes AM, Gu H, … Piven J (2014). Longitudinal patterns of repetitive behavior in toddlers with autism. Journal of Child Psychology and Psychiatry, 55(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Boyd BA, & Elison JT (2016). A quantitative measure of restricted and repetitive behaviors for early childhood. Journal of Neurodevelopmental Disorders, 8(1), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Swanson MR, Elison JT, Gerig G, Pruett JR, Styner MA, … Gu H (2017). Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Molecular Autism, 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.