Abstract
BACKGROUND.
Recombinant leptin therapy reverses nonalcoholic steatohepatitis (NASH) in leptin-deficient lipodystrophy. We inquired if leptin therapy would improve nonalcoholic steatohepatitis in more common forms of this heterogeneous condition.
METHODS.
Nine male patients with relative leptin deficiency (level < 25th percentile of body mass index- and gender-matched United States population) and biopsy-proven NASH and 23 patients with partial lipodystrophy and NASH were recruited for two distinctive open-label trials. Participants received leptin therapy in the form of metreleptin for 12 months. The primary endpoints were the global nonalcoholic steatohepatitis scores from paired liver biopsies scored blindly.
FINDINGS.
Of 9 participants recruited in the relative leptin deficiency treatment study, 7 completed 12-months of therapy. Mean global NASH scores were reduced from 8 ± 3 to 5 ± 2 (range: from 1 to 6, P = 0.004). In the partial lipodystrophy study, 19 of 22 subjects completed 12 months of treatment, and 18 completed a second liver biopsy. Global NASH scores also reduced significantly from 6 ± 2 to 5 ± 2 (range: from −2 to 4, P = 0.008). In both studies, the predominant changes were in steatosis and hepatic injury scores.
CONCLUSION.
Our findings show that patients with NASH associated with both relative leptin deficiency and partial lipodystrophy have reductions in hepatic steatosis and injury in response to exogenous leptin therapy. Moreover, leptin deficiency may have regulatory effects in mediating fat deposition and ensuing injury in the liver.
TRIAL REGISTRATION. ClinicalTrials.gov NCT00596934 and NCT01679197.
Keywords: Fatty Liver Disease, Partial Lipodystrophy, Insulin Resistance, Obesity, Leptin
eTOC blurb
In two different clinical settings with relative leptin deficiency, Akinci et al show that exogenous leptin therapy resulted in significant decreases in liver fat content on MRI and global nonalcoholic steatohepatitis scores on liver biopsies, suggesting the therapeutic window for leptin therapy may be wider than severe leptin deficiency.
Graphical Abstract
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) covers a spectrum of diseases that ranges from simple hepatic steatosis to the more aggressive nonalcoholic steatohepatitis (NASH), which involves inflammation and/or fibrosis 1. NAFLD is an emerging public health problem that is being recognized in up to one-third of the general population 2–4, including teenagers and young adults. NAFLD is now considered the leading cause of abnormal liver function tests and chronic liver disease in adults from the United States 1; 3.
An estimated 20 to 30 % of patients with simple steatosis progress to NASH, which in turn can progress to cirrhosis, hepatocellular cancer, and liver failure 1; 5. Hepatic steatosis also appears to forecast future diabetes and cardiovascular disease 6. Currently, there is no approved drug for the treatment of NAFLD, and lifestyle modifications remain the standard of care. Diet and exercise in association with weight loss appear to be effective in ameliorating steatosis in humans with or without diabetes, but long-term adherence to lifestyle interventions is difficult and costly even if successful 7; 8. While vitamin E and thiazolidinediones have shown some encouraging preliminary results, there are potential drawbacks for long-term use 9; 8. Obeticholic acid, a selective farnesoid X receptor (FXR) agonist, demonstrated improved histological features of NASH over 72 weeks in a phase 2 study of patients with non-cirrhotic NASH 10; however, obeticholic acid is not currently approved for this indication 11. Given that there are no approved therapies to slow or halt the progression of NASH, and that the extent of the affected portion of the population is quite large, new therapies are urgently needed to treat these conditions.
Leptin plays a central role in regulation of body weight and adaptations to caloric restriction 12. In addition to its crucial role in regulating energy expenditure, it also plays an important role in the regulation of insulin sensitivity 13; 14. We and others have previously shown that patients with non-HIV-lipodystrophy, a rare heterogeneous cluster of syndromes characterized by a paucity of adipose tissue, insulin resistance, and hypertriglyceridemia due to either genetic or other acquired reasons, showed a marked amelioration in NASH scores 15; 16 in addition to the metabolic improvement when treated with recombinant leptin 17.
In this study, we primarily sought to determine if leptin could play a role in the treatment of more commonly observed forms of NASH. Because the past therapeutic experience of leptin in obesity was disappointing due to postulated leptin resistance 18, our approach was to identify NASH patients with relative leptin deficiency (RLD) among a cohort of biopsy-proven NASH patients followed at our institution who were nondiabetic and non-cirrhotic. RLD was defined as a leptin level in the lower 25th percentile for BMI. We then initiated a 12-month, open-label, prospective pilot study of therapy with recombinant methionyl-human leptin (metreleptin [Myalept®] in 9 male patients with NASH and RLD and assessed clinical and histological responses. We also provide data on potential molecular pathways in the liver following metreleptin treatment. Results from this small study may provide a rationale to investigate the therapeutic utility for this peptide or other analogs for the treatment of NASH on a larger scale.
In a separate cohort, we examined the efficacy of metreleptin in NASH associated with PL. Most prior studies evaluating the effects of metreleptin on metabolic and hepatic parameters include only those patients with baseline low leptin levels. We aimed to further characterize the effects of metreleptin therapy on the liver disease associated with PL in a more diverse group of patients than previously studied. The simultaneous presentation of both cohorts provides comprehensive documentation of systematic liver biopsy investigations in humans who have been treated with metreleptin for 12 months.
