Summary
Background
Among patients with type 2 diabetes, minority racial/ethnic groups have a higher burden of cardiovascular disease, chronic kidney disease, and hypoglycaemia. These groups may especially benefit from newer diabetes medication classes, but high cost may limit access. We examined the association of race/ethnicity with the initiation of newer diabetes medications (GLP-1 receptor agonists, DPP-4 inhibitors, SGLT-2 inhibitors).
Methods
We conducted a secondary analysis of the Look AHEAD (Action for Health in Diabetes) trial including participants with at least one study visit after April 28, 2005. Cox proportional hazards models were used to estimate the association between race/ethnicity and socioeconomic factors with time to initiation of any newer diabetes medication from April 2005 to February 2020. Models were adjusted for demographic and clinical characteristics.
Findings
Among 4,892 participants, 63.6%, 15.7%, 12.6%, 5.2%, and 2.9% were White, Black, Hispanic, American Indian or Alaskan Native (AI/AN), or other race/ethnicity, respectively. During a median follow-up of 8.3 years, 2,180 (45.2%) participants were initiated on newer diabetes medications. Race/ethnicity was associated with newer diabetes medication initiation (p=.019). Specifically, initiation was lower among Black (HR 0.81, 95% CI 0.70–0.94) and AI/AN participants (HR 0.51, 95% CI 0.26–0.99). Yearly family income was inversely associated with initiation of newer diabetes medications (HR 0.78, 95% CI 0.62–0.98) comparing the lowest and highest income groups. Findings were mostly driven by GLP-1 receptor agonists.
Interpretation
These findings provide evidence of racial/ethnic disparities in the initiation of newer diabetes medications, independent of socioeconomic factors, which may contribute to worse health outcomes.
Funding source
NIDDK, NIH
Keywords: Racial disparities, Diabetes care, Newer medications, Medication initiation, Socioeconomic disparities, Diabetes outcomes
Research in context.
Evidence before this study
There is strong and consistent observational evidence for racial/ethnic disparities in health outcomes for patients with diabetes, and barriers to accessing medications for chronic conditions. Clinical practice guidelines recommend the preferential use of newer classes of diabetes medications in my clinical scenarios, especially with, or at high risk for, cardiovascular and renal complications. We therefore sought to examine whether there are differences by race/ethnicity in initiation of newer diabetes medications in the US during the period after these drugs came to market. We searched PubMed, Embase and Web of Science and found no studies reporting the association between race/ethnicity and newer diabetes medication use.
Added value of this study
In this study, we provide the first data to our knowledge examining racial/ethnic differences in the initiation of newer classes of diabetes medications. We were able to show that individuals of all minority race/ethnicities had lower initiation of newer diabetes medications compared to white individuals and initiation of newer diabetes medications was significantly lower for black and American Indian or Alaskan Native individuals. This effect was independent of socioeconomic status and clinical factors (glycemic control and intensity of diabetes therapy). While income was inversely associated with initiation of newer diabetes medications, adjustment for income and other socioeconomic factors did not substantially attenuate the effect of race/ethnicity, suggesting that there are factors beyond medication cost contributing to lower initiation in racial/ethnic minorities.
Implications of all the available evidence
Racial/ethnic disparities in the initiation of newer diabetes medications have important clinical consequences. These groups may especially benefit from the use of newer diabetes medications given their reno- and cardio-protective effects. Lack of access to newer diabetes medications could widen the existing disparities in diabetes care and contribute to the worse outcomes experienced by minority race/ethnicity groups. Further study is needed to understand if these differences are driven by systemic health systems, physician-specific or patient-specific factors. Doing so may help in the design of interventions that reduce barriers to care and improve diabetes outcomes for racial/ethnic minorities.
Alt-text: Unlabelled box
Introduction
Racial/ethnic minorities with type 2 diabetes mellitus have worse glycaemic control and higher rates of diabetes complications and mortality.1, 2, 3 Socioeconomic status has also been found to impact diabetes outcomes such that individuals with lower income or educational attainment have worse glycaemic control and higher diabetes-related mortality.4,5 Further, among patients with diabetes, minority race/ethnicity and lower socioeconomic status are associated with greater cost-limited access of diabetes medications.6,7
In the past 20 years, three newer classes of diabetes medications became available in the U.S.: glucagon-like peptide receptor agonists (GLP-1RAs) in 2005, dipeptidyl peptidase 4 inhibitors (DPP-4Is) in 2006, and sodium/glucose cotransporter 2 inhibitors (SGLT-2Is) in 2013.8 Given the evidence for cardiovascular and renal benefits, GLP-1RAs and SGLT-2Is are the preferred second-line medication classes for patients with atherosclerotic cardiovascular disease, heart failure or chronic kidney disease.8 In addition, all newer diabetes medications classes have a substantially lower risk of hypoglycaemia and weight gain compared to sulfonylureas or insulin.8 However, these newer medications are expensive, creating concerns about equitable access 6,8,9.
Racial/ethnic minorities, and lower income and educational attainment groups, have a higher burden of chronic kidney disease, worse cardiovascular outcomes, and higher rates of severe hypoglycaemia.2,3,10, 11, 12 Therefore, these groups may especially benefit from the use of newer diabetes medications. Lack of access to newer diabetes medications could widen the existing disparities in diabetes care.
