Introduction
Von Willebrand Disease (VWD), the most common inherited bleeding disorder, is defined by decreased activity of von Willebrand Factor (VWF) activity in the blood. This can be secondary to a quantitative or qualitative defect. VWF is a multimeric glycoprotein synthesized in endothelial cells and megakaryocytes and then stored within Weibel Palade bodies and alpha granules respectively. It is cleared by macrophages in the liver and spleen. Lack of VWF function, either through quantitative or qualitative defects, leads to a bleeding phenotype due to its critical role in primary and secondary hemostasis. Within primary hemostasis, VWF binds extracellular matrix proteins such as collagen, as well as platelets through the glycoprotein Ib receptor. In secondary hemostasis, VWF acts as a chaperone to factor VIII (FVIII) to prevent premature clearance and degradation.
History
VWD was originally identified in 1926 by Dr. Erik Adolf von Willebrand who described a familial pedigree of 58 individuals from two interrelated families spanning four generations. The proband was a five-year-old girl who presented with recurrent severe mucosal bleeding and on testing demonstrated normal clotting time and clot retraction, but marked prolongation of her bleeding time (1,2). Within the pedigree, both males and females were affected, and bleeding was severe, with multiple female family members, including the proband, dying secondary to mucosal bleeding. Diagnostic testing available in the early to mid-1900s was non-specific, cumbersome, not reproducible, and time consuming. Measurement of FVIII coagulation activity (FVIII:C) was first available in the mid-1900s and led to diagnostic confusion as deficiency was seen in classical hemophilia as well as the condition later defined as VWD (3,4). This led to the label of “pseudo-hemophilia” though others called the disorder “vascular hemophilia” as it was hypothesized that the prolonged bleeding time could be secondary to capillary defects (5,6). Correction of the bleeding time defect with normal, as well as hemophilic plasma, confirmed that the defect lay outside of FVIII in the late 1950s (7) but it was not until the mid-1970s that VWF antigen testing was possible through immunoprecipitation(8). A plethora of diagnostic assays have been developed and development continues as currently available assays each have their own unique limitations.
Prevalence/Inheritance
The prevalence of VWD based on abnormal laboratory parameters alone is approximately 1 in 100, while the clinical prevalence, taking into consideration only those patients with bleeding symptoms, is likely closer to 1 in 1000 (9,10,11). VWD is inherited in an autosomal manner so males and females are affected equally. However, women are more likely to have symptomatic bleeding given the additional hemostatic challenges of menstruation and childbirth (12). Most commonly, VWD inheritance is autosomal dominant, but autosomal recessive inheritance is seen in Type 3 and Type 2N (13). Type 1 VWD is the most common subtype, seen in 70–80% of patients with VWD. Type 2 is seen in approximately 20% of patients and Type 3 is rare, occurring in <5% of cases (14).
Symptoms
Mucocutaneous bleeding is common in patients with VWD although symptoms are variable based on the subtype, level of VWF activity, age and sex. The most commonly reported symptoms of VWD are outlined in Table 1(15,16) and should be specifically elucidated in the patient history. Particular attention should be paid to details of menses as women may not recognize bleeding as abnormal, especially in the setting of a family history of similar bleeding.
Table 1.
Most commonly reported symptoms in patients with VWD
| Symptom | Proportion of Patients |
|---|---|
| Heavy Menstrual Bleeding | 75–100% |
| Excessive Bruising | 62–81% |
| Oropharyngeal Bleeding | 64% |
| Epistaxis | 56% |
| Post Dental Procedure Bleeding | 26% |
| Post-Surgical Bleeding | 24% |
| Excessive Bleeding from Wounds | 24–58% |
In addition to mucocutaneous bleeding, patients with VWD may suffer from gastrointestinal bleeding or joint bleeding. Recurrent gastrointestinal bleeding is a distinctive, difficult to treat, symptom of VWD which is often associated with angiodysplasia. It is more common in the elderly and in patients with type 2A and type 3 VWD. Joint bleeding is seen in 5–10% of patients with type 1 and type 2, and approximately 50% of patients with type 3 (17). Risk for joint bleeding is largely dependent on FVIII activity level and can lead to significant arthropathy (18).
Standardized bleeding assessment tools (BATs) have been validated to help identify significant bleeding phenotypes (19, 20) though their utility is limited in younger patients who may not have encountered hemostatic challenges or in patients treated prophylactically. They also rely on cumulative scoring so that if a patient has previously had a lot of bleeding but is no longer having symptoms, their score will remain elevated. Lastly, they are easily saturable in that one episode of very severe bleeding within a subtype of bleeding will result in the same score as someone who has frequent episodes. Use of BATs is recommended when there is a low suspicion for VWD to help determine which patients require laboratory testing, but if a high suspicion for VWD exists, current guidelines suggest proceeding directly to laboratory testing (21).
Classification
VWD can be due to a quantitative or qualitative deficiency of VWF and is subdivided into types based on the underlying defect. Type 1 and 3 are quantitative defects with Type 1 defined as a partial deficiency and Type 3 resulting from an absolute deficiency of VWF. Type 1 includes Type 1C, a subtype defined by increased clearance of VWF. Type 2 comprises qualitative defects and is further divided into multiple subtypes. Type 2A results from a defect in multimerization while types 2B,2N, and 2M are secondary to abnormal ligand binding: increased binding to GPIb, defective binding to FVIII, and defective binding with normal multimers respectively.
Diagnosis
Testing for VWD should only be performed in patients with a personal or family history of bleeding. Once a personal or family history of bleeding has been established, laboratory testing is recommended. No single laboratory assay can definitively diagnose VWD. Diagnosis is further complicated by low penetrance, variable expressivity, lab variability, and the large number of modifiers affecting an individual’s VWF level. The use of laboratories that utilize off-site processing are subject to many potential pre-analytical variables that affect VWF assays and can lead to falsely low levels and misdiagnosis of VWD (22). VWF levels are influenced by genetic, environmental, hormonal and pathologic processes. As an acute phase reactant, inflammatory processes cause elevation (23,24). Similar increases are seen in pregnancy, aging, exercise, oral contraceptives, and exposure to cigarette smoke or air pollution (25–30). As such, interpretation of VWF assays must be approached with caution and multiple evaluations are typically needed.
Screening assays such as a complete blood count, activated partial thromboplastin time (aPTT) and prothrombin time, are likely to be normal in the majority of patients with VWD and thus are of little utility. The aPTT may be prolonged in more severe cases and patients with type 2B may exhibit thrombocytopenia. Initial testing for VWD should include quantitative measurement with VWF antigen (VWF:Ag), a platelet binding assay such as the VWF-ristocetin cofactor assay (VWF: RCo), and FVIII:C. A functional assay is necessary as antigen testing alone will miss some type 2 patients, and in order to classify types appropriately. Within these first-tier tests, clinical variability is significant at 30% for VWF:RCo and 20% for VWF:Ag (31, 32) although some observed differences in the VWF:Ag may be due to changes with age or stress.
