Supplemental Digital Content is available in the text.
Keywords: rIPD, pneumococcal vaccine, pneumococcus, IMPACT
Background:
Invasive pneumococcal disease due to Streptococcus pneumoniae can cause mortality and severe morbidity due to sepsis, meningitis and pneumonia, particularly in young children and the elderly. Recurrent invasive pneumococcal disease is rare yet serious sequelae of invasive pneumococcal disease that is associated with the immunocompromised and leads to a high mortality rate.
Method:
This retrospective study reviewed recurrent invasive pneumococcal disease cases from the Canadian Immunization Monitoring Program, ACTive (IMPACT) between 1991 and 2019, an active network for surveillance of vaccine-preventable diseases and adverse events following immunization for children ages 0–16 years. Data were collected from 12 pediatric tertiary care hospitals across all 3 eras of public pneumococcal conjugate vaccine implementation in Canada.
Results:
The survival rate within our cohort of 180 recurrent invasive pneumococcal disease cases was 98.3%. A decrease of 26.4% in recurrent invasive pneumococcal disease due to vaccine serotypes was observed with pneumococcal vaccine introduction. There was also a 69.0% increase in the rate of vaccination in children with preexisting medical conditions compared with their healthy peers.
Conclusion:
The decrease in recurrent invasive pneumococcal disease due to vaccine-covered serotypes has been offset by an increase of non-vaccine serotypes in this sample of Canadian children.
Streptococcus pneumoniae is a Gram-positive encapsulated bacterium that can cause mortality and severe morbidity from clinical syndromes of sepsis, meningitis and pneumonia, particularly in young children and the elderly. Invasive pneumococcal disease (IPD) is associated with primary immunodeficiency (PID)1 or other established risk factors such as prematurity, asplenia, chronic cardiopulmonary conditions, HIV infection, hemoglobinopathies, traumatic cerebrospinal fluid leaks or cochlear implant.
Current literature shows about 1.2%-10.5% of children infected with IPD experience recurrent IPD (rIPD) (defined as 2 or more episodes >30 days apart).1–5 The most frequent immune defects associated with rIPD described include B cell dysfunction, complement deficiencies and defects in toll-like receptor signaling pathways.3–5 With the increasing use of next-generation sequencing in diagnosing immunologic disorders to complement functional immune assessments, there has been a dramatic increase in recognition and diagnosis of inborn errors of immunity associated with rIPD.6,7 Furthermore, optimizing the care of immunocompromised children or other predisposing factors also includes the consideration of preventing vaccine-preventable diseases (VPDs).
The aims of this study were to investigate the presenting clinical syndromes and outcomes of rIPD in the paediatric population in Canada. We also studied the effects of pneumococcal vaccine (PCV) on the prevalence of vaccine-covered serotypes.
METHODS
Study Design
This study was a retrospective review of rIPD cases from the Canadian Immunization Monitoring Program, ACTive (IMPACT); an active, sentinel surveillance network for childhood vaccine-preventable diseases and vaccine adverse events in existence for more than 30 years and spanning all 3 eras (pre-2001, 2002–2010 and post 2011) of public pneumococcal conjugate vaccine (PCV) implementation in Canada.