RESULTS
The “Relative Leptin Deficiency (RLD)” study
Demographics and disease characteristics of RLD
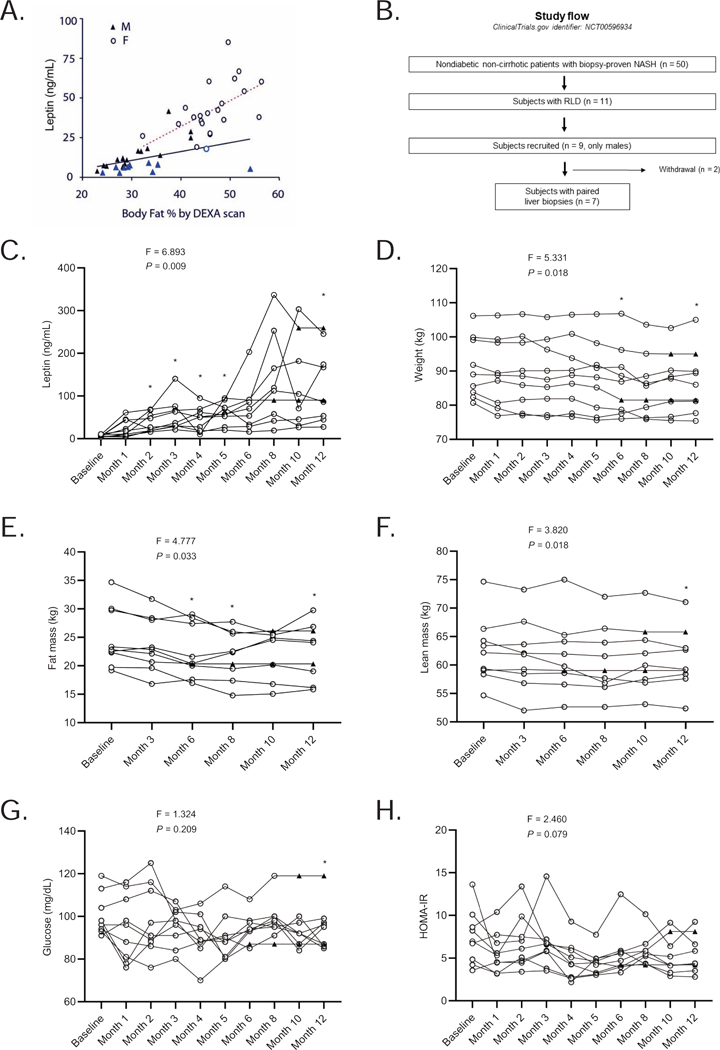
Fifty nondiabetic, non-cirrhotic subjects with biopsy-proven NASH who were followed in our health care system (University of Michigan Medical School, Ann Arbor, Michigan) on a regular basis were identified from 2005 – 2009. Most of the cohort (47/50) were Caucasian. The mean age was 38 ± 9 (range 29 – 63) years; 27 (54 %) were male and 23 (46 %) were female. The mean BMI was 31.6 ± 5.5 kg/m2 (range 24.5 – 42.0 kg/m2), and the mean leptin level was 26.2 ± 14.0 ng/mL (range 2.1 – 72.9 ng/mL). Figure 1A demonstrates the relationship for the entire cohort between leptin levels and body adiposity as measured by dual-energy x-ray absorptiometry (DEXA) scan. As in many other populations, leptin levels demonstrate a significant relationship with body adiposity in both men (r = 0.430, P = 0.030) and women (r = 0.540, P = 0.010) with NASH.
Figure 1. Correlation of circulating leptin levels with adiposity in patients with NASH, the RLD study design, and the effect of metreleptin therapy on metabolic parameters in the RLD study.
(A) Serum leptin levels against total body fat percentage determined by DEXA scan in 50 NASH patients. F = females (r = 0.540, P = 0.010), M = males (r = 0.430, P = 0.030). Blue symbols show 11 patients with RLD; (B) The RLD study design. For metabolic and body composition parameters, data carried forward. For liver related parameters, a decision was made to only conduct completer analysis since liver biopsy was done only at baseline and at month 12; and withdrawal reasons may have confounded the observations; (C) Effect of metreleptin therapy on leptin levels throughout the study period. Note that levels after month 3 may be confounded by circulating anti-drug antibodies; (D) Changes from baseline in weight; (E) fat percentage; (F) lean body mass percentage; (G) glucose; and (H) HOMA-IR studied at the indicated times. We report the F-statistic and P value from a repeated-measures ANOVA. *These P values are marked if they are significant versus baseline with post hoc paired sample t-test after multiplicity correction. Paired t-test was used to compare month-12 values to baseline (without multiplicity correction) as the change at 12 months vs. baseline was a prespecified endpoint. ▲ shows specific data points where the last observation was carried forward.
Subjects with RLD (n = 11 [22%]) were defined based on criteria in Table S1. This group classification was based on leptin levels measured by the historical Linco radioimmunoassay (RIA) (see methods). Subjects with RLD were mostly male (10/11) with lower subcutaneous fat volumes on a single-slice CT study. There was a trend towards higher levels of circulating free fatty acid levels in the RLD group, but the differences were not statistically significant (data not shown).
These results established the feasibility of a pilot intervention study in the RLD group. It was interesting that when subjects with NASH were evaluated for leptinemia, almost 40 percent of our predominantly Caucasian male population met the definition of RLD; whereas there was only one female who met the definition. This finding of sexual dimorphism suggested that there was a difference in the threshold for hepatic fat deposition and subsequent NASH between the two sexes and confirmed to us that the two sexes should be studied separately. Given that it was feasible to treat a small cohort among the males, we decided to focus on just males in the subsequent intervention study.
Study design, population baseline characteristics, and flow through the study
Nine male subjects from the RLD group with biopsy-proven NASH in the above cross-sectional study were included in the open-label pilot treatment study who were the only recruitment pool used for this study (Figure 1B). The definition of NASH grading is presented in Table S2. The hypothesis to be tested was that metreleptin would ameliorate the global NASH scores after 12 months of treatment in the RLD population with NASH. Eight participants would have given us 80 percent power with an alpha of 0.05 to detect the same degree of improvement noted in the NIH cohort 17 with a mean effect size of 3-point reduction in global NASH score and an SD of 2. We had aimed to recruit 10 of the 11 subjects (all males) identified to display RLD, but we could only enroll nine from 2009 to 2011. One patient from the recruitment pool did not want to participate as he wanted to pursue metabolic surgery for his high BMI. The study was completed in 2012. Treatment was delivered at a dose of 0.1 mg/kg/day in an open-label fashion via daily subcutaneous, patient self-administered injections. The maximum dose was 10 mg/day. The mean metreleptin dose was 9.02 ± 0.74 mg/day. Secondary outcomes were hepatic fat percent via proton density fat fraction as well as spectroscopy, body weight, and body fat. Other endpoints of interest were metabolic parameters of insulin sensitivity, fasting glucose, and resting energy expenditure. Molecular changes in the liver, incretin hormone levels and markers of inflammation as well as circulating free fatty acid species were studied as exploratory outcomes.