The VWF:RCo assay is the most commonly used platelet binding assay and exploits the ristocetin induced interaction between VWF and GPIbM, which results in platelet agglutination. Although widely available, the VWF:RCo assay is limited by poor reproducibility, high coefficient of variation, low sensitivity at very low VWF levels, as well as falsely low VWF activity seen with certain benign sequence variations (32,33). The p.D1472H variant has been seen in up to 67% of subjects with African ancestry and 20% of subjects with Caucasian backgrounds (33). Given these limitations, other platelet binding assays have been developed. The VWF:GPIbM assay allows VWF to bind platelets spontaneously in vitro without the use of ristocetin by introduction two gain of function mutations into GPIbα (34). Although results from VWF:RCo and VWF:GPIbM demonstrate a degree of correlation, GPIbM is more precise with a lower limit of detection of 2 IU/dL and a coefficient of variation of 5.6% (35) but is less readily available in many places. Neither assay is physiologic as shear stress is not used to induce the VWF platelet interaction. The latest ASH/ISTH/NHF/WFH guidelines published earlier this year reviewed VWF:RCo, VWF:GPIbM, and VWF:GPIbR to reach a low certainty recommendation to use GPIbM. Although all three assays were judged to have comparable test accuracy in terms of sensitivity and specificity, the lower coefficient of variation, increased reproducibility, and lack of false positives due to benign sequence variations contributed to this recommendation (21).
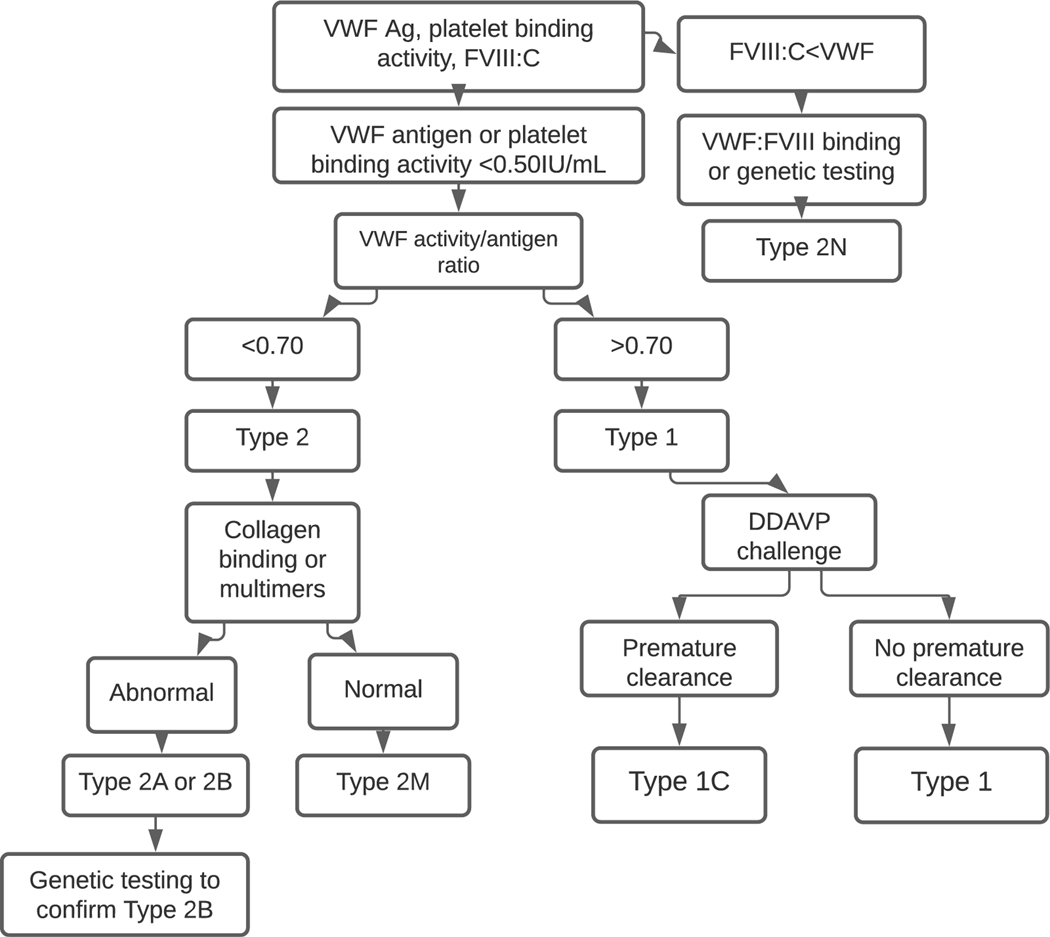
The platelet binding activity/VWF antigen ratio is used to differentiate between type 1 and type 2 VWD, with type 2 characterized by an activity/antigen ratio <0.7 (21). In addition to differentiating between type 1 and type 2 VWD, this ratio is essential in diagnosis as some patients with type 2 VWD will have normal platelet binding activity and VWF antigen but an abnormal ratio. If the platelet binding activity/VWF antigen ratio is normal, consistent with a diagnosis of Type 1 VWD, a desmopressin trial or the VWF propeptide (pp)/VWF antigen ratio can be used to evaluate for type 1C. Recent guidelines (21) recommend a desmopressin trial as the VWFpp/VWF:Ag ratio can be normal in the setting of rapid clearance (36). If platelet binding activity/VWF:Ag ratio is <0.7, additional testing is necessary to identify the type 2 subtype. Multimer evaluation is performed using qualitative visual assessment and quantitative densitometric assessment by gel electrophoresis. Findings on multimer evaluation for VWD types are seen in Figure 1. In addition to binding of FVIII and platelets, VWF also binds exposed collagen following vascular injury. VWF binds to multiple different collagen types and is dependent on high molecular weight (HMW) multimers. As binding to collagen types I and III is particularly reliant on HMW multimers, testing for this binding can serve as a surrogate for multimer analysis (37). As such, this testing serves dual purposes of investigating for collagen defects, as well as evaluating multimer status. Of note, collagen binding defects have also been described in patients with type 1 VWD as well and are associated with a more severe phenotype (38). Collagen binding or multimer analysis should be performed in patients suspected of having types 2A, 2B and 2M. Abnormal VWF:CB or multimers is consistent with types 2A and 2B, whereas normal VWF:CB and multimers indicates a type 2M diagnosis.
Fig. 1.
An algorithmic approach to diagnosis.
Patients with type 2B VWD may have thrombocytopenia secondary to increased binding of VWF to platelets leading to premature clearance. Historically, ristocetin induced platelet aggregation (RIPA) has been used to differentiate between types 2A and 2B. Using an aggregometer, various concentrations of ristocetin are added, and platelet aggregation measured. Aggregation at concentrations <0.7 mg/mL or less are consistent with increased VWF binding to GPIb. Pathogenic variants which cause type 2B are found in exon 28, so targeted genetic testing can also be used. Patients with type 2N VWD can have normal or low normal levels of VWF in addition to low FVIII levels. The FVIII/VWF:Ag ratio can be used to help identify type 2N, as this group will have a FVIII/VWF:Ag ratio <0.6. As patients with hemophilia A will have a similar ratio, and FVIII is decreased, further testing should be performed to differentiate type 2N from hemophilia A. An ELISA assay is available to evaluate VWF FVIII binding using an exogenous source of FVIII, as well as identify asymptomatic carriers (39). Alternately, targeted genetic testing can be performed (21). Given significant laboratory variability in type 2 diagnosis (40) and the importance of accurate subtyping, genetic testing has emerged as an adjunct for patients suspected of having type 2 VWD.