Active surveillance was conducted by 12 IMPACT centers in the Canadian provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, Nova Scotia and Newfoundland. Ethics or hospital approvals for surveillance were acquired by all centers. The network covers approximately 90% of tertiary care pediatric beds in Canada and receives referrals from all regions covering 50% of children in Canada.8 Nurse monitors are employed at each center to identify cases through reviewing daily admission lists, visiting inpatient units and interacting with clinical medical staff. Nurse monitors work with an infectious diseases specialist who acts as a lead site investigator.8
Case Definition
Over 6000 laboratory-confirmed inpatient and outpatient cases of IPD in children 0–16 years of age, identified between 1991 (start of IMPACT invasive pneumococcal surveillance) and 2019 were identified. Laboratory confirmed cases were defined as positive culture (body fluid from sterile site) and/or PCR. Pneumococcal isolates were serotyped at the National Centre for Streptococcus (1991–2009) and the National Microbiology Laboratory (2010–2019). Serotyping was performed by Quellung reaction using pool, group, type and factor specific antisera (Statens Serum Institut, Copenhagen, Denmark).9 Recurrent IPD was defined as a case with previous IPD based on history alone, as determined at the time of subsequent IPD infection (more than 30 days apart). A total of 180 rIPD cases were identified at IMPACT centers between 1991 and 2019 and included in the analysis. Pneumococcal immunization history was available for each case through public health and family physician records. Immunization status for each case was determined according to age at time of vaccination, number of doses administered and dates of vaccination. This was compared with the provincial vaccination schedules (Table, Supplemental Digital Content 1, http://links.lww.com/INF/E639) in place at the time the child was vaccinated to determine the immunization status (appropriately vaccinated, program not available and not/under vaccinated).10 Cases were also stratified by the number of vaccines doses received in a separate analysis as younger cases can be considered appropriately vaccinated for their age but still have not received a full complement of PCV doses.
Statistical Analysis
Patients were grouped into 3 cohorts, matching the 3 phases of public PCV introduction, based on year of admission as follows: 1991–2001 [pre-pneumococcal 7-valent conjugate vaccine (PCV7)], 2002–2010 (PCV7-10 valent vaccine) and 2011–2019 [pneumococcal 13-valent conjugate vaccine (PCV13)].
Statistical analysis was carried out using SAS Studio (SAS Institute, Cary, NC) and Microsoft Excel (Microsoft Corporation, Redmond, WA). Statistical significance for associated variables was determined using the χ2 test and analysis of variance (ANOVA). A two-tailed 95% confidence interval for P value < 0.05 was used as a threshold for statistical significance, not adjusted for cases of multiple comparisons. A Z score test was used to compare the proportion of male and female cases with a P value < 0.05 used as a threshold for statistical significance. Data used in this study are not available for public access.
RESULTS
Patient Demographics and Outcomes
Among the 180 rIPD cases, males accounted for a significantly larger proportion than females (63.2% vs. 36.7% of sample, respectively, P value: 0.00017). Age at the current admission was available for all cases and the median age was 4.88 years overall. Age at the first episode was available for 157 cases and the median was 3.09 years of age at the first admission with a median interval of 1.61 year between admissions. After the recurrent episode, a total of 177 cases (98.3%) survived (including 4 lost to follow-up) and 3 (1.7%) died. In total, 27 children (15%) were admitted into an IMPACT center intensive care unit (ICU) as a result of rIPD. Median ICU stay was 3 days with longer stays in cases recorded during more recent vaccine eras (ANOVA P value = 0.03). These data are summarized in Table, Supplemental Digital Content 2, http://links.lww.com/INF/E640.
IPD Syndromes and Signs of Severe Illness
Bacteremia was the most common IPD syndrome in this patient population, both at the first episode and at recurrence (35.6% and 44.4%, respectively). Incidence of disease syndromes and other signs of severe illness are presented in Table 1 for both the current and first presentations. Overall, 174 cases (96.7%) presented with at least 1 syndrome or sign of severe illness.
TABLE 1.
Clinical Syndromes of rIPD Compared with the First IPD Presentation.