In this secondary analysis of Look AHEAD (Action for Health in Diabetes), we aimed to determine the association of race/ethnicity and socioeconomic factors with the initiation of newer classes of diabetes medications. Look AHEAD is a multicentre randomized controlled trial of an intensive lifestyle intervention for adults with type 2 diabetes that followed participants over the period when newer diabetes medication classes became available on the U.S. market. We hypothesized that participants of minority race/ethnicity would have lower initiation of newer diabetes medication.13
Methods
Study population
The Look AHEAD trial enrolled 5145 adults with type 2 diabetes and body mass index (BMI) ≥25 kg/m2 (≥27 kg/m2 if using insulin) from 16 U.S. centres with recruitment from 2001 to 2004.14 For this study, we examined the 4892 participants with at least one follow-up visit after April 28, 2005, the date when the first newer classes of diabetes medications entered the U.S. market. Eligibility criteria for Look AHEAD included age 45–76 years, HbA1c <11% (97 mmol/mol), having a primary healthcare provider, and able to complete a maximal exercise test at baseline 14,15. Exclusion criteria included serum creatinine >1.4 mg/dL (women) or 1.5 mg/dL (men), 4+ proteinuria, need for dialysis, or recent or exercise-limiting cardiovascular disease.14,15
Participants were randomized 1:1 to an intensive lifestyle intervention (ILI) or diabetes support and education (DSE), the latter being the control group. The objective of the ILI was to reduce participants’ initial body weight by 7% through sessions that encouraged increased physical activity and reduced caloric intake, self-monitoring, and, sometimes, pharmacologic weight loss interventions.16 Diabetes care during the study was provided by participants’ outside physicians. Participants in the ILI arm taking insulin, sulfonylureas, or meglitinides had additional monitoring and, when needed, temporary adjustment of diabetes medications by trial staff to prevent hypoglycaemia.16 The DSE arm received information on nutrition and physical activity and social support delivered in group classes up to three times a year.15 The primary outcome of Look AHEAD was time to occurrence of a combined cardiovascular outcome.14,15 Due to futility for the primary outcome, the intervention was terminated in September 2012 with a median follow-up of 9.6 years.15 Participants continue to be followed, and this study uses data through February 2020.
Study outcome
The primary outcome of this study is time to first use of a newer class of diabetes medication: a DPP-4I, GLP-1RA, or SGLT-2I. Medication use was determined at annual study visits using a medication inventory form completed by trained study staff with participants instructed to bring in their home medications for review. When participants did not bring their medications, staff ascertained medication changes and placed follow-up phone calls when necessary.14,15
Primary and secondary exposure variables
The predictors of interest in this study were race/ethnicity and socioeconomic measures. Race/ethnicity was self-reported in categories of White, Black, Hispanic, American Indian or Alaskan Native (AI/AN), Asian or Pacific Islander, and other. Asian or Pacific Islander were included in “other” for these analyses due to few participants in this group. Socioeconomic measures were assessed by standardized interviewer-administered questionnaires at study baseline. Yearly family income was analysed in five categories from less than $20,000 to greater than $80,000 or missing. Highest level of education was analysed in categories of less than high school, high school or equivalent, vocational school or some college, bachelor's degree or post-graduate degree. Employment status was analysed in categories of working full or part time, homemaker, unemployed, or missing. Health insurance was analysed in categories of individual or partner's insurance, government insurance (Medicare, Medicaid, Veterans Affairs, or Indian Health Services), other insurance, or uninsured. Source of medical care was analysed in categories of private doctor's office, hospital clinic or outpatient department, community health center, or other.
Other characteristics
Other variables added to the model include HbA1c, estimate glomerular filtration rate (eGFR, CKD-Epi equation), hypertension, BMI, and cardiovascular disease. HbA1c and eGFR were measured annually through study year four and on alternating years thereafter. Hypertension (defined as systolic blood pressure >140 mmHg or use of blood pressure lowering medications) and BMI were measured yearly using standardized protocols. History of cardiovascular disease was self-reported at baseline. Subsequent records were defined using the prespecified the Look AHEAD trial primary cardiovascular outcome: composite of myocardial infarction, stroke, or hospitalized angina ascertained through regular telephone calls to participants and adjudicated hospital records.
Statistical analysis
All continuous variables were categorized into clinically relevant groups determined a-priori. All categorical variables were analysed as nominal (non-ordered). For descriptions, see Supplemental Table 1.
Baseline characteristics were described as means or proportions and compared across categories of race/ethnicity using one-way analysis of variance for continuous variables or chi-squared tests for categorical variables. Cox proportional hazards models were used to examine the association of race/ethnicity and socioeconomic measures with the primary outcome. The time scale was calendar time from the first study visit after April 28, 2005 until the occurrence of the outcome or censoring at the date of their last study contact through February 2020. Participants with gaps due to missing study visit medication data as determined by an absent medication form were excluded from analysis for the duration of the gap and did not accrue time at risk for that period. Two multivariable models were used to assess the relationship of race/ethnicity with the primary outcome, without (Model 1) and with adjustment for socioeconomic factors (Model 2). Both models were adjusted for demographic and clinical characteristics hypothesized to have potential roles in diabetes medication selection described in “Other Characteristics” (see Supplemental Table 1), as well as Look AHEAD treatment arm and study site. A 2-sided P <0.05 was considered statistically significant. The proportional hazards assumption was checked by visual inspection of log-log hazards curves. We examined the interaction between race/ethnicity and yearly family income where we treated income as continuous. All analyses were performed using SAS software version 9.4 (Cary, NC).
Subgroup and sensitivity analysis
We conducted exploratory subgroup analyses using the fully adjusted model (Model 2). We assessed for multiplicative interactions of our primary association, performing stratified analyses when merited, by factors in clinical guidelines that may affect the selection of diabetes medications including: age (<65 years, ≥65 years), gender, diabetes duration (<10 years, ≥10 years), and the presence of cardiovascular disease and chronic kidney disease.8 We conducted four sensitivity analyses using the fully adjusted model: 1) examining initiation of each newer diabetes medication class individually; 2) stratifying by intervention arm; 3) adjusting for whether participants brought their home medications to the study visit for review; and 4) modeling death as a competing risk using the Fine and Gray approach.17
Role of the funding source
The study was primarily supported by the NIDDK and the NIH. The funding sources had no role in designing or conducting the study or in the reporting of results.
Results
Participant characteristics
The baseline (April 2005) characteristics of the 4892 included participants are shown in Table 1. The mean age was 58.7 years, 59.8% of participants were female, and 63.6%, 15.7%, 12.6%, 5.2%, and 2.9% of participants were White, Black, Hispanic, AI/AN, or other race/ethnicity, respectively. White participants were more likely to be male and had an older average age than minority race/ethnicity participants. There were differences by race/ethnicity in clinical characteristics with White participants having lower HbA1c, more thiazolidinedione use, and lower insulin use compared to minority participants. White participants were also more likely to have a history of cardiovascular disease and lower eGFR compared to Black, Hispanic, and AI/AN participants. White participants had greater yearly family income, higher levels of education, and were more likely to have health insurance compared to Black and Hispanic participants. Hispanic participants were substantially more likely to be uninsured than White participants (32.6% vs. 2.2%). The majority of participants brought medications to the visit for review (Supplemental Table 5).