Genetic testing is being increasingly utilized in the diagnosis of VWD with increasing availability and decreasing costs. As with all testing, genetic testing is not without flaws. One drawback is the considerable variability seen in the VWF gene even within healthy cohorts (41). For type 1, the most common subtype, there is weak correlation between sequence variants and disease (42). A number of variants outside the VWF locus have also been implicated as modifiers of VWF levels including ABO blood group, CLEC4M, SCARA5, STXBP5, and UFM1 (43).
Controversies
One topic that has been the source of controversy is the laboratory threshold to meet diagnostic criteria for VWD. As previously discussed, approximately 1% of the population demonstrate low VWF levels on testing, but the prevalence of clinically relevant disease is likely much lower. As such, there is a good proportion of the population with low VWF levels without accompanying bleeding symptomatology. Conversely, mild bleeding symptoms are also commonly reported in healthy populations (44). Patients with VWF levels < 30 IU/dL are more likely to have VWF coding mutations and autosomal dominant family inheritance (45) compared to those with levels of 30–50 IU/dL. Given the differences in these two groups, some have advocated that patients with VWF levels between 30–50 IU/dL be labelled as having low VWF, a modest risk factor for bleeding, rather than diagnosed with a disease. The most recent guidelines strongly recommend a diagnosis of VWD in patients with a bleeding history and levels <50 IU/dL. Of note, these guidelines referenced data demonstrating similar bleeding across the range of VWF levels (46,47), as well as data demonstrating significant bleeding in the majority of patients with VWF levels between 30–50IU/dL (48). Clinician judgement remains an important consideration, and patients with modest decrease in VWF level may require evaluation for additional bleeding disorders such as platelet function defects that could contribute to the bleeding phenotype.
Another area of dispute relates to the increase in VWF levels that is seen with normal aging (49). Given this, patients, especially those with modestly low levels at diagnosis, may have levels in the normal range at older ages. Observational studies have shown that this normalization occurs in ~43% of patients (21). It is not known whether this normalization of levels translates to decreased risk for bleeding. One study examining bleeding symptoms in type 1 VWD patients found that patients older than 65 years of age experienced as much bleeding as those 18–65 years of age (50). As such, the normal increase in levels seen with age may not correlate with normalization of bleeding. The recent VWD guidelines addressed the question of whether patients whose levels normalize with age should still carry a diagnosis of VWD and recommended reconsidering the diagnosis, rather than removing it (21). Interestingly, type 2 VWD patients may see an increase in antigen levels but still have dysfunctional protein and report increased bleeding with age (50). Conversely, one study that evaluated VWF levels in young children prior to tonsillectomy found decreased levels to be relatively common and not predictive of bleeding risk (51), suggesting bleeding history may be as important as the absolute VWF level.
Treatment
Treatment of VWD centers around replacing the deficient or defective VWF. For patients with a history of frequent severe bleeding, long term prophylaxis with VWF replacement is recommended (52). A randomized controlled trial comparing prophylaxis to on demand treatment found extended time to first bleed, reduced bleeding episode risk, and decreased epistaxis with prophylaxis (53). Observational trials have similarly demonstrated reduced bleeding episodes, hospitalizations and heavy menstrual bleeding with prophylaxis (54–62). Current products available for VWF replacement include plasma derived and recombinant products, as well as products with and without FVIII. Commercially available products and dosing recommendations can be found in Table 2. When using combined VWF/FVIII products, it is important to note that once infused, the half-life of FVIII is longer than that of VWF. In addition to the exogenously infused FVIII, the infused VWF will stabilize endogenous FVIII which can lead to accumulation. Elevated FVIII levels are a risk factor for the development of venous thromboembolism (VTE) (63) and cases of VTE have been described in VWD patients with elevated FVIII secondary to treatment (64). Replacement products with VWF alone may be preferable in patients with other risk factors for thrombosis (65). For patients with VWD and associated low FVIII levels, replacement products with VWF alone require a FVIII priming dose or sufficient time for endogenous levels to rise. Compiled recommendations for surgical prophylaxis can be found in Table 3.
Table 2.
Currently approved VWF concentrates in the United States
| Product | Type | VWF RCo:VWF Ag ratio | VWF RCo:FVIII ratio | Mean half life (hours) |
|---|---|---|---|---|
| Alphanate | Plasma derived | 0.47 ± 0.1 | 1.33 ± 0.26 | 17.9 ± 9.6 |
| Humate | Plasma derived | 0.59 ± 0.1 | 2.45 ± 0.3 | ~12.2 |
| Vonvendi | Recombinant | 1.16 ± 0.25 | No FVIII | 21.9 ± 8.36 |
| Wilate | Plasma derived | NA | 1.0 | 19.6 ± 6.9 |
Table 3.
| Pre-operative VWF:RCo | Pre-operative FVIII:C | Post-operative VWF:RCo | Post-operative FVIII:C | |
|---|---|---|---|---|
| Major Surgery | ||||
| Nichols et al | >100 | >100 | ≥50 for 7–10 days | ≥50 for 7–10 days |
| Laffan et al | >100 | >100 | >50 | |
| Castaman et al | 80–100 | 80–100 | 80–100 for 36h, >50 for 5–10 days | |
| Windyga et al | >50 | >80–100 | >30 for days 1–14 | >50 for days 1–7, >30 for days 8–14 |
| Flood et al | ≥50 for at least 3 days | ≥50 for at least 3 days | ||
| Minor Surgery | ||||
| Nichols et al | >30, preferably >50 | >30, preferably >50 | >30, preferably >50 for 3–5 days | >30, preferably >50, for 3–5 days |
| Laffan et al | >50 | >50 | ||
| Castman et al | >30 | >30 2–4 d | ||
| Windyga et al | >50 | >80–100 | >30 for 3–5 days | >50 for 3–5 days |
| Flood et al | ≥50 + TXA or TXA alone in Type 1 VWD patient with levels >30, mild bleeding phenotype and minor mucosal procedure |
In many patients with type 1 and type 2 VWD, desmopressin, a synthetic analog of vasopressin, can be used to increase VWF levels without VWF replacement and is the most widely used drug in VWD (66). Desmopressin stimulates release of endogenous VWF and FVIII from endothelial cells, leading to a transient increase in levels. It is available in intravenous (IV), subcutaneous (SC), and intranasal (IN) preparations. Recommended IV and SC dosing is 0.3 μg per kilogram of body weight (up to 20 mcg), and IN dosing is 150 μg (one spray) in patients <50 kg or 300 μg (2 sprays) in patients >50 kg. Subcutaneous and intravenous formulations increase VWF and FVIII levels 2–4 times baseline within 30–60 minutes of administration (67) and can be repeated every 12 to 24 hours. Tachyphylaxis (reduced response to successive doses) occurs after 2–3 days. The intranasal formulation has variable absorption and results in a more modest increase in levels. Not all patients with VWD will respond to desmopressin. Given its mechanism of action, it requires a pool of endogenous VWF and therefore is not effective or recommended in type 3 VWD. Desmopressin is generally contraindicated in patients with type 2B VWD, as increasing defective VWF can lead to increased binding of VWF and platelets, worsening thrombocytopenia. While many patients with type 2A and 2M VWD may have improvement of minor symptoms with desmopressin, VWF levels typically do not have a sufficient rise with desmopressin to allow its use for surgery or major bleeds. Patients with type 1C VWD are characterized by premature clearance of desmopressin so identification of these patients is imperative as they are likely to demonstrate an initial response followed by a drop to baseline within 2–4 hours.
A desmopressin trial is recommended to diagnose type 1C and prior to use in patients with type 1 and levels <3 0IU/dL (21,52). The majority of type 1 patients are responsive to desmopressin (68,69). In adults, patients with levels >30 IU/dL typically respond to desmopressin (52) so may not require a trial prior to use. There is no consensus for how a desmopressin trial is conducted but FVIII and VWF levels are commonly assessed at baseline, 1 hour post infusion, and 4 hours post infusion. Response has also been defined variably with a recent proposal to define it as an increase of at least 2 times baseline VWF activity level and sustained increase of FVIII and VWF above 50 IU/dL for at least 4 hours (70). As this level is inadequate for some procedures, desmopressin responsive patients may at times require VWF concentrate. Adverse effects of desmopressin are typically mild (tachycardia, flushing, headache) but occur frequently. Due to its antidiuretic effect, there is a risk of hyponatremia and fluid overload. As this risk is greatest in the youngest children, its use is not recommended in children less than 2 years of age (52). For all others, fluid restriction and electrolyte monitoring when repeated doses are given is recommended. Desmopressin is not recommended for use in patients with active cardiovascular disease, seizure disorders, women with preeclampsia, and patients with type 1C VWD in situations which require sustained response (52).
Supportive treatments such as hormonal treatments and antifibrinolytics are commonly used in patients with VWD depending on the type of bleeding. Tranexamic acid and ε-aminocaproic acid are antifibrinolytics which act by blocking plasminogen binding sites to prevent fibrin degradation. Due to high fibrinolytic activity in mucosal tissue, antifibrinolytics are particularly helpful for mucosal bleeding (71). Antifibrinolytics also play a role in the treatment of heavy menstrual bleeding. Tranexamic acid (TXA) has been shown to decrease menstrual blood loss (MBL) by 50% (72) and in a randomized controlled cross over trial demonstrated greater efficacy than desmopressin in reducing MBL in patients with VWD(73). Due to their low cost and favorable side effect profile, they are often used as an adjunct in other settings. Antifibrinolytics can be given systemically, via intravenous or oral formulations, or topically. They should not be used in patients with urinary tract bleeding as this can lead to clotting and ureteral obstruction(74).
Given the high rate of HMB in women with VWD, hormonal treatments are also commonly used. In women not desiring pregnancy, combined hormonal contraceptives (CHC) and the levonorgestrel intrauterine device (IUD) have shown benefit in decreasing MBL. CHCs lead to non-ovulatory bleeding and decrease bleeding during the placebo week when taken cyclically and greatly reduce days of bleeding when taken continuously (75). The IUD decreases MBL by increasing capillary thrombosis and suppressing endometrium and spiral arteriole growth. A systematic review found that in comparison to TXA and CHC, IUDs decreased MBL by both objective and subjective measures (76). In patients with HMB desiring pregnancy, TXA is recommended (52). Women with HMB should also be assessed and treated for iron deficiency and iron deficiency anemia.
In addition to heavy menstrual bleeding, women with VWD are at risk for bleeding associated with pregnancy and delivery. In the case of pregnancy in a patient with VWD, a multidisciplinary approach should be utilized with input from hematology, pediatric hematology, obstetrics, and anesthesiology. During pregnancy, women undergo physiologic changes to tip the balance of hemostasis toward a procoagulant state to prepare for delivery (77). Many changes occur including an increase in VWF and FVIII which both peak shortly after birth and return to normal by twelve weeks postpartum(78). In patients with Type 1 VWD VWF and FVIII levels increase over the course of pregnancy, particularly during the third trimester to 2–3 times their baseline level at delivery(79). Unfortunately, not all women, even those with mild disease, mount this physiologic response. In Type 2 patients, VWF:Ag and FVIII levels may increase but function remains abnormal. In patients with type 3 VWD, VWF and FVIII remain low throughout the pregnancy. Given the variable increase in FVIII and VWF during pregnancy, VWF testing including functional activity, antigen, and FVIII should be done between 30–34 weeks gestation (80). Prior guidelines suggest treating with desmopressin or factor concentrate for functional VWF levels <50 IU/dL (81). However, this has been debated in recent years given data on Type 1 VWD with postpartum hemorrhage (PPH) despite normalization of endogenous VWF and FVIII levels (82). Furthermore, in another study, PPH was seen in 50% of VWD patients treated with VWF concentrate targeting levels >100 IU/dL, suggesting that women with VWD may need higher levels in the postpartum setting (83).
Women with VWD who desire or require neuraxial anesthesia during delivery should receive VWF concentrate to achieve VWF levels of 50 to 150 IU/dL (52) although higher levels may be required to prevent PPH. TXA has also been shown to decrease the risk of PPH in women with and without bleeding disorders (84, 85). TXA is recommended postpartum for women with Type 1 VWD, as well as possibly Type 2 and Type 3 VWD (52) with recommendations to continue for 7–15 days depending on bleeding risk (86).
Complications
A rare complication of VWD is the development of alloantibodies. It is estimated that 5–10% of patients with Type 3 VWD will develop antibodies following VWF factor concentrate administration (87). Patients with partial or complete gene deletions are at highest risk (88). The development of inhibitors renders treatment with VWF concentrate ineffective. Recombinant FVIII products have shown to be effective for procedural hemostasis, but prophylactic use is limited given the incredibly short half-life in the absence of VWF (89). Bypassing agents such as recombinant factor VIIa are also used (90), and recent reports demonstrate efficacy with emicizumab, a bispecific antibody FVIII mimetic (91).
Another challenging situation is the development of comorbidities which require the use of antiplatelet or anticoagulant therapy. Despite bleeding risk, patients with VWD requiring antiplatelet or anticoagulation therapy should receive this treatment. Patients at severe risk for bleeding may require specific VWF replacement to mitigate this risk (52). Careful clinical monitoring is indicated with regular reassessment of risk.
Summary
Optimal treatment for patients with VWD requires suspicion of the diagnosis since specific testing is required. Understanding the available laboratory tests is also important to determine if a patient has VWD and if so, what type of VWD is present as that will help determine appropriate treatment. Accurate treatment of VWD ameliorates bleeding symptoms and improves quality of life for patients with VWD.
Fig. 2.
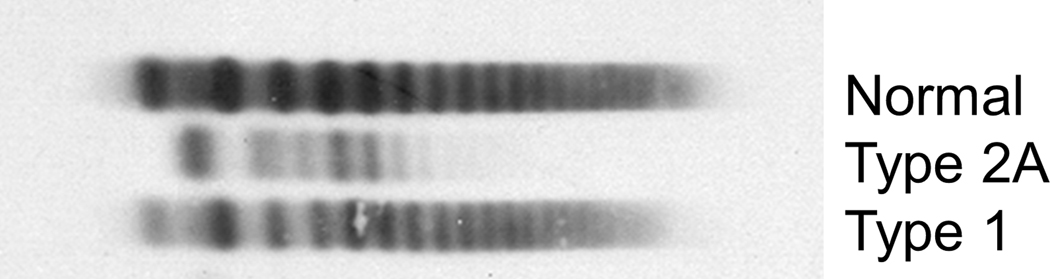
Findings on multimer evaluation for VWD types.
Key points:
VWD is the most common inherited bleeding disorder.
Multiple laboratory tests are needed for an accurate diagnosis of VWD.
Treatment of patients with VWD includes desmopressin, antifibrinolytics, and VWF concentrates.
Synopsis
Von Willebrand disease (VWD) is a common bleeding disorder, affecting males and females equally, that often manifests in mucosal bleeding. VWD can be secondary to a quantitative (Type 1 and Type 3) or qualitative (Type 2) defects in Von Willebrand factor. Initial testing includes VWF antigen, as well as a platelet binding assay to differentiate between qualitative and quantitative defects. Further subtyping requires additional testing and is needed to ensure appropriate treatment. Desmopressin, antifibrinolytics, hormonal treatments for heavy menstrual bleeding, and VWF concentrates are commonly used in the treatment of VWD.
Clinics Care Points
Patients with significant history of mucosal bleeding merit evaluation for von Willebrand disease
Accurate diagnosis requires evaluation of VWF antigen as well as platelet binding activity in order to determine if a patient has a quantitative (type 1 or type 3) or a qualitative (type 2) defect in VWF.
While most patients with type 1 VWD respond well to desmopressin, there are patients with clearance defects and a short VWF half-life that require treatment with VWF concentrate.
Footnotes
Disclosure Statement: the authors have nothing to disclose.
Contributor Information
Angela C. Weyand, Department of Pediatrics, University of Michigan Medical School, Ann Arbor, MI.
Veronica H. Flood, Department of Pediatrics, Medical College of Wisconsin and Versiti Blood Research Institute, Milwaukee, WI.
References
- 1.von Willebrand AE. Hereditar pseudohemofili. Finska Lakarsall- skapetes Handl 1926;67:7–112 [Google Scholar]
- 2.Lassila R, Lindberg O. Erik von Willebrand. Haemophilia 2013; 19(5):643–647 [DOI] [PubMed] [Google Scholar]
- 3.Biggs R, Eveling J, Richards G. The assay of antihaemophilic- globulin activity. Br J Haematol 1955;1(1):20–34 [DOI] [PubMed] [Google Scholar]
- 4.Hardisty RM, MacPherson JC. A one-stage factor VIII (antihae- mophilic globulin) assay and its use on venous and capillary plasma. Thromb Diath Haemorrh 1962;7:215–228 [PubMed] [Google Scholar]
- 5.Alexander B, Goldstein R. Dual hemostatic defect in pseudohe- mophilia. J Clin Invest 1953;32:551 [Google Scholar]
- 6.Erlandson M, Fort E, Lee RE, Schulman I, Smith CH. Vascular hemophilia; a familial hemorrhagic disease in males and females characterized by combined antihemophilic globulin deficiency and vascular abnormality. Pediatrics 1956;18(3):347–361 [PubMed] [Google Scholar]
- 7.Cornu P, Larrieu MJ, Caen J, Bernard J. Transfusion studies in von Willebrand’s disease: effect on bleeding time and factor VIII. Br J Haematol 1963;9:189–202 [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman TS, Hoyer LW, Dickson L, Edgington TS. Determina- tion of the von Willebrand’s disease antigen (factor VIII-related antigen) in plasma by quantitative immunoelectrophoresis. J Lab Clin Med 1975;86(1):152–159 [PubMed] [Google Scholar]
- 9.Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von WillebrandÕs disease. Blood 1987; 69: 454–9. [PubMed] [Google Scholar]
- 10.Werner EJ, Broxson EH, Tucker EL, Giroux DS, Shults J, Abshire TC. Prevalence of von Willebrand disease in children: a multiethnic study. J Pediatr 1993; 123: 893–8. [DOI] [PubMed] [Google Scholar]
- 11.Bowman M, Hopman WM, Rapson D, Lillicrap D, James P. The prevalence of symptomatic von Willebrand disease in primary care practice. J Thromb Haemost. 2010. Jan;8(1):213–6. doi: 10.1111/j.1538-7836.2009.03661.x. Epub 2009 Oct 23. PMID: 19874468. [DOI] [PubMed] [Google Scholar]
- 12.De Wee EM, Knol HM, Mauser-Bunschoten EP, van der Bom JG, Eikenboom JC, Fijnvandraat K, et al. Gynaecological and obstetric bleeding in moderate and severe von Willebrand disease. Thromb Haemost. 2011;106:885–92. [DOI] [PubMed] [Google Scholar]
- 13.Itzhar-Baikian N, Boisseau P, Joly B, Veyradier A. Updated overview on von Willebrand disease: focus on the interest of genotyping. Expert Rev Hematol. 2019. Dec;12(12):1023–1036. doi: 10.1080/17474086.2019.1670638. Epub 2019 Oct 6. PMID: 31536379. [DOI] [PubMed] [Google Scholar]
- 14.Leebeek FW, Eikenboom JC. Von Willebrand’s Disease. N Engl J Med. 2016. Nov 24;375(21):2067–2080. doi: 10.1056/NEJMra1601561. PMID: 27959741. [DOI] [PubMed] [Google Scholar]
- 15.James PD, Notley C, Hegadorn C, Leggo J, Tuttle A, Tinlin S, Brown C, Andrews C, Labelle A, Chirinian Y, O’Brien L, Othman M, Rivard G, Rapson D, Hough C, Lillicrap D. The mutational spectrum of type 1 von Willebrand disease: Results from a Canadian cohort study. Blood. 2007. Jan 1;109(1):145–54. doi: 10.1182/blood-2006-05-021105.. PMID: 17190853. [DOI] [PubMed] [Google Scholar]
- 16.Sanders YV, Fijnvandraat K, Boender J, Mauser-Bunschoten EP, van der Bom JG, de Meris J, Smiers FJ, Granzen B, Brons P, Tamminga RY, Cnossen MH, Leebeek FW; WiN Study Group. Bleeding spectrum in children with moderate or severe von Willebrand disease: Relevance of pediatric-specific bleeding. Am J Hematol. 2015. Dec;90(12):1142–8. doi: 10.1002/ajh.24195. Epub 2015 Nov 17. PMID: 26375306. [DOI] [PubMed] [Google Scholar]
- 17.van Galen KP, Sanders YV, Vojinovic U, et al. ; WiN Study Group. Joint bleeds in von Willebrand disease patients have significant impact on quality of life and joint integrity: a cross-sectional study. Haemophilia 2015;21(03):e185–e192 [DOI] [PubMed] [Google Scholar]
- 18.van Galen KPM, Mauser-Bunschoten EP, Leebeek FWG. Hemophilic arthropa- thy in patients with von Willebrand dis- ease. Blood Rev 2012;26:261–6. [DOI] [PubMed] [Google Scholar]
- 19.Rodeghiero F, Tosetto A, Abshire T, Arnold DM, Coller B, James P, Neunert C, Lillicrap D; ISTH/SSC joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010. Sep;8(9):2063–5. doi: 10.1111/j.1538-7836.2010.03975.x. PMID: 20626619. [DOI] [PubMed] [Google Scholar]
- 20.Bowman ML, James PD. Bleeding Scores for the Diagnosis of von Willebrand Disease. Semin Thromb Hemost. 2017 Jul;43(5):530–539. doi: 10.1055/s-0036-1597289. Epub 2017 Feb 14. PMID: 28196380. [DOI] [PubMed] [Google Scholar]