Pre-treatment characteristics of participants are presented in Table 1. The mean age of the subjects at enrollment was 43 ± 6, ranging from 32 to 53 years. The mean BMI was 28.2 ± 1.6 kg/m2, ranging from 26.4 to 30.8 kg/m2 (i.e. overweight or grade 1 obesity). Baseline circulating leptin levels as measured on an enzyme-linked immunosorbent assay (ELISA) (see methods) ranged from 3.6 to 10.9 ng/dL; values from the cross-sectional RLD study were slightly lower as is typical for the change in methodology. Seven of the nine subjects were on lipid-lowering agents (fibrates and/or statin or fish oil) for dyslipidemia. Three of the nine participants had impaired fasting glucose levels, while one patient was treated with metformin for impaired glucose tolerance. Two of the nine subjects were on antihypertensive treatment. Baseline medications were kept constant during the 1-year study period except for incidental use of analgesics and/or antibiotics for minor non-study-related intercurrent events. Seven of the nine participants completed one year of the study while two discontinued the treatment at 6 and 10 months of the protocol respectively (Figure 1B). One patient withdrew due to the inability to comply with daily injections and study visits, and the other due to the discovery of exclusion criterion (substantial alcohol intake) by the study team.
Table 1.
Characteristics of subjects at baseline and 12 months after metreleptin treatment in the RLD study (n = 9)
| Parameters | Baseline | Month 12 | P value |
|---|---|---|---|
| Body weight (kg) | 90.9 ± 8.7 | 86.8 ± 9.3 | 0.012 |
| Total fat mass (kg) | 24.9 ± 5.3 | 22.5 ± 4.9 | 0.048 |
| Total lean mass (kg) | 62.5 ± 5.8 | 61.0 ± 5.4 | 0.010 |
| Leptin (ng/mL) | 7.0 ± 2.4 | 127.5 ± 87 | 0.003 |
| Glucose (mg/dL) | 100 ± 10 | 95 ± 11 | 0.038 |
| Triglycerides (mg/dL) | 129 ± 66 | 148 ± 69 | 0.440 |
| HOMA-IR | 7.43 ± 3.16 | 5.45 ± 2.17 | 0.097 |
| FFA (mEq/L) | 0.44 ± 0.15 | 0.55 ± 0.31 | 0.414 |
| REE (kcal) | 1746 ± 307 | 1795 ± 247 | 0.475 |
| RQ | 0.80 ± 0.05 | 0.80 ± 0.05 | 0.710 |
| Adiponectin (μg/mL) | 6.44 ± 2.35 | 6.42 ± 2.32 | 0.962 |
| GIP (pg/mL) | 52.68 ± 37.29 | 27.77 ± 7.60 | 0.044 |
| GLP-1 (pg/mL) | 4.81 ± 2.16 | 4.62 ± 1.83 | 0.420 |
| Ghrelin (pg/mL) | 645.26 ± 497.24 | 786.20 ± 457.40 | 0.256 |
| IL-6 (ng/mL) | 1.71 ± 1.20 | 1.34 ± 0.99 | 0.373 |
| sLEPR (ng/mL) | 19.90 ± 6.1 | 19.83 ± 4.84 | 0.957 |
Mean ± SD values are shown (even for non-normally distributed data). P values are calculated by using a paired sample t-test. Log transformation was applied if needed. FFA: Free fatty acids, HOMA-IR: Homeostatic Model Assessment of Insulin Resistance, GIP: glucose-dependent insulinotropic peptide, GLP-1: Glucagon-like peptide 1, REE: Resting energy expenditure, RQ: Respiratory quotient, sLEPR: soluble leptin receptor.
Effects of metreleptin on body composition and vital signs
Table 1 also compares the characteristics of subjects before and 12 months after metreleptin. Metreleptin therapy resulted in an increase in measured leptin levels when compared to baseline (Figure 1C), though these levels are confounded with the presence of anti-drug antibodies after month 3 (Table S3). Participants lost an average of 4.4 ± 2.7 % and 4.5 ± 3.9 % of body weight at 6 and 12 months, respectively. Weight loss was statistically significant at month 6 (P = 0.002), and 12 (P = 0.012) when compared to baseline (Figure 1D). The predominant weight loss was from the fat compartment (P = 0.002 at month 6, P = 0.009 at month 8, and P = 0.048 at month 12; Figure 1E), but there was loss of lean mass as well (P = 0.010 at month 12; Figure 1F). Blood pressure and heart rate were not impacted by metreleptin therapy though some subjects were on blood pressure medications (data not shown).
Effects of metreleptin on metabolic parameters, circulating hormones, and cytokines
Leptin slightly reduced fasting glucose levels (P = 0.038 at month 12; Figure 1G). A slight improvement was observed in insulin sensitivity as evidenced by a reduction in HOMA-IR (Figure 1H); however, it was not statistically significant. Reported food intake on average was reduced by 19 % from baseline at 3 and 6 months, but this did not reach statistical significance due to the large variation in the food intake (data not shown in figures, but available in Data S1). Despite the decrease in body weight and improvement in insulin sensitivity, resting energy expenditure (REE) and respiratory quotient (RQ) remained similar throughout the treatment period (Table 1).
The incretin glucose-dependent insulinotropic peptide (GIP) showed a trend towards time-dependent decline (P = 0.044 at 12 months; Figure S1A). Notably, another gut hormone, ghrelin, demonstrated an increase at 6 months (P = 0.006; Figure S1B). There was a trend towards an increase in adiponectin levels (Figure S1C). The change in IL-6 was not statistically significant (Figure S1D). No significant change was observed in soluble leptin receptor (sLEPR) levels (Table 1).
Effects of metreleptin on liver parameters and histologic features
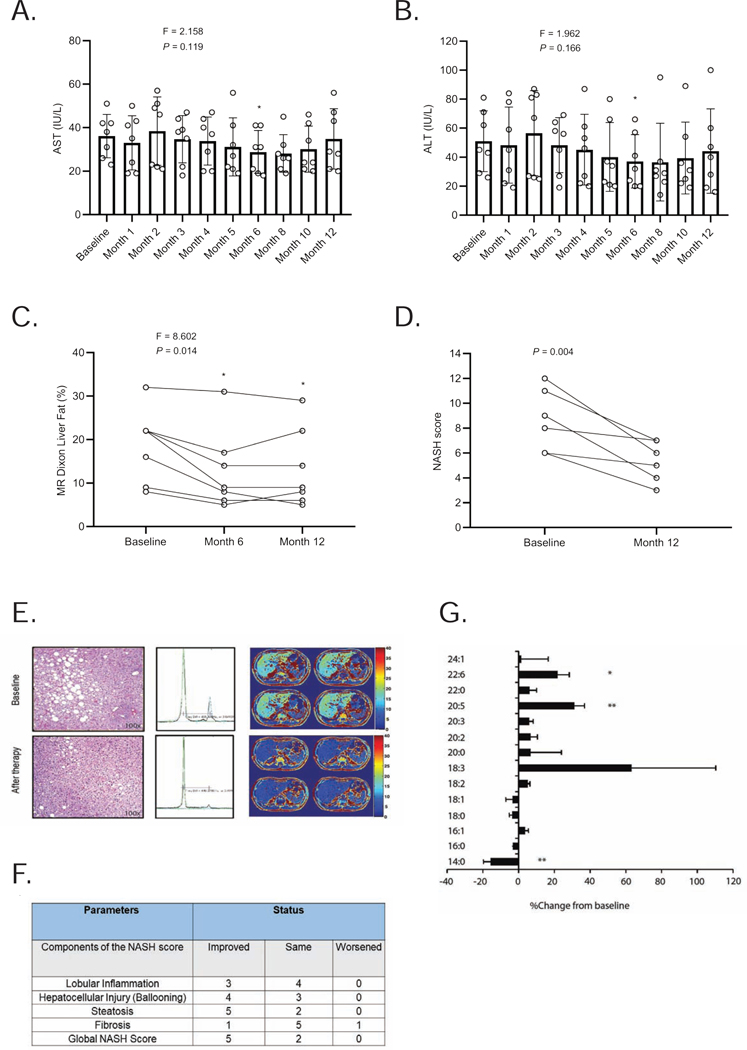
As mentioned above, seven participants underwent paired liver biopsies at baseline and month 12 (Figure 1B). Serum liver enzymes aspartate aminotransferase (AST) (P = 0.002 at month 6, Figure 2A), and alanine aminotransferase (ALT) (P < 0.001 at month 6; Figure 2B) were significantly lower at 6 months but not significantly different at the end of the study. The mean levels of AST and ALT were 36 ± 10 IU/L and 51 ± 21 IU/L at baseline and 35 ± 14 IU/L and 44 ± 29 IU/L at month 12, respectively. There were statistically significant decreases in hepatic fat content by magnetic resonance imaging (MRI) measurements at both 6 (P = 0.009) and 12 months (P = 0.034) (Figure 2C). The mean levels of hepatic fat (using MRI Dixon method) were 19 ± 8 % at baseline and 13 ± 9 % at month 12. The mean NASH scores in the 7 subjects at baseline and month 12 were 8 ± 3 and 5 ± 2, respectively, with a range of decrease by 1 to 6 points (P = 0.004, Figure 2D). All subjects demonstrated a decrease in NASH scores (Tables 2 and 3). Five of the seven subjects who completed 12 months of therapy had a minimum 3-point reduction in their global total NASH scores. An example of paired biopsies from a representative patient is shown along with matched MRI and MRS examinations in Figure 2E. In addition to improvement in steatosis with weight loss, improvement of inflammation scores was observed even in participants without weight loss. One of the 7 subjects demonstrated an improvement of fibrosis and one experienced worsening of fibrosis (Figure 2F). The findings of key trial outcomes reported as mean percent changes from baseline are presented in Table S4. The full trial clinical database is available in the attached data file labeled Data S1.
Figure 2. Effect of metreleptin therapy on liver parameters in the RLD study.
Effect of metreleptin on (A) aspartate aminotransferase (AST); (B) alanine aminotransferase (ALT); and (C) liver fat percentage as determined by MRI over time; (D) individual NASH scores at baseline and 12 months. We report the F-statistic and P value from a repeated-measures ANOVA. *These P values are marked if they are significant versus baseline with post hoc paired sample t-test after multiplicity correction. Paired t-test was used to compare month-12 values to baseline (without multiplicity correction) as the change at 12 months vs. baseline was a prespecified endpoint. (E) H&E stains of liver biopsies before and after 1 year of therapy with leptin. Note the marked improvement in steatosis on biopsy and magnetic resonance images and spectroscopy (15 % fat at baseline vs. 5 % fat after treatment with metreleptin); (F) Components of NASH score at 12 months compared to baseline; (G) Percent change in different free fatty acid species relative to total free fatty acid levels compared to baseline after 6 months of metreleptin therapy. *P < 0.05 and **P < 0.01 versus baseline.
Table 2.
Comparison of study parameters at baseline and 12 months after metreleptin treatment in the PL study
| Parameters | n* | Baseline | Month 12 | P value |
|---|---|---|---|---|
| Body weight (kg) | 19 | 75.6 ± 21.8 | 75.0 ± 23.1 | 0.494 |
| Body mass index (kg/m2) | 19 | 26.8 ± 5.8 | 26.5 ± 6.3 | 0.270 |
| Waist-to-hip ratio | 19 | 1.00 ± 0.08 | 0.98 ± 0.08 | 0.079 |
| FMR (%fat trunk/ %fat legs) | 19 | 1.8 ± 0.5 | 1.7 ± 0.7 | 0.537 |
| Systolic blood pressure (mmHg) | 19 | 126 ± 16 | 128 ± 17 | 0.583 |
| Diastolic blood pressure (mmHg) | 19 | 74 ± 11 | 73 ± 12 | 0.653 |
| Hemoglobin A1c (%) | 19 | 8.9 ± 1.9 | 8.2 ± 1.9 | 0.077 |
| Glucose (mg/dL) | 19 | 193 ± 84 | 164 ± 67 | 0.055 |
| # Triglyceride (mg/dL) | 19 | 576 (327 – 1016) | 301 (206 – 441) | 0.014 |
| # AST (IU/L) | 19 | 42 ± 29 | 30 ± 14 | 0.043 |
| # ALT (IU/L) | 19 | 51 ± 33 | 36 ± 23 | 0.004 |
| REE (kcal) | 19 | 1851 ± 333 | 1719 ± 369 | 0.004 |
| RQ | 19 | 0.77 ± 0.07 | 0.75 ± 0.06 | 0.305 |
| Liver fat (Dixon MR method) (%) | 19 | 13 ± 7 | 8 ± 5 | 0.001 |
| NAS | 18 | 5 ± 1 | 4 ± 1 | < 0.001 |
| NASH score | 18 | 6 ± 2 | 5 ± 2 | 0.008 |
| **Total intake (grams) | 18 | 2791 ± 1141 | 2668 ± 675 | 0.809 |
| Total energy intake (kcal) | 18 | 1710 ± 412 | 1540 ± 531 | 0.253 |
| Total fat intake (grams) | 18 | 73 ± 26 | 64 ± 24 | 0.175 |
| Total carbohydrate intake (grams) | 18 | 184 ± 49 | 166 ± 74 | 0.372 |
| Total protein intake (grams) | 18 | 88 ± 18 | 84 ± 28 | 0.802 |
Number of participants with data available at baseline and 12 months for each outcome. Nineteen participants completed 1 year of metreleptin treatment and 18 completed a second liver biopsy. One subject did not complete the second biopsy due to use of anticoagulation. Energy intake was assessed in 18 subjects as one subject (patient 1) did not return baseline food records. ALT: alanine aminotransferase, AST: aspartate aminotransferase, FMR: fat mass ratio, REE: resting energy expenditure, RQ: respiratory quotient. NAS: non-alcoholic fatty liver disease activity score, the sum of scores for steatosis, lobular inflammation, and ballooning, NASH score: nonalcoholic steatohepatitis score equals the sum of scores for steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis.
Total intake includes food, water and other beverages. Levels are compared by using paired sample t-test.
Tests are run on log-transformed data. Data are presented as mean ± standard deviation (SD). Triglycerides are shown as geometric mean with 95% confidence intervals.
Effects of metreleptin on hepatic gene expression and fatty acid analysis
To explore potential changes in liver gene expression following leptin treatment, we subjected messenger ribonucleic acid (mRNA) isolated from liver biopsies from 6 individuals (one sample did not yield high-quality RNA) to quantitative profiling using microarrays. Enriched pathways following leptin treatment were identified by LR path 19. Pathways associated with protease activity and proteosome were found to be significantly (FDR < 0.1) upregulated following leptin treatment, while G-protein coupled olfactory receptor signaling pathways were down-regulated (Figure S2A). Full dataset is also available as an additional file labeled Data S2. We also used quantitative PCR to directly assess the mRNA levels of several genes previously identified as being regulated by leptin in the mouse model 20. Sterol regulatory element-binding protein (SREBP) 1 is a transcription factor that regulates several genes associated with de novo lipogenesis, including stearoyl‐CoA desaturase −1 (SCD-1) that causes the mono-desaturation of palmitic and stearic acid. There was a trend to decreased SREBP1 mRNA levels and SCD-1 levels (Figure S2B), but these did not reach significance (P > 0.05 for all). In addition, in keeping with reduced lipogenesis, there was a trend towards decreased ATP citrate lyase and fatty acid synthase expression. Further, we interrogated the FXR/RXR pathway due to its importance in NAFLD pathophysiology, which showed a numerical decrease in average expression of CYP27A1, but it did not reach statistical significance. We also assessed total fatty acids derived from total lipids in the peripheral plasma of subjects at baseline, 1 and 6 months of metreleptin treatment. Data at 1 month did not reveal significant changes compared to baseline. We found a decrease in 14:0 fatty acids and increases in polyunsaturated fatty acids at 6 months (Figure 2G). There were minimal changes in the 16:1/16:0 and 18:1/18:0 ratios, suggesting that overall SCD-1 activity was not changed in response to metreleptin. These results suggest that metreleptin treatment has a minimal direct effect on these parameters in these subjects likely due to dietary variability and treatment for hyperlipidemia.
Adverse events
Two of the nine participants exposed to metreleptin treatment in this study experienced transient skin reactions at the injection sites. One patient had moderate, and one patient had mild localized reactions that occurred within 2 to 4 weeks of treatment and abated spontaneously within 6 weeks of emergence. Leptin-binding antibodies and neutralizing activity were measured from samples collected at baseline and 3, 6, and 12 months using an assay developed by Amylin Pharmaceuticals 21 after the trial was completed. All subjects had evidence for leptin-binding antibodies by 6 months and these were sustained at 12 months (Table S3). None of the samples demonstrated in vitro neutralizing activity. One patient developed lymphadenopathy on the right side of the neck and axillae emerging at 11 months of treatment. Biopsy and clinical evaluation determined that the patient developed acute toxoplasmosis due to presumptive exposure to stray cats; the patient completed 12 months of the study treatment period.
The Partial Lipodystrophy (PL) study
Study design, population baseline characteristics, and flow through the study
An NIDDK funded open-label prospective 1 year-intervention study was conducted to determine systematically whether leptin improves NASH histopathology associated with PL (University of Michigan Medical School, Ann Arbor, Michigan). For this study, we a priori defined at least 2 points of improvement in total NASH score as a clinical response in 12 months. With a difference of 2, a baseline mean value of 8 and with a baseline SD of 2, a total of 17 subjects yielded 80% power with 5% of the significance level. Assuming a 15% possible dropout rate, we aimed to recruit a minimum of 20 and no more than 24 subjects.
Twenty-three participants with physician-diagnosed PL were enrolled and completed baseline study procedures. A detailed description of the baseline parameters of these subjects was previously published 22. One patient was excluded as her “baseline” liver biopsy did not meet the histopathological definition of NASH. The remaining 22 participants had biopsy-proven NASH and continued the protocol, receiving at least one dose of metreleptin (Supplementary data). Baseline characteristics of the participants receiving at least one dose of the drug in the study are presented in Supplementary data file. The mean age of 22 subjects (17 females and 5 males) was 43 ± 16 years (range 12–64). Nineteen subjects were Caucasian, two related subjects were Hispanic with mixed racial background and one subject was African American. Twenty-one had familial partial lipodystrophy (FPLD) and one subject had acquired partial lipodystrophy (APL). Pathogenic variants in the lamin A/C (LMNA) gene were found in 7 of 22 participants receiving at least one dose of the drug. Two related subjects had the p.E1067K variant of the polymerase (DNA-directed), delta 1, catalytic subunit-1 (POLD1) gene as previously reported 22. Additionally, several subjects had variants of unknown significance noted in several genes such as fibrillin-1 (FBN1), sterol regulatory element-binding transcription factor 1 (SERBF1), and dual-specificity tyrosine phosphorylation-regulated kinase 1b (DYRK1B) as shown in Supplementary data file.
Median baseline leptin levels were 16.2 ng/mL (range 4.8 – 67.1 ng/ml) with mean ± SD of 22.3 ± 16.6 ng/mL. These levels were measured by an ELISA (see methods) and these subjects did not have severe leptin deficiency analogous to generalized lipodystrophy but instead had a broader range of leptinemia.
The mean baseline total NASH score was 6 ± 2 (details of the NASH scoring system are presented in Table S2). Individual-level data are further presented in the additional data file labeled as Supplementary Data File.
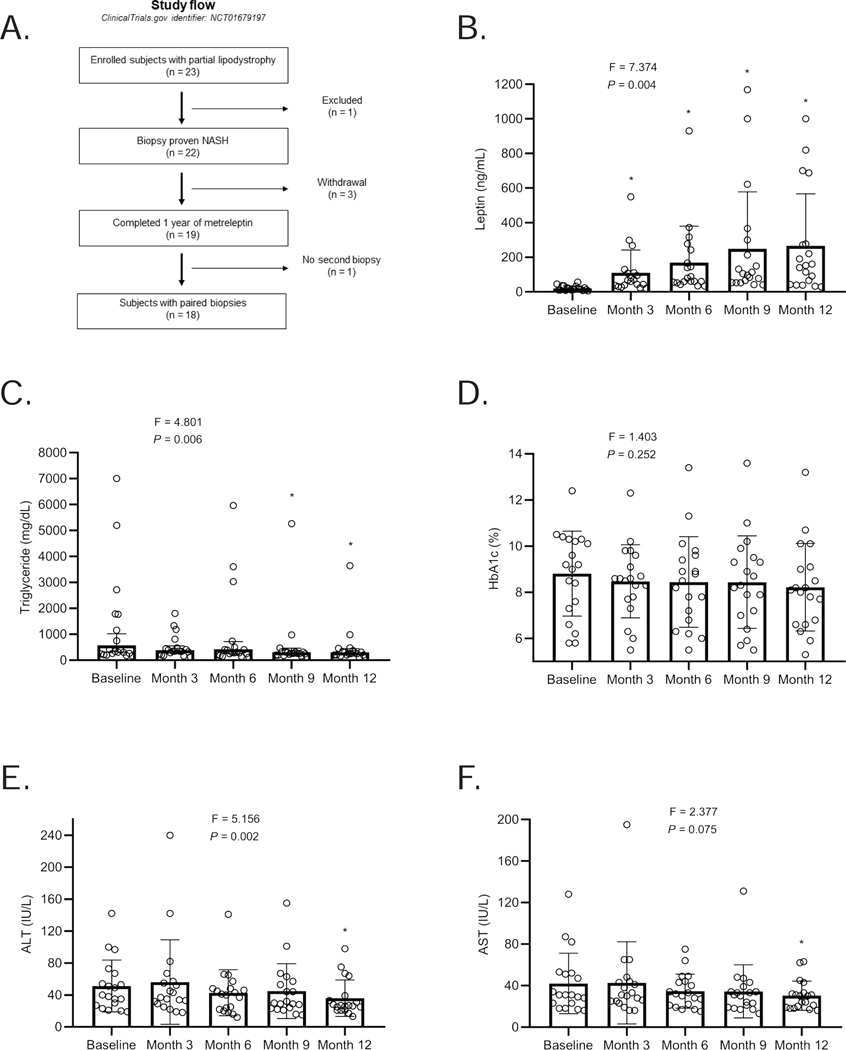
Nineteen subjects completed 12 months of metreleptin treatment (mean age: 43 ± 17 years, range: 12–64; 16 females and 3 males), and 18 completed a second liver biopsy (mean age: 43 ± 17 years, range: 12–64; 15 females and 3 males). One participant did not complete the second biopsy due to initiation of anticoagulation during the study (Figure 3A).
Figure 3. Leptin levels, study design, and the effect of metreleptin on metabolic parameters and liver enzymes over the 12-month treatment period in the PL study.
(A) Leptin levels throughout the PL study period. Leptin levels were measured from three samples measured 30 minutes apart at baseline, and 3, 6, 9, and 12 months after metreleptin. The levels shown are average leptin levels. The F-statistic and P value are reported from a repeated-measures ANOVA. *These P values are marked if they are significant versus baseline with post hoc paired sample t-test after multiplicity correction. Paired t-test was used to compare month-12 values to baseline (without multiplicity correction) as the change at 12 months vs. baseline was a prespecified endpoint; (B) Patient progression through the PL study. A total of 23 patients with partial lipodystrophy were enrolled and 22 had biopsy-proven NASH. Of the 22 patients, 3 withdrew from the study and 19 completed 1 year of metreleptin treatment. 18 patients completed the 12-month post-treatment biopsy; (C) Triglycerides; (D) HbA1c; (E) ALT; and (F) AST levels in subjects with partial lipodystrophy treated with metreleptin for 1 year. The F-statistic and P value are reported from a repeated-measures ANOVA. *These P values are marked if they are significant versus baseline with post hoc paired sample t-test after multiplicity correction. Paired t-test was used to compare month-12 values to baseline (without multiplicity correction) as the change at 12 months vs. baseline was a prespecified endpoint. Tests are run on log transformed data for triglycerides, ALT and AST. Triglycerides are shown geometric mean with 95 % confidence intervals (CI), otherwise, the data are reported as mean ± SD. The last observed non-missing values (month 6) is used to fill in missing values at month 9 visit in one subject (subject ID: 21) who missed month 9 visit but completed the study protocol.
The starting dose of metreleptin was 2.5 mg daily in the male subjects and 5 mg in the female subjects. Average metreleptin dose used per subject across the study was 7.04 ± 1.99 mg/day. The maximum dose was 10 mg/day. We made every attempt to keep the metabolic therapy stable with the exception of down-titration of metabolic treatments to avoid hypoglycemia. There were a few cases who had minor increases in their glucose-lowering treatments, as detailed in Supplementary Data File. Given the complicated multi-system disease, some patients did initiate additional therapies for intercurrent illness, such as the discovery of CAD in one subject (patient 22) necessitating the initiation of anticoagulant medication.
Effects of metreleptin on metabolic parameters
Nineteen participants were treated with metreleptin for 12 months (Figure 3A and B). Table 2 shows metabolic parameters before and 12 months after metreleptin treatment. Temporal changes in several metabolic parameters over 12 months in response to metreleptin are shown in Figure 3C-F. We observed a significant decrease in fasting triglycerides, liver enzymes (ALT and AST), and resting energy expenditure (REE) 12 months after metreleptin. Although fluctuations were observed on an individual basis, the decrease in triglyceride levels was significant both during and at the end of the treatment period (Figure 3C). Fasting glucose and HbA1c levels also tended to be decreased after treatment but the effect was not statistically significant (Table 2 and Figure 3D). Liver enzymes showed mild reductions over time, and the differences were significant at month 12 (Figure 3E and F). REE decreased after metreleptin therapy (P = 0.004, Table 2).
Effects of metreleptin on liver parameters and histologic features
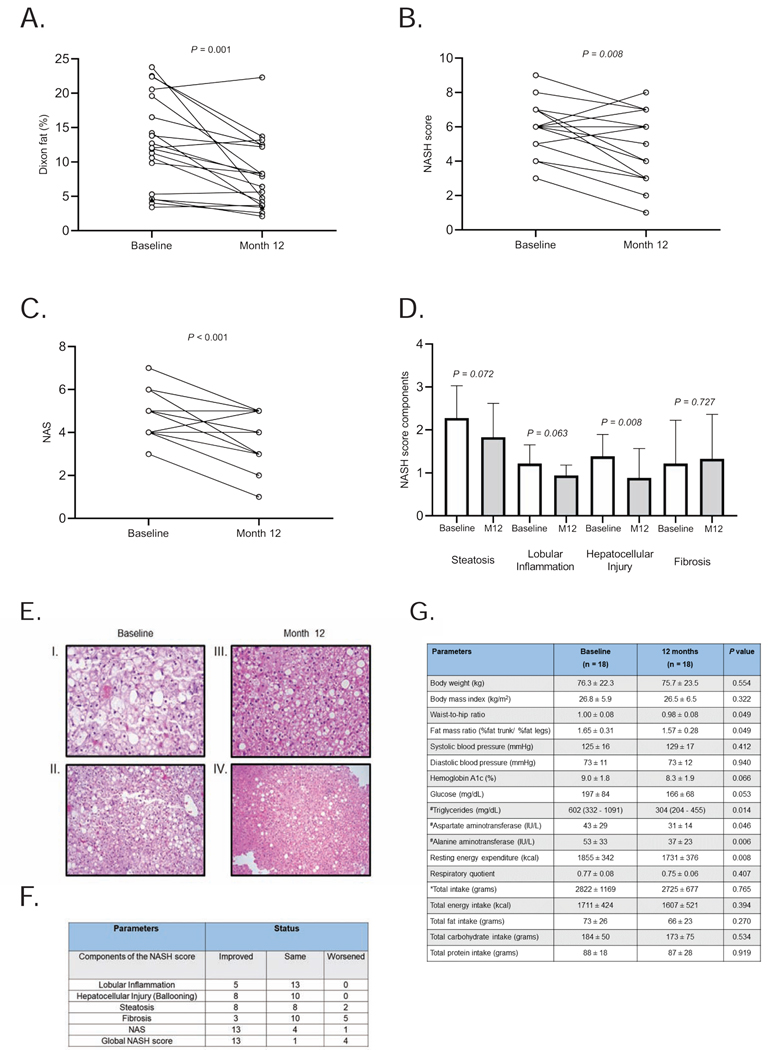
In the evaluable 19 patients with paired MRIs, liver fat (quantified by MRI Dixon method), decreased from baseline mean 13 ± 7 % to 8 ± 5 % after 12 months of metreleptin (n = 19; P = 0.001; Table 2 and Figure 4A). NASH scores (the primary endpoint for this study) were compared in 18 subjects who completed the baseline and 1-year biopsies and showed a significant decrease from 6 ± 2 to 5 ± 2 (P = 0.008; Figure 4B). NAFLD activity scores (NAS) also decreased from 5 ± 2 to 4 ± 1 (P < 0.001; Figure 4C). Figure 4D shows the change in the components of the NASH score after metreleptin. Trends for reduction in steatosis (P = 0.072) and lobular inflammation (P = 0.063) as well as significant reduction in hepatocellular injury (P = 0.008) were noted while fibrosis score did not change significantly (P = 0.727) after treatment with metreleptin. An illustrative case is presented in Figure 4E. Of 18 subjects who completed 12 months of therapy, 13 had improvements in NAS and NASH scores (Figure 4F). Steatosis and hepatocellular injury improved in 8 participants and lobular inflammation improved in 5 subjects, although 2 subjects showed worsening of steatosis. Three of the 18 subjects demonstrated an improvement of fibrosis and 5 experienced worsening of fibrosis (none equaled to or more than 2 points of worsening). Individual liver biopsies are presented in Supplementary Data File.
Figure 4. Liver fat quantification and histopathological features of NASH after 1-year of metreleptin therapy in subjects with PL.
A) Liver fat quantification, using magnetic resonance (MR) Dixon method, decreased from baseline 13 ± 7 % to 8 ± 5 % after 12 months of metreleptin in subjects who completed 1 year of follow up (n = 19; P = 0.001). Liver fat of 18 subjects with paired liver biopsies showed a reduction from baseline 13 ± 7 % to 8 ± 5 % after 12 months of metreleptin (n = 18, P = 0.001); (B) NASH score comparisons at baseline and after 12 months of metreleptin therapy of 18 subjects with 1-year biopsies show a decrease in global NASH scores from 6 ± 2 to 5 ± 2 (P = 0.008); (C) During the 1 year follow up, NAS scores also decreased from 5 ± 1 to 4 ± 1 (P < 0.001); (D) Different components of the NASH score before and after metreleptin treatment. P values are obtained with Wilcoxon matched-pairs signed rank test; (E) H&E staining of the liver biopsy specimens from a 14-year-old female participant. I. Baseline biopsy at 200x magnification. II. 12-month biopsy at 200X magnification. III. Baseline biopsy at 100x magnification. IV.12-month biopsy at 100x magnification. Note the marked steatosis and hepatocyte ballooning at baseline state, which significantly improve by 12 months; (F) Components of NASH score at 12 months compared to baseline in the PL study (n =18); (G) Metabolic parameters before and after metreleptin in PL patients with baseline and 12-month liver biopsies. Energy intake is reported in 17 patients. *Total intake includes food, water and other beverages. Levels are compared by using paired sample t-test. #Tests are run on log-transformed data. Data are presented as mean ± standard deviation (SD). Triglycerides are reported.as geometric mean and 95% confidence intervals (CI).
In the 18 subjects with paired liver biopsies available, liver fat as measured by the MRI Dixon method decreased from baseline 13 ± 7 % to 8 ± 5 % after 12 months of metreleptin (P = 0.001). The summary of key efficacy measurements with percent change from baseline is presented in Table S5. Mean changes in specific metabolic characteristics of these 18 participants with paired liver biopsies are presented in Figure 4G.
Adverse events
All participants who received at least one dose of metreleptin (n = 22) reported some type of adverse event during the treatment period, although only 13 of the 22 subjects had at least one adverse event that was possibly or probably related to metreleptin treatment (Supplementary data). Eleven subjects experienced an upper respiratory tract infection, 6 had hypoglycemia, 5 had diarrhea and 4 had reactions at the site of metreleptin injections. Three participants each also experienced urinary tract infections, dizziness, asthma exacerbation, abdominal pain, and nausea. Three subjects experienced skin rashes at other sites away from the immediate injection sites. One of these participants, who had remote history of skin granulomas prior to the start of the drug developed recurrent granulomas starting at 6 weeks of exposure and withdrew from the study of her own volition. She was ultimately diagnosed with systemic sarcoidosis. This event was deemed unlikely related to metreleptin by subspecialty physicians who evaluated her (and not by primary investigator) owing in part to the presence of the history of dermal granulomatous disease prior to the initiation of the drug. Events that were deemed to be likely or definitely related to metreleptin treatment included hypoglycemia and injection site reactions. No serious hematologic events or development of neutralizing antibodies to leptin were observed during the reported 12-month treatment period even though one participant subsequently developed neutralizing antibody during the extension phase at 18 months of treatment and her complicated clinical course is reported elsewhere 23.
DISCUSSION
In this paper evaluating the effects of exogenous leptin therapy in the metabolic liver diseases of two distinctive groups of participants, we saw an amelioration in the histopathological features of nonalcoholic steatohepatitis both in a group of males with common NASH and RLD without lipodystrophy as well as in a group of participants with PL irrespective of their circulating leptin levels. These results document improvements particularly in the degree of steatosis, and hepatic injury, and provide further support for the hypothesis that the therapeutic utility of exogenous leptin therapy may extend beyond severe leptin deficiency. In addition, limited liver gene expression studies demonstrated a profile that was largely confirmatory of earlier observations in the rodent studies.
Do the subgroups we defined as RLD and PL lie across a continuous metabolic spectrum?
One noticeable characteristic of the RLD subgroup was relative reduction in the subcutaneous fat and preservation of the visceral fat. Although the RLD study historically strived to enroll subjects with NASH and RLD of both genders, we were not able to identify sufficient number of female subjects who met this definition among patients with biopsy-proven NASH without obvious clinical lipodystrophy. This is in contrast to the overwhelming female preponderance among individuals who are correctly diagnosed with partial lipodystrophy. PL refers to a heterogeneous cluster of diseases characterized by relative paucity of peripheral fat depots coupled with insulin resistance and metabolic dyslipidemia. It is much easier clinically to appreciate the absence of peripheral (and especially lower extremity) fat in females. Our suspicion is that females who are more in the RLD category may have already received a diagnosis of PL by their metabolic specialists while making such a diagnosis is much harder in males. Also, having no diabetes was a prerequisite to be included in the cross-sectional RLD study. Therefore, female patients with NASH may have already developed diabetes, as we know that fat loss from the peripheral compartments have far more severe metabolic consequences in females compared to males 24. To that end, we have worked hard to come up with objective diagnostic criteria for PL. Currently having a mid-thigh skinfold thickness of less than 11 mm in males and 22 mm in females is accepted as sufficient evidence for the presence of PL 25; 26. However, a quick glance at the fat distribution patterns shown by the fat shadow images of our PL patients (presented in Supplementary data file) highlights the heterogeneity of fat distribution patterns as well as the degree of residual fat in the PL population. While none of the cases included in our RLD cohort met the formal diagnostic criteria for PL, their body fat distribution characteristics suggested a more central deposition and as such they may represent a cluster of cases who resemble the PL cases in some metabolic features. If one can view common truncal obesity and metabolic syndrome/type 2 diabetes as a continuous spectrum, the PL cases with known single gene causes likely represent the most extreme form of this phenotype.
In fact, females are reported to be protected from common NASH in younger ages and there appears to be ethnic and racial factors that determine adipose tissue storage capacity and expandability that in turn may govern the threshold for NASH 24; 27. Further studies are needed to really underpin the mechanisms that determine how NASH develops in the two sexes across different racial and ethnic groups and how leptin availability may play a role in the different subgroups as well as how genotypic factors can modify the clinical presentations and treatments.
Observations in the RLD cohort: where do they fit?
It was of particular importance that in the RLD cohort we observed a reduction in body weight, and in both fat and lean mass. The earlier phase 2 studies with metreleptin in obesity demonstrated that there was heterogeneity in how individuals responded to exogenous leptin therapy 28; 18, with the challenge lying in predicting those who would demonstrate a biological response. Published phase 2 studies with monotherapy were not large enough to determine predictors of response 29; 18; 30; 31. However, a post hoc pooled subgroup analysis showed that metreleptin reduced weight in adults with low baseline leptin levels that was confirmed in a subsequent study in adults with low baseline leptin and BMI 27.5–38.0 kg/m2 28. Other data from a trial using a combination of metreleptin with pramlintide suggested that individuals with lower grade obesity (specifically those with BMI < 35 kg/m2) respond more robustly with weight loss 32. This relatively lower BMI is concordant with the notion that the group that benefits the most from metreleptin therapy may have a limited capacity for adipose tissue expansion. However, other factors may influence leptin levels that include hormonal state 33, iron stores 34, other genetic influences of mitochondrial function 35, and other factors that provide transcriptional control 36. The decrease in body weight and adiposity was evident quite early on in the treatment in the 5 participants in our RLD cohort who demonstrated this response (as early as 3 months). Pursuant to weight loss, we also observed an increase in circulating ghrelin concentration at 6 months and a decrease in GIP levels by 12 months. These limited observations suggest that there may be specific regulators of the therapeutic response to metreleptin as well as adaptive changes. While these points deserve further evaluation, we could not identify a baseline or early predictor of treatment response outside of the clinical parameters tested possibly due to our small sample size.
The hepatic gene expression analysis is also quite limited by our small sample size though we did find a significant increase in protease/proteasome pathway, but the mechanism for the change is unclear. In mouse models of fatty liver disease, proteasome function is decreased significantly in association with fat accumulation 37. The increase we noted after metreleptin treatment may either be a primary effect of metreleptin therapy or a secondary adaptation to changes in metabolic improvement or weight reduction. Regulated cellular protein turnover via the ubiquitin-proteasome system can affect multiple homeostatic systems, including glucose and lipid metabolism 38; 39 The NAFLD risk variant of PNPLA3 has been suggested to disrupt the degradation of the wild-type protein, resulting in impaired mobilization of triglycerides from lipid droplets 40. We also observed a significant decrease in olfactory receptors and G-protein signaling. These receptors are found in multiple tissues outside of the olfactory system and are evolutionarily conserved and may be related to control of meta-inflammation 41. The latter may involve adaptations to environmental changes including energy control or conservation pathways 42.
The trend towards a decrease in hepatic gene expression of SREBP-1c and SCD-1 in response to metreleptin therapy is consistent with previously published results 43; 20; 44. SREBPs comprise a subclass of basic-helix–loop–helix–leucine zipper transcription factors that regulