Abstract
Objectives:
To assess 1-week and 1-month efficacy of Systane iLux thermal pulsation treatment for meibomian gland dysfunction (MGD).
Methods:
This prospective, nonrandomized, open-label, multicenter study enrolled 30 adult patients (60 eyes) who had a Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire score greater than 6 and total meibomian gland secretion (MGS) score equal to or less than 12 in each eye. All participants received thermal pulsation treatment bilaterally. Primary efficacy measures included MGS score (sum of grades for 15 glands graded on a scale of 0–3; 0 [no secretion], 1 [inspissated], 2 [cloudy], and 3 [clear liquid]) and tear breakup time (TBUT). Secondary efficacy measures were SPEED and Ocular Surface Disease Index (OSDI) scores.
Results:
The mean age of patients was 52.9±11.9 years. After 1 week, the mean MGS score improved significantly from 4.1±3.1 to 15.8±7.1 (right eye, OD) and 3.7±3.1 to 16.7±7.6 (left eye, OS); mean TBUT improved significantly from 4.9±4.1 to 8.4±3.6 (OD) and 5.2±4.2 to 8.9±3.9 (OS); and mean SPEED and OSDI scores improved significantly from 16.1±5.3 to 7.2±6.1 and 45.2±21.3 to 19.0±16.8, respectively (all P<0.001). After 1 month, the mean MGS score improved to 18.3±8.2 (OD) and 18.6±7.3 (OS); mean TBUT improved to 9.7±3.8 (OD) and 9.6±3.5 (OS); and mean SPEED and OSDI scores improved to 7.0±5.6 and 16.7±14.5, respectively (all P<0.001). No adverse events were reported.
Conclusions:
Systane iLux thermal pulsation treatment for MGD resulted in a statistically significant increase in meibomian gland secretion, improvement in tear film stability, and reduction in dry eye symptoms as early as both 1 week and 1 month.
Key Words: iLux thermal pulsation treatment, Meibomian gland dysfunction, MGD, Dry eye disease, Systane iLux
Meibomian gland dysfunction (MGD) is believed to be the leading cause of dry eye disease and is characterized by terminal duct obstruction and reduced quality or quantity of meibum that can lead to signs and symptoms of dry eye.1,2 The prevalence of MGD in the normal population is reported to be as high as 70% but is often undiagnosed and untreated.1,3–7 It is considered to be the most predominant form of dry eye disease, with more than 86% of patients with dry eye presenting with clinical signs of MGD.2 The presence of MGD can negatively affect many aspects of ocular surface health and may interfere with quality of life, work performance, and productivity.8
Although the pathophysiology of MGD remains unclear, increased viscosity of the meibum and hyperkeratinization of the ductal epithelium have been observed and are believed to cause duct obstruction, cystic dilation, and eventual acinar atrophy and gland dropout.9,10 The mainstay treatment of MGD involves the application of therapeutic levels of heat and pressure to the eyelids to melt altered meibum and to clear meibomian gland obstruction, leading to the improved meibum quality, tear film stability, and dry eye symptoms.11 Home therapy warm compresses have had varying degrees of success because of their inability to reach or maintain a therapeutic temperature, lack of standardization in regimen, or patient noncompliance.1 In-office treatment of MGD with thermal pulsation systems that apply simultaneous heat and pressure to melt and express meibum has been reported to effectively clear meibomian gland obstruction and improve dry eye symptoms by as early as two weeks and lasting for as long as 12 months.12–15
Although the duration of the effect is important, time to effect can be particularly relevant for severely symptomatic and presurgical patients with MGD seeking quick relief. This study aimed to evaluate the efficacy of the Systane iLux thermal pulsation system (Alcon, Fort Worth, TX) at both 1 week and 1 month for patients with MGD by assessing the meibomian gland secretion score and tear breakup time, as well as symptoms using standardized dry eye questionnaires, including the SPEED questionnaire.
MATERIALS AND METHODS
This prospective, nonrandomized, open-label, multicenter study (ClinicalTrials.gov identifier: NCT03055650) was conducted at three clinical sites in California (Gordon Schanzlin New Vision Institute, La Jolla, CA; Encinitas Optometry, Encinitas, CA; and Total Vision Care, San Diego, CA). The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the Aspire IRB, Santee, CA. All subjects provided written informed consent before any study-related procedures.
Subjects
Subjects aged 18 years or older of any sex or race were enrolled if they reported dry eye symptoms for three months before the study enrollment; had a Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire score greater than 6 of a maximum of 28; had evidence of meibomian gland obstruction (total meibomian gland secretion score ≤12, of a maximum score of 45, for 15 glands [five nasal, five medial, and five temporal] in each lower eyelid); had a need for regular use of artificial tears, lubricants, or rewetting drops in both eyes; and were able to return for all study visits. Subjects were excluded if they had a history of ocular surgery or isotretinoin (e.g., Accutane) use within the past year; ocular trauma, herpetic keratitis, or recurrent ocular inflammation within the past 3 months; cyclosporine A (Restasis) use within the past 2 months; contact lens wear, fluctuations in systemic or ophthalmic medication dose, or use of another investigational device or agent within the past month; or use of topical medications other than nonpreserved artificial tears within 2 weeks. Other exclusion criteria included systemic disease conditions that cause dry eye (e.g., Sjogren syndrome), active ocular infection, anterior blepharitis, lid abnormalities that affect lid function, limbal stem cell deficiency, pregnancy, or lactation.
Study Treatment
Treatment with the Systane iLux thermal pulsation device was delivered under topical anesthesia as previously described.15 Both eyes were treated on the same day with the right eye treated before the left eye. The medial temporal zone of the lower eyelid was treated first followed by the medial nasal zone. The eyelid margin was viewed throughout the procedure using the built-in magnifying lens on the device. If clear meibum was successfully expressed across the entire treatment zone, treatment was stopped; otherwise, heat was applied again (40–50 sec) and compression or decompression was repeated up to three additional times. Once clear meibum was visible across the treatment zone or at the end of four compression cycles, treatment was stopped. Postprocedure, the subject could use only nonpreserved artificial tears, as needed, until the 1-month study outcome assessment was completed.
Primary efficacy outcomes were changes from baseline in the meibomian gland secretion score and tear breakup time evaluated 1 week and 1 month after the study procedure. A Meibomian Gland Evaluator (MGE 1000; Johnson & Johnson Vision, Santa Ana, CA) was used to express a total of 15 meibomian glands in the nasal, medial, and temporal regions (5 glands each) of the lower eyelid, and the meibomian gland secretion score was determined using a scale of 0 (no secretion), 1 (inspissated), 2 (cloudy), and 3 (clear liquid secretion) for each gland. Tear breakup time was measured after instilling fluorescein in the lower fornix. The time (in seconds) from the blink that evenly distributed the fluorescein to the appearance of the first tear breakup was evaluated. The average of three consecutive measurements was recorded for each eye.
The secondary efficacy outcomes were changes from baseline in Standard Patient Evaluation of Eye Dryness (SPEED) and Ocular Surface Disease Index (OSDI) scores at 1 week and 1 month.16–18 Changes from the baseline of subscores for SPEED (frequency and severity of dry eye symptoms) and OSDI (vision-related symptoms, ocular symptoms, and environmental triggers) were also calculated.
Safety parameters included assessments of best-corrected visual acuity, manifest refraction, intraocular pressure, slitlamp examination for anterior segment health, and nondilated fundus examination. Reporting of adverse events included assessment of pain and patient discomfort during and after the treatment, lid margin abnormalities, and corneal surface staining. Pain or discomfort was assessed using a subjective pain scale with descriptors provided for the subject as previously described.19 The scale ranged from 0 to 10 and included the following: 0 = no discomfort or pain, 2 = slight or transient awareness of pressure without pain, 4 = moderate discomfort with minimal pain, 6 = moderate pain, 8 = severe pain, and 10 = intolerable pain. The scale had descriptors comparative experiences to provide context and promote consistency. Lid margin abnormalities were scored from 0 to 4 based on the number of abnormalities: irregular lid margin, vascular engorgement, plugged meibomian gland orifices, and anterior or posterior placement of the mucocutaneous junction. Corneal fluorescein staining was evaluated using the National Eye Institute corneal grading scale.20 Serious adverse events involving life-threatening or sight-threatening conditions that may have required medical or surgical intervention were also assessed.
Statistical Analysis
Based on results from relevant published clinical studies, a sample size of 30 patients (60 eyes) was needed to detect a clinically meaningful change for both primary outcomes,15 meibomian gland secretion (5 points) and tear breakup time (2.5 sec), using t tests assuming an (two-sided) α of 0.05. The change from the baseline of primary and secondary efficacy outcome was assessed at 1 week posttreatment. Secondary outcomes were planned to be analyzed at 1 month only if both primary outcomes were significant at 1 month in both eyes. All subjects enrolled in the study were included as part of the safety analysis. Descriptive statistics were calculated as appropriate including mean, SD, and 95% confidence intervals. To assess the treatment effect between baseline and 1-week and between baseline and 1-month visits in separate analyses, t tests were used. Statistical significance was set at α=0.05. For measurements made on each eye separately, statistics were calculated for right and left eyes separately. All analyses were performed using Statistical Analysis Software version 9.4 (SAS Institute Inc, Cary, NC).
Given that four comparisons were planned for each primary outcome measure—two eyes and two posttreatment assessments—the criterion for statistical significance (α) should be adjusted from 0.05 to 0.0125 (=0.05/4).
RESULTS
Thirty subjects met the eligibility criteria and were enrolled in the study. There were 20 women and 10 men, and the mean (±SD) age of all subjects was 52.9±11.9 years (range: 25–74 years). Twenty of the subjects (67%) were White, four (13%) Native American, two Asian, two Hispanic, one Persian, and one of unknown race. All subjects completed the study. Of the 29 right eyes where treatment data were available, 11 required only one compression to express clear meibum, whereas 12 received the maximum four treatment cycles. For the 30 left eyes, 14 required only one compression, whereas 12 underwent four cycles.
There was a statistically significant improvement in primary (mean meibomian gland secretion score and mean tear breakup time) and secondary (mean SPEED and mean OSDI scores) efficacy outcomes at 1 week and 1 month after treatment compared with baseline (all P<0.001).
Primary Efficacy Outcomes: Meibomian Gland Secretion and Tear Breakup Time
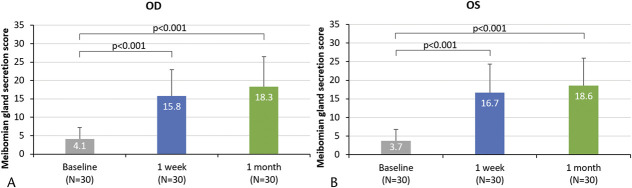
The mean meibomian gland secretion score increased by at least 11 points on the 45-point scale in each eye and at each posttreatment assessment (all P<0.001; Table 1 and Fig. 1). The mean tear breakup time also increased by at over 3 seconds in each eye and at each posttreatment assessment (all P<0.001; Table 1).
TABLE 1.
Mean Meibomian Gland Secretion Score for Right (OD) and Left (OS) Eyes and Tear Breakup Time (in Seconds) at Each Study Visit
| Baseline | 1 Week | Change From Baseline to 1 week | P a | 1 Month | Change From Baseline to 1 Month | P b | |||
| Mean±SD | Mean±SD | Mean±SD | 95% CI (Lower, Upper) | Mean±SD | Mean±SD | 95% CI (Lower, Upper) | |||
| N=30 | N=30 | N=30 | N=30 | N=30 | N=30 | N=30 | |||
| Meibomian gland secretion score | |||||||||
| OD | 4.1±3.1 | 15.8±7.1 | 11.6±6.9 | +9.06, +14.20 | <0.001 | 18.3±8.2 | 14.2±8.2 | +11.10, +17.24 | <0.001 |
| OS | 3.7±3.1 | 16.7±7.6 | 13.0±7.3 | +10.24, +15.69 | <0.001 | 18.6±7.3 | 14.8±7.7 | +11.97, +17.69 | <0.001 |
| Tear breakup time | |||||||||
| OD | 4.9±4.1 | 8.4±3.6 | 3.5±2.7 | +2.46, +4.47 | <0.001 | 9.7±3.8 | 4.7±3.3 | +3.48, +5.96 | <0.001 |
| OS | 5.2±4.2 | 8.9±3.9 | 3.7±3.5 | +2.43, +5.04 | <0.001 | 9.6±3.5 | 4.4±2.9 | +3.31, +5.47 | <0.001 |
Change from baseline to 1 week and 1 month are also shown.
Change from baseline to 1 week.
Change from baseline to 1 month.
FIG. 1.
Mean meibomian gland secretion score (out of 45) at baseline, posttreatment 1 week, and posttreatment 1 month in (A) right eyes and (B) left eyes. Error bars represent the positive SD. P values for change in the meibomian gland secretion score from baseline to 1 week and baseline to 1 month are presented.
Secondary Efficacy Outcomes: Standard Patient Evaluation of Eye Dryness and Ocular Surface Disease Index
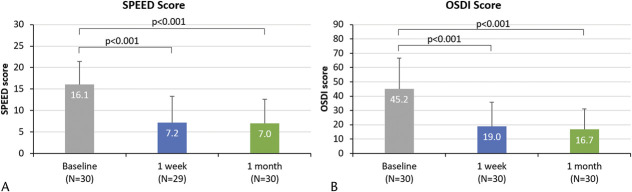
The mean (±SD) SPEED scores reduced from 16.1±5.3 at baseline to 7.2±6.1 at 1 week (mean change of −8.6±7.0; 95% CI: −11.2, −5.9; P<0.001) and 7.0±5.6 at 1 month (mean change of −9.1±6.6; 95% CI: −11.6, −6.6; P<0.001) (Fig. 2A). Similarly, SPEED subscores of symptoms of burning, dryness, eye fatigue, and soreness also showed a statistically significant reduction in frequency and severity (all P<0.001, Table 2).
FIG. 2.
Mean Standard Patient Evaluation of Eye Dryness (SPEED) score (out of 28) and mean Ocular Surface Disease Index (OSDI) total score (out of 100) at baseline, posttreatment 1 week, and posttreatment 1 month. Error bars represent the SDs. P values for change in SPEED score and OSDI score from baseline to 1 week and baseline to 1 month are presented.
TABLE 2.
Mean Standard Patient Evaluation of Eye Dryness (SPEED) Score (Individual Symptoms) at Each Study Visit
| Symptom | Baseline | 1 week | Change from Baseline to 1 week | P a | 1 month | Change from Baseline to 1 month | P b |
| Mean | Mean | Mean | Mean | Mean | |||
| N=30 | N=29 | N=29 | N=30 | N=30 | |||
| Frequency of burning | 1.67 | 0.79 | −0.86 | <0.001 | 0.73 | −0.93 | <0.001 |
| Frequency of dryness | 2.13 | 1.24 | −0.90 | <0.001 | 1.17 | −0.97 | <0.001 |
| Frequency of eye fatigue | 1.43 | 0.66 | −0.76 | <0.001 | 0.83 | −0.60 | <0.001 |
| Frequency of soreness | 1.77 | 0.76 | −1.00 | <0.001 | 0.57 | −1.20 | <0.001 |
| Severity of burning | 2.20 | 0.86 | −1.28 | <0.001 | 0.90 | −1.30 | <0.001 |
| Severity of dryness | 2.70 | 1.31 | −1.34 | <0.001 | 1.23 | −1.47 | <0.001 |
| Severity of eye fatigue | 1.90 | 0.72 | −1.10 | <0.001 | 0.93 | −0.97 | <0.001 |
| Severity of soreness | 2.27 | 0.90 | −1.31 | <0.001 | 0.60 | −1.67 | <0.001 |
Change from baseline to 1 week and 1 month are also shown.
Change from baseline to 1 week.
Change from baseline to 1 month.
The mean OSDI scores reduced from 45.2±21.3 at baseline to 19.0±16.8 at 1 week (mean change of −26.3±24.3; 95% CI: −35.3, −17.2; P<0.001) and 16.7±14.5 at 1 month (mean change of −28.5±22.0; 95% CI: −36.7, −20.3; P<0.001) (Fig. 2B). The OSDI subscores for vision-related symptoms (questions 1–5), ocular symptoms (questions 6–9), and environmental triggers (questions 10–12) also demonstrated a statistically significant reduction at both posttreatment visits from baseline (all P<0.001; Table 3). Similarly, when each of the 12 OSDI questions was individually analyzed, significant reductions were observed at posttreatment 1 week and 1 month (all P<0.002).
TABLE 3.
Mean Ocular Surface Disease Index (OSDI) Score (Individual Symptoms) at Each Study Visit
| Symptom | Baseline | 1 week | Change from Baseline to 1 week | P a | 1 month | Change from Baseline to 1 month | P b | ||
| Mean±SD | Mean±SD | Mean±SD | 95% CI (Lower, Upper) | Mean±SD | Mean±SD | 95% CI (Lower, Upper) | |||
| N=30 | N=30 | N=30 | N=30 | N=30 | N=30 | N=30 | |||
| Vision-related symptoms | 42.0±22.2 | 18.0±17.4 | −24.0±26.9 | −34.1, −14.0 | 0.001 | 14.5±12.2 | −27.5±23.9 | −36.3, −18.4 | <0.001 |
| Ocular symptoms | 37.5±27.9 | 13.8±17.0 | −23.8±25.1 | −33.1, −14.4 | 0.001 | 12.5±16.5 | −25.0±24.6 | −33.9, −15.6 | <0.001 |
| Environmental triggers | 60.8±27.8 | 27.5±26.4 | −33.3±32.7 | −45.5, −21.2 | 0.002 | 25.8±27.7 | −35.0±32.6 | −47.4, −23.1 | <0.001 |
Change from baseline to 1 week and 1 month are also shown.
Change from baseline to 1 week.
Change from baseline to 1 month.
Safety Results
No adverse or serious adverse events were reported during the study. Eight subjects reported discomfort scores of 4 (moderate discomfort with minimal pain) or 6 (moderate pain) during the treatment, but mean discomfort scores returned to baseline levels or below by one day posttreatment and further improved by the 1-week and 1-month follow-up visits. Corneal fluorescein staining improved significantly from the baseline mean of 1.4±2.0 to 0.8±1.7 (right eye, P=0.002), 1.7±2.2 to 0.9±1.5 (left eye, P=0.001) at 1 week and to 1.0±2.1 (right eye, P=0.037) and 0.7±1.1 (left eye, P=0.001) at 1 month. There was no statistically significant change in intraocular pressure or lid abnormalities from baseline to 1 week or 1 month.
DISCUSSION
Thermal pulsation systems, including Systane iLux, have been well documented to clear meibomian gland obstruction and improve dry eye symptoms at both 2 and 4 weeks posttreatment.12–15,19,21,22 This study found that a single 8- to 10-minute treatment with the Systane iLux thermal pulsation system produced statistically significant and clinically relevant improvements in key study outcomes at both 1 week and 1 month after treatment. Specifically, the mean meibomian gland secretion score and tear breakup time significantly improved, and subjective symptom scores (OSDI and SPEED) were markedly reduced at all study time points after treatment. Statistical significance was easily reached, even when adjusting criteria for multiple comparisons.
The 1-month efficacy results are consistent with those from the Systane iLux pivotal study.15 The statistically significant improvement of the meibomian gland secretion score of almost 15 points (45-point scale) in this study was similar to the improvement of almost 18 points in the pivotal study (all P<0.001). The magnitude of these changes suggests that, on average, Systane iLux treatment yielded the equivalent of clear meibum from additional 5 to 6 glands by 1 month posttreatment. Similarly, the 1-month improvement of approximately 4.5 seconds in the mean tear breakup time in this study was greater than the clinically meaningful improvement of 2.5 seconds.15 The pivotal study also reported a clinically and statistically significant improvement of almost 3 seconds in the mean tear breakup time (all P<0.001).15 SPEED scores, which reduced from approximately 16 to 7 (28-point scale), suggest that, on average, patients improved from severe to moderate dry eye classification by 1 month after treatment.23 The OSDI scores in this study (28.5-point change) were also similar to the pivotal study (30-point change) at 1 month posttreatment (all P<0.001). For reference, it is estimated that a 4.5 to 7.3 unit change in OSDI is meaningful for subjects with mild-to-moderate symptoms and that a 7.3 to 13.4 unit change is meaningful for patients with severe symptoms.24 Therefore, the observed mean decreases in both SPEED and OSDI suggest a clinically relevant improvement and that, on average, participants improved from having severe symptoms at baseline to having mild-to-moderate symptoms by 1 month after a single Systane iLux treatment.