Table 1.
Baseline characteristics overall and by race/ethnicity.
| Characteristic* | Overall (n = 4892) | White (n = 3111) | Black (n = 766) | Hispanic (n = 614) | AI/AN (n = 255) | Other (n = 146) | p-value† |
|---|---|---|---|---|---|---|---|
| Intensive Lifestyle Intervention Arm (%) | 2461 (50.3) | 1561 (50.2) | 384 (50.1) | 309 (50.3) | 129 (50.6) | 78 (53.4) | 0.96 |
| Age, mean (SD), years | 58.7 (6.8) | 59.5 (6.8) | 58.0 (6.7) | 57.4 (6.3) | 55.3 (7.2) | 58.4 (6.9) | <0.001 |
| Age category (%) | <0.001 | ||||||
| 45–54 years | 1185 (24.2) | 651 (20.9) | 196 (25.6) | 173 (28.2) | 127 (49.8) | 38 (26.0) | |
| 55–64 years | 2700 (55.2) | 1723 (55.4) | 438 (57.2) | 362 (59.0) | 96 (37.7) | 81 (55.5) | |
| 65–76 years | 1007 (20.6) | 737 (23.7) | 132 (17.2) | 79 (12.9) | 32 (12.6) | 27 (18.5) | |
| Female (%) | 2927 (59.8) | 1607 (51.7) | 583 (76.1) | 445 (72.5) | 201 (78.8) | 91 (62.3) | <0.001 |
| Yearly family income (%) | <0.001 | ||||||
| > $80,000 | 1302 (26.6) | 1048 (33.7) | 126 (16.5) | 67 (10.9) | 82 (32.2) | 11 (7.5) | |
| $60,000–80,000 | 725 (14.8) | 500 (16.1) | 114 (14.9) | 64 (10.4) | 61 (23.9) | 27 (18.5) | |
| $40,000–60,000 | 910 (18.6) | 584 (18.8) | 143 (18.7) | 108 (17.6) | 49 (19.2) | 26 (17.8) | |
| $20,000–40,000 | 932 (19.1) | 484 (15.6) | 177 (23.1) | 183 (29.8) | 21 (8.2) | 26 (17.8) | |
| <$20,000 | 538 (11.0) | 161 (5.2) | 107 (14.0) | 177 (28.8) | 18 (7.1) | 43 (29.5) | |
| Missing | 485 (9.9) | 334 (10.7) | 99 (12.9) | 15 (2.4) | 24 (9.4) | 13 (8.9) | |
| Highest level of education (%) | <0.001 | ||||||
| Post Graduate degree | 937 (19.6) | 722 (23.8) | 125 (16.6) | 45 (7.4) | 8 (3.3) | 37 (26.6) | |
| Bachelor's degree | 1069 (22.4) | 798 (26.3) | 149 (19.7) | 66 (10.9) | 16 (6.6) | 40 (28.8) | |
| Vocational / some college | 1816 (38.0) | 1088 (35.8) | 343 (45.4) | 208 (34.4) | 122 (50.2) | 55 (39.6) | |
| High School or equivalent | 648 (13.6) | 389 (12.8) | 103 (13.6) | 100 (16.5) | 51 (21.0) | 5 (3.6) | |
| Less than high school | 310 (6.5) | 41 (1.4) | 35 (4.6) | 186 (30.7) | 46 (18.9) | 2 (1.4) | |
| Employment status (%) | <0.001 | ||||||
| Working full or part time | 3128 (63.9) | 2059 (66.2) | 473 (61.8) | 346 (56.4) | 150 (58.8) | 100 (68.5) | |
| Homemaker | 837 (17.1) | 469 (15.1) | 116 (15.1) | 174 (28.3) | 53 (20.8) | 25 (17.1) | |
| Unemployed | 381 (7.8) | 240 (7.7) | 72 (9.4) | 36 (5.9) | 24 (9.4) | 9 (6.2) | |
| Missing | 546 (11.2) | 343 (11.0) | 105 (13.7) | 58 (9.5) | 28 (11.0) | 12 (8.2) | |
| Type of health insurance (%) | <0.001 | ||||||
| Private insurance | 3808 (78.0) | 2665 (85.8) | 587 (76.9) | 329 (53.6) | 111 (43.5) | 116 (79.5) | |
| Government | 616 (12.6) | 324 (10.4) | 114 (14.9) | 66 (10.8) | 102 (40.0) | 10 (6.9) | |
| Other insurance | 88 (1.8) | 47 (1.5) | 13 (1.7) | 19 (3.1) | 3 (1.2) | 6 (4.1) | |
| Uninsured | 371 (7.6) | 69 (2.2) | 49 (6.4) | 200 (32.6) | 39 (15.3) | 14 (9.6) | |
| Source of medical care (%) | <0.001 | ||||||
| Private doctor's office | 3619 (74.2) | 2622 (84.4) | 533 (69.9) | 319 (52.0) | 39 (15.5) | 106 (72.6) | |
| Hospital clinic or outpatient department | 613 (12.6) | 237 (7.6) | 119 (15.6) | 88 (14.3) | 149 (59.1) | 20 (13.7) | |
| Community health center | 357 (7.3) | 75 (2.4) | 48 (6.3) | 169 (27.5) | 54 (21.4) | 11 (7.5) | |
| Other | 291 (6.0) | 171 (5.5) | 63 (8.3) | 38 (6.2) | 10 (4.0) | 9 (6.2) | |
| HbA1c, mean (SD),% | 7.3 (1.2) | 7.2 (1.1) | 7.5 (1.3) | 7.5 (1.3) | 7.5 (1.3) | 7.2 (1.1) | <0.001 |
| HbA1c category (%) | <0.001 | ||||||
| < 6.0% / 42 mmol/mol | 365 (7.5) | 265 (8.5) | 39 (5.1) | 36 (5.9) | 17 (6.7) | 8 (5.5) | |
| 6.0–6.4% / 42–46 mmol/mol | 865 (17.7) | 565 (18.2) | 126 (16.5) | 100 (16.3) | 43 (16.9) | 31 (21.2) | |