- 21.James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH Guidelines on the diagnosis of von Willebrand disease. Blood Advances. 2021. Jan 12;5(1):280–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffray J, Staber JM, Malvar J, Sidonio R, Haley KM, Stillings A, Weyand A, Hege K, Jain S, Gupta S, Agnew C, Wheeler A, Pawar A, Sharma M, Chitlur M, OʼBrien SH, Kouides P. Laboratory misdiagnosis of von Willebrand disease in post-menarchal females: A multi-center study. Am J Hematol. 2020. Sep;95(9):1022–1029. doi: 10.1002/ajh.25869. Epub 2020 Jun 20. PMID: 32419248. [DOI] [PubMed] [Google Scholar]
- 23.Pottinger B, Read R, Paleolog E, Higgins P, Pearson J. von Willebrand factor is an acute phase reactant in man. Thromb Res 1989; 53: 387–94. [DOI] [PubMed] [Google Scholar]
- 24.Kremer Hovinga JA, Zeerleder S, Kessler P, Romani de Wit T, van Mourik JA, Hack CE, ten Cate H, Reitsma PH, Wuillemin WA, Lammle B. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost 2007; 5: 2284–90. [DOI] [PubMed] [Google Scholar]
- 25.Castaman G. Changes of von Willebrand factor during pregnancy in women with and without von Willebrand disease. Mediterr J Hematol Infect Dis 2013; 5: e2013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro J, Almeida-Dias A, Ascens~ao A, Magalh~aes J, Oliveira AR, Carlson J, Mota J, Appell HJ, Duarte J. Hemostatic response to acute physical exercise in healthy adolescents. J Sci Med Sport 2007; 10: 164–9. [DOI] [PubMed] [Google Scholar]
- 27.Timm A, Fahrenkrug J, Jørgensen HL, Sennels HP, Goetze JP. Diurnal variation of von Willebrand factor in plasma: the Bis- pebjerg study of diurnal variations. Eur J Haematol 2014; 93: 48–53. [DOI] [PubMed] [Google Scholar]
- 28.Al-Awadhi AM, Alfadhli SM, Mustafa NY, Sharma PN. Effects of cigarette smoking on hematological parameters and von Wille- brand factor functional activity levels in asymptomatic male and female Arab smokers. Med Princ Pract 2008; 17: 149–53. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Z,Chen Y,Zhang Y,Liu H,Liu Q,Zhao J,Hu M, Huang W, Wang G, Zhu T, Zhang J, Zhu P. Changes of plasma vWF level in response to the improvement of air qual- ity: an observation of 114 healthy young adults. Ann Hematol 2013; 92: 543–8. [DOI] [PubMed] [Google Scholar]
- 30.Kouides PA. Aspects of the laboratory identification of von Willebrand disease in women. Semin Thromb Hemost 2006; 32: 480–4. [DOI] [PubMed] [Google Scholar]
- 31.Hayes TE, Brandt JT, Chandler WL, et al. External peer review quality assurance testing in von Willebrand disease: the re- cent experience of the United States College of American Pathologists proficiency testing program. Semin Thromb Hemost. 2006;32:499–504. [DOI] [PubMed] [Google Scholar]
- 32.Kitchen S, Jennings I, Woods TA, et al.Laboratory tests for measurement of von Willebrand factor show poor agreement among different centers: results from the United Kingdom national external quality assessment scheme for blood coagulation. Semin Thromb Hemost, 32 (2006), pp. 492–498 [DOI] [PubMed] [Google Scholar]
- 33.Flood VH, Gill JC, Morateck PA, et al.Common VWF exon 28 polymorphisms in African Americans affecting the VWF activity assay by ristocetin cofactor. Blood, 116 (2010), pp. 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patzke J, Budde U, Huber A, et al. Performance evaluation and multicentre study of a von Willebrand factor activity assay based on GPIb binding in the absence of ristocetin. Blood Coagul Fibrinolysis. 2014;25(8):860–870. [DOI] [PubMed] [Google Scholar]
- 35.Graf L, Moffat KA, Carlino SA, et al. Evaluation of an automated method for measuring von Willebrand factor activity in clinical samples without ristocetin. Int J Lab Hematol. 2014;36(3): 341–351. [DOI] [PubMed] [Google Scholar]
- 36.Haberichter SL, Castaman G, Budde U, et al. Identification of type 1 von Willebrand disease patients with reduced von Willebrand factor survival by assay of the VWF propeptide in the European study: molecular and clinical markers for the diagnosis and management of type 1 VWD (MCMDM-1VWD). Blood. 2008;111(10):4979–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Favaloro EJ. An update on the von Willebrand factor collagen binding assay: 21 years of age and beyond adolescence but not yet a mature adult. Semin Thromb Hemost. 2007;33(8):727–744. [DOI] [PubMed] [Google Scholar]
- 38.Flood VH, Schlauderaff AC, Haberichter SL, et al. ; Zimmerman Program Investigators. Crucial role for the VWF A1 domain in binding to type IV collagen. Blood. 2015;125(14):2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casonato A, Pontara E, Zerbinati P, Zucchetto A, GirolamiThe A. evaluation of factor VIII binding activity of von Willebrand factor by means of an ELISA method: significance and practical implications Am. J. Clin. Pathol, 109 (1998), pp. 347–352 [DOI] [PubMed] [Google Scholar]
- 40.DiGiandomenico S, Christopherson PA, Haberichter SL, Abshire TC, Montgomery RR, Flood VH; Zimmerman Program Investigators. Laboratory variability in the diagnosis of type 2 VWD variants. J Thromb Haemost. 2021. Jan;19(1):131–138. doi: 10.1111/jth.15129. Epub 2020 Nov 10. PMID: 33049112; PMCID: PMC7790985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellissimo DB, Christopherson PA, Flood VH, et al. VWF muta- tions and new sequence variations identified in healthy controls are more frequent in the African-American population. Blood. 2012;119:2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flood VH, Christopherson PA, Gill JC, Friedman KD, Haberichter SL, Bellissimo DB, Udani RA, Dasgupta M, Hoffmann RG, Ragni MV, Shapiro AD, Lusher JM, Lentz SR, Abshire TC, Leissinger C, Hoots WK, Manco-Johnson MJ, Gruppo RA, Boggio LN, Montgomery KT, Goodeve AC, James PD, Lillicrap D, Peake IR, Montgomery RR. Clinical and laboratory variability in a cohort of patients diagnosed with type 1 VWD in the United States. Blood. 2016. May 19;127(20):2481–8. doi: 10.1182/blood-2015-10-673681. Epub 2016 Feb 9. PMID: 26862110; PMCID: PMC4874228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma R, Flood VH. Advances in the diagnosis and treatment of Von Willebrand disease. Blood. 2017. Nov 30;130(22):2386–2391. doi: 10.1182/blood-2017-05-782029. PMID: 29187375; PMCID: PMC5709787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadler JE. Low von Willebrand factor: sometimes a risk factor and sometimes a disease. Hematology Am Soc Hematol Educ Program. 2009:106–12. doi: 10.1182/asheducation-2009.1.106. PMID: 20008188. [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell JS. Low VWF: insights into pathogenesis, diagnosis, and clinical management. Blood Adv. 2020. Jul 14;4(13):3191–3199. doi: 10.1182/bloodadvances.2020002038. PMID: 32663299; PMCID: PMC7362371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavin M, Aguila S, Schneppenheim S, et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017; 130(21):2344–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flood VH, Christopherson PA, Gill JC, et al. Clinical and laboratory variability in a cohort of patients diagnosed with type 1 VWD in the United States. Blood. 2016;127(20):2481–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bucciarelli P, Siboni SM, Stufano F, et al. Predictors of von Willebrand disease diagnosis in individuals with borderline von Willebrand factor plasma levels. J Thromb Haemost. 2015;13(2):228–236. [DOI] [PubMed] [Google Scholar]
- 49.Rydz N, Grabell J, Lillicrap D, James PD. Changes in von Willebrand factor level and von Willebrand activity with age in type 1 von Willebrand disease. Haemo- philia 2015;21:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders YV, Giezenaar MA, Laros-van Gorkom BAP, et al. Von Willebrand dis- ease and aging: an evolving phenotype. J Thromb Haemost 2014;12:1066–75. [DOI] [PubMed] [Google Scholar]
- 51.Gill JC, Conley SF, Johnson VP, Christopherson PA, Haberichter SL, Diaz CD, Strong TC, Zhang J, Simpson P, Abshire TC, Montgomery RR, Flood VH. Low VWF levels in children and lack of association with bleeding in children undergoing tonsillectomy. Blood Adv. 2020. Jan 14;4(1):100–105. doi: 10.1182/bloodadvances.2019000992. PMID: 31905240; PMCID: PMC6960478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Connell NT, Flood VH, Brignardello-Peterson R, et al. ASH ISTH NHF WFH guidelines on the management of von Willebrand disease. Blood Advances. 2021. Jan 12;5(1):301–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peyvandi F, Castaman G, Gresele P, De Cristofaro R, Schinco P, Bertomoro A, Morfini M, Gamba G, Barillari G, Jiménez-Yuste V, Königs C, Iorio A, Federici AB. A phase III study comparing secondary long-term prophylaxis versus on-demand treatment with vWF/FVIII concentrates in severe inherited von Willebrand disease. Blood Transfus. 2019. Sep;17(5):391–398. doi: 10.2450/2019.0183-18. Epub 2019 Feb 4. PMID: 30747707; PMCID: PMC6774924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berntorp E, Petrini P. Long-term prophylaxis in von Willebrand disease. Blood Coagul Fibrinolysis. 2005;16(suppl 1):S23–S26. [DOI] [PubMed] [Google Scholar]
- 55.Berntorp E, Windyga J; European Wilate Study Group. Treatment and prevention of acute bleedings in von Willebrand disease–efficacy and safety of Wilate, a new generation von Willebrand factor/factor VIII concentrate. Haemophilia. 2009;15(1):122–130. [DOI] [PubMed] [Google Scholar]
- 56.Borel-Derlon A, Federici AB, Roussel-Robert V, et al. Treatment of severe von Willebrand disease with a high-purity von Willebrand factor concentrate (Wilfactin): a prospective study of 50 patients. J Thromb Haemost. 2007;5(6):1115–1124. [DOI] [PubMed] [Google Scholar]
- 57.Federici AB, Barillari G, Zanon E, et al. Efficacy and safety of highly purified, doubly virus-inactivated VWF/FVIII concentrates in inherited von Willebrand’s disease: results of an Italian cohort study on 120 patients characterized by bleeding severity score. Haemophilia. 2010;16(1):101–110. [DOI] [PubMed] [Google Scholar]
- 58.Holm E, Abshire TC, Bowen J, et al. Changes in bleeding patterns in von Willebrand disease after institution of long-term replacement therapy: resultsfrom the von Willebrand Disease Prophylaxis Network. Blood Coagul Fibrinolysis. 2015;26(4):383–388. [DOI] [PubMed] [Google Scholar]
- 59.Abshire T, Cox-Gill J, Kempton CL, et al. Prophylaxis escalation in severe von Willebrand disease: a prospective study from the von Willebrand DiseaseProphylaxis Network. J Thromb Haemost. 2015;13(9):1585–1589. [DOI] [PubMed] [Google Scholar]
- 60.Abshire TC, Federici AB, Alv ´ arez MT, et al. ; VWD PN. Prophylaxis in severe forms of von Willebrand’s disease: results from the von Willebrand DiseaseProphylaxis Network (VWD PN). Haemophilia. 2013;19(1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holm E, Carlsson KS, Lö vdahl S, Lail AE, Abshire TC, Berntorp E. Bleeding-related hospitalization in patients with vonWillebrand disease and the impact of prophylaxis: Results from national registers in Sweden compared with normal controls and participants in the von Willebrand Disease Prophylaxis Network. Haemophilia. 2018;24(4):628–633. [DOI] [PubMed] [Google Scholar]
- 62.Federici AB. Highly purified VWF/FVIII concentrates in the treatment and prophylaxis of von Willebrand disease: the PRO. WILL study. Haemophilia.2007;13(suppl 5):15–24. [DOI] [PubMed] [Google Scholar]
- 63.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, Folsom AR. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). Am J Med. 2002. Dec 1;113(8):636–42. doi: 10.1016/s0002-9343(02)01345-1. PMID: 12505113. [DOI] [PubMed] [Google Scholar]
- 64.Makris M, Colvin B, Gupta V, Shields ML, Smith MP. Venous thrombosis following the use of intermediate purity FVIII concentrate to treat patients with von Willebrand’s disease. Thromb Haemost. 2002. Sep;88(3):387–8. PMID: 12353065. [PubMed] [Google Scholar]
- 65.Weyand AC, Jesudas R, Pipe SW. Advantage of recombinant von Willebrand factor for peri-operative management in paediatric acquired von Willebrand syndrome. Haemophilia. 2018. May;24(3):e120–e121. doi: 10.1111/hae.13436. Epub 2018 Feb 8. PMID: 29418043. [DOI] [PubMed] [Google Scholar]
- 66.Heijdra JM, Cnossen MH, Leebeek FWG. Current and Emerging Options for the Management of Inherited von Willebrand Disease. Drugs. 2017. Sep;77(14):1531–1547. doi: 10.1007/s40265-017-0793-2. PMID: 28791655; PMCID: PMC5585291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castaman G. How I treat von Willebrand disease. Thromb Res. 2020. Dec;196:618–625. doi: 10.1016/j.thromres.2020.07.051. Epub 2020 Aug 3. PMID: 32819724. [DOI] [PubMed] [Google Scholar]
- 68.Ben-Ami T, Revel-Vilk S. The use of DDAVP in children with bleeding disorders. Pediatr Blood Cancer. 2013;60 Suppl 1:S41–3. doi: 10.1002/pbc.24335. Epub 2012 Oct 25. PMID: 23109357. [DOI] [PubMed] [Google Scholar]
- 69.Federici AB. The use of desmopressin in von Willebrand disease: the experience of the first 30 years (1977–2007). Haemophilia. 2007;14(suppl 1):5–14. [DOI] [PubMed] [Google Scholar]
- 70.Connell NT, James PD, Brignardello-Petersen R, Abdul-Kadir R, Ameer B, Arapshian A, Couper S, Di Paola J, Eikenboom J, Giraud N, Grow JM, Haberichter S, Jacobs-Pratt V, Konkle BA, Kouides P, Laffan M, Lavin M, Leebeek FWG, McLintock C, McRae S, Montgomery R, O’Brien SH, O’Donnell JS, Ozelo MC, Scappe N, Sidonio R, Tosetto A, Weyand AC, Kalot MA, Husainat N, Mustafa RA, Flood VH. von Willebrand disease: proposing definitions for future research. Blood Adv. 2021. Jan 26;5(2):565–569. doi: 10.1182/bloodadvances.2020003620. PMID: 33496750; PMCID: PMC7839375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sindet-Pedersen S. Haemostasis in oral surgery—the possible pathogenetic implications of oral fibrinolysis on bleeding. Experimental and clinical studies of the haemostatic balance in the oral cavity, with particular reference to patients with acquired and congenital defects of the coagulation system Review. Dan Med Bull. 1991;38(6):427–443. [PubMed] [Google Scholar]
- 72.Bryant-Smith AC, Lethaby A, Farquhar C, Hickey M. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kouides PA, Byams VR, Philipp CS, Stein SF, Heit JA, Lukes AS, Skerrette NI, Dowling NF, Evatt BL, Miller CH, Owens S, Kulkarni R. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009. Apr;145(2):212–20. doi: 10.1111/j.1365-2141.2009.07610.x. Epub 2009 Feb 19. PMID: 19236375. [DOI] [PubMed] [Google Scholar]
- 74.Koo JR, Lee YK, Kim YS, Cho WY, Kim HK, Won NH. Acute renal cortical necrosis caused by an antifibrinolytic drug (tranexamic acid) Nephrol Dial Transplant. 1999;14(3):750–752. doi: 10.1093/ndt/14.3.750. [DOI] [PubMed] [Google Scholar]
- 75.Kwiecien M, Edelman A, Nichols MD, Jensen JT. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67(1):9–13. doi: 10.1016/S0010-7824(02)00445-6. [DOI] [PubMed] [Google Scholar]
- 76.Bofill Rodriguez M, Lethaby A, Jordan V. Progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2020. Jun 12;6(6):CD002126. doi: 10.1002/14651858.CD002126.pub4. PMID: 32529637; PMCID: PMC7388184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brenner B. Haemostatic changes in pregnancy.Thromb Res.2004; 114(5–6): 409–414. [DOI] [PubMed] [Google Scholar]
- 78.Nowak-Göttl U, Limperger V, Kenet G, et al. Developmental hemostasis: a lifespan from neonates and pregnancy to the young and elderly adult in a European white population. Blood Cells Mol Dis. 2017; 67:2–13 [DOI] [PubMed] [Google Scholar]
- 79.Sánchez-Luceros A, Meschengieser SS, Marchese C, et al. Factor VIII and von Willebrand factor changes during normal pregnancy and puerperium. Blood Coagul Fibrinolysis. 2003;14(7):647–651. [DOI] [PubMed] [Google Scholar]
- 80.Leebeek FWG, Duvekot J, Kruip MJHA. How I manage pregnancy in carriers of hemophilia and patients with von Willebrand disease. Blood. 2020. Nov 5;136(19):2143–2150. doi: 10.1182/blood.2019000964. PMID: 32797211. [DOI] [PubMed] [Google Scholar]
- 81.Laffan MA, Lester W, O’Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol. 2014;167(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Punt MC, Waning ML, Mauser-Bunschoten EP, et al. Maternal and neonatal bleeding complications in relation to peripartum management in women with Von Willebrand disease: a systematic review. Blood Rev. 2020;39:100633. [DOI] [PubMed] [Google Scholar]
- 83.Stoof SC, van Steenbergen HW, Zwagemaker A, et al. Primary postpartum haemorrhage in women with von Willebrand disease or carriership of haemophilia despite specialised care: a retrospective survey. Haemophilia. 2015;21(4):505–512. [DOI] [PubMed] [Google Scholar]
- 84.WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10084):2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kouides PA. Antifibrinolytic therapy for preventing VWD-related postpartum hemorrhage: indications and limitations. Blood Adv. 2017;1(11):699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castaman G, James PD. Pregnancy and delivery in women with von Willebrand disease. Eur J Haematol. 2019. Aug;103(2):73–79. doi: 10.1111/ejh.13250. Epub 2019 May 31. PMID: 31107984; PMCID: PMC7604852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.James PD, Lillicrap D, Mannucci PM. Alloantibodies in von Willebrand disease. Blood. 2013;122(5):636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Federici AB. Clinical and molecular markers of inherited von Willebrand disease type 3: are deletions of the VWF gene associated with alloantibodies to VWF? J Thromb Haemost. 2008;6(10):1726–1728. [DOI] [PubMed] [Google Scholar]
- 89.Pergantou H, Xafaki P, Adamtziki E, Koletsi P, Komitopoulou A, Platokouki H. The challenging management of a child with type 3 von Willebrand disease and antibodies to von Willebrand factor. Haemophilia. 2012;18(3):e66–e67. [DOI] [PubMed] [Google Scholar]
- 90.Franchini M, Mannucci PM. Alloantibodies in von Willebrand Disease. Semin Thromb Hemost. 2018. Sep;44(6):590–594. doi: 10.1055/s-0037-1607440. Epub 2017 Nov 17. PMID: 29165738. [DOI] [PubMed] [Google Scholar]
- 91.Weyand AC, Flood VH, Shavit JA, Pipe SW. Efficacy of emicizumab in a pediatric patient with type 3 von Willebrand disease and alloantibodies. Blood Adv. 2019. Sep 24;3(18):2748–2750. doi: 10.1182/bloodadvances.2019000656. PMID: 31540901; PMCID: PMC6759735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, Rick ME, Sadler JE, Weinstein M, Yawn BP. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008. Mar;14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x. PMID: 18315614. [DOI] [PubMed] [Google Scholar]
- 93.Laffan MA, Lester W, O’Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol. 2014;167(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castaman G, Goodeve A, Eikenboom J; European Group on von Willebrand Disease. Principles of care for the diagnosis and treatment of von Willebrand disease. Haematologica. 2013;98(5):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Windyga J, Dolan G, Altisent C, Katsarou O, López Fernández MF, Zülfikar B; EHTSB. Practical aspects of factor concentrate use in patients with von Willebrand disease undergoing invasive procedures: a European survey. Haemophilia. 2016;22(5):739–751. [DOI] [PubMed] [Google Scholar]