| First Episode | Current Episode | Change in Frequency | ||||
|---|---|---|---|---|---|---|
| Syndrome | ||||||
| Bacteremia only | 64 | (35.6%) | 80 | (44.4%) | 16 | (8.9%) |
| Skin and soft tissue | 0 | (0.0%) | 15 | (8.3%) | 15 | (8.3%) |
| CNS (excluding meningitis) | 0 | (0.0%) | 2 | (1.1%) | 2 | (1.1%) |
| Meningitis | 32 | (17.8%) | 26 | (14.4%) | -6 | (-3.3%) |
| Osteoarticular infection | 2 | (1.1%) | 6 | (3.3%) | 4 | (2.2%) |
| Pneumonia | 27 | (15.0%) | 33 | (18.3%) | 6 | (3.3%) |
| Other sterile site | 7 | (3.9%) | 12 | (6.7%) | 5 | (2.8%) |
| Signs of severe illness | ||||||
| Seizure | 3 | (1.7%) | 6 | (3.3%) | 3 | (1.7%) |
| Shock | 8 | (4.4%) | 10 | (5.6%) | 2 | (1.1%) |
| Petechial rash | 0 | (0.0%) | 2 | (1.1%) | 2 | (1.1%) |
| No syndromes or signs of severe illness | 41 | (22.8%) | 13 | (7.2%) | −29 | (−16.1%) |
Number does not total 180 as cases may have more than one syndrome or sign.
CNS indicates central nervous system.
Medical Conditions and Immunocompromise
A total of 124/180 (68.9%) cases were diagnosed with at least one underlying medical condition, including 78/180 (43.3%) diagnosed with an immunocompromising condition and 24/180 (13.3%) who were immunosuppressed due to medication (Table 2). The most common preexisting medical conditions were chronic neurologic conditions and chronic heart/lung conditions (10.5% and 8.3%, respectively). Secondary immunodeficiency (31.6%), largely a consequence of neoplasms (19.4% including both those undergoing treatment and those who are not), was twice as prevalent as primary immunodeficiency (14.4%) in this population with rIPD. Iatrogenic immunosuppression was a less frequent cause of immunosuppression (13.3%) with cancer treatment being the main cause (7.9%).
TABLE 2.
Prevalence of Medical Conditions, Including Immunocompromise, in the rIPD Cases Studied
| Medical Condition | Frequency | |
|---|---|---|
| Chronic neurologic condition | 19 | (10.5%) |
| Chronic heart/lung condition* | 15 | (8.3%) |
| Chronic liver condition | 11 | (6.1%) |
| Nephrotic syndrome | 10 | (5.5%) |
| CSF leak | 9 | (5.0%) |
| Reactive airway disease/asthma | 9 | (4.9%) |
| Chronic kidney disease | 8 | (4.4%) |
| Sickle cell disease or hemoglobinopathy | 2 | (1.1%) |
| Diabetes mellitus | 1 | (0.5%) |
| Immunocompromising condition(s) | 78 | (43.3%) |
| Secondary | 57 | (31.6%) |
| Neoplasm | 35 | (19.4%) |
| Solid organ transplant | 12 | (6.6%) |
| Stem cell transplant | 7 | (3.8%) |
| HIV/AIDS | 6 | (3.3%) |
| Asplenia | 4 | (2.2%) |
| Other secondary immunodeficiency | 2 | (1.1%) |
| Primary | 26 | (14.4%) |
| Immunocompromising medication(s) † | 24 | (13.3%) |
| Cancer treatment | 14 | (7.9%) |
| Immunosuppressive medication for other indication | 10 | (5.6%) |
Including: valvular heart disease, double outlet right ventricle, partial anomalous venous pulmonary return as well as non-specified conditions.
Including: cyclophosphamide, methotrexate, tacrolimus, and prednisone.
Vaccination Status
Vaccination data were available for 175/180 (97%) cases and were analyzed in 3 cohorts based on year of admission as follows: 1991–2001 (pre-PCV7, vaccine not available, n = 93), 2002–2010 (PCV7-10, n = 42) and 2011–2019 (PCV13, n = 45) as shown in Table, Supplemental Digital Content 2, http://links.lww.com/INF/E640. The proportion of patients with no prior PCV were 45.2% and 17.7%, in the latter 2 vaccine eras respectively. During the period of PCV7-10 immunization, 6/42 (14.2%) cases were fully up-to-date with their age-appropriate number of pneumococcal vaccines. This increased to 26/45 (57.8%) cases in the PCV13 cohort (χ2 P value = 0.000013). Using data from the 42/45 cases with known age and vaccination status in the PCV13 era to compare vaccine coverage between patients with no preexisting medical conditions or immune compromise and those with at least one such condition, we observe the following trends: among the rIPD patients with no preexisting medical conditions, none were appropriately vaccinated for age in contrast with 26/42 (66.7%) of those with one or more of the medical conditions (χ2 P value: 0.022); among the rIPD patients with no chronic medical conditions which were immunocompromising 8/15 (53.3%) were up-to-date with age-appropriate scheduled vaccinations. This proportion increased to 18/27 (66.7%) patients with one or more immunocompromising condition. The difference between those with and without immunocompromising conditions was not statistically significant (χ2 P value = 0.39).
Serotype
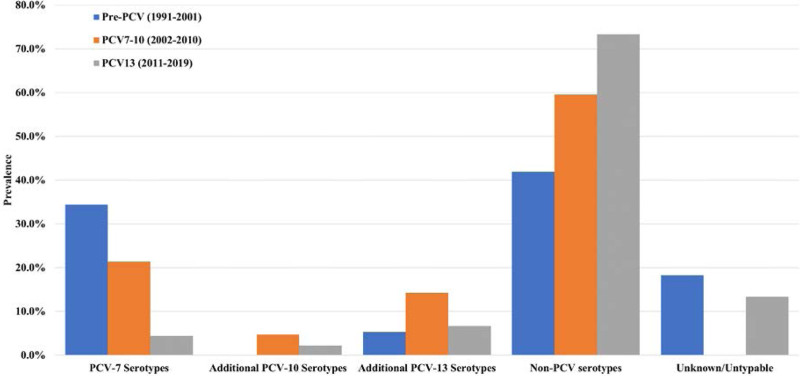
Pneumococcal serotype data were available for 157/180 cases (87.2%), at the most recent episode (Figure, Table, Supplemental Digital Content 3, http://links.lww.com/INF/E641). Among the cases with known serotype and immunization status, 59 were infected with PCV13 serotypes including: serotype 14 (n = 13), 23F (n = 10), 18C (n = 9), 19F (n = 7), 6A (n = 6), 19A (n = 95), 4 (n = 4), 7F (n = 2), 3 (n = 2) and 1 (n = 1). Among the 59 patients with PCV13 serotypes, 6 were appropriately immunized for age, the rest were under or not vaccinated (Figure,Table, Supplemental Digital Content 4, http://links.lww.com/INF/E642). Of these 6 immunized cases, 4 were male, 5 were immunocompromised, all 6 were treated in the inpatient setting and all 6 survived their rIPD episode. Serotypes of these 6 cases included 19A (n = 3) 3 (n = 1) and 4 (n = 1) 1 case was of unknown serotype.
FIGURE 1.
Serotype distribution at the most recent rIPD episode across the three vaccine eras covered by the dataset.
FIGURE 2.
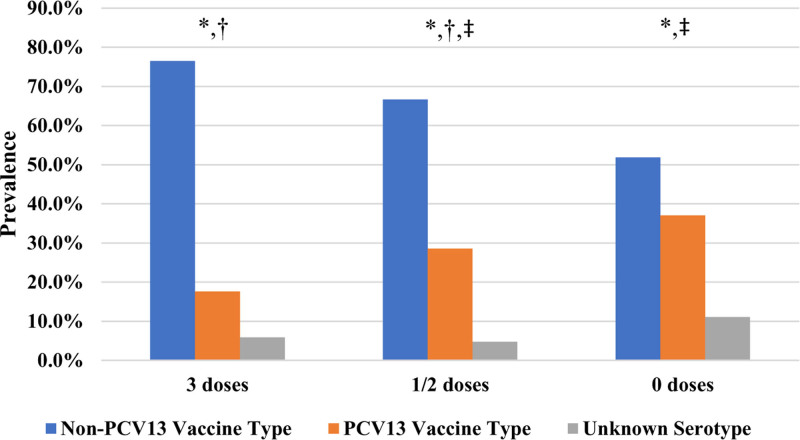
Pneumococcal conjugate vaccine status and infection serotype. * P value: 0.17, † P value: 0.20, ‡ P value: 0.17.