| 6.5–6.9% / 48–52 mmol/mol | 1018 (20.8) | 697 (22.4) | 140 (18.3) | 108 (17.6) | 45 (17.7) | 28 (19.2) | |
| 7.0–7.9% / 53–63 mmol/mol | 1511 (30.9) | 963 (31.0) | 238 (31.1) | 189 (30.8) | 73 (28.6) | 48 (32.9) | |
| 8.0–8.9% / 64–74 mmol/mol | 742 (15.2) | 422 (13.6) | 143 (18.7) | 111 (18.1) | 44 (17.3) | 22 (15.1) | |
| ≥ 9.0% / 75 mmol/mol | 391 (8.0) | 199 (6.4) | 80 (10.4) | 70 (11.4) | 33 (12.9) | 9 (6.2) | |
| eGFR, mean (SD), mL/min/1.73m2 | 89.7 (16.0) | 87.1 (15.0) | 95.5 (18.2) | 94.3 (14.0) | 93.6 (16.9) | 87.0 (16.8) | <0.001 |
| eGFR category (%) | <0.001 | ||||||
| ≥ 90 | 2742 (56.2) | 1567 (50.5) | 480 (63.2) | 448 (73.1) | 171 (67.1) | 76 (52.1) | |
| 60–90 | 1898 (38.9) | 1364 (43.9) | 254 (33.4) | 147 (24.0) | 71 (27.8) | 62 (42.5) | |
| < 60 | 239 (4.9) | 174 (5.6) | 26 (3.4) | 18 (2.9) | 13 (5.1) | 8 (5.5) | |
| BMI, mean (SD), kg/m2 | 35.9 (5.9) | 36.0 (5.9) | 36.6 (6.0) | 35.3 (5.7) | 35.8 (6.3) | 34.7 (5.8) | <0.001 |
| BMI categories (%) | 0.001 | ||||||
| 25–29 | 732 (15.0) | 455 (14.6) | 96 (12.5) | 106 (17.3) | 40 (15.8) | 35 (24.0) | |
| 30–34 | 1723 (35.3) | 1101 (35.4) | 247 (32.3) | 239 (39.0) | 90 (35.6) | 46 (31.5) | |
| 35–39 | 1336 (27.3) | 852 (27.4) | 227 (29.6) | 153 (25.0) | 62 (24.5) | 42 (28.8) | |
| ≥ 40 | 1097 (22.4) | 702 (22.6) | 196 (25.6) | 115 (18.8) | 61 (24.1) | 23 (15.8) | |
| Diabetes duration, mean (SD), years | 6.8 (6.6) | 6.6 (6.3) | 6.6 (6.5) | 7.2 (6.9) | 8.6 (8.5) | 7.5 (7.1) | <0.001 |
| Diabetes duration, categories (%) | 0.037 | ||||||
| 0–4 | 2210 (45.5) | 1411 (45.6) | 361 (47.3) | 278 (45.6) | 97 (39.8) | 63 (44.1) | |
| 5–9 | 1345 (27.9) | 896 (29.0) | 205 (26.8) | 151 (24.8) | 63 (25.8) | 39 (27.3) | |
| ≥ 10 | 1289 (26.6) | 785 (25.4) | 198 (25.9) | 181 (29.7) | 84 (34.4) | 41 (28.7) | |
| Cardiovascular disease (%) | 667 (13.6) | 485 (15.6) | 74 (9.7) | 56 (9.1) | 25 (9.8) | 27 (18.5) | <0.001 |
| Hypertension (%) | 4073 (83.3) | 2595 (83.4) | 676 (88.3) | 486 (79.2) | 195 (76.5) | 121 (82.9) | <0.001 |
| Insulin use (%) | 741 (15.2) | 422 (13.6) | 140 (18.3) | 108 (17.6) | 49 (19.2) | 22 (15.1) | 0.001 |
| Metformin use (%) | 2965 (60.6) | 1904 (61.2) | 445 (58.1) | 372 (60.6) | 161 (63.1) | 83 (56.9) | 0.401 |
| Sulfonylurea use (%) | 2210 (45.2) | 1364 (43.8) | 350 (45.7) | 310 (50.5) | 122 (47.8) | 64 (43.8) | 0.038 |
| Thiazolidinedione use (%) | 1271 (26.0) | 873 (28.1) | 207 (27.0) | 116 (18.9) | 39 (15.3) | 36 (24.7) | <0.001 |
| No. of diabetes medications (%) | <0.001 | ||||||
| 0 | 647 (13.4) | 447 (14.5) | 82 (10.8) | 62 (10.2) | 34 (13.6) | 22 (15.3) | |
| 1 | 1912 (39.5) | 1171 (38.0) | 309 (40.7) | 277 (45.6) | 103 (41.0) | 52 (36.1) | |
| 2 | 1601 (33.1) | 995 (32.3) | 284 (37.4) | 191 (31.4) | 83 (33.1) | 48 (33.3) | |
| ≥ 3 | 684 (14.1) | 469 (15.2) | 84 (11.1) | 78 (12.8) | 31 (12.4) | 22 (15.3) | |
| Diabetes Treatment Intensity (%) | <0.001 | ||||||
| No medications | 721 (14.7) | 489 (15.7) | 94 (12.3) | 72 (11.7) | 39 (15.3) | 27 (18.5) | |
| 1 non-insulin medication | 1669 (34.1) | 1054 (33.9) | 251 (32.8) | 233 (38.0) | 86 (33.7) | 45 (30.8) | |
| 2 non-insulin medications | 1358 (27.8) | 856 (27.5) | 227 (29.6) | 167 (27.2) | 68 (26.7) | 40 (27.4) | |
| 3+ non-insulin medications | 403 (8.2) | 290 (9.3) | 54 (7.1) | 34 (5.5) | 13 (5.1) | 12 (8.2) | |
| Insulin (with or without other meds) | 741 (15.2) | 422 (13.6) | 140 (18.3) | 108 (17.6) | 49 (19.2) | 22 (15.1) |
N (% of column) or mean (SD)
† Tested using chi-squared tests for categorical variables or one-way analysis of variance (ANOVA) for continuous variables
AI/AN, American Indian or Alaskan Native.
Association of race/ethnicity and initiation of newer diabetes medications
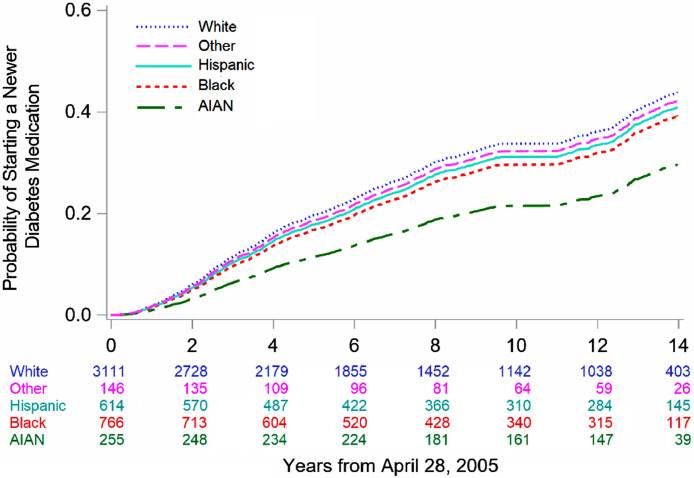
The median follow-up time for participants was 8.3 years with a total of 41,318 person-years at risk accrued. Overall, 2211 participants (45.2%) initiated a newer diabetes medication during follow-up. This included 48.0% of White, 44.2% of Black, 41.4% of Hispanic, 21.6% of AI/AN, and 41.6% of other race/ethnicity participants, respectively. The results of the Cox proportional hazards models for the association of race/ethnicity and socioeconomic factors with initiation of a newer diabetes medication are shown in Tables 2 and 3, and fully-adjusted time-to-event curves by race/ethnicity are shown in Fig. 1. In the fully adjusted analysis, race/ethnicity was significantly associated with initiation of newer diabetes medications (p=.019) with all minority race/ethnicities having a lower hazard ratio (HR) for initiation compared to Whites. This association was strongest among Black (HR 0.81, 95% CI 0.70–0.94) and AI/AN participants (0.51, 95% CI 0.26–0.99); the CI for other race/ethnicities crossed the null. The association of race/ethnicity and initiation of newer diabetes medication was slightly attenuated after adjustment for socioeconomic factors but was significant in both models. Notably, without adjustment for socioeconomic factors, Hispanic participants had a CI that did not cross the null (HR 0.82, 95% CI 0.68–0.99). There was no significant interaction between race/ethnicity and yearly family income (p= .30).
Table 2.
Unadjusted and adjusted hazard ratios for initiation of any newer class of diabetes medication by race/ethnicity and socioeconomic factors.
| Unadjusted results | Model 1* | Model 2* | ||||
|---|---|---|---|---|---|---|
| Characteristics | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Race/ethnicity | <0.001 | <0.001 | 0.019 | |||
| White | Reference | Reference | Reference | |||
| Black | 0.82 (0.73–0.92) | 0.73 (0.64–0.84) | 0.81 (0.70–0.94) | |||
| Hispanic | 0.77 (0.68–0.88) | 0.82 (0.68–0.99) | 0.88 (0.72–1.07) | |||
| American Indian or Alaskan Native | 0.33 (0.25–0.44) | 0.53 (0.29–0.98) | 0.51 (0.26–0.99) | |||
| Other | 0.79 (0.61–1.02) | 0.89 (0.68–1.17) | 0.93 (0.70–1.24) | |||
| Yearly family income | <0.001 | 0.008 | ||||
| > $80,000 | Reference | Reference | ||||
| $60,000–80,000 | 0.99 (0.87–1.13) | 0.96 (0.83–1.10) | ||||
| $40,000–60,000 | 0.87 (0.77–0.99) | 0.86 (0.75–0.98) | ||||
| $20,000–40,000 | 0.73 (0.64–0.84) | 0.77 (0.65–0.90) | ||||
| <$20,000 | 0.61 (0.49–0.75) | 0.78 (0.62–0.98) | ||||
| Missing | 0.76 (0.64–0.90) | 0.77 (0.64–0.93) | ||||
| Highest level of education | 0.960 | 0.48 | ||||
| Masters, doctorate or professional degree | Reference | Reference | ||||
| BA or some graduate school | 0.97 (0.86–1.10) | 0.96 (0.83–1.09) | ||||
| Vocational, some college, associate degree | 0.98 (0.87–1.11) | 0.92 (0.81–1.05) | ||||
| High school diploma or equivalent | 0.96 (0.82–1.13) | 0.86 (0.72–1.02) | ||||
| Less than high school | 0.92 (0.72–1.17) | 0.85 (0.63–1.15) | ||||
| Employment status | 0.022 | 0.37 | ||||
| Working full, part time, or student | Reference | Reference | ||||
| Homemaker | 0.97 (0.86–1.10) | 1.11 (0.97–1.28) | ||||
| Unemployed | 0.84 (0.71–1.01) | 0.99 (0.81–1.21) | ||||
| Missing | 0.81 (0.70–0.95) | 0.94 (0.79–1.12) | ||||
| Type of health insurance | 0.003 | 0.32 | ||||
| Private insurance | Reference | Reference | ||||
| Government insurance | 0.84 (0.72–0.99) | 0.96 (0.79–1.15) | ||||
| Other insurance | 1.34 (0.99–1.82) | 1.05 (0.75–1.47) | ||||
| Uninsured | 0.76 (0.60–0.96) | 0.78 (0.59–1.03) | ||||
| Source of medical care | <0.001 | <0.001 | ||||
| Private doctor's office | Reference | Reference | ||||
| Hospital clinic or outpatient department | 0.67 (0.58–0.78) | <0.001 | 0.78 (0.66–0.93) | |||
| Community health center | 0.65 (0.52–0.82) | <0.001 | 0.78 (0.60–1.01) | |||
| Other | 0.56 (0.46–0.69) | <0.001 | 0.66 (0.53–0.83) | |||
Cox proportional hazards models adjusted for race/ethnicity, socioeconomic factors, study site, intervention arm, age, gender, HbA1c, diabetes duration, diabetes treatment intensity, eGFR, BMI, and history of cardiovascular disease.
Table 3.
Use of newer diabetes medication classes during the study period, overall and by race/ethnicity.
| Medication class | Overall (N = 4892) | White (N = 3111) | Black (N = 766) | Hispanic (N = 614) | AI/AN* (N = 255) | Other (N = 146) |
|---|---|---|---|---|---|---|
| First newer diabetes medication class used | ||||||
| GLP-1 receptor agonist (%) | 976 (20.0) | 724 (23.3) | 120 (15.7) | 97 (15.8) | 13 (5.1) | 22 (15.1) |
| DPP-4 inhibitor (%) | 1154 (23.6) | 712 (22.9) | 206 (26.9) | 157 (25.6) | 42 (16.5) | 37 (25.3) |
| SGLT-2 inhibitor (%) | 81 (1.7) | 56 (1.8) | 12 (1.6) | 12 (2.0) | 0 (0.0) | 1 (1.2) |
| Any use of diabetes medication class during the study period | ||||||
| GLP-1 receptor agonist (%) | 1215 (24.8) | 886 (28.5) | 152 (19.8) | 131 (21.3) | 15 (5.9) | 31 (21.2) |
| DPP-4 inhibitor (%) | 1384 (28.3) | 878 (28.2) | 239 (31.2) | 181 (29.5) | 44 (17.3) | 42 (28.8) |
| SGLT-2 inhibitor (%) | 309 (6.3) | 219 (7.0) | 36 (4.7) | 40 (6.5) | 3 (1.2) | 11 (7.5) |
AI/AN, American Indian or Alaskan Native.
Fig. 1.
Adjusted time-to-event curve for initiation of any newer class of diabetes medication by race/ethnicity.
Association of socioeconomic factors and use of newer diabetes medications
In the fully adjusted analysis, yearly family income had a graded inverse relationship with initiation of newer diabetes medications (p=.008) with a HR of 0.78 (95% CI 0.62–0.98) comparing the lowest to highest income categories (Table 2). Source of medical care was also significantly associated with initiation of newer diabetes medications (p<.001) with participants who received care in hospital-based practices or other settings being significantly less likely to initiate newer medications, compared to receiving care in private offices. Educational achievement, employment status, and type of health insurance were not significantly associated with the outcome.
Use of newer diabetes medications by medication class
Table 3 shows the frequency of use of each newer diabetes medication class, including the frequency of each medication class being the first newer diabetes medication initiated, and the frequency of use at any time during the study period. DPP-4Is were the most frequently used newer diabetes medication class, both as the first class initiated (23.6%) and any use during the study period (28.3%). These were followed closely by GLP-1Ras (20.0% first use, 24.8% any use). SGLT-2Is were used relatively infrequently (1.7% first use, 6.3% any use).
Subgroup analyses
There were no significant interactions between race/ethnicity and the primary outcome by age, gender, diabetes duration, or the presence of cardiovascular disease or chronic kidney disease (Supplemental Fig. 1).
Sensitivity analysis
The association of race/ethnicity and socioeconomic factors with initiation of GLP-1Ras only was consistent with the primary analysis (Supplemental Table 2). Race/ethnicity and socioeconomic factors were not significantly associated with initiation of SGLT-2Is or DPP-4Is. Finding in each intervention arm were consistent with the primary analysis of both arms combined (Supplemental Table 3). There were no substantive differences from the primary analysis after adjustment for whether participants brought their home medications for review (Supplemental Tables 4 and 5) or accounting for competing risk of mortality (Supplemental Table 6).
Discussion
In this study among adults with type 2 diabetes in the Look AHEAD trial, we examined racial/ethnic differences in the initiation of newer diabetes medications from their entry onto the U.S. market in April 2005 until February 2020. We found that individuals of all minority race/ethnicities had lower initiation of newer diabetes medications compared to White participants, with initiation of newer diabetes medications being significantly lower for Black and AI/AN participants. This finding was mostly driven by GLP-1RAs. Among the socioeconomic factors examined, lower yearly family income and receiving medical care at a hospital clinic or outpatient department were significantly associated with lower initiation of newer diabetes medications. Adjustment for socioeconomic factors minimally attenuated the association of race/ethnicity with initiation of newer diabetes medications. These findings suggest that minorities with diabetes may experience barriers to initiating newer diabetes medications. Given that newer diabetes medications are especially beneficial for patients with cardiovascular disease and chronic kidney disease, and racial/ethnic minorities are disproportionately affected by these conditions, differences in the initiation of newer diabetes medications may be an important contributing factor to racial/ethnic disparities in diabetes outcomes.
This is the first study to our knowledge to examine racial/ethnic differences in the initiation of newer classes of diabetes medications. Prior studies examining racial/ethnic disparities in diabetes care have focused on medication underuse and found that Black and Hispanic groups reported greater cost and income-related medication underuse.6,18 In this study, we adjusted for the participants’ glycaemic control and intensity of diabetes therapy so that our findings reflect differences in the classes of diabetes medications initiated, independent of the aggressiveness of diabetes treatment. Our findings show that Black and AI/AN individuals had a 19% and 49% lower risk of initiating newer diabetes medications, respectively. This finding suggests that individuals of minority race/ethnicity are less likely to initiate newer diabetes medication classes than their White counterparts of similar socioeconomic status and diabetes management.
Racial/ethnic disparities in the initiation of newer diabetes medications have important clinical consequences. There is evidence from clinical trials that GLP-1Ras and SGLT-2Is have beneficial effects on cardiovascular and renal outcomes compared to other classes of diabetes medications.19,20 As racial/ethnic minorities with diabetes have a higher burden of chronic kidney disease and worse cardiovascular outcomes,2,3 they may have a greater indication for initiation of GLP-1Ras and SGLT-2Is, which is incongruous with our findings. There is also evidence that all newer diabetes medication classes, compared to sulfonylureas or insulin, have lower risk for hypoglycaemia,21 which occurs more often among racial/ethnic minorities.12,22 Therefore, reduced access to newer diabetes medications in minority race/ethnic groups who may benefit most could contribute to diabetes health disparities.
