Supplemental Digital Content is available in the text.
Keywords: adipose tissue, heart failure, magnetic resonance imaging, obesity, prognosis
Background:
Epicardial adipose tissue (EAT) accumulation is thought to play a role in the pathophysiology of heart failure (HF) with mid-range and preserved ejection fraction, but its effect on outcome is unknown. We evaluated the prognostic value of EAT volume measured with cardiac magnetic resonance in patients with HF with mid-range ejection fraction and HF with preserved ejection fraction.
Methods:
Patients enrolled in a prospective multicenter study that investigated the value of implantable loop-recorders in HF with mid-range ejection fraction and HF with preserved ejection fraction were analyzed. EAT volume was quantified with cardiac magnetic resonance. Main outcome was the composite of all-cause mortality and first HF hospitalizations. Hazard ratios (HR) and 95% CI are described per SD increase in EAT.
Results:
We studied 105 patients (mean age 72±8 years, 50% women, and mean left ventricular ejection fraction 53±8%). During median follow-up of 24 (17–25) months, 31 patients (30%) died or were hospitalized for HF. In univariable analysis, EAT was significantly associated with a higher risk of the composite outcome (HR, 1.76 [95% CI, 1.24–2.50], P=0.001), and EAT remained associated with outcome after adjustment for age, sex, and body mass index (HR, 1.61 [95% CI, 1.13–2.31], P=0.009), and after adjustment for New York Heart Association functional class and N-terminal of pro-brain natriuretic peptide (HR, 1.53 [95% CI, 1.04–2.24], P=0.03). Furthermore, EAT was associated with all-cause mortality alone (HR, 2.06 [95% CI, 1.26–3.37], P=0.004) and HF hospitalizations alone (HR, 1.54 [95% CI, 1.04–2.30], P=0.03).
Conclusions:
EAT accumulation is associated with adverse prognosis in patients with HF with mid-range ejection fraction and HF with preserved ejection fraction. This finding supports the importance of EAT in these patients with HF.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01989299.
What Is New?
This study found that accumulation of epicardial adipose tissue (EAT) is strongly associated with poor prognosis in patients with heart failure with mid-range and preserved ejection fraction.
Patients with obesity with increased EAT had a significantly higher relative event rate compared to patients with obesity with low EAT.
What are the Clinical Implications?
These findings support that measurement of EAT should be considered in patients with heart failure with mid-range ejection fraction and heart failure with preserved ejection fraction as part of clinical work-up.
Future studies should focus on specifically reducing the amount of EAT.
Heart failure (HF) with mid-range or with preserved ejection fraction (HFmrEF; HFpEF, respectively) is an increasingly large health problem with high morbidity and mortality and is thought to become the predominant form of HF in the coming years.1–3 However, to date, there are no specific therapies to reduce mortality in these patients, resulting in a 5-year survival rate of <50%.4,5 The incidence of obesity in this population is high, and obesity is one of the strongest predictors for HFpEF and HFmrEF.6–10
A specific fat depot of interest in the pathophysiology of HF is epicardial adipose tissue (EAT). EAT is the regional fat depot surrounding the myocardium within the pericardial sac, and it was demonstrated that patients with HFmrEF/HFpEF have higher volumes of EAT compared with matched controls without HF, despite similar body mass index (BMI).11 Furthermore, EAT has been linked to biomarkers of myocardial damage, ventricular hypertrophy, increased cardiac filling pressures, and worse exercise capacity,11–14 which are all hallmark features of this type of HF. Very recently, it has been shown that EAT was also predictive of new-onset HFmrEF and HFpEF.15,16 Whereas these associations suggest that EAT is an important factor in the pathophysiology and symptomatology of HFmrEF/HFpEF, it remains unclear whether EAT is indeed associated with poor prognosis. We, therefore, investigated the prognostic value of EAT volume measured with gold-standard cardiac magnetic resonance imaging in patients with HFmrEF and HFpEF.
Methods
Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. All patients who were enrolled in a recent prospective study, in which the diagnostic value of an implantable loop recorder in patients with HFmrEF and HFpEF was investigated, were part of the present study.17 Patients were enrolled between January 2015 and December 2019 and were seen every 6 months at the outpatient clinic for two years according to the study protocol. The inclusion and exclusion criteria have been previously described.17 In brief, patients had mild to moderate HF (New York Heart Association functional class II–III) in combination with a hospitalization or emergency room visit for HF or symptom relief with diuretics in the past 12 months. Patients in this study were all well-characterized, which included an NT-proBNP (N-terminal pro-brain natriuretic peptide) >300 pg/mL if in sinus rhythm, or >900 pg/mL if in atrial fibrillation. Left ventricular (LV) ejection fraction had to be >40% on echocardiography, and patients were required to have echocardiographic evidence of functional or structural alterations, including septal or posterior wall thickness ≥11 mm, or LV diastolic dysfunction (mean septal and lateral e′<9 cm/s, or E/e′≥13), or left atrial dilatation (left atrial volume index ≥34 mL/m2) or a combination of these alterations.4 As part of the study protocol, all patients also underwent a technetium 99m hydroxydiphosphonate scan for the detection of wild-type cardiac amyloidosis. For a minority of patients, the technetium 99m hydroxydiphosphonate scan turned out to be positive after inclusion into the study, and patients who tested positive were not excluded from the present analysis. Patients who had a myocardial infarction, percutaneous intervention, or coronary artery bypass grafting within the last 3 months were excluded, as well as patients with an internal cardiac defibrillator or pacemaker, patients with complex congenital heart disease, or patients with known genetic or infiltrative cardiomyopathies. The study was approved by the ethics committee of the University Medical Center Groningen and the study conforms to the Declaration of Helsinki. All patients provided written informed consent.
Cardiac Magnetic Resonance Imaging Protocol and Analysis
Cardiac magnetic resonance imaging was performed using a standard protocol for the acquisition of cardiac volume, function, and mass, as previously described by our group.11,18 In brief, all cardiac magnetic resonance studies were performed using a 1.5 Tesla scanner (Philips, Amsterdam, the Netherlands and Siemens, Erlangen, Germany). ECG-triggered cine loop images were obtained during breath-hold at end-expiration, using a retrospectively gated cine steady-state, free-precession sequence. Approximately 15 short-axis slices from base to apex were obtained, including the atria.
