Abstract
The 9-t-butylglycylamido derivative of minocycline (TBG-MINO) is a recently synthesized member of a novel group of antibiotics, the glycylcyclines. This new derivative, like the first glycylcyclines, the N,N-dimethylglycylamido derivative of minocycline and 6-demethyl-6-deoxytetracycline, possesses activity against bacterial isolates containing the two major determinants responsible for tetracycline resistance: ribosomal protection and active efflux. The in vitro activities of TBG-MINO and the comparative agents were evaluated against strains with characterized tetracycline resistance as well as a spectrum of recent clinical aerobic and anaerobic gram-positive and gram-negative bacteria. TBG-MINO, with an MIC range of 0.25 to 0.5 μg/ml, showed good activity against strains expressing tet(M) (ribosomal protection), tet(A), tet(B), tet(C), tet(D), and tet(K) (efflux resistance determinants). TBG-MINO exhibited similar activity against methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant streptococci, and vancomycin-resistant enterococci (MICs at which 90% of strains are inhibited, ≤0.5 μg/ml). TBG-MINO exhibited activity against a wide diversity of gram-negative aerobic and anaerobic bacteria, most of which were less susceptible to tetracycline and minocycline. The in vivo protective effects of TBG-MINO were examined against acute lethal infections in mice caused by Escherichia coli, S. aureus, and Streptococcus pneumoniae isolates. TBG-MINO, administered intravenously, demonstrated efficacy against infections caused by S. aureus including MRSA strains and strains containing tet(K) or tet(M) resistance determinants (median effective doses [ED50s], 0.79 to 2.3 mg/kg of body weight). TBG-MINO demonstrated efficacy against infections caused by tetracycline-sensitive E. coli strains as well as E. coli strains containing either tet(M) or the efflux determinant tet(A), tet(B), or tet(C) (ED50s, 1.5 to 3.5 mg/kg). Overall, TBG-MINO shows antibacterial activity against a wide spectrum of gram-positive and gram-negative aerobic and anaerobic bacteria including strains resistant to other chemotherapeutic agents. The in vivo protective effects, especially against infections caused by resistant bacteria, corresponded with the in vitro activity of TBG-MINO.
Tetracycline antibiotics were first isolated at Lederle Laboratories in 1945 and represented a significant advancement in the treatment of many infections (4, 7). However, due to an increased incidence of resistance among many bacteria (27), the use of the tetracyclines has been relegated to second- and third-line drug categories for most clinical indications (16, 25). The synthesis of new derivatives containing the N,N-dimethylglycylamido (DMG) substitution at the 9 position of minocycline and of 6-demethyl-6-deoxytetracycline (DMDOT) represented a significant advance in the tetracycline class of antibiotics (29). These new derivatives were named the glycylcyclines and were shown to be active against a wide spectrum of gram-positive and gram-negative bacteria, including resistant strains (5, 9, 12, 22, 31, 33, 34).
Derivatives in the minocycline series were found to be better tolerated than the DMDOT series in studies with rats (data not shown). In the present study we investigated the in vitro activity and in vivo efficacy of a new member of the glycylcyclines, TBG-MINO, the 9-t-butylglycylamido derivative of minocycline (Fig. 1), which was selected on the basis of its better tolerability and improved activity against tetracycline-resistant strains compared with those of DMG-DMDOT. The activity of TBG-MINO was determined against strains harboring characterized tetracycline resistance determinants and recent clinical isolates. The activities were compared with those of DMG-DMDOT, DMG-MINO, minocycline, tetracycline, and other antimicrobial agents. The efficacy of TBG-MINO was compared with those of DMG-DMDOT and minocycline against murine systemic infections caused by bacterial strains harboring characterized tetracycline resistance determinants, laboratory strains, and recent clinical isolates adapted for murine infection.
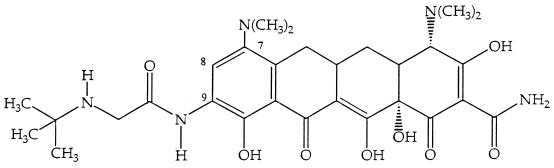
FIG. 1.
Chemical structure of TBG-MINO.
MATERIALS AND METHODS
Organisms.
Routine clinical isolates were collected from various medical centers in the United States and Canada between 1989 and 1994. Identification of each culture was done by conventional methods, as follows: gram-negative rods with the API 20E system (Analytab Products, Plainville, N.Y.) and the NF system (Remel, Lenexa, Kans.), anaerobes by the procedure outlined in the Wadsworth Anaerobic Bacteriology Manual (30), enterococci by biochemical tests as recommended by Facklam and Collins (6), streptococci with the API 20 Strep system (Analytab Products), and staphylococci with the Staph Trac system (Analytab Products). Staphylococcus aureus was also confirmed by a coagulase-test. Methicillin-resistance in S. aureus was determined with a plate containing oxacillin at 6 μg/ml, as described in the Manual of Clinical Microbiology (28). Penicillin-resistant (MICs, ≥2 μg/ml) Streptococcus pneumoniae isolates were obtained from A. Barry, Clinical Microbiology Institute, Tualatin, Oreg., and S. Block, Bardstown, Ky. Strains with tetracycline resistance determinants and the vancomycin-resistant enterococci were obtained from the sources described previously (31). All isolates were stored frozen in skim milk at −70°C.
Antibiotics.
Standard powders of TBG-MINO, DMG-MINO, DMG-DMDOT, vancomycin, minocycline, and tetracycline were obtained from Wyeth-Ayerst Laboratories, Pearl River, N.Y.; erythromycin was obtained from Sigma Chemical Co., St. Louis, Mo.; ciprofloxacin was obtained from Bayer Laboratories, West Haven, Conn.; ceftazidime was obtained from Glaxo Group Research, Ware, Herts, United Kingdom; and imipenem was obtained from Merck & Co., West Point, Pa.
In vitro susceptibility testing.
The activities of the antibiotics were determined by the agar dilution method by following the recommendations of the National Committee for Clinical Laboratory Standards (20, 21). Mueller-Hinton II agar was used to test nonfastidious aerobic bacteria. The medium was supplemented with 5% sheep blood for the testing of streptococcal isolates and 15 μg of β-NAD per ml, 15 μg of hematin per ml, and 5 mg of yeast extract per ml for the testing of Haemophilis influenzae and Moraxella catarrhalis. GC agar supplemented with 1% hemoglobin and 1% IsoVitaleX was used to test Neisseria gonorrhoeae. Anaerobic bacteria were tested on Wilkins Chalgren agar supplemented with 5% lysed sheep blood and 0.001% vitamin K. The inocula, which were adjusted to the recommended densities (107 CFU/ml for aerobes and 108 CFU/ml for anaerobes), were applied to the surfaces of the agar plates with a Steers replicator. Test plates were incubated at 35°C for 18 to 24 h in ambient air for nonfastidious aerobic bacteria and streptococci and in CO2 for N. gonorrhoeae, H. influenzae, and M. catarrhalis. Anaerobic bacteria were incubated in an anaerobic chamber (Coy Laboratories, Ann Arbor, Mich.) at 35°C for 48 h. The MIC was defined as the lowest concentration of the antimicrobial agent that completely inhibited the growth of the organism as detected by the unaided eye.
In vivo efficacy against murine infections.
The therapeutic effects of the antibiotics were determined against acute lethal infections in mice (3) caused by minocycline-susceptible and minocycline-resistant gram-positive and gram-negative bacteria. Female CD-1 mice from Charles River Laboratories (weight, 20 ± 2 g each) were challenged by intraperitoneal injection of 0.5 ml of a bacterial suspension in either 5% hog gastric mucin or broth (10 to 100 50% lethal doses). Five to six doses of the antibiotic in phosphate-buffered saline (0.01 M; pH 7.4) were administered intravenously (0.2 ml) or orally (0.5 ml) at 0.5 h postinfection. For mice infected with Escherichia coli JC3272 Tcr tet(B), a second dose of the antibiotic was given 3 h later. In each test, five animals were treated with each dose. All the untreated controls died within 48 h of infection. The median effective dose (ED50) was determined by probit analysis of the 7-day survival ratios pooled from three separate tests (8).
RESULTS
In vitro activity against tetracycline-resistant strains.
The in vitro activity of TBG-MINO against prototype strains possessing characterized tetracycline resistance mechanisms is summarized in Table 1. TBG-MINO had similar activity (MICs, ≤0.5 μg/ml) against tetracycline-susceptible and tetracycline-resistant E. coli strains carrying the efflux resistance determinants tet(A), tet(B), tet(C), and tet(D) and the strain carrying the ribosomal protection resistance determinant tet(M). TBG-MINO had activity similar to those of DMG-MINO and DMG-DMDOT against E. coli strains containing the tet(B) and tet(D) efflux resistance determinant and the ribosomal protection resistance determinant tet(M); however, TBG-MINO was more active than DMG-MINO and DMG-DMDOT against E. coli strains containing efflux resistance determinants tet(A) and tet(C). Minocycline demonstrated poorer activity (MIC range, 4 to >32 μg/ml) against all of the E. coli strains carrying the resistance determinants. TBG-MINO, with MICs of ≤0.5 μg/ml, was as active as DMG derivatives against the tet(K) (efflux)- and tet(M)-containing S. aureus strains. Minocycline was slightly more active than the glycylcyclines against tet(K)-containing S. aureus but had poorer activity against the three S. aureus strains containing tet(M).
TABLE 1.
In vitro activities of TBG-MINO, DMG-MINO, DMG-DMDOT, minocycline, and tetracycline against strains with characterized tetracycline resistance determinants
| Organism | Strain | Resistance determinant | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|---|
| TBG-MINO | DMG-MINO | DMG-DMDOT | Minocycline | Tetracycline | |||
| E. coli | UBMS 88-1 | tet(B) | 0.5 | 0.5 | 0.5 | 16 | >32 |
| E. coli | MC4100 | tet(B) | 0.5 | 0.5 | 0.5 | 8 | >32 |
| E. coli | J3272, pRP1 | tet(A) | 0.5 | 2 | 2 | 4 | 32 |
| E. coli | J3272, pBR322 | tet(C) | 0.25 | 2 | 2 | 4 | >32 |
| E. coli | J3272, pRA1 | tet(D) | 0.25 | 0.25 | 0.25 | 8 | >32 |
| E. coli | UBMS 90-4 | tet(M) | 0.25 | 0.25 | 0.25 | >32 | >32 |
| E. coli | UBMS 90-5 | Sensitive | 0.25 | 0.5 | 0.25 | 1 | 1 |
| E. coli | ATCC 25922 | Control | 0.25 | 0.25 | 0.25 | 0.5 | 1 |
| S. aureus | UBMS 88-7 | tet(K) | 0.5 | 1 | 1 | 0.25 | >32 |
| S. aureus | UBMS 88-5 | tet(M) | 0.5 | 0.25 | 0.25 | 4 | >32 |
| S. aureus | UBMS 90-1 | tet(M) | 0.25 | 0.25 | 0.12 | 4 | >32 |
| S. aureus | UBMS 90-2 | tet(M) | 0.25 | 0.25 | 0.25 | 2 | 32 |
| S. aureus | UBMS 90-3 | Sensitive | 0.25 | 0.25 | 0.12 | 0.06 | 0.12 |
| S. aureus | ATCC 29213 | Control | 0.5 | 0.25 | 0.25 | 0.06 | 0.25 |
| S. aureus | Smith | Sensitive | 0.25 | 0.25 | 0.12 | 0.06 | 0.12 |
| E. faecalis | UBMS 90-6 | tet(M) | 0.25 | 0.12 | 0.25 | 16 | >32 |
| E. faecalis | ATCC 29212 | Control | 0.25 | 0.12 | 0.12 | 1 | 8 |
| N. gonorrhoeae | 6418 | tet(M) | 1 | 1 | 1 | 16 | >32 |
In vitro activity against recent clinical isolates.
TBG-MINO showed good activity against isolates of methicillin-resistant S. aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (MICs at which 90% of isolates are inhibited [MIC90s], ≤1 μg/ml). This activity was similar to that of minocycline and was 2 to 3 dilutions lower than those of DMG-MINO and DMG-DMDOT (Table 2). Against methicillin-susceptible staphylococci, the three glycylcycline derivatives had equivalent activities (MICs, ≤0.5 μg/ml). TBG-MINO and the DMG derivatives demonstrated activity against Enterococcus faecalis and Enterococcus faecium, including vancomycin-resistant strains (MIC90s, ≤0.5 μg/ml). The three glycylcyclines, minocycline, and tetracycline exhibited good activity against Streptococcus pyogenes and penicillin-susceptible S. pneumoniae; however, TBG-MINO and the DMG derivatives were 32 to 64 times more active than minocycline against Streptococcus agalactiae and penicillin-resistant S. pneumoniae. No differences in the activity of TBG-MINO between penicillin-susceptible and penicillin-resistant S. pneumoniae isolates were noted. In general, TBG-MINO, with MICs of ≤1 μg/ml, displayed greater activity than the other comparative antibiotics, vancomycin, ciprofloxacin, and erythromycin, against most of the staphylococcal and enterococcal isolates tested.
TABLE 2.
In vitro activities of TBG-MINO and comparative antibiotics against gram-positive isolates
| Organism (no. of isolates) | Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Staphylococcus aureus, methicillin resistant (12) | TBG-MINO | 0.25–1 | 0.5 | 0.5 |
| DMG-MINO | 0.12–2 | 0.25 | 2 | |
| DMG-DMDOT | 0.25–2 | 0.5 | 2 | |
| Minocycline | 0.03–4 | 0.12 | 1 | |
| Tetracycline | 0.25–>32 | 0.5 | >32 | |
| Ciprofloxacin | 0.25–>32 | 8 | 32 | |
| Vancomycin | 0.5–2 | 1 | 1 | |
| Erythromycin | 4–>32 | >32 | >32 | |
| Staphylococcus aureus, methicillin susceptible (13) | TBG-MINO | 0.5 | 0.5 | 0.5 |
| DMG-MINO | 0.25–0.5 | 0.5 | 0.5 | |
| DMG-DMDOT | 0.5 | 0.5 | 0.5 | |
| Minocycline | 0.12 | 0.12 | 0.12 | |
| Tetracycline | 0.5 | 0.5 | 0.5 | |
| Ciprofloxacin | 0.5–1 | 0.5 | 1 | |
| Vancomycin | 0.5–1 | 1 | 1 | |
| Erythromycin | 0.5–1 | 0.5 | 0.5 | |
| Coagulase-negative staphylococci, methicillin resistant (13) | TBG-MINO | 0.25–2 | 0.5 | 1 |
| DMG-MINO | 0.25–8 | 0.5 | 4 | |
| DMG-DMDOT | 0.12–8 | 1 | 8 | |
| Minocycline | 0.12–1 | 0.5 | 1 | |
| Tetracycline | 0.25–>32 | 4 | >32 | |
| Ciprofloxacin | 0.25–>32 | 0.5 | 32 | |
| Vancomycin | 1–2 | 2 | 2 | |
| Erythromycin | 0.12–>32 | >32 | >32 | |
| Coagulase-negative staphylococci, methicillin susceptible (16) | TBG-MINO | 0.12–0.5 | 0.25 | 0.5 |
| DMG-MINO | 0.12–0.5 | 0.25 | 0.5 | |
| DMG-DMDOT | 0.12–1 | 0.25 | 0.5 | |
| Minocycline | 0.06–0.5 | 0.12 | 0.25 | |
| Tetracycline | 0.12–32 | 0.5 | 32 | |
| Ciprofloxacin | 0.12–1 | 0.25 | 0.5 | |
| Vancomycin | 1–2 | 1 | 2 | |
| Erythromycin | 0.12–>32 | 0.12 | 1 | |
| Enterococcus faecalis (11) | TBG-MINO | 0.12–0.5 | 0.25 | 0.5 |
| DMG-MINO | 0.06–0.25 | 0.25 | 0.25 | |
| DMG-DMDOT | 0.12–0.5 | 0.25 | 0.25 | |
| Minocycline | 0.06–16 | 8 | 8 | |
| Tetracycline | 0.25–32 | 32 | 32 | |
| Ciprofloxacin | 1–32 | 1 | 2 | |
| Vancomycin | 1–4 | 2 | 2 | |
| Erythromycin | 1–16 | 1 | 8 | |
| Enterococcus faecium (11) | TBG-MINO | 0.12–0.25 | 0.25 | 0.25 |
| DMG-MINO | 0.06–0.12 | 0.12 | 0.12 | |
| DMG-DMDOT | 0.12–0.25 | 0.12 | 0.12 | |
| Minocycline | 0.06–16 | 0.06 | 4 | |
| Tetracycline | 0.25–>32 | 0.25 | 32 | |
| Ciprofloxacin | 1–4 | 4 | 4 | |
| Vancomycin | 0.25–2 | 1 | 2 | |
| Erythromycin | 0.5–>32 | 4 | >32 | |
| Enterococcus spp., vancomycin resistant (10) | TBG-MINO | 0.12–0.25 | 0.25 | 0.25 |
| DMG-MINO | 0.06–0.25 | 0.12 | 0.25 | |
| DMG-DMDOT | 0.12–0.25 | 0.12 | 0.25 | |
| Minocycline | 0.06–8 | 0.06 | 8 | |
| Tetracycline | 0.25–>32 | 0.25 | >32 | |
| Ciprofloxacin | 0.5–4 | 4 | 4 | |
| Vancomycin | >32 | >32 | >32 | |
| Erythromycin | >32 | >32 | >32 | |
| Streptococcus pneumoniae, penicillin resistant (10) | TBG-MINO | 0.06–0.25 | 0.12 | 0.12 |
| DMG-MINO | 0.03–0.12 | 0.06 | 0.06 | |
| DMG-DMDOT | 0.06–0.12 | 0.12 | 0.12 | |
| Minocycline | 0.12–8 | 4 | 4 | |
| Tetracycline | 0.5–32 | 32 | 32 | |
| Vancomycin | 0.25–0.5 | 0.5 | 0.5 | |
| Streptococcus pneumoniae, penicillin susceptible (10) | TBG-MINO | 0.06–0.12 | 0.06 | 0.12 |
| DMG-MINO | 0.06–0.25 | 0.06 | 0.12 | |
| DMG-DMDOT | 0.06–0.5 | 0.06 | 0.12 | |
| Minocycline | 0.06–0.5 | 0.12 | 0.12 | |
| Tetracycline | 0.12–4 | 0.25 | 0.5 | |
| Vancomycin | 0.12–1 | 0.5 | 0.5 | |
| Streptococcus pyogenes (10) | TBG-MINO | 0.12–0.5 | 0.12 | 0.25 |
| DMG-MINO | 0.06–0.12 | 0.12 | 0.12 | |
| DMG-DMDOT | 0.12 | 0.12 | 0.12 | |
| Minocycline | 0.06–0.25 | 0.06 | 0.12 | |
| Tetracycline | 0.25–16 | 0.25 | 0.25 | |
| Vancomycin | 0.5 | 0.5 | 0.5 | |
| Streptococcus agalactiae (10) | TBG-MINO | 0.12–0.5 | 0.12 | 0.25 |
| DMG-MINO | 0.12–0.5 | 0.12 | 0.5 | |
| DMG-DMDOT | 0.12–1 | 0.12 | 0.25 | |
| Minocycline | 0.12–16 | 16 | 16 | |
| Tetracycline | 0.25–32 | 32 | 32 | |
| Vancomycin | 0.5–1 | 0.5 | 0.5 | |
TBG-MINO, with a range of MICs of 0.5 to 8 μg/ml, was 4 to 32 times more active than minocycline against clinical isolates of E. coli, Shigella spp., Citrobacter diversus, Salmonella spp., Providencia spp., Morganella morganii, and N. gonorrhoeae (Table 3). TBG-MINO was generally as active or more active than minocycline against most strains of Klebsiella spp., Citrobacter freundii, Enterobacter spp., Serratia marcescens, Proteus mirabilis, Proteus vulgaris, Burkholderia cepacia, and Pseudomonas aeruginosa. In general, the three glycylcyclines demonstrated similar activities against gram-negative isolates; however, greater activity was observed with TBG-MINO than with DMG-MINO or DMG-DMDOT (MIC90s, ≤0.5 versus 4 μg/ml, respectively) against E. coli strains for which minocycline MICs were elevated (MIC90, 16 μg/ml). TBG-MINO, DMG-MINO, and DMG-DMDOT were generally less active than ciprofloxacin, imipenem, and ceftazidime against most gram-negative bacteria. However, organisms resistant to these antibiotics showed no cross-resistance with the glycylcyclines.
TABLE 3.
In vitro activities of TBG-MINO and comparative antibiotics against gram-negative isolates
| Organism (no. of isolates) | Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Escherichia coli (minocycline MIC, ≥1 μg/ml (32) | TBG-MINO | 0.25–1 | 0.5 | 0.5 |
| DMG-MINO | 0.25–4 | 0.5 | 4 | |
| DMG-DMDOT | 0.25–4 | 1 | 4 | |
| Minocycline | 1–32 | 8 | 16 | |
| Tetracycline | 2–>32 | >32 | >32 | |
| Ciprofloxacin | 0.008–32 | 0.008 | 0.015 | |
| Imipenem | 0.06–0.25 | 0.12 | 0.12 | |
| Ceftazidime | 0.06–1 | 0.12 | 0.25 | |
| Escherichia coli (minocycline MIC, ≤0.5 μg/ml) (14) | TBG-MINO | 0.25–0.5 | 0.5 | 0.5 |
| DMG-MINO | 0.25–0.5 | 0.25 | 0.5 | |
| DMG-DMDOT | 0.5 | 0.5 | 0.5 | |
| Minocycline | 0.25–0.5 | 0.5 | 0.5 | |
| Tetracycline | 1–2 | 1 | 2 | |
| Ciprofloxacin | ≤0.004–0.25 | ≤0.004 | 0.03 | |
| Imipenem | 0.06–0.12 | 0.12 | 0.12 | |
| Ceftazidime | 0.06–0.25 | 0.12 | 0.12 | |
| Shigella spp. (26) | TBG-MINO | 0.12–0.5 | 0.25 | 0.5 |
| DMG-MINO | 0.12–1 | 0.25 | 0.5 | |
| DMG-DMDOT | 0.12–1 | 0.5 | 0.5 | |
| Minocycline | 0.25–16 | 2 | 4 | |
| Tetracycline | 1–>32 | >32 | >32 | |
| Ciprofloxacin | ≤0.004–0.015 | ≤0.004 | 0.008 | |
| Imipenem | 0.06–0.5 | 0.12 | 0.25 | |
| Ceftazidime | 0.06–0.12 | 0.12 | 0.12 | |
| Klebsiella pneumoniae (10) | TBG-MINO | 0.5–2 | 1 | 2 |
| DMG-MINO | 0.5–1 | 1 | 1 | |
| DMG-DMDOT | 0.5–1 | 1 | 1 | |
| Minocycline | 1–4 | 2 | 4 | |
| Tetracycline | 1–4 | 2 | 2 | |
| Ciprofloxacin | 0.008–0.03 | 0.03 | 0.03 | |
| Imipenem | 0.12–0.5 | 0.12 | 0.25 | |
| Ceftazidime | 0.06–0.5 | 0.12 | 0.12 | |
| Klebsiella oxytoca (10) | TBG-MINO | 1 | 1 | 1 |
| DMG-MINO | 1 | 1 | 1 | |
| DMG-DMDOT | 1 | 1 | 1 | |
| Minocycline | 2–8 | 2 | 2 | |
| Tetracycline | 2–>32 | 2 | 2 | |
| Ciprofloxacin | 0.008–0.03 | 0.015 | 0.015 | |
| Imipenem | 0.12–0.25 | 0.12 | 0.25 | |
| Ceftazidime | 0.06–0.5 | 0.12 | 0.12 | |
| Citrobacter freundii (10) | TBG-MINO | 0.5–8 | 1 | 2 |
| DMG-MINO | 0.5–8 | 1 | 2 | |
| DMG-DMDOT | 1–8 | 1 | 1 | |
| Minocycline | 1–32 | 4 | 4 | |
| Tetracycline | 1–16 | 2 | 2 | |
| Ciprofloxacin | ≤0.004–16 | 0.015 | 0.12 | |
| Imipenem | 0.25–2 | 0.5 | 1 | |
| Ceftazidime | 0.12–>32 | 0.5 | 8 | |
| Citrobacter diversus (10) | TBG-MINO | 0.5–2 | 1 | 1 |
| DMG-MINO | 0.5–2 | 1 | 1 | |
| DMG-DMDOT | 1–2 | 1 | 1 | |
| Minocycline | 1–4 | 2 | 4 | |
| Tetracycline | 2–8 | 2 | 4 | |
| Ciprofloxacin | ≤0.004–0.06 | 0.008 | 0.06 | |
| Imipenem | 0.06–12 | 0.06 | 0.12 | |
| Ceftazidime | 0.12–0.5 | 0.12 | 0.5 | |
| Salmonella spp. (14) | TBG-MINO | 0.25–2 | 1 | 1 |
| DMG-MINO | 0.5–4 | 0.5 | 1 | |
| DMG-DMDOT | 0.5–4 | 0.5 | 1 | |
| Minocycline | 0.5–32 | 2 | 16 | |
| Tetracycline | 1–>32 | 2 | >32 | |
| Ciprofloxacin | ≤0.004–0.03 | 0.015 | 0.03 | |
| Imipenem | 0.06–0.25 | 0.12 | 0.25 | |
| Ceftazidime | 0.12–0.5 | 0.25 | 0.5 | |
| Serratia marcescens (10) | TBG-MINO | 4–8 | 4 | 4 |
| DMG-MINO | 4–8 | 4 | 8 | |
| DMG-DMDOT | 4–8 | 4 | 4 | |
| Minocycline | 4–8 | 8 | 8 | |
| Tetracycline | 8–>32 | 32 | >32 | |
| Ciprofloxacin | 0.008–2 | 0.12 | 0.25 | |
| Imipenem | 0.25–2 | 0.5 | 2 | |
| Ceftazidime | 0.12–1 | 0.25 | 0.5 | |
| Enterobacter cloacae (10) | TBG-MINO | 1–2 | 1 | 2 |
| DMG-MINO | 1–2 | 1 | 2 | |
| DMG-DMDOT | 1–2 | 1 | 2 | |
| Minocycline | 2–4 | 4 | 4 | |
| Tetracycline | 2–4 | 4 | 4 | |
| Ciprofloxacin | ≤0.004–0.06 | 0.03 | 0.03 | |
| Imipenem | 0.25 | 0.25 | 0.25 | |
| Ceftazidime | 0.12–>32 | 0.25 | >32 | |
| Enterobacter aerogenes (10) | TBG-MINO | 1 | 1 | 1 |
| DMG-MINO | 1 | 1 | 1 | |
| DMG-DMDOT | 1 | 1 | 1 | |
| Minocycline | 2 | 2 | 2 | |
| Tetracycline | 2 | 2 | 2 | |
| Ciprofloxacin | ≤0.004–0.03 | 0.015 | 0.03 | |
| Imipenem | 0.25–2 | 0.25 | 2 | |
| Ceftazidime | 0.12–>32 | 0.12 | >32 | |
| Providencia spp. (10) | TBG-MINO | 4–8 | 4 | 8 |
| DMG-MINO | 2–8 | 8 | 8 | |
| DMG-DMDOT | 4–8 | 4 | 8 | |
| Minocycline | 4–>32 | 16 | >32 | |
| Tetracycline | 4–>32 | >32 | >32 | |
| Ciprofloxacin | ≤0.004–0.25 | 0.03 | 0.25 | |
| Imipenem | 0.25–2 | 1 | 2 | |
| Ceftazidime | 0.03–4 | 0.06 | 4 | |
| Proteus mirabilis (15) | TBG-MINO | 2–8 | 4 | 8 |
| DMG-MINO | 1–16 | 4 | 8 | |
| DMG-DMDOT | 0.12–2 | 1 | 1 | |
| Minocycline | 2–32 | 8 | 16 | |
| Tetracycline | 1–32 | 16 | 32 | |
| Ciprofloxacin | 0.008–0.06 | 0.06 | 0.06 | |
| Imipenem | 0.003–0.12 | 0.06 | 0.12 | |
| Ceftazidime | 0.015–0.06 | 0.03 | 0.03 | |
| Proteus vulgaris (15) | TBG-MINO | 1–4 | 4 | 4 |
| DMG-MINO | 0.5–4 | 1 | 2 | |
| DMG-DMDOT | 0.25–1 | 0.5 | 1 | |
| Minocycline | 0.5–8 | 2 | 4 | |
| Tetracycline | 0.5–>32 | 8 | 32 | |
| Ciprofloxacin | 0.008–0.25 | 0.015 | 0.12 | |
| Imipenem | 0.03–0.12 | 0.06 | 0.12 | |
| Ceftazidime | 0.015–0.25 | 0.03 | 0.06 | |
| Morganella morganii (10) | TBG-MINO | 2–8 | 4 | 4 |
| DMG-MINO | 1–4 | 4 | 4 | |
| DMG-DMDOT | 1–4 | 2 | 2 | |
| Minocycline | 2–>32 | 4 | 16 | |
| Tetracycline | 2–>32 | 2 | >32 | |
| Ciprofloxacin | ≤0.004–1 | 0.008 | 0.03 | |
| Imipenem | 2–4 | 2 | 4 | |
| Ceftazidime | 0.06–32 | 0.12 | 32 | |
| Pseudomonas aeruginosa (10) | TBG-MINO | 8–16 | 16 | 16 |
| DMG-MINO | 4–8 | 8 | 8 | |
| DMG-DMDOT | 4–16 | 8 | 8 | |
| Minocycline | 2–8 | 8 | 8 | |
| Tetracycline | 8–>32 | 16 | >32 | |
| Ciprofloxacin | 0.12–2 | 0.25 | 2 | |
| Imipenem | 0.5–2 | 1 | 1 | |
| Ceftazidime | 0.5–32 | 2 | 16 | |
| Burkholderia cepacia (10) | TBG-MINO | 0.5–4 | 2 | 4 |
| DMG-MINO | 0.5–4 | 1 | 4 | |
| DMG-DMDOT | 0.5–4 | 2 | 4 | |
| Minocycline | 0.06–2 | 0.5 | 2 | |
| Tetracycline | 1–>32 | 2 | 4 | |
| Ciprofloxacin | 0.03–4 | 0.12 | 2 | |
| Imipenem | 0.06–8 | 4 | 8 | |
| Ceftazidime | 0.5–4 | 2 | 4 | |
| Stenotrophomonas malto-philia (10) | TBG-MINO | 1–4 | 2 | 4 |
| DMG-MINO | 0.5–4 | 1 | 2 | |
| DMG-DMDOT | 2–8 | 4 | 8 | |
| Minocycline | 0.06–0.5 | 0.12 | 0.25 | |
| Tetracycline | 8–16 | 16 | 16 | |
| Ciprofloxacin | 1–4 | 2 | 4 | |
| Imipenem | >32 | >32 | >32 | |
| Ceftazidime | 4–>32 | 8 | >32 | |
Continued on following page
TBG-MINO and the other glycylcycline derivatives, with a range of MICs of 0.12 to 2 μg/ml, were more active than minocycline against Bacteroides spp., Prevotella spp., Clostridium difficile, and anaerobic gram-positive cocci (Table 4). For some members of the Bacteroides fragilis group, the MICs of TBG-MINO but not those of DMG-MINO or DMG-DMDOT were found to be elevated (1 to 2 μg/ml). In general, the three glycylcyclines were more active than cefoxitin but were less active than imipenem against most of the anaerobic bacteria tested.
TABLE 4.
In vitro activities of TBG-MINO and comparative antibiotics against anaerobic bacteria
| Organism (no. of isolates) | Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Bacteroides fragilis group (12) | TBG-MINO | 0.25–2 | 0.5 | 2 |
| DMG-MINO | 0.25–0.5 | 0.25 | 0.5 | |
| DMG-DMDOT | 0.25–0.5 | 0.25 | 0.5 | |
| Minocycline | 0.06–4 | 2 | 4 | |
| Cefoxitin | 2–>32 | 16 | >32 | |
| Imipenem | ≤0.06–2 | 0.12 | 2 | |
| Bacteroides fragilis (14) | TBG-MINO | 0.5–8 | 2 | 2 |
| DMG-MINO | 0.25–2 | 1 | 1 | |
| DMG-DMDOT | 0.5–2 | 1 | 2 | |
| Minocycline | ≤0.06–8 | 8 | 8 | |
| Cefoxitin | 1–8 | 8 | 8 | |
| Imipenem | ≤0.06–0.25 | ≤0.06 | 0.12 | |
| Prevotella spp. (11) | TBG-MINO | 0.12–1 | 0.5 | 1 |
| DMG-MINO | ≤0.06–0.5 | 0.25 | 0.5 | |
| DMG-DMDOT | ≤0.06–2 | 0.5 | 2 | |
| Minocycline | ≤0.06–16 | 8 | 16 | |
| Cefoxitin | 0.25–4 | 1 | 2 | |
| Imipenem | ≤0.06 | ≤0.06 | ≤0.06 | |
| Clostridium difficile (10) | TBG-MINO | ≤0.06–0.25 | 0.12 | 0.12 |
| DMG-MINO | ≤0.06–0.12 | 0.12 | 0.12 | |
| DMG-DMDOT | ≤0.06–0.12 | 0.12 | 0.12 | |
| Minocycline | ≤0.06–4 | 0.03 | 4 | |
| Cefoxitin | >32 | >32 | >32 | |
| Imipenem | 2–16 | 4 | 4 | |
| Clostridium perfringens (10) | TBG-MINO | 0.12–4 | 0.5 | 1 |
| DMG-MINO | 0.12–4 | 0.25 | 2 | |
| DMG-DMDOT | 0.12–4 | 0.25 | 2 | |
| Minocycline | ≤0.06–8 | ≤0.06 | 4 | |
| Cefoxitin | 0.25–1 | 0.5 | 1 | |
| Imipenem | ≤0.06 | ≤0.06 | ≤0.06 | |
| Anaerobic gram-positive cocci (15) | TBG-MINO | ≤0.06–0.25 | 0.12 | 0.25 |
| DMG-MINO | ≤0.06–0.12 | ≤0.06 | 0.12 | |
| DMG-DMDOT | ≤0.06–0.5 | 0.12 | 0.5 | |
| Minocycline | 0.12–16 | 4 | 16 | |
| Cefoxitin | ≤0.06–16 | 0.12 | 16 | |
| Imipenem | ≤0.06–1 | ≤0.06 | 1 | |
In vivo efficacy.
Administered as a single intravenous dose, TBG-MINO showed efficacy against infections caused by tetracycline-susceptible and tetracycline-resistant S. aureus and E. coli strains in mice (Table 5 and 6). Against an infection with S. aureus Smith, a tetracycline-susceptible strain, all three compounds, TBG-MINO, DMG-DMDOT, and minocycline, displayed efficacy (ED50s, 0.64, 0.51, and 0.53 mg/kg of body weight, respectively) when they were administered intravenously; however, when they were administered orally, TBG-MINO and DMG-DMDOT were 40- to 60-fold less efficacious (Table 5). In contrast, when administered orally minocycline exhibited efficacy equivalent to that achieved when it was administered intravenously against S. aureus Smith infection (ED50, 0.52 mg/kg). Due to the poor efficacy in mice noted when the drugs were given by the oral route, other in vivo tests were performed with only intravenous administration. TBG-MINO and DMG-DMDOT were moderately more efficacious than minocycline against an infection with S. aureus UBMS 90-2 [a tet(M) (ribosomal protection)-containing strain] (Table 6). TBG-MINO, DMG-DMDOT, and minocycline had comparable efficacies against an infection caused by S. aureus UBMS 88-7, a tet(K) efflux resistance determinant-containing strain (ED50s, 2.1, 3.1, and 2.0 mg/kg, respectively). TBG-MINO and DMG-DMDOT showed protective efficacy against an infection caused by S. aureus NEMC 89-4 (a tetracycline-susceptible, methicillin-resistant strain), but minocycline was slightly more effective. Against infections caused by an MRSA strain containing the tet(M) resistance determinant (strain ID 4729) and an MRSA strain carrying both tet(M) and tet(K) resistance determinants (strain ID 2371), TBG-MINO and DMG-DMDOT showed efficacies which exceeded that of minocycline by approximately two and five times, respectively. Comparable efficacies against infections caused by S. pneumoniae were obtained with TBG-MINO and DMG-DMDOT, regardless of the strain’s susceptibility to penicillin (range of ED50s, 0.53 to 1.9 mg/kg). Minocycline was slightly less effective against infections caused by penicillin-susceptible S. pneumoniae and was >30 times less effective than the glycylcyclines against a penicillin-resistant S. pneumoniae infection (ED50, 20 mg/kg).
TABLE 5.
In vivo activities of TBG-MINO, DMG-DMDOT, and minocycline against experimental acute lethal S. aureus Smith infection in micea
| Antibiotic | Route | ED50 (mg/kg) (95% confidence limit) | MIC (μg/ml) |
|---|---|---|---|
| TBG-MINO | Intravenous | 0.64 (0.51–0.80) | 0.25 |
| TBG-MINO | Oral | 36 (28–45) | 0.25 |
| DMG-DMDOT | Intravenous | 0.51 (0.41–0.64) | 0.12 |
| DMG-DMDOT | Oral | 21 (16–26) | 0.12 |
| Minocycline | Intravenous | 0.53 (0.40–0.70) | 0.06 |
| Minocycline | Oral | 0.52 (0.40–0.69) | 0.06 |
Challenge dose, 6.2 × 105 CFU/mouse.
TABLE 6.
In vivo activities of TBG-MINO, DMG-DMDOT, and minocycline against experimental acute lethal infections in mice
| Infection (resistance determinant or resistance; challenge dose [CFU/mouse]) | Intravenous treatment | ED50 (mg/kg) (95% confidence limit) | MIC (μg/ml) | |
|---|---|---|---|---|
| Staphylococcus aureus UBMS 90-2 (tet(M), ribosomal protection; 7.9 × 107) | TBG-MINO | 1.0 (0.87–1.3) | 0.12 | |
| DMG-DMDOT | 0.68 (0.56–0.81) | 0.12 | ||
| Minocycline | 1.8 (1.5–2.2) | 2.0 | ||
| Staphylococcus aureus UBMS 88-7,649(pUB111) (tet(K), efflux; 9.0 × 107) | TBG-MINO | 2.1 (1.8–2.6) | 0.5 | |
| DMG-DMDOT | 3.1 (2.5–3.7) | 1.0 | ||
| Minocycline | 2.0 (1.6–2.4) | 0.25 | ||
| Staphylococcus aureus NEMC 89-4 (MRSA; 5.3 × 107) | TBG-MINO | 0.79 (0.64–0.97) | 0.50 | |
| DMG-DMDOT | 0.48 (0.39–0.59) | 0.25 | ||
| Minocycline | 0.31 (0.25–0.38) | 0.12 | ||
| Staphylococcus aureus ID 4729 (MRSA, tet(M); 1.3 × 108) | TBG-MINO | 0.84 (0.69–1.0) | 0.5 | |
| DMG-DMDOT | 0.53 (0.43–0.64) | 0.25 | ||
| Minocycline | 1.6 (1.3–2.0) | 4.0 | ||
| Staphylococcus aureus ID 2371 (MRSA, tet(M), tet(K); 1.3 × 108) | TBG-MINO | 2.3 (1.9–2.7) | 1.0 | |
| DMG-DMDOT | 3.0 (2.4–3.6) | 2.0 | ||
| Minocycline | 16 (13–20) | 4.0 | ||
| Streptococcus pneumoniae ATCC 6301 (penicillin susceptible; 3.3 × 101) | TBG-MINO | 1.3 (1.1–1.6) | 0.12 | |
| DMG-DMDOT | 1.3 (1.1–1.6) | 0.06 | ||
| Minocycline | 3.9 (3.2–4.8) | 0.12 | ||
| Streptococcus pneumoniae ATCC 10015 (penicillin susceptible; 1.5 × 101) | TBG-MINO | 1.7 (1.4–2.2) | 0.12 | |
| DMG-DMDOT | 1.9 (1.5–2.4) | 0.12 | ||
| Minocycline | 3.5 (2.8–4.4) | 0.12 | ||
| Streptococcus pneumoniae GS 1894 (penicillin resistant; 3.7 × 101) | TBG-MINO | 0.61 (0.48–0.77) | 0.12 | |
| DMG-DMDOT | 0.53 (0.42–0.67) | 0.12 | ||
| Minocycline | 20 (16–26) | 4 | ||
| Escherichia coli 311 (susceptible; 2.3 × 106) | TBG-MINO | 1.7 (1.4–2.1) | 0.5 | |
| DMG-DMDOT | 1.5 (1.2–1.8) | 0.5 | ||
| Minocycline | 3.2 (2.6–4.0) | 1.0 | ||
| Escherichia coli J3272 (pRP1) (tet(A), efflux; 1.6 × 107) | TBG-MINO | 1.6 (1.0–2.6) | 0.5 | |
| DMG-DMDOT | 4.6 (2.9–7.5) | 2.0 | ||
| Minocycline | 16.0 (9.8–26.0) | 4.0 | ||
| Escherichia coli J3272(pBR322) (tet(C), efflux; 2.6 × 107) | TBG-MINO | 1.5 (1.3–1.9) | 0.25 | |
| DMG-DMDOT | 5.0 (4.1–6.4) | 2.0 | ||
| Minocycline | 14.0 (11.0–17.0) | 4.0 | ||
| Escherichia coli UBMS 90-4 (tet(M), ribosomal protection; 6.6 × 107) | TBG-MINO | 3.5 (2.8–4.3) | 0.25 | |
| DMG-DMDOT | 2.1 (1.8–2.6) | 0.25 | ||
| Minocycline | >32.0 | >32.0 | ||
| Escherichia coli UBMS 88-1, J3272TcR (tet(B), efflux; 3.9 × 107) | TBG-MINO | 3.9 (3.2–4.9) | 0.5 | |
| DMG-DMDOT | 3.1 (2.5–3.8) | 0.5 | ||
| Minocycline | >32.0 | 32.0 | ||
| Escherichia coli NEMC 87-30 (minocycline resistant; 5.3 × 107) | TBG-MINO | 1.6 (1.3–1.9) | 0.5 | |
| DMG-DMDOT | 2.0 (1.7–2.4) | 0.5 | ||
| Minocycline | >32.0 | 32.0 | ||
| Organism (no. of isolates) | Antibiotic | MIC (μg/ml) | ||
| Range | 50% | 90% | ||
| Moraxella catarrhalis (14) | TBG-MINO | 0.12–0.25 | 0.12 | 0.25 |
| DMG-MINO | 0.06–0.12 | 0.12 | 0.12 | |
| DMG-DMDOT | 0.12–0.25 | 0.12 | 0.25 | |
| Minocycline | 0.008–0.06 | 0.03 | 0.06 | |
| Tetracycline | 0.06–0.25 | 0.12 | 0.25 | |
| Ciprofloxacin | 0.03–0.06 | 0.03 | 0.06 | |
| Imipenem | 0.008–0.06 | 0.015 | 0.06 | |
| Ceftazidime | 0.015–0.12 | 0.015 | 0.06 | |
| Neisseria gonorrhoeae (22) | TBG-MINO | 0.25–1 | 0.5 | 1 |
| DMG-MINO | 0.12–1 | 0.25 | 0.5 | |
| DMG-DMDOT | 0.25–1 | 0.5 | 1 | |
| Minocycline | 0.25–>32 | 0.5 | 32 | |
| Tetracycline | 0.5–>32 | 1 | >32 | |
| Ciprofloxacin | ≤0.004 | ≤0.004 | ≤0.004 | |
| Imipenem | 0.015–0.12 | 0.06 | 0.12 | |
| Ceftazidime | 0.015–0.25 | 0.03 | 0.06 | |
| Haemophilus influenzae (15) | TBG-MINO | 0.25–1 | 0.5 | 1 |
| DMG-MINO | 0.25–0.5 | 0.25 | 0.5 | |
| DMG-DMDOT | 0.25–0.5 | 0.5 | 0.5 | |
| Minocycline | 0.12–0.25 | 0.12 | 0.25 | |
| Tetracycline | 0.12–8 | 0.25 | 0.5 | |
| Ciprofloxacin | ≤0.004–0.03 | 0.015 | 0.03 | |
| Imipenem | 1–8 | 2 | 4 | |
| Ceftazidime | 0.015–0.25 | 0.12 | 0.12 | |
TBG-MINO, DMG-DMDOT, and minocycline were observed to have similar efficacies against an infection caused by the tetracycline-susceptible strain E. coli 311, with ED50s of 1.7, 1.5, and 3.2 mg/kg, respectively. Against infections caused by E. coli strains containing tet(A) or tet(C) efflux resistance determinants, TBG-MINO (ED50s, 1.6 and 1.5 mg/kg, respectively) exhibited efficacy that was approximately three times that of DMG-DMDOT and more than nine times that of minocycline. Against an infection caused by E. coli UBMS 90-4, a laboratory strain in which the tet(M) resistance determinant mechanism was inserted, both TBG-MINO and DMG-DMDOT, with ED50s of 3.5 and 2.1 mg/kg, respectively, demonstrated good efficacy, while minocycline was not therapeutically effective at doses of up to 32 mg/kg. Intravenous administration of TBG-MINO or DMG-DMDOT resulted in good efficacy against an infection caused by E. coli UBMS 88-1, a strain carrying the tet(B) efflux resistance determinant, while minocycline was not efficacious. Both TBG-MINO and DMG-DMDOT showed efficacy (ED50s, ≤2.0 mg/kg) against an infection caused by a minocycline-resistant E. coli clinical isolate (NEMC 87-30).
DISCUSSION
Previous studies (5, 9, 12, 22, 31, 33, 34) demonstrated that the DMG modification of the 9 position of the tetracycline molecule (29), i.e., DMG-MINO and DMG DMDOT, resulted in drugs that have the ability to overcome the two major mechanisms responsible for tetracycline resistance, i.e., ribosomal protection or active efflux of drug out of the bacterial cell (1, 2, 13–15, 24, 26, 27). TBG-MINO, the 9-t-butylglycylamido derivative of minocycline, a recently synthesized member of the glycylcycline family of compounds, possesses a spectrum of activity similar to those DMG-MINO and DMG-DMDOT against most of the strains carrying the tetracycline resistance determinants. However, TBG-MINO has improved in vitro and in vivo activities against E. coli strains carrying the tet(A) or tet(C) resistance determinant.
The activity of TBG-MINO matched the activities of DMG-MINO and DMG-DMDOT against recent clinical gram-negative and -positive aerobic and anaerobic isolates, including minocycline- and tetracycline-resistant isolates. Differences in activities between TBG-MINO, DMG-MINO, and DMG-DMDOT were noted against some strains of E. coli, against which TBG-MINO was more active than DMG-MINO or DMG-DMDOT. Because TBG-MINO demonstrated better activity when it was tested against prototype strains of E. coli with tet(A) or tet(C) resistance determinants, it is possible that some of these clinical isolates may contain one or both of these resistance determinants. The MIC90s of TBG-MINO for MRSA and methicillin-resistant coagulase-negative staphylococci were also lower. The MICs of DMG-DMDOT and DMG-MINO were elevated for two of the clinical MRSA strains, which contained both tet(K) and tet(M) resistance determinants, but these strains were more sensitive to TBG-MINO (data not shown). Because all three glycylcyclines showed good activities against tet(M)-carrying strains, the slightly improved activity of TBG-MINO might reflect the slightly better inherent activity noted against tet(K)-containing strains. TBG-MINO and DMG-MINO were less active than DMG-DMDOT against Proteus spp. and M. morganii.
The improved in vitro activity of TBG-MINO was also observed in vivo when its activity against acute lethal infections in mice was tested. When it was dosed intravenously, TBG-MINO was as effective as minocycline against infections caused by minocycline-susceptible bacteria including MRSA and tet(K)-containing S. aureus. However, the ED50s of TBG-MINO and DMG-DMDOT against infections caused by MRSA that also contained tet(M) were lower than those of minocycline. Infections caused by E. coli strains carrying tet(A), tet(B), tet(C), or tet(M) were more responsive to treatment with TBG-MINO or DMG-DMDOT than to treatment with minocycline. The activity of TBG-MINO, however, exceeded the activity of DMG-DMDOT against infections caused by the tet(A)- and tet(C)-containing strains, thus reflecting the improved in vitro activity of TBG-MINO over that of DMG-DMDOT. Both TBG-MINO and DMG-DMDOT had poor efficacies when they were administered orally.
The ability of TBG-MINO to overcome the major tetracycline resistance mechanisms and extend its spectrum of activity to include multidrug-resistant staphylococci, penicillin-resistant S. pneumoniae, vancomycin-resistant enterococci, anaerobes, and minocycline-resistant bacteria while retaining activity against minocycline-susceptible microorganisms makes it an attractive new antibacterial agent. Resistance among S. pneumoniae, Enterococcus spp., and MRSA is becoming an increasing medical problem worldwide (10, 11, 17, 18, 19, 23, 32), with reduced therapeutic options and an increased need for new antimicrobial agents. TBG-MINO at concentrations of ≤0.5 μg/ml inhibited all strains of penicillin-resistant S. pneumoniae, vancomycin-resistant Enterococcus spp., and MRSA. Therefore, additional evaluation of TBG-MINO is warranted.
REFERENCES
- 1.Chopra I, Hawkey P M, Hilton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992;29:245–277. doi: 10.1093/jac/29.3.245. [DOI] [PubMed] [Google Scholar]
- 2.Chopra I, Shales S, Ball P. Tetracycline resistant determinants from groups A to D vary in their ability to confer decreased accumulation of tetracycline derivatives by Escherichia coli. J Gen Microbiol. 1982;128:689–692. doi: 10.1099/00221287-128-4-689. [DOI] [PubMed] [Google Scholar]
- 3.Cleeland R, Squires E. Evaluation of new antimicrobials in vitro and in experimental animal infections. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 752–783. [Google Scholar]
- 4.Duggar B M. Aureomycin: a product of the continuing search for new antibiotics. Ann N Y Acad Sci. 1948;51:177–181. doi: 10.1111/j.1749-6632.1948.tb27262.x. [DOI] [PubMed] [Google Scholar]
- 5.Eliopoulos G, Wennersten C, Cole G, Moellering R. In vitro activities of two glycylcyclines against gram-positive bacteria. Antimicrob Agents Chemother. 1994;38:534–541. doi: 10.1128/aac.38.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Facklam R, Collins D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finland M. Twenty-fifth anniversary of the discovery of aureomycin: the place of the tetracyclines in antimicrobial therapy. Clin Pharmacol Ther. 1974;15:3–8. doi: 10.1002/cpt19741513. [DOI] [PubMed] [Google Scholar]
- 8.Finney D J. Probit analysis. 3rd ed. London, United Kingdom: Cambridge University Press; 1971. [Google Scholar]
- 9.Goldstein F W, Kitzis M D, Acar J F. N,N-Dimethylglycylamido derivative of minocycline and 6-demethly-6-desoxytetracycline, two new glycylcyclines highly effective against tetracycline-resistant gram-positive cocci. Antimicrob Agents Chemother. 1994;38:2218–2220. doi: 10.1128/aac.38.9.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayson M, Eliopoulos G, Wennersten C, Ruoff K, Girolami P, Ferraro M, Moellering R. Increasing resistance to β-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob Agents Chemother. 1991;35:2180–2184. doi: 10.1128/aac.35.11.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Singh K, Murray B, Wolff J, Waters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin and gentamicin. Clin Infect Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 12.Kenny G E, Cartright F D. Susceptibilities of Mycoplasma hominis, Mycoplasma pneumoniae, and Ureaplasma urealyticum to new glycylcyclines in comparison with those to older tetracyclines. Antimicrob Agents Chemother. 1994;38:2628–2632. doi: 10.1128/aac.38.11.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy S B. Resistance to the tetracyclines. In: Bryan L E, editor. Antimicrobial drug resistance. New York, N.Y: Academic Press, Inc.; 1984. pp. 191–240. [Google Scholar]
- 14.Levy S B. Evolution and spread of tetracycline resistance determinants. J Antimicrob Chemother. 1989;24:1–3. doi: 10.1093/jac/24.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Levy S B, McMurry L M, Burdett V, Courvalin P, Hillen W, Roberts M C, Taylor D E. Nomenclature for tetracycline resistance determinants. Antimicrob Agents Chemother. 1989;33:1373–1374. doi: 10.1128/aac.33.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandell G L. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingston Inc.; 1993. [Google Scholar]
- 17.Mason E, Kaplan S, Lamberth L, Tillman J. Increased rate of isolation of penicillin-resistant Streptococcus pneumoniae in a childrens hospital and in vitro susceptibilities to antibiotics of potential therapeutic use. Antimicrob Agents Chemother. 1992;36:1703–1707. doi: 10.1128/aac.36.8.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan M, Murray-Leisure K, Ribner B, Standiford H, John J, Korvick J, Kauffman C, Yu V. Methicillin-resistant Staphylococcus aureus: a concensus review of the microbiology, pathogenesis and epidemiology with implications for prevention and management. Am J Med. 1993;94:313–328. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 19.Murray B. The life and times of the enterococci. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4, vol. 17, no. 2. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard M11-A4, vol. 17, no. 22. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 22.Nord C, Lindmark A, Persson I. In vitro activity of DMG-Mino and DMG-Dmdot, two new glycylcyclines, against anaerobic bacteria. Eur J Clin Microbiol Infect Dis. 1993;12:784–786. doi: 10.1007/BF02098471. [DOI] [PubMed] [Google Scholar]
- 23.Sahm D, Kissinger J, Gilmore M, Murray P, Mulder R, Solliday J, Clarke B. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 1989;33:1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salyers A A, Spear B S, Shoemaker N G. New perspectives on tetracycline resistance. Mol Microbiol. 1990;4:151–156. doi: 10.1111/j.1365-2958.1990.tb02025.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanford J. Guide to antimicrobial therapy. West Bethesda, Md: Antimicrobial Therapy, Inc.; 1997. [Google Scholar]
- 26.Shales S W, Chopra I, Ball P R. Evidence of more than one mechanism of plasmid-determined tetracycline resistance in Escherichia coli. J Gen Microbiol. 1980;121:221–229. doi: 10.1099/00221287-121-1-221. [DOI] [PubMed] [Google Scholar]
- 27.Speer B S, Shoemaker N B, Salyers A A. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev. 1992;5:387–399. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratton C W, Cooksey R C. Susceptibility tests: special tests. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 1153–1165. [Google Scholar]
- 29.Sum P E, Lee V J, Testa R T, Hlavka J J, Ellestad G A, Bloom J D, Gluzman Y, Tally F P. Glycylcyclines. I. A new generation of potent antibacterial agents through modification of 9-aminotetracyclines. J Med Chem. 1993;37:184–188. doi: 10.1021/jm00027a023. [DOI] [PubMed] [Google Scholar]
- 30.Sutter V L, Citron D M, Edelstein M A C, Finegold S M. Wadsworth anaerobic bacteriology manual. 4th ed. Belmont, Calif: Star Publishing Co.; 1985. [Google Scholar]
- 31.Testa R T, Petersen P J, Jacobus N V, Sum P E, Lee V J, Tally F T. In vitro and in vivo antibacterial activities of the glycylcyclines, a new class of semisynthetic tetracyclines. Antimicrob Agents Chemother. 1993;37:2270–2277. doi: 10.1128/aac.37.11.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uttley A H C, Collins C H, Naidoo J, George K C. Vancomycin resistant enterococci. Lancet. 1988;i:57–58. doi: 10.1016/s0140-6736(88)91037-9. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 33.Wexler H M, Molitoris E, Finegold S M. In vitro activities of two new glycylcyclines, N,N-dimethylglycylamido derivatives of minocycline and 6-demethyl-6-deoxytetracycline, against 339 strains of anaerobic bacteria. Antimicrob Agents Chemother. 1994;38:2513–2515. doi: 10.1128/aac.38.10.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wise R, Andrews J M. In vitro activity of two glycylcyclines. Antimicrob Agents Chemother. 1994;38:1096–1102. doi: 10.1128/aac.38.5.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]