Reasons for the racial/ethnic differences in initiation of newer diabetes medications may include differences in insurance coverage, provider treatment patterns, and patient preference. In this study we were not able to distinguish between these potential causes. However, adjustment for multiple socioeconomic factors only minimally attenuated the racial/ethnic differences observed, and prior studies have found that racial and ethnic disparities in diabetes management occur even among individuals with similar income and healthcare access.23 This suggests that there may be other factors beyond medication access that are contributing to differences in initiation of newer diabetes medications which require further study. For example, patient attitudes about treatment, which differ by race/ethnicity, may contribute to medication underuse.24
We examined socioeconomic factors because they are tightly linked to race/ethnicity and may mediate observed differences.6 We found that participants with lower yearly family income had lower initiation of newer diabetes medications. This finding is likely explained by the higher cost of these newer diabetes medication with monthly national average drug acquisition costs of $175-$456, $284-$499, and $706-$930 for DPP-4Is, SGLT-2Is and GLP-1Ras, respectively.8 Notably, we found that there was significantly lower initiation of newer diabetes medications for participants earning a yearly family income of less than $60,000, which is similar to the median yearly household income in the U.S. during the study period of $63,179 in 2018.25 This suggests a substantial portion of U.S. patients with type 2 diabetes could be experiencing lower access to newer diabetes medications.
Access to newer diabetes medications may be influenced by insurance formulary coverage and out of pocket costs. We found no significant differences by major categories of health insurance providers. However, we lacked detailed insurance data on formulary coverage, and few participants were uninsured, limiting our ability to examine these associations. Previous studies have found that cost sharing, formulary restrictions and Medicaid expansion are associated with utilization of newer diabetes medications.7 The relationship between insurance coverage and access to newer diabetes medications requires further study.
We also found that significant differences in initiation of newer diabetes medications by participants’ primary source of medical care such that those receiving care from hospital-affiliated clinics or community health centres had lower initiation compared to those receiving care from a private doctor's office. This could be due to differences in diabetes medication prescribing patterns of the participants’ primary care physicians or access to endocrinologists at different types of practices.26 There may be differences in practice characteristics such as regional variation in treatment preferences that were not accounted for. Further, it was not until 2019 that ADA standard of care guidelines for type 2 diabetes recommended the use of newer diabetes medications for patients with pre-existing cardiovascular and renal disease, likely resulting in increased practice variation prior to this time;8 however, our study found no significant interactions among these subgroups. Also, limited inclusion of racial/ethnic minorities in cardiovascular outcomes trials could lead to clinician concerns about initiating new medications in minorities.27 Overall, these findings suggest that there may be practice-level variation in initiating newer diabetes medications that should be examined further.
Findings in this study were largely driven by lower initiation of GLP-1Ras among racial/ethnic minorities. Race/ethnicity was not associated with initiation of DPP4-Is or SLGT2-I's, although the latter were used infrequently during the study period. The null findings for DPP4-Is suggest that access to this class may be fundamentally different than GLP-1Ras. Previous analysis has shown that the diffusion of GLP-1 RA use after approval was slower than DPP4-Is and more concentrated in a few high prescribing practices.28 The different mode of administration and clinical profile of the DPP4-Is and GLP-1Ras may be contributing to this.19,20,28
The strengths of this study include a large, well characterized population with good representation of racial and ethnic minorities from multiple study sites across the U.S. Participants were followed for a median of 8.3 years with little loss to follow-up. The study also ascertained medication use, socioeconomic and clinical data using standardized assessments by trained staff.
Limitations include the possibility of unmeasured confounding as detailed information on participants’ health insurance plans were not available. Therefore, associations between specific health insurance plans and race/ethnicity could not be accounted for. As such, differences observed may reflect differences in eligibility for health insurance with different formularies and benefits. Further, only 7.6% of participants were uninsured and so it was not possible to examine the effect of insurance status on low-income groups specifically. This study did adjust for study site, but there may be regional variations in prescribing patterns not accounted for, notably AI/AN participants who were concentrated in three Southwest centres and predominantly received care from the Indian Health Service. Medication data was also ascertained annually which raises the possibility that newer medications were initiated and discontinued within that period. Non-pharmacologic diabetes treatment during the trial may also have differed by race/ethnicity; in Look AHEAD White participants responded most favourably to the study intervention.29 Since this study examined participants enrolled in a clinical trial with primary care at baseline, there may be differences in diabetes care relative to the general population. However, trends in diabetes medication use among participants were similar to trends in the US population over the same timeframe.30
Conclusions
In summary, this study provides evidence of racial/ethnic and socioeconomic disparities in the initiation of newer diabetes medication among adults with overweight/obesity and type 2 diabetes. The association between race/ethnicity and initiation of newer diabetes medications persisted after accounting for differences in socioeconomic factors. These findings warrant attention as disparities in access to newer diabetes medications may exacerbate existing racial/ethnic disparities in diabetes care. Further research to understand the drivers of this disparity are needed to inform interventions that increase equitable access to diabetes treatment.
Data sharing statement
All deidentified participant data and the data dictionary through the end of the intervention period are currently available as public use datasets through the NIDDK. Look Ahead A-C and Look Ahead E data which are included in this manuscript are still in preparation for release with no set date. Data will be made available to anyone requesting the data for any purpose and without investigator support.
CRediT authorship contribution statement
Ahmed Elhussein: Data curation, Formal analysis, Conceptualization, Methodology, Validation, Writing – review & editing, Writing – original draft. Andrea Anderson: Formal analysis, Methodology, Validation, Writing – review & editing, Visualization.Michael P Bancks: Methodology, Validation, Writing – review & editing. Mace Coday: Methodology, Validation, Writing – review & editing. William C Knowler: Methodology, Validation, Writing – review & editing. Anne Peters: Methodology, Validation, Writing – review & editing. Elizabeth M Vaughan: Methodology, Validation, Writing – review & editing. Nisa M. Maruthur: Methodology, Validation, Writing – review & editing. Jeanne M Clark: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision. Scott Pilla: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors report no conflict of interest.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The Indian Health Service (IHS) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the IHS or other funding sources. Dr. Bancks and Ms. Anderson were supported by NIH/NIDDK grant U01DK57136–18. Dr. Coday was supported by NIH/NIDDK grant U01-DK057078–21. Dr. Peters was supported by NIH/NIDDK grant U01DK057219–22. Dr. Vaughan was supported by NIH/NIDDK grant K23DK110341–04. Dr. Maruthur and Dr. Clark were supported by NIH/NIDDK grant U01DK057149–17. Dr. Pilla was supported by NIH grant KL2TR003099. The funding sources had no role in designing or conducting the study or in the reporting of results.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lana.2021.100111.