The proportion of cases affected by non-PCV13 covered serotypes increased significantly over this time period, from 41.9% to 59.5% and 73.3% of all cases in the pre PCV7, PCV7-10 and post PCV13 cohorts, respectively (Table, Supplemental Digital Content 2, http://links.lww.com/INF/E640, P value: 0.0022). In the most recent (post PCV13) cohort, the non-PCV13 covered serotypes were recorded: 15B (n = 5), 23A (n = 4), 35B (n = 4), 22F (n = 3), 15A (n = 2), 23B (n = 2), 9N (n = 2), 11A (n = 2) as well as 10A, 13, 15C, 16F, 20, 21, 24F, 27, 33F (n = 1) each as shown in Table, Supplemental Digital Content 3, http://links.lww.com/INF/E641.
The total under-vaccinated and nonvaccinated population was 50 cases (excluding pre-PCV cases), 46 of which were infected by a known serotype. Of these serotypes, 16 of 46 (34.8%) were infected by PCV-13 covered serotypes compared with 6 of 30 (20.0%) appropriately vaccinated patients (Table, Supplemental Digital Content 4, http://links.lww.com/INF/E642, P value: 0.17). Extrapolating from this by assuming a fixed 20% (6 of 30 known serotypes for appropriately vaccinated cases in Table, Supplemental Digital Content 4, http://links.lww.com/INF/E642) prevalence of PCV-13 covered serotypes, we estimate that 8 of these 16 cases could have been prevented with appropriate levels of vaccination.
Partial vaccination, defined as 1 or 2 doses of any PCV, was investigated by comparing the prevalence of PCV-13 serotypes in cases with different numbers of vaccine doses received as shown in Figure, Table, Supplemental Digital Content 4, http://links.lww.com/INF/E642. PCV-13 serotypes accounted for 37.0% of all cases having received no PCV vaccine, 28.6% of all cases having received 1 or 2 doses and 17.6% of cases having received 3 doses. These differences were not shown to be statistically significant with χ2 P values > 0.05 as shown in Figure, Table, Supplemental Digital Content 4, http://links.lww.com/INF/E642.
DISCUSSION
The patient survival rate in this rIPD population was 98.3% (including those lost to follow-up) and the mortality rate was 1.6%. Mortality due to nonrecurrent IPD is not studied in this cohort. The hospitalization rate was 89.4% and the ICU admission rate was 15% with a median duration of 3 days which increased over time. Recent studies from Barcelona, Spain and Suzhou, China showed an IPD mortality rate of 1.7%, comparable with the one shown here, and 17.5%, respectively in pediatric IPD populations.2,11 Mortality has been shown to be similar in IPD and rIPD cases.12 However, pediatric rIPD mortality rates as low as 0% have been reported.13 A recent Australian study showed a mortality rate of 5.3% in a cohort covering the time period of 1991–2016, similar to this study.14 In our study, isolated bacteremia was the most frequent clinical syndrome, both at the first episode and at recurrence (35.6% and 44.4%, respectively).
Males accounted for 63.2% of this cohort. This is consistent with previous rIPD reports and with male sex being identified as an IPD risk factor.15 No explanation for this has yet been provided in the literature. Immunocompromised patients accounted for 43.3% of this rIPD sample. The rate of immunocompromise among rIPD cases is variable in the literature (40%–92%) and it is hypothesized that such variability is due to the variability in testing for these conditions across healthcare systems and institutions.5 Primary immunodeficiency has been shown to be more prevalent in pediatric rIPD cases than all pediatric IPD cases (66.7% vs. 1.3–10.5% in a 2019 systematic review).16 With respect to primary IPD, children over the age of 2 years are more likely to have a primary immunodeficiency.16 Recurrent IPD, particularly in vaccinated children, has been suggested to be used as an indication for immunodeficiency screening.1,5 Certainly, in our data, we found rIPD to be a good indicator for further testing given the high prevalence of immune compromise in this population, similar to the case with the studies above. Testing for immunodeficiency after the first IPD presentation is already considered best practice at some centers.
We found an increase in the proportion of rIPD patients who received an age-appropriate number of PCV doses from 14.2% to 57.8% in the PCV7-10 and PCV13 cohorts, respectively. This was accompanied by an increase in the proportion of cases infected with non-PCV13 serotypes. The increased prevalence of non-PCV13 serotypes in IPD has been previously described in the literature.13,17 The non-PCV-covered serotype 8, in particular, has been associated with rIPD.18 Further monitoring of this serotype is important as it may have implications on vaccine development.
The vaccine failure rate of 12.5% reported here (cases involving a PCV-covered serotype and vaccinated with 3 or more PCV-13 doses) is plausible given the high prevalence of immunocompromise in this population. Partial vaccination, defined as 1 or 2 doses of any PCV, was shown to be less protective against PCV-covered serotypes in this cohort when compared with 3 doses but these results were not statistically significant (Table, Supplemental Digital Content 4, http://links.lww.com/INF/E642).
Limitations of this study include that some patients, particularly younger ones, may have other relevant existing medical conditions not yet diagnosed at the time of reporting. Data collection was performed during acute illness and therefore may be incomplete and would not capture any diagnoses (eg, primary immune deficiency) made after discharge. Similar cases have previously been reported in the literature.1 The first IPD episode reported was based on medical history and/or previous admission records and did not employ a serotype or molecular marker to distinguish if the two IPD episodes were due to a relapsed infection or a recurrence.
Strengths of this study include the relatively large sample size over 3 vaccine eras. The data were recorded over 3 decades on standardized case report forms using standardized procedures. This provides for a long, standardized, population-level follow-up period over multiple vaccine eras and a lower degree of variability in reporting practices. The dataset also includes cases from across Canada making it more robust against the effects of local infection serotype and antimicrobial resistance.
CONCLUSION
This study described the demographics, clinic presentation, outcome and vaccine coverage in Canadian children affected by rIPD. These data show patient demographics and outcomes consistent with previous reports. Significantly, we show a decrease in cases associated with PCV13 covered serotypes after the introduction of this higher-valent PCV. This is especially important for patients with preexisting medical conditions who are shown to be more likely to be vaccinated than their healthy peers. The overall occurrence of rIPD, both in our sample and from the literature, appears to be largely unchanged. This is due to the increase in rIPD due to non-PCV serotypes which acts to offset the decrease in rIPD due to PCV serotypes. The implications of this change in predominant rIPD serotypes remains to be seen as the trend continues.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the expert assistance of the Monitor Liaisons (Heather Samson, Annick Audet), IMPACT nurse monitors, staff of the IMPACT Data Center (Kim Marty, Jen Mark) and the Canadian Paediatric Society (Melanie Laffin).
* Investigators participating in this IMPACT project included:
N. Bridger MD, Cheryl Foo MD
Janeway Children’s Health & Rehabilitation Centre, St. John’s, NL
S.A. Halperin MD, K.A. Top MD
IWK Health Centre, Halifax, NS
R. Thibeault MD
Centre Mère-Enfant de Québec, CHUL, Quebec City, PQ
D. Moore MD, J. Papenburg MD
The Montreal Children’s Hospital, Montreal, PQ
M. Lebel MD
CHU Ste-Justine, Montreal, PQ
N. Le Saux MD
Children’s Hospital of Eastern Ontario, Ottawa, ON
S. Morris MD
The Hospital for Sick Children, Toronto, ON
J. Embree MD
Winnipeg Children’s Hospital, Winnipeg, MB
B. Tan M, Athena McConnell MD
Jim Pattison Children’s Hospital, Saskatoon, SK
T. Jadavji MD, C. Constantinescu MD
Alberta Children’s Hospital, Calgary, AB
W. Vaudry MD
Stollery Children’s Hospital, Edmonton, AB
D. Scheifele MD, M. Sadarangani BM BCh DPhil, J. Bettinger PhD, L. Sauvé MD
BC Children’s Hospital, Vancouver, BC
Supplementary Material
Footnotes
The Canadian Immunization Monitoring Program, Active (IMPACT) invasive pneumococcal surveillance is a national surveillance initiative managed by the Canadian Paediatric Society and conducted by the IMPACT network of pediatric investigators on behalf of the Public Health Agency of Canada’s Centre for Immunization and Respiratory Infectious Diseases.
S.H. has received funding for clinical trials and have served on ad hoc advisory boards for Pfizer, the manufacturer of PCV7 and PCV13, unrelated to pneumococcal disease. M.S. is supported via salary awards from the BC Children’s Hospital Foundation, the Canadian Child Health Clinician Scientist Program and the Michael Smith Foundation for Health Research. M.S. has been an investigator on projects funded by GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments. G.T. has received funding for pneumococcal studies from Merck and has served on a Pfizer advisory committee for vaccines. S.M. has participated on an advisory board for Pfizer, is an investigator on an investigator-led project funded by Pfizer, and has received personal fees from Johnson and Johnson China and GlaxoSmithKline outside the submitted work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com)
Contributor Information
N. Bridger, Janeway Children’s Health & Rehabilitation Centre, St. John’s, NL.
Cheryl Foo, Janeway Children’s Health & Rehabilitation Centre, St. John’s, NL.
S.A. Halperin, IWK Health Centre, Halifax, NS.
K.A. Top, IWK Health Centre, Halifax, NS.
R. Thibeault, Centre Mère-Enfant de Québec, CHUL, Quebec City, PQ.
D. Moore, The Montreal Children’s Hospital, Montreal, PQ.
J. Papenburg, The Montreal Children’s Hospital, Montreal, PQ.
M. Lebel, CHU Ste-Justine, Montreal, PQ.
N. Le Saux, Children’s Hospital of Eastern Ontario, Ottawa, ON.
S. Morris, The Hospital for Sick Children, Toronto, ON.
J. Embree, Winnipeg Children’s Hospital, Winnipeg, MB.
B. Tan, Jim Pattison Children’s Hospital, Saskatoon, SK.
Athena McConnell, Jim Pattison Children’s Hospital, Saskatoon, SK.
T. Jadavji, Alberta Children’s Hospital, Calgary, AB.
C. Constantinescu, Alberta Children’s Hospital, Calgary, AB.
W. Vaudry, Stollery Children’s Hospital, Edmonton, AB.
D. Scheifele, BC Children’s Hospital, Vancouver, BC.
M. Sadarangani, BC Children’s Hospital, Vancouver, BC.
J. Bettinger, BC Children’s Hospital, Vancouver, BC.
L. Sauvé, BC Children’s Hospital, Vancouver, BC.
Collaborators: * Investigators participating in this IMPACT project included:, N. Bridger, Cheryl Foo, S.A. Halperin, K.A. Top, R. Thibeault, D. Moore, J. Papenburg, M. Lebel, N. Le Saux, S. Morris, J. Embree, B. Tan, Athena McConnell, T. Jadavji, C. Constantinescu, W. Vaudry, D. Scheifele, M. Sadarangani, J. Bettinger, and L. Sauvé
REFERENCES
- 1.Gaschignard J, Levy C, Chrabieh M, et al. Invasive pneumococcal disease in children can reveal a primary immunodeficiency. Clin Infect Dis. 2014;59:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsina L, Basteiro MG, de Paz HD, et al. Recurrent invasive pneumococcal disease in children: underlying clinical conditions, and immunological and microbiological characteristics. PLoS One. 2015;10:e0118848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason EO, Jr, Wald ER, Tan TQ, et al. Recurrent systemic pneumococcal disease in children. Pediatr Infect Dis J. 2007;26:480–484. [DOI] [PubMed] [Google Scholar]
- 4.Einarsdöttir HM, Erlendsdóttir H, Kristinsson KGG, et al. Nationwide study of recurrent invasive pneumococcal infections in a population with a low prevalence of human immunodeficiency virus infection. Clin Microbiol Infect. 2005;11:744–749. [DOI] [PubMed] [Google Scholar]
- 5.Ingels H, Schejbel L, Lundstedt AC, et al. Immunodeficiency among children with recurrent invasive pneumococcal disease. Pediatr Infect Dis J. 2015;34:644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picard C, Bobby Gaspar H, Al-Herz W, et al. International union of immunological societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J Clin Immunol. 2018;38:96–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheifele D. IMPACT after 17 years: lessons learned about successful networking. Can J Infect Dis Med Microbiol. 2009;20:12–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Scheifele DW, Halperin SA; CPS/Health Canada, Immunization Monitoring Program, Active (IMPACT). Immunization Monitoring Program, Active: a model of active surveillance of vaccine safety. Semin Pediatr Infect Dis. 2003;14:213–219. [DOI] [PubMed] [Google Scholar]
- 9.Austrian R. The quellung reaction, a neglected microbiologic technique. Mt Sinai J Med. 1976;43:699–709. [PubMed] [Google Scholar]
- 10.National Advisory Committee on Immunization. Update on pediatric invasive pneumococcal disease and recommended use of conjugate pneumococcal vaccines. Canada Commun Dis Rep. 2010;36:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S, Guo X, Xu Z, et al. Early clinical predictors for the prognosis of invasive pneumococcal disease. BMC Infect Dis. 2020;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King MD, Whitney CG, Parekh F, et al. ; Active Bacterial Core Surveillance Team/Emerging Infections Program Network. Recurrent invasive pneumococcal disease: a population-based assessment. Clin Infect Dis. 2003;37:1029–1036. [DOI] [PubMed] [Google Scholar]
- 13.Ingels H, Lambertsen L, Harboe ZB, et al. Recurrent invasive pneumococcal disease in children: epidemiological, microbiological, and clinical aspects from a Danish 33-year nationwide survey (1980–2013). Scand J Infect Dis. 2014;46:265–271. [DOI] [PubMed] [Google Scholar]
- 14.Malo JA, Ware RS, Lambert SB. Estimating the risk of recurrent invasive pneumococcal disease in Australia, 1991–2016. Vaccine. 2021;39:5748–5756. [DOI] [PubMed] [Google Scholar]
- 15.Mufson MA, Hao JB, Stanek RJ, et al. Clinical features of patients with recurrent invasive Streptococcus pneumoniae disease. Am J Med Sci. 2012;343:303–309. [DOI] [PubMed] [Google Scholar]
- 16.Butters C, Phuong LK, Cole T, et al. Prevalence of immunodeficiency in children with invasive pneumococcal disease in the pneumococcal vaccine era: a systematic review. JAMA Pediatr. 2019;173:1084–1094. [DOI] [PubMed] [Google Scholar]
- 17.Bettinger JA, Scheifele DW, Kellner JD, et al. The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000–2007. Vaccine. 2010;28:2130–2136. [DOI] [PubMed] [Google Scholar]
- 18.Sanz JC, Rodríguez-Avial I, Ríos E, et al. Recurrent pneumococcal invasive disease in the region of Madrid during a five-year period. Infection. 2014;42:475–483. [DOI] [PubMed] [Google Scholar]