Although the 1-month results of this study further support previous findings that Systane iLux can provide an extended period of comfort, the 1-week efficacy demonstrates potential benefits for patients seeking quicker relief, including patients with severe dry eye and patients planning to undergo refractive or cataract surgery. Nearly 50% of patients with cataract have signs and symptoms of MGD, and an estimated 38% to 75% of patients scheduled for refractive surgery have dry eye symptoms.25,26 Because tear film instability of patients with dry eye can affect the repeatability of keratometry and biometry measurements, preoperative patients can benefit from MGD treatments that more rapidly stabilize the tear film to yield optimal ocular surface health and symptoms and more reliable presurgical plans.27
Although 2-week efficacy results have been reported for Systane iLux treatment,15 this is the first report of 1-week efficacy results. Similar to 1-month results, there were significant improvements in clinical signs and symptoms of MGD as soon as 1 week after treatment. In this study, the mean meibomian gland secretion score increased by approximately 10 points on a 45-point scale at 1 week compared with baseline, which in this case was equivalent to approximately three additional clear meibum-secreting glands. In addition, tear breakup time increased by an average of 3.6 seconds from a baseline mean of 5.1 seconds. The improved gland function and tear film stability were accompanied by a significant reduction in dry eye symptoms, represented by a decrease in the OSDI score of approximately 26 units. On average, patients went from having severe symptoms at baseline (mean±SD=45.2±21.3) to having mild symptoms 1 week after treatment (19.0±16.8). Of note, at 1 week posttreatment, the change in the meibomian gland secretion score was approximately 84% of that achieved at 1 month, suggesting that Systane iLux can effectively and rapidly clear the obstruction of meibomian glands. Similarly, the 1-week change in tear breakup time and OSDI were approximately 74% and 92% of that achieved at 1 month posttreatment. The continued improvement between 1 week and 1 month supports a previous hypothesis that clearing of the obstruction may help to gradually restore downregulated meibomian glands.19 Further research is needed to understand the physiological changes to the meibomian glands during this period of continued improvement.
In this study, both OSDI and SPEED questionnaires were used to assess symptoms, and the analysis was completed for both the composite scores and subscores. As discussed above, the change in SPEED and OSDI suggests an average reduction in overall symptom severity at both 1 week and 1 month based on the composite score and the subscores. Vision-related symptom assessment (OSDI questions 1–5) suggests significant improvements in light sensitivity, gritty sensation, pain or soreness, blurred vision, and perception of poor vision. Ocular symptom assessment (OSDI questions 6–9) suggests significant improvements with reading, night driving, computer or ATM, and television watching tasks. Finally, environmental triggers (OSDI questions 10–12), such as windy, low humidity, and air conditioning environments, were significantly less influential to patient comfort. SPEED scores also significantly improved substantially from baseline at 1 week and 1 month posttreatment. When evaluating the subscores at both 1 week and 1 month, the overall composite score improvement could be attributed to reductions in frequency and severity of burning, dryness, eye fatigue, and soreness. Both the OSDI and SPEED score results suggest that the reduction in frequency and severity of symptoms was key to driving the improved overall comfort, which was associated with reduced problems with completing key daily tasks and increased tolerability to environmental triggers.
One-month efficacy of the LipiFlow thermal pulsation system has been shown to provide a statistically significant improvement in the mean meibomian gland secretion score (range: 5.9–11.5) and tear breakup time (range: 1.1–4.9 seconds) and reductions in SPEED (range: 4.1–8.5) and OSDI scores (range: 12.4–21.5).12–14,19,21,28–30 When Systane iLux was compared with the LipiFlow thermal pulsation system in the pivotal trial, the Systane iLux system was shown to be noninferior to LipiFlow by 4 weeks after a single treatment.15 However, a direct comparison of the 1-week efficacy between LipiFlow and Systane iLux is not available. For LipiFlow, 1-week efficacy was evaluated in two separate studies. Friedland et al.19 reported a statistically significant improvement from baseline to 1 week posttreatment in the mean meibomian gland secretion score (3.4±3.2–8.8±3.0), tear breakup time (5.2±2.6–10.1±7.6 seconds), SPEED (16.2±5.4–10.2±4.2), and OSDI scores (37.0±23.8–25.6±21.7). Although Zhao et al.22 did not evaluate the mean change in the meibomian gland secretion score, the authors reported a statistically significant increase from baseline to 1 week in the mean tear breakup time (2.5±0.8–3.2±1.0 seconds) and reduction in SPEED scores (11.2±4.9–7.4±5.2) posttreatment. The results of OSDI score were consistent with SPEED scores. Although the changes after 1 week of treatment with LipiFlow seem to be less than those seen with Systane iLux after the same period in this study, it is unclear whether these devices would perform similarly in a head-to-head 1-week study.
Other in-office eyelid heating devices are available on the market, but do not offer the simultaneous heating and expression that thermal pulsation provides. For example, the TearCare System (Sight Sciences, Inc, Menlo Park, CA) delivers heat to the eyelid for several minutes, after which manual meibomian gland expression with a pair of forceps is recommended to clear the gland ducts. In a recent published study, TearCare improved tear breakup time, meibomian gland secretion score, and symptoms (OSDI) after 1 week and 1 month following a single treatment involving 15 min of heating and two rounds of comprehensive gland expression on all four eyelids.31 It is unclear whether efficacy is different between TearCare and Systane iLux at 1 week or 1 month, but the additional time needed to conduct two rounds of gland expression separately along all four eyelids might be a drawback for practices that prefer shorter treatment times.
Another commonly used in-office MGD heating treatment is intense pulsed light (IPL) therapy. Although the precise mechanism of IPL is not clearly understood, it has been hypothesized that thermal heating of the glands causes melting of the thickened meibum and dilates the glands to facilitate effective clinical expression.32,33 Studies evaluating the safety and effectiveness of IPL reported significant improvement in meibum quality, tear breakup time, and ocular surface symptom scores at 4 to 8 weeks after three to eight treatment sessions.32,34–36 Although the results suggest IPL to be effective, the reported time to efficacy may be longer than desired for patients needing quick relief or preparing for surgery.
This study is not without its limitations. Although the objective measures of meibomian gland secretion and tear breakup time were assessed on each eye, the assessment of subjective symptom scores was made on individual patients and, as a result, may be driven by the better eye or the worse eye or some combination of the two. Nevertheless, the improvements in both objective measures were similar for right and left eyes. Frequency of at-home therapies, such as artificial tears, warm compresses, and lid hygiene, was not monitored by the study and can potentially affect key study outcomes. Both the investigator and participants were unmasked, which may bias assessments; however, the results in this study were consistent with previously published studies. In contrast to a recent clinical trial,15 this study was limited by the absence of a control group.
CONCLUSION
MGD treatment with the Systane iLux thermal pulsation system was found to be effective in relieving meibomian gland obstruction by as early as 1 week and lasting through at least 1 month as represented by statistically significant and clinically relevant improvements in meibomian gland secretion score, tear breakup time, and patient-reported dry eye symptom scores.
ACKNOWLEDGMENTS
Editorial assistance in the preparation of this article was provided by IrisARC - Analytics, Research & Consulting, Chandigarh, India.
Footnotes
D. Schanzlin has received consulting fees from Refocus Group (Dallas, TX). J.P. Owen has been a consultant or received honorarium from ScienceBased Health and Alcon Vision, LLC. S. Klein has received honoraria or consulting fees from AMO, Alcon, CooperVision, J&J, Tear Film Innovations, SynergEyes, and Zeiss. He is not an equity holder in any ophthalmic industry companies. T.N. Yeh and M.M. Merchea are employees of Alcon Vision, LLC. M.A. Bullimore is a consultant for Alcon Research, Apellis, Arctic Vision, AsclepiX, CooperVision, CorneaGen, Essilor, Euclid Systems, Eyenovia, Genentech, Johnson & Johnson Vision, Lentechs, Novartis, Oculus, Paragon Vision Sciences, and Presbia.
Contributor Information
David Schanzlin, Email: dschanz123@aol.com.
James P. Owen, Email: encinitasod@gmail.com.
Steve Klein, Email: steve.klein@totalvisionllc.com.
Thao N. Yeh, Email: thao.yeh@alcon.com.
Mohinder M. Merchea, Email: mo.merchea@Alcon.com.
REFERENCES
- 1.Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2011;52:2050–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea 2012;31:472–478. [DOI] [PubMed] [Google Scholar]
- 3.Amano S, Inoue K. Estimation of prevalence of meibomian gland dysfunction in Japan. Cornea 2017;36:684–688. [DOI] [PubMed] [Google Scholar]
- 4.Murali A, Krishnaswamy M. Study of meibomian gland dysfunction in patients undergoing cataract surgery from rural ophthalmology camps. Delhi J Ophthalmol 2016;27:227–229. [Google Scholar]
- 5.Rabensteiner DF, Aminfar H, Boldin I, et al. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol 2018;96:707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaumberg DA, Nichols JJ, Papas EB, et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52:1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang XB, Ding YH, He W. The association between demodex infestation and ocular surface manifestations in meibomian gland dysfunction. Int J Ophthalmol 2018;11:589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Wang Y, Dong N, et al. Meibomian gland dysfunction determines the severity of the dry eye conditions in visual display terminal workers. PLoS One 2014;9:105575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: The role of gland dysfunction in dry eye disease. Ophthalmology 2017;124:S20–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Richards SM, Lo K, et al. Changes in gene expression in human meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2011;52:2727–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabeti S, Kheirkhah A, Yin J, et al. Management of meibomian gland dysfunction: A review. Surv Ophthalmol 2020;65:205–217. [DOI] [PubMed] [Google Scholar]
- 12.Blackie CA, Coleman CA, Holland EJ. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol 2016;10:1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greiner JV. Long-term (12-month) improvement in meibomian gland function and reduced dry eye symptoms with a single thermal pulsation treatment. Clin Exp Ophthalmol 2013;41:524–530. [DOI] [PubMed] [Google Scholar]
- 14.Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea 2012;31:396–404. [DOI] [PubMed] [Google Scholar]
- 15.Tauber J, Owen J, Bloomenstein M, et al. Comparison of the iLUX and the LipiFlow for the treatment of meibomian gland dysfunction and symptoms: A randomized clinical trial. Clin Ophthalmol 2020;14:405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korb DR, Herman JP, Greiner JV, et al. Lid wiper epitheliopathy and dry eye symptoms. Eye Contact Lens 2005;31:2–8. [DOI] [PubMed] [Google Scholar]
- 17.Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea 2013;32:1204–1210. [DOI] [PubMed] [Google Scholar]
- 18.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol 2000;118:615–621. [DOI] [PubMed] [Google Scholar]
- 19.Friedland BR, Fleming CP, Blackie CA, et al. A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res 2011;36:79–87. [DOI] [PubMed] [Google Scholar]
- 20.Lemp MA. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J 1995;21:221–232. [PubMed] [Google Scholar]
- 21.Finis D, Hayajneh J, Konig C, et al. Evaluation of an automated thermodynamic treatment (LipiFlow(R)) system for meibomian gland dysfunction: A prospective, randomized, observer-masked trial. Ocul Surf 2014;12:146–154. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Xie J, Li J, et al. Evaluation of monocular treatment for meibomian gland dysfunction with an automated thermodynamic system in elderly Chinese patients: A contralateral eye study. J Ophthalmol 2016;2016:9640643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asiedu K, Kyei S, Mensah SN, et al. Ocular surface disease index (OSDI) versus the standard patient evaluation of eye dryness (SPEED): A study of a nonclinical sample. Cornea 2016;35:175–180. [DOI] [PubMed] [Google Scholar]
- 24.Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol 2010;128:94–101. [DOI] [PubMed] [Google Scholar]
- 25.Cochener B, Cassan A, Omiel L. Prevalence of meibomian gland dysfunction at the time of cataract surgery. J Cataract Refract Surg 2018;44:144–148. [DOI] [PubMed] [Google Scholar]
- 26.Cohen E, Spierer O. Dry eye post-laser-assisted in situ keratomileusis: Major review and latest updates. J Ophthalmol 2018;2018:4903831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matossian C. Impact of thermal pulsation treatment on astigmatism management and outcomes in meibomian gland dysfunction patients undergoing cataract surgery. Clin Ophthalmol 2020;14:2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greiner J; LipiFlow Study Group. Treatment of meibomian gland dysfunction (MGD) with the novel LipiFlow® Thermal Pulsation System restores meibomian gland function. Invest Ophthalmol Vis Sci 2010;51:6282. [Google Scholar]
- 29.Greiner JV. A single LipiFlow® Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res 2012;37:272–278. [DOI] [PubMed] [Google Scholar]
- 30.Liang Q, Liu H, Guo Y, et al. [Clinical evaluation of a thermodynamic treatment system for meibomian gland dysfunction]. Zhonghua Yan Ke Za Zhi 2015;51:924–931. [PubMed] [Google Scholar]
- 31.Karpecki P, Wirta D, Osmanovic S, et al. A prospective, post-market, multicenter trial (CHEETAH) suggested TearCare® system as a safe and effective blink-assisted eyelid device for the treatment of dry eye disease. Clin Ophthalmol 2020;14:4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albietz JM, Schmid KL. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin Exp Optom 2018;101:23–33. [DOI] [PubMed] [Google Scholar]
- 33.Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg 2015;33:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arita R, Mizoguchi T, Fukuoka S, et al. Multicenter study of intense pulsed light therapy for patients with refractory meibomian gland dysfunction. Cornea 2018;37:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig JP, Chen YH, Turnbull PR. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2015;56:1965–1970. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X, Lv H, Song H, et al. Evaluation of the safety and effectiveness of intense pulsed light in the treatment of meibomian gland dysfunction. J Ophthalmol 2016;2016:1910694. [DOI] [PMC free article] [PubMed] [Google Scholar]