Appendix. Supplementary materials
References
- 1.Rosenstock S., Whitman S., West J.F., Balkin M. Racial disparities in diabetes mortality in the 50 most populous US cities. J Urban Health. 2014;91(5):873–885. doi: 10.1007/s11524-013-9861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peek M.E., Cargill A., Huang E.S. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64(5 Suppl):56S–101S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanting L.C., Joung I.M.A., Mackenbach J.P., Lamberts S.W.J., Bootsma A.H. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005;28(9):2280–2288. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- 4.Saydah S., Lochner K. Socioeconomic status and risk of diabetes-related mortality in the U.S. Public Health Rep. 2010;125(3):377–388. doi: 10.1177/003335491012500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grintsova O., Maier W., Mielck A. Inequalities in health care among patients with type 2 diabetes by individual socio-economic status (SES) and regional deprivation: a systematic literature review. Int J Equity Health. 2014;13:43. doi: 10.1186/1475-9276-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng C.W., Tierney E.F., Gerzoff R.B., Dudley R.A., Waitzfelder B., Ackermann R.T., et al. Race/ethnicity and economic differences in cost-related medication underuse among insured adults with diabetes: the translating research into action for diabetes study. Diabetes Care. 2008;31(2):261–266. doi: 10.2337/dc07-1341. [DOI] [PubMed] [Google Scholar]
- 7.Sumarsono A., Buckley L.F., Machado S.R., Wadhera R.K., Warraich H.J., Desai R.J., et al. Medicaid expansion and utilization of antihyperglycemic therapies. Diabetes Care. 2020;43(11):2684–2690. doi: 10.2337/dc20-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes - 2021. Diabetes Care. 2021;44(Supplement 1):S111–S124. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 9.Ham S.A., Nathan A., Laiteerapong N., Sargis R.M., Quinn M.T., Huang E. Cost-related barriers to new diabetes medications-a national physician survey. Diabetes. 2018;67(Supplement 1) 149-LB. [Google Scholar]
- 10.Schultz W.M., Kelli H.M., Lisko J.C., Varghese T., Shen J., Sandesara P., et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stringhini S., Carmeli C., Jokela M., Avendaño M., Muennig P., Guida F., et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1•7 million men and women. Lancet. 2017;389(10075):1229–1237. doi: 10.1016/S0140-6736(16)32380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra-Hebert A.D., Pantalone K.M., Ji X., Milinovich A., Dey T., Chagin K.M. Patient characteristics associated with severe hypoglycemia in a type 2 diabetes cohort in a large, integrated health care system from 2006 to 2015. Diabetes Care. 2018;41(6):1164–1171. doi: 10.2337/dc17-1834. [DOI] [PubMed] [Google Scholar]
- 13.Snider J.T., Seabury S., Lopez J., McKenzie S., Wu Y., Goldman D.P. Impact of type 2 diabetes medication cost sharing on patient outcomes and health plan costs. Am J Manag Care. 2016;22(6):433–440. [PubMed] [Google Scholar]
- 14.Espeland M. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 15.Wing R.R., Bolin P., Brancati F.L., Bray G.A., Clark J.M., Coday M., et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadden T.A. The look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 18.Kirk J.K., D’Agostino R.B., Bell R.A., Passmore L.V., Bonds D.E., Karter A.J., et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2006;29(9):2130–2136. doi: 10.2337/dc05-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelniker T.A., Braunwald E. Clinical benefit of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(4):435–447. doi: 10.1016/j.jacc.2019.11.036. Elsevier USA. [DOI] [PubMed] [Google Scholar]
- 20.Sachinidis A., Nikolic D., Stoian A.P., Papanas N., Tarar O., Rizvi A.A., et al. Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metab Clin Exp. 2020;111 doi: 10.1016/j.metabol.2020.154343. W.B. Saunders. [DOI] [PubMed] [Google Scholar]
- 21.Jensen M.H., Kjolby M., Hejlesen O., Jakobsen P.E., Vestergaard P. Risk of major adverse cardiovascular events, severe hypoglycemia, and all-cause mortality for widely used antihyperglycemic dual and triple therapies for type 2 diabetes management: a cohort study of all Danish users. Diabetes Care. 2020;43(6):1209–1218. doi: 10.2337/dc19-2535. [DOI] [PubMed] [Google Scholar]
- 22.Seligman H.K., Bolger A.F., Guzman D., López A., Bibbins-Domingo K. Health Aff. 1st. Vol. 33. Milwood; 2014. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia; pp. 33–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heisler M., Smith D.M., Hayward R.A., Krein S.L., Kerr E.A. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care. 2003;41(11):1221–1232. doi: 10.1097/01.MLR.0000093421.64618.9C. [DOI] [PubMed] [Google Scholar]
- 24.Piette J.D., Heisler M., Harand A., Juip M. Beliefs about prescription medications among patients with diabetes: variation across racial groups and influences on cost-related medication underuse. J Health Care Poor Underserved. 2010;21(1):349–361. doi: 10.1353/hpu.0.0247. [DOI] [PubMed] [Google Scholar]
- 25.Semega J., Kollar M., Creamer J., Mohanty A. Income and poverty in the United States: 2018 [Internet]. [cited 2020 Jun 28]. Available from: https://www.census.gov/library/publications/2019/demo/p60-266.html
- 26.Ackermann R.T., Wallia A., O’brien M.J., Kang R., Cooper A., Moran M.R., et al. Correlates of second-line type 2 diabetes medication selection in the USA. BMJ Open Diabetes Res Care. 2017;5:e000421. doi: 10.1136/bmjdrc-2017-000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Home P. Cardiovascular outcome trials of glucose-lowering medications: an update. Diabetologia. 2019;62(3):357–369. doi: 10.1007/s00125-018-4801-1. [DOI] [PubMed] [Google Scholar]
- 28.Gilstrap L.G., Blair R.A., Huskamp H.A., Zelevinsky K., Normand S.L. Assessment of second-generation diabetes medication initiation among medicare enrollees from 2007 to 2015. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadden T.A., West D.S., Neiberg R., Wing R.R., Ryan D.H., Johnson K.C., et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity. 2009;17(4):713. doi: 10.1038/oby.2008.637. (Silver Spring) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le P., Chaitoff A., Misra-Hebert A.D., Ye W., Herman W.H., Rothberg M.B. Use of antihyperglycemic medications in U.S. adults: an analysis of the national health and nutrition examination survey. Diabetes Care. 2020;43(6):1227–1233. doi: 10.2337/dc19-2424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.