Cine loop images were analyzed offline by 2 observers (G.W. and T.M.G.) using dedicated software (QMass 7.6 and 8.1, QStrain 2.0, Medis, Leiden, the Netherlands), as previously described.11 Endocardial and epicardial borders of the left and right ventricle (RV) were manually delineated on the end-diastolic and end-systolic phases on the short-axis stacks. End-diastolic volumes and end-systolic volumes were automatically calculated by the summation of slices multiplied by slice thickness method. Volumetric measurements were indexed for body surface area, according to the Dubois formula.19 Strain was measured as the total deformation of the myocardium from its baseline length to its maximum length and is expressed as a percentage. LV longitudinal strain was measured on the long-axis cine images. Using the long-axis slices, left and right atrial volumes were measured by tracing the area and length of both atria in end-systole and end-diastole. Atrial volume was approximated using the area-length method.20 Atrial reservoir strain were subsequently assessed.
Epicardial Adipose Tissue
EAT was manually delineated on end-diastolic short-axis slices, working from the most basal slice around the atria towards the most apical slice around the ventricles, and was defined as the adipose tissue situated between the outer wall of the myocardium and the visceral layer of the pericardium.21 The mitral valve annulus position was used to differentiate between atrial and ventricular EAT. EAT volumes were calculated by summation of EAT volume of each slice using the modified Simpson rule.22 The presence of EAT was verified by comparing the precontrast and postcontrast T1 times of the EAT with T1 times of the subcutaneous fat using T1 mapping at mid-ventricular level, as described previously.11 All measurements were performed by 1 experienced investigator (G.W.) and were visually checked in a random fashion by 2 other investigators (B.D.W and T.P.W.), all blinded for patient characteristics. In addition, interobserver and intraobserver variability for measuring EAT was previously assessed (intraclass coefficient >0.90).14
Echocardiography
Echocardiographic parameters were assessed according to the current recommendations for cardiac chamber quantification and included: LV ejection fraction, e′ septal and lateral wall E/e′ ratio, and LV diastolic dysfunction grading.23 To determine the RV systolic pressure, the peak velocity of the tricuspid valve gradient signal was converted to a pressure gradient using the modified Bernoulli equation.23 Pulmonary artery systolic pressure was calculated by adding RV systolic pressure to an estimation of right atrial pressure obtained from the diameter and collapsibility of the inferior vena cava. For an inferior vena cava with diameter <2.1 cm that collapses ≥50% with a sniff, the right atrial pressure value of 3 mm Hg was used; an inferior vena cava with diameter ≥2.1 cm that collapses <50% suggests right atrial pressure of 15 mm Hg. If inferior vena cava diameter and collapse did not fit this scenario, an intermediate value of 8 mm Hg was used.23 In addition, the absence of pericardial effusion to ensure the reliability of EAT measurements was also verified on echocardiography.
Outcome
The main outcome in this study was the composite of all-cause mortality and first HF hospitalizations. HF hospitalization was defined as follows: hospital admission with at least one overnight stay with HF being the main reason for hospitalization and that required intravenous diuretics or an increase in diuretic dose. Secondary outcomes were all-cause mortality and HF hospitalizations separately. Follow-up time was defined as the time between start of the study and the occurrence of death or HF hospitalization or the end-of-study visit within 2 years follow-up, whichever occurred first, as per protocol of the main study.17 Due to the coronavirus disease 2019 (COVID-19) pandemic, the end-of-study visit was postponed for some patients, and in these patients, the total follow-up was longer than 2 years, with a maximum of 31 months. As a sensitivity analysis, we also assessed death due to cardiovascular causes, which was defined as death due to end-stage HF, acute coronary syndrome, sudden cardiac death, or stroke. No patients were lost to follow-up.
Statistical Analysis
Data are presented as numbers (percentage), means±SD, or medians with interquartile ranges, depending on the distribution. Differences between groups were analyzed using the independent samples t test, the Wilcoxon rank-sum test, 1-way ANOVA, the Kruskal-Wallis test, the χ2 test, or the Fisher exact test where appropriate. Correlations were analyzed using a Pearson correlation. Associations with outcome were assessed using univariable and multivariable Cox proportional hazard regression models. Covariates that were univariably associated with outcome were then adjusted using 4 different multivariable Cox proportional hazard regression models. We constructed the following multivariable Cox proportional hazard regression models for the main outcome (composite of all-cause mortality and HF hospitalizations), adjusting for (1) age, sex, and BMI; (2) HF severity (ie, New York Heart Association functional class and NT-proBNP); (3) comorbidities (ie, previous myocardial infarction, atrial fibrillation and renal dysfunction)24–27; and (4) all univariably associated variables in a backward selection model. If a covariate was used both as a covariate of interest, as well as an adjustment covariate, then we did not report this hazard ratio. As a sensitivity analysis, we also assessed the association between EAT and outcome with forced entry of BMI in all models. Lastly, the relation between EAT with outcome was also assessed after adjustment for baseline HF medications. The Cox proportional hazards assumption was tested using the cox.zph() function in R. This function checks for proportionality assumption, by checking whether the Schoenfeld residuals have a trend in time. NT-proBNP, LV E/e′, and regional wall thickness were all non-normally distributed and were log-transformed before the analysis. Hazard Ratios (HR) and 95% CI for normally distributed variables are shown per SD increase or decrease. To assess the goodness of fit for EAT predicting outcome, Harrell C statistic was calculated. To discriminate between patients at increased risk for outcome, a receiver operating characteristic curve was plotted, and area under the curve was calculated within a fixed follow-up time of 2 years. Kaplan-Meier plots with Log Rank tests were used to display the relation between EAT and outcome. Statistical analyses were performed using SPSS (Version 23, Chicago, Illinois) and R (Version 4.0.2, Vienna, Austria). Statistical significance was considered achieved at a P<0.05.
Results
Patient Characteristics
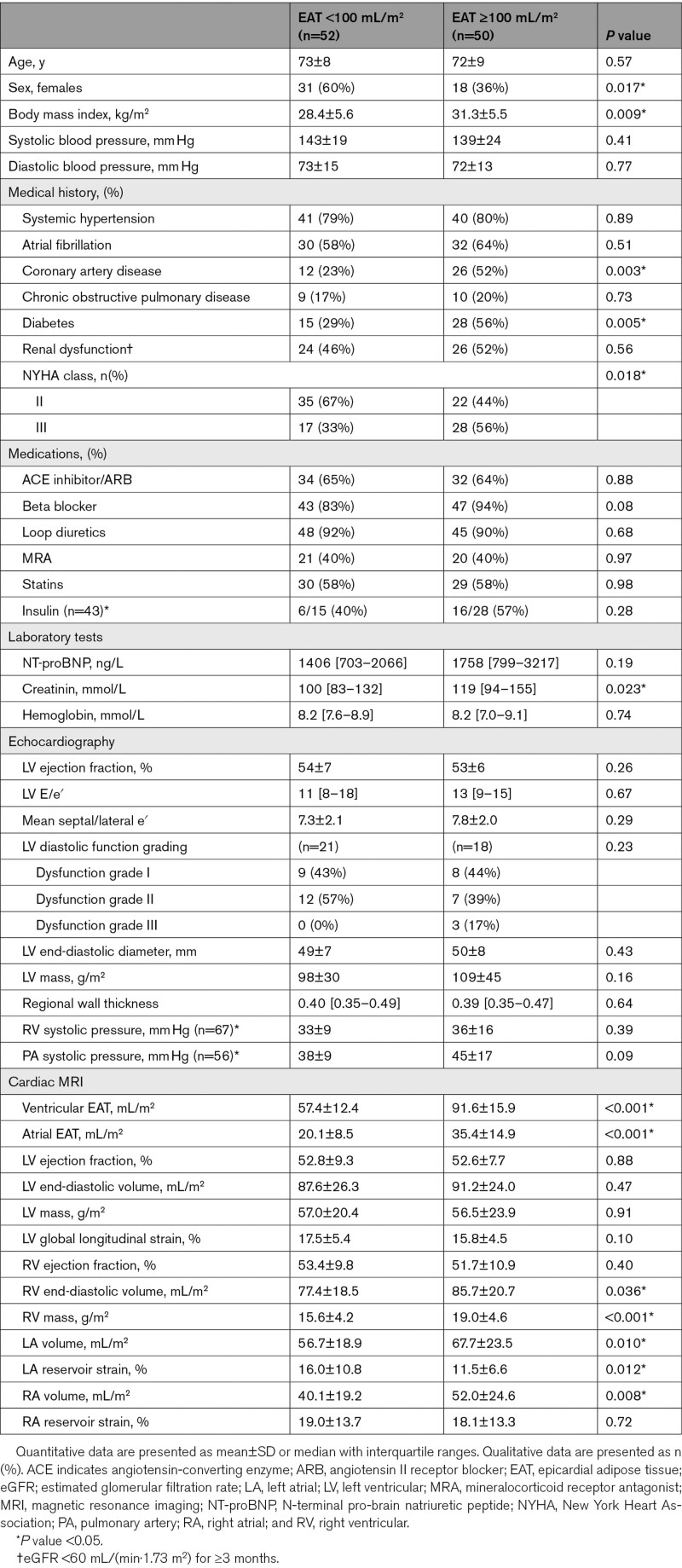
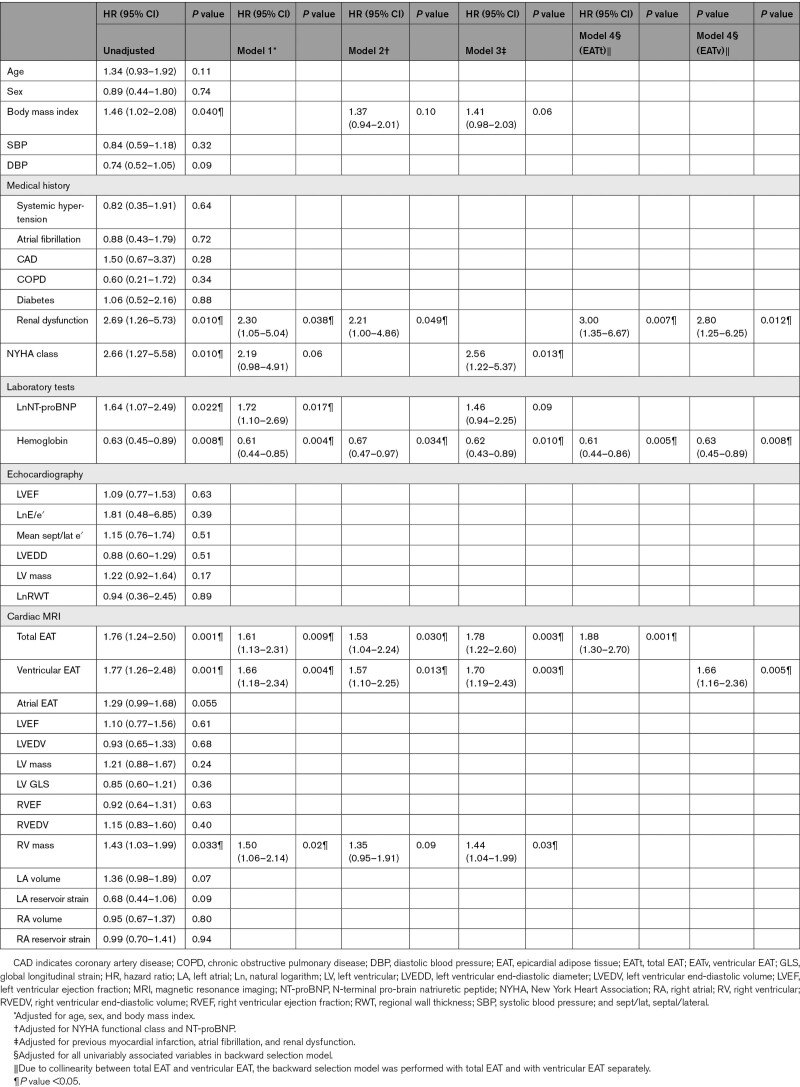
Among the 113 patients with HF who participated in the main study, cardiac magnetic resonance imaging scans were not available in eight patients, leaving 105 patients for the present analysis (Table S1). In 2 patients, the atria were not included in the short-axis measurements. In one patient, ventricular EAT could not reliably be measured. Analyses regarding total EAT were, therefore, based on 102 patients, for ventricular EAT on 104 patients, and for atrial EAT on 103 patients. Table 1 depicts the patient characteristics based on EAT volume higher or lower than 100 mL/m2. Patients with high EAT volume were on average more often men and had a higher BMI compared with patients with low EAT volume. In addition, patients with high EAT volume had more often coronary artery disease, diabetes mellitus and were more often diagnosed with severe HF. Typical examples of high- and low-EAT volume are shown in Figure 1A. EAT was significantly associated with lower LV global longitudinal strain (r=−0.21, P=0.038) and higher pulmonary artery systolic pressure (r=0.27, P=0.043).
Table 1.
Patient Characteristics Based on High or Low EAT Volume
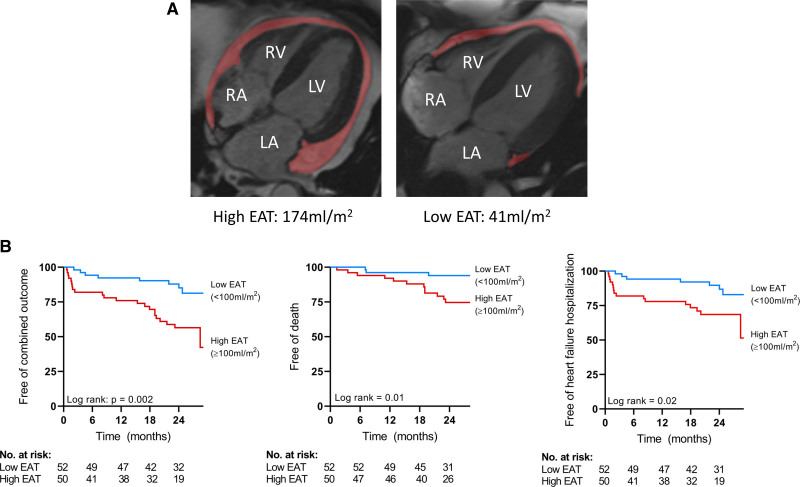
Figure 1.
Typical examples of high and low epicardial adipose tissue (EAT) and Kaplan-Meier curves based on high or low EAT.
A, Typical examples of high EAT volume and low EAT volume on cardiac magnetic resonance imaging. B, Kaplan-Meier curves for the combined outcome, all-cause mortality, and heart failure hospitalization stratified for EAT ≥100 mL/m2. LA indicates left atrium; LV, left ventricle; No., number, RA, right atrium; and RV, right ventricle.
Association Between EAT and Prognosis
During a median follow-up of 24 (17–25) months, 24 patients (23%) were hospitalized for HF and 16 patients (15%) died. The majority of deaths (10 patients, 63%) were due to cardiovascular causes. EAT volume was associated with a higher risk of all-cause mortality and HF hospitalizations (Table 2). Other factors associated with all-cause mortality and HF hospitalizations were higher BMI, renal dysfunction, worse New York Heart Association functional class, higher levels of NT-proBNP, and RV mass. No violation of the proportional hazards assumption was observed. Total EAT volume remained associated with all-cause mortality and HF hospitalizations after adjustment for age, sex, and BMI (model 1). EAT was also predictive of all-cause mortality and HF hospitalizations after adjustment for HF severity (model 2) and comorbidities (model 3; Table 2). In the backward selection model, EAT also remained significantly associated with all-cause mortality and HF hospitalizations (model 4). After forced entry of BMI in all models, EAT remained significantly associated with outcome (Table S2). EAT remained significantly associated with outcome after adjustment for the baseline HF medications (HR, 1.91 [95% CI, 1.30–2.83], P=0.001). No significant interaction for the association with the main composite outcome between men and women was observed (P for interaction=0.9). There was also no interaction between EAT and BMI for the association with outcome (P for interaction=0.5) EAT was also associated with all-cause mortality (HR, 2.06 [95% CI, 1.26–3.37], P=0.004) and HF hospitalizations (HR, 1.54 [95% CI, 1.04–2.30], P=0.03) in separate, unadjusted analyses. Lastly, total EAT was also associated with a composite of cardiovascular death and HF hospitalizations (HR, 1.61 [95% CI, 1.11–2.34], P=0.01).
Table 2.
Cox Regression Analysis
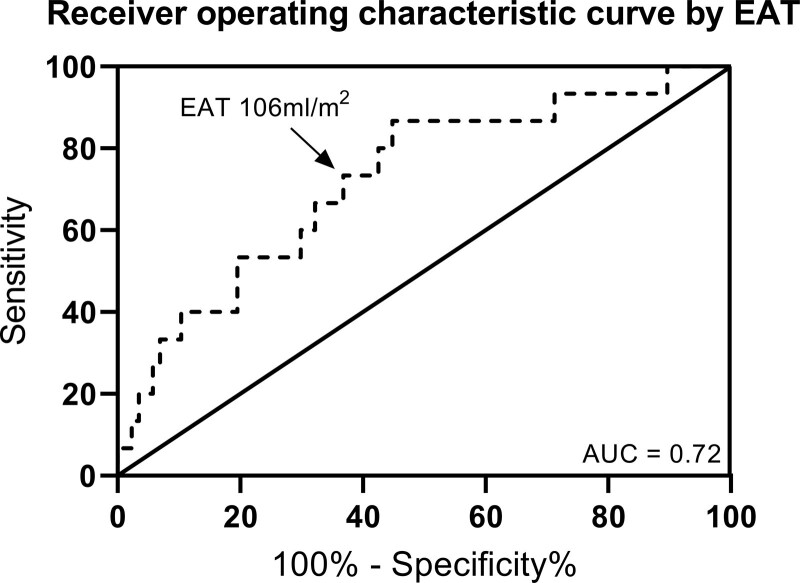
Harrell C statistic for EAT to predict the composite outcome was 0.67, for all-cause mortality 0.70, and for HF hospitalizations 0.65. The area under the curve of total EAT volume to identify patients with HF at increased risk for the composite outcome was 0.71, for all-cause mortality 0.72, and for HF hospitalizations 0.65. Receiver operating characteristic analysis demonstrated a sensitivity of 73% and a specificity of 63% for total EAT of 106 mL/m2 to detect patients with HF with increased risk for mortality (Figure 2). We chose to use 100 mL/m2 EAT for further analysis for practical purposes. Figure 1B depicts the Kaplan-Meier curves when patients were divided according to total EAT volume 100 mL/m2.
Figure 2.
Receiver operating characteristic curve showing the identification of patients at increased risk of all-cause mortality by epicardial adipose tissue (EAT) volume.
AUC indicates area under the curve.
Differences Between Obesity and EAT Regarding Clinical Characteristics and Prognosis
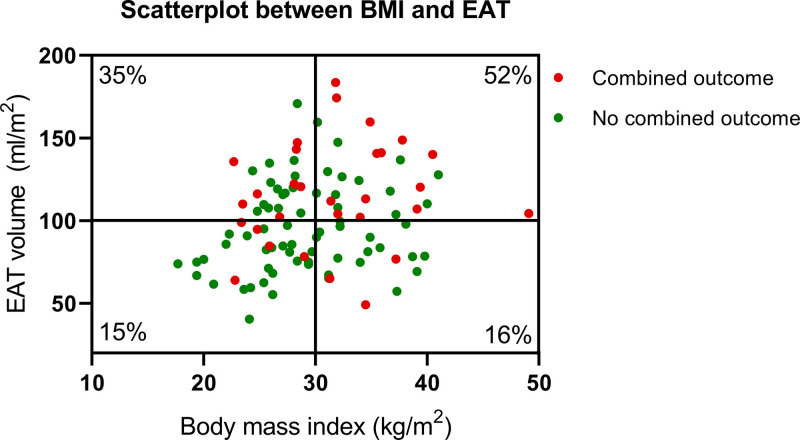
A significant but weak association was observed between BMI and EAT (r=0.24, P=0.017). Patients were grouped into 4 categories based on the presence of obesity (BMI ≥30 kg/m2) and EAT volume above or below 100 mL/m2 (Figure 3). Twenty-three (22%) patients were classified as nonobese but had high EAT volume, whereas 19 (19%) patients were classified as obese but had low EAT volume (Table S3). Patients with obesity with high EAT had a significantly higher relative event rate versus patients with obesity with low EAT (52% versus 16%, respectively, Log Rank, P=0.02). Patients without obesity with high EAT volume more often had coronary artery disease, type II diabetes, and had higher left atrial volume and lower left atrial strain compared to patients without obesity with low EAT volume. Interestingly, right atrial volume was also higher in patients with HF with high EAT volume, both in patients with obesity and without obesity. Of note, patients without obesity with high EAT volume still had slightly higher BMI compared to patients without obesity with low EAT volume.
Figure 3.
Scatterplot between body mass index (BMI) and epicardial adipose tissue (EAT) volume, rendering 4 groups based on obesity and EAT volume.
Percentages are relative event rates for the composite of all-cause mortality and heart failure hospitalizations per group.
Discussion
In this study, we found that increased EAT volume was significantly associated with the composite outcome of all-cause mortality and HF hospitalization in patients with HFmrEF and HFpEF, independent of BMI, HF severity, and several comorbidities. In addition, patients with obesity and high EAT had a significantly higher relative event rate compared to patients with obesity and low EAT. These data support the concept that accumulation of EAT is important in the pathophysiology of HFmrEF and HFpEF and the assessment of EAT may therefore be considered in the work-up of these patients with HF.
In concordance with our study, Pugliese et al28 very recently also reported that higher EAT was associated with adverse prognosis in patients with HFpEF. In the study by Pugliese et al,28 EAT was measured as thickness on echocardiography, rather than total volume quantification, and may have led to an underestimation or overestimation of total EAT volume, as a 3-dimensional structure was estimated with a 2-dimensional measurement. Nevertheless, the finding by us and Pugliese et al28 further supports the notion that EAT is an important prognosticator in these patients with HF. The finding that EAT volume is associated with poor outcome in patients with HFmrEF and HFpEF is in line with other studies investigating the association between EAT and prognosis in patients with type II diabetes and coronary artery disease but also in individuals without cardiovascular disease.15,16,29–32 The mechanistic link between EAT accumulation and impaired outcome in HFmrEF and HFpEF is unknown, yet multiple mechanisms have been proposed.33 EAT accumulation may contribute to myocardial steatosis, but may also be related to the infiltration of adipose tissue into the adjacent myocardium.34 EAT and the underlying myocardium are intricately intertwined, as the relation with the microcirculation is not obstructed by a basal layer.35 Therefore, when systemic inflammation and metabolic disorders cause EAT to proliferate, this may lead to a shift from normal functional EAT, to proinflammatory functioning EAT that may harm the underlying myocardium in a local, paracrine manner causing microvascular dysfunction and fibrosis.35 However, the accumulation of EAT could also have mechanical consequences, for instance by causing pseudo pericardial constriction.12,13 It has been shown that increased EAT in HFpEF patients with obesity is associated with increased LV eccentricity index, indicating pericardial restraint.13 Compared with HFpEF patients without obesity, HFpEF patients with obesity have higher right-to-left-sided filling pressures in addition to increased wedge pressure. This constrictive pattern correlates with increased EAT within a fixed pericardial space.13 Additionally, EAT has been shown to release proinflammatory adipokines that have the capacity to affect the adjacent myocardium.36,37 The accumulation of EAT has been associated not only with ventricular hypertrophy, diastolic dysfunction, and increased cardiac filling pressures but also with endothelial dysfunction and atrial fibrillation, all of which are highly prevalent in HFmrEF and HFpEF.12–14,34,38–40
EAT may have a different impact on HFpEF and HFmrEF. The study by Kenchaiah et al15 showed that EAT accumulation was particularly associated with new-onset HFpEF and to a lesser degree to HFmrEF. However, in an earlier study by our group, we did not observe significant differences in EAT between these patient populations.11 Considering HFmrEF patients are suggested to behave more similar to patients with HFrEF than patients with HFpEF in terms of prognosis,41 EAT may have a different pathophysiological pathway in HFpEF than HFmrEF, in which the burden of visceral adiposity and its impact on the heart may be lighter in HFmrEF. However, whether there indeed is a pathophysiological difference in EAT accumulation between patients with HFpEF and HFmrEF needs to be established.
EAT has been related to atrial fibrillation in the literature, also in the setting of HFmrEF/HFpEF.11,42 A recent study found that accumulation of EAT is strongly related to electroanatomical alterations, as well as increased atrial fibrosis.34 In the present study, we found that patients with a low BMI but increased EAT had higher atrial volumes and worse atrial function. In addition, patients with low BMI but high EAT also had more often coronary artery disease and diabetes. These findings suggest that a patient with normal bodyweight but with increased EAT volume may be at a higher risk for developing atrial fibrillation compared to a patient with normal bodyweight and low EAT volume. Therefore, directly targeting EAT beyond overall obesity for therapy may positively impact atrial fibrillation, even in patients with a normal BMI. Of course, these hypothesis-generating results need further study in this regard.
BMI is limited in providing information about a patient’s visceral fat status. A previous study found that visceral adiposity was associated with incident HF, especially HFpEF, after adjusting for BMI in a multiethnic cohort.43 In line with this, we observed a rather weak association between BMI and EAT volume, supporting that BMI is not a specific estimator of EAT volume. Moreover, one-fifth of our population was classified as not being obese, whereas EAT volume was substantial. In addition, patients without obesity and increased EAT volume more often had type II diabetes and coronary artery disease compared to patients without obesity and low EAT volume. These data are in line with a large post hoc analysis from the TOPCAT trial (Treatment of Preserved Cardiac Function in HF with Aldosterone Antagonist), which showed that patients with abdominal adiposity had the highest risk for all-cause mortality.44 The question still remains whether EAT is a surrogate marker of overall adiposity in patients with obesity or whether EAT plays an active role in the pathophysiology of patients with HFmrEF and HFpEF and needs to be further elucidated.
Interestingly, patients with high EAT volume also had increased right atrial volumes compared to patients with low EAT volume. Furthermore, EAT was positively associated with increased pulmonary artery systolic pressure. These findings are in line with previous data that showed an association between EAT and right-sided filling pressures and may suggest that an abundance of EAT tissue surrounding the heart may lead to right heart overload in particular.12 This is important, as RV dysfunction is strongly associated with worse outcome in these patients with HF.45
Clinical Implications
Our data show that EAT accumulation is associated with worse outcome in patients with HFmrEF and HFpEF, and measurement of EAT may, therefore, be considered in the work-up and clinical follow-up of these patients with HF. Future studies should focus on therapies specifically designed for reducing the amount of EAT.15,35 These potential therapies include intense lifestyle changes leading to significant weight reduction, specific drugs that reduce visceral adiposity such as GLP-1 (glucagon-like peptide 1) receptor agonists, SGLT-2 (sodium-glucose co-transporter 2) inhibitors, bariatric surgery, or direct surgical resection specifically for patients without obesity with disproportionate high EAT.35,46–49 Statins and metformin use may potentially ameliorate the proinflammatory character of EAT.35,50 Randomized clinical trials are urgently needed to evaluate the potential benefits of such therapies. Clearly, at this moment, it is unknown whether these approaches will be able to improve treatment and outcomes in HFpEF/HfmrEF, and it needs to be further investigated whether therapies that specifically reduce EAT indeed improve outcomes.
Limitations
First, this was a study with a relatively small sample size, therefore we could not investigate extensive multivariable associations with outcome. Second, when measuring EAT, we could not entirely rule out the presence of pericardial effusion. However, the T1 values of the EAT depots corresponded with the T1 times of subcutaneous fat and not water. Third, the cutoff value for when EAT is increased may differ in other HF populations and in other countries and needs external validation in other cohorts. Fourth, due to enrollment of both patients with a recent hospitalization for HFpEF/HFmrEF, as well as patients with chronic HFpEF/HFmrEF, the generalizability of these results may be limited. Fifth, this was a small region cohort with limited inclusion of other ethnic groups. Sixth, although sleep-disordered breathing is an important factor in HFpEF, especially in the obese phenotype, we did not routinely perform polysomnography at baseline. Lastly, to adjust for the potential confounding effects of global adiposity, the gold-standard would be measuring abdominal visceral adipose tissue using magnetic resonance imaging or computed tomography imaging. However, we did not have abdominal images at our disposal and chose to use BMI as a surrogate for global adiposity instead.
Conclusions
EAT accumulation is associated with adverse prognosis in patients with HFmrEF and HFpEF. This finding underscores the importance of EAT in these patients with HF.
Article Information
Sources of Funding
The ventricular tachyarrhythmia detection by implantable loop recording in patients with heart failure and preserved ejection fraction study was supported by an unrestricted grant from Abbott-Netherlands to the University Medical Center Groningen. Abbott-Netherlands was neither involved in the conduction of the study, nor in the writing of this article.
Disclosures
None.
Supplemental Material
Tables S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BMI
- body mass index
- EAT
- epicardial adipose tissue
- GLP-1
- glucagon-like peptide 1
- HF
- heart failure
- HFmrEF
- heart failure with mid-range ejection fraction
- HFpEF
- heart failure with preserved ejection fraction
- HR
- hazard ratio
- LV
- left ventricle
- NT-proBNP
- N-terminal of pro-brain natriuretic peptide
- RV
- right ventricle
- SGLT-2
- sodium-glucose co-transporter 2
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.121.009238.
For Sources of Funding and Disclosures, see page 253.
Contributor Information
Gijs van Woerden, Email: g.van.woerden@umcg.nl.
Dirk J. van Veldhuisen, Email: d.j.van.veldhuisen@umcg.nl.
Olivier C. Manintveld, Email: o.manintveld@erasmusmc.nl.
Vanessa P.M. van Empel, Email: vanessa.van.empel@mumc.nl.
Tineke P. Willems, Email: t.p.willems@umcg.nl.
Rudolf A. de Boer, Email: r.a.de.boer@umcg.nl.
Michiel Rienstra, Email: michielrienstra@hotmail.com.
B. Daan Westenbrink, Email: b.d.westenbrink@umcg.nl.
References
- 1.Lam CSP, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018;39;2780–2792. doi: 10.1093/eurheartj/ehy301 [DOI] [PubMed] [Google Scholar]
- 2.Bekfani T, Bekhite Elsaied M, Derlien S, Nisser J, Westermann M, Nietzsche S, Hamadanchi A, Fröb E, Westphal J, Haase D, et al. Skeletal muscle function, structure, and metabolism in patients with heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. Circ Heart Fail. 2020;13;e007198. doi: 10.1161/CIRCHEARTFAILURE.120.007198 [DOI] [PubMed] [Google Scholar]
- 3.Weiss K, Schär M, Panjrath GS, Zhang Y, Sharma K, Bottomley PA, Golozar A, Steinberg A, Gerstenblith G, Russell SD, et al. Fatigability, exercise intolerance, and abnormal skeletal muscle energetics in heart failure. Circ Heart Fail. 2017;10;e004129. doi: 10.1161/CIRCHEARTFAILURE.117.004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al.; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18;891–975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 5.Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29;339–347. doi: 10.1093/eurheartj/ehm554 [DOI] [PubMed] [Google Scholar]
- 6.Sorimachi H, Obokata M, Takahashi N, Reddy YNV, Jain CC, Verbrugge FH, Koepp KE, Khosla S, Jensen MD, Borlaug BA. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J. 2021;42;1595–1605. doi: 10.1093/eurheartj/ehaa823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6;701–709. doi: 10.1016/j.jchf.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhambhani V, Kizer JR, Lima JAC, van der Harst P, Bahrami H, Nayor M, de Filippi CR, Enserro D, Blaha MJ, Cushman M, et al. Predictors and outcomes of heart failure with mid-range ejection fraction. Eur J Heart Fail. 2018;20;651–659. doi: 10.1002/ejhf.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabbah MS, Fayyaz AU, de Denus S, Felker GM, Borlaug BA, Dasari S, Carter RE, Redfield MM. Obese-inflammatory phenotypes in heart failure with preserved ejection fraction. Circ Heart Fail. 2020;13;e006414. doi: 10.1161/CIRCHEARTFAILURE.119.006414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, et al. Predicting heart failure with preserved and reduced ejection fraction: the International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9;e003116. doi: 10.1161/CIRCHEARTFAILURE.115.003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. 2018;20;1559–1566. doi: 10.1002/ejhf.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorter TM, van Woerden G, Rienstra M, Dickinson MG, Hummel YM, Voors AA, Hoendermis ES, van Veldhuisen DJ. Epicardial adipose tissue and invasive hemodynamics in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8;667–676. doi: 10.1016/j.jchf.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 13.Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8;657–666. doi: 10.1016/j.jchf.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Woerden G, van Veldhuisen DJ, Gorter TM, van Empel VPM, Hemels MEW, Hazebroek EJ, van Veldhuisen SL, Willems TP, Rienstra M, Westenbrink BD. Importance of epicardial adipose tissue localization using cardiac magnetic resonance imaging in patients with heart failure with mid-range and preserved ejection fraction. Clin Cardiol. 2021;44;987–993. doi: 10.1002/clc.23644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenchaiah S, Ding D, Carr JJ, Allison MA, Budoff MJ, Tracy RP, Burke GL, McClelland RL, Arai AE, Bluemke DA. Pericardial fat and the risk of heart failure. J Am Coll Cardiol. 2021;77;2638–2652. doi: 10.1016/j.jacc.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao VN, Bush CG, Mongraw-Chaffin M, Hall ME, Clark D, 3rd, Fudim M, Correa A, Hammill BG, O’Brien E, Min YI, et al. Regional adiposity and risk of heart failure and mortality: the Jackson Heart Study. J Am Heart Assoc. 2021;10;e020920. doi: 10.1161/JAHA.121.020920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Veldhuisen DJ, van Woerden G, Gorter TM, van Empel VPM, Manintveld OC, Tieleman RG, Maass AH, Vernooy K, Westenbrink BD, van Gelder IC, et al. Ventricular tachyarrhythmia detection by implantable loop recording in patients with heart failure and preserved ejection fraction: the VIP-HF study. Eur J Heart Fail. 2020;22;1923–1929. doi: 10.1002/ejhf.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Te Rijdt WP, Ten Sande JN, Gorter TM, van der Zwaag PA, van Rijsingen IA, Boekholdt SM, van Tintelen JP, van Haelst PL, Planken RN, de Boer RA, et al. Myocardial fibrosis as an early feature in phospholamban p.Arg14del mutation carriers: phenotypic insights from cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2019;20;92–100. doi: 10.1093/ehjci/jey047 [DOI] [PubMed] [Google Scholar]
- 19.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5;303–3. discussion 312-3. doi: 10.1001/archinte.1916.00080130010002 [PubMed] [Google Scholar]
- 20.Sievers B, Kirchberg S, Addo M, Bakan A, Brandts B, Trappe HJ. Assessment of left atrial volumes in sinus rhythm and atrial fibrillation using the biplane area-length method and cardiovascular magnetic resonance imaging with TrueFISP. J Cardiovasc Magn Reson. 2004;6;855–863. doi: 10.1081/jcmr-200036170 [DOI] [PubMed] [Google Scholar]
- 21.Doesch C, Streitner F, Bellm S, Suselbeck T, Haghi D, Heggemann F, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure due to dilated cardiomyopathy. Obesity (Silver Spring). 2013;21;E253–E261. doi: 10.1002/oby.20149 [DOI] [PubMed] [Google Scholar]
- 22.Flüchter S, Haghi D, Dinter D, Heberlein W, Kühl HP, Neff W, Sueselbeck T, Borggrefe M, Papavassiliu T. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity (Silver Spring). 2007;15;870–878. doi: 10.1038/oby.2007.591 [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28;1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 24.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34;1424–1431. doi: 10.1093/eurheartj/eht066 [DOI] [PubMed] [Google Scholar]
- 25.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61;1498–1506. doi: 10.1016/j.jacc.2012.12.044 [DOI] [PubMed] [Google Scholar]
- 26.Cunningham JW, Vaduganathan M, Claggett BL, John JE, Desai AS, Lewis EF, Zile MR, Carson P, Jhund PS, Kober L, et al. Myocardial infarction in heart failure with preserved ejection fraction: pooled analysis of 3 Clinical Trials. JACC Heart Fail. 2020;8;618–626. doi: 10.1016/j.jchf.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 27.Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68;2217–2228. doi: 10.1016/j.jacc.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 28.Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, Mengozzi A, Virdis A, Nesti L, Taddei S, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail. Published online August 23, 2021. doi: 10.1002/ejhf.2337 [DOI] [PubMed] [Google Scholar]
- 29.Christensen RH, von Scholten BJ, Hansen CS, Jensen MT, Vilsbøll T, Rossing P, Jørgensen PG. Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes. Cardiovasc Diabetol. 2019;18;114. doi: 10.1186/s12933-019-0917-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah RV, Anderson A, Ding J, Budoff M, Rider O, Petersen SE, Jensen MK, Koch M, Allison M, Kawel-Boehm N, et al. Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2017;10;1016–1027. doi: 10.1016/j.jcmg.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen BA, Laughlin GA, Saad SD, Barrett-Connor E, Allison MA, Wassel CL. Pericardial fat is associated with all-cause mortality but not incident CVD: the Rancho Bernardo Study. Atherosclerosis. 2015;239;470–475. doi: 10.1016/j.atherosclerosis.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tscharre M, Hauser C, Rohla M, Freynhofer MK, Wojta J, Huber K, Weiss TW. Epicardial adipose tissue and cardiovascular outcome in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Eur Heart J Acute Cardiovasc Care. 2017;6;750–752. doi: 10.1177/2048872616680609 [DOI] [PubMed] [Google Scholar]
- 33.Nattel S, Aguilar M. Electrophysiological effects of atrial epicardial adipose tissue: keep your friends close and your enemies closer. J Am Coll Cardiol. 2020;76;1212–1214. doi: 10.1016/j.jacc.2020.07.031 [DOI] [PubMed] [Google Scholar]
- 34.Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, Montgomery MK, Binny S, Watts T, Joshi SB, et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol. 2020;76;1197–1211. doi: 10.1016/j.jacc.2020.07.017 [DOI] [PubMed] [Google Scholar]
- 35.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71;2360–2372. doi: 10.1016/j.jacc.2018.03.509 [DOI] [PubMed] [Google Scholar]
- 36.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108;2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5 [DOI] [PubMed] [Google Scholar]
- 37.Shaihov-Teper O, Ram E, Ballan N, Brzezinski RY, Naftali-Shani N, Masoud R, Ziv T, Lewis N, Schary Y, Levin-Kotler LP, et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation. 2021;143;2475–2493. doi: 10.1161/CIRCULATIONAHA.120.052009 [DOI] [PubMed] [Google Scholar]
- 38.Mancio J, Azevedo D, Fragao-Marques M, Falcao-Pires I, Leite-Moreira A, Lunet N, Fontes-Carvalho R, Bettencourt N. Meta-analysis of relation of epicardial adipose tissue volume to left atrial dilation and to left ventricular hypertrophy and functions. Am J Cardiol. 2019;123;523–531. doi: 10.1016/j.amjcard.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 39.Nerlekar N, Muthalaly RG, Wong N, Thakur U, Wong DTL, Brown AJ, Marwick TH. Association of volumetric epicardial adipose tissue quantification and cardiac structure and function. J Am Heart Assoc. 2018;7;e009975. doi: 10.1161/JAHA.118.009975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aydin H, Toprak A, Deyneli O, Yazici D, Tarçin O, Sancak S, Yavuz D, Akalin S. Epicardial fat tissue thickness correlates with endothelial dysfunction and other cardiovascular risk factors in patients with metabolic syndrome. Metab Syndr Relat Disord. 2010;8;229–234. doi: 10.1089/met.2009.0080 [DOI] [PubMed] [Google Scholar]
- 41.Lam CS, Solomon SD. Fussing over the middle child: heart failure with mid-range ejection fraction. Circulation. 2017;135;1279–1280. doi: 10.1161/CIRCULATIONAHA.117.027324 [DOI] [PubMed] [Google Scholar]
- 42.Packer M. Disease-treatment interactions in the management of patients with obesity and diabetes who have atrial fibrillation: the potential mediating influence of epicardial adipose tissue. Cardiovasc Diabetol. 2019;18;121. doi: 10.1186/s12933-019-0927-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS, Michos ED. Adiposity and incident heart failure and its subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail. 2018;6;999–1007. doi: 10.1016/j.jchf.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70;2739–2749. doi: 10.1016/j.jacc.2017.09.1111 [DOI] [PubMed] [Google Scholar]
- 45.Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. 2016;18;1472–1487. doi: 10.1002/ejhf.630 [DOI] [PubMed] [Google Scholar]
- 46.Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, Emungania O, Alessi MC, Clément K, Bernard M, et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60;1381–1389. doi: 10.1016/j.jacc.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 47.Smail H, Baciu A, Dacher JN, Litzler PY. Surgical resection of circumferential epicardial adipose tissue hypertrophy: case report and systematic review of the literature. J Thorac Cardiovasc Surg. 2016;151;e27–e30. doi: 10.1016/j.jtcvs.2015.08.083 [DOI] [PubMed] [Google Scholar]
- 48.Iacobellis G, Villasante Fricke AC. Effects of semaglutide versus dulaglutide on epicardial fat thickness in subjects with type 2 diabetes and obesity. J Endocr Soc. 2020;4;bvz042. doi: 10.1210/jendso/bvz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, Atallah-Lajam F, Giannarelli C, Macaluso F, Lala A, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM Study. JACC Heart Fail. 2021;9;578–589. doi: 10.1016/j.jchf.2021.04.014 [DOI] [PubMed] [Google Scholar]
- 50.Alehagen U, Benson L, Edner M, Dahlström U, Lund LH. Association between use of statins and mortality in patients with heart failure and ejection fraction of ≥50. Circ Heart Fail. 2015;8;862–870. doi: 10.1161/CIRCHEARTFAILURE.115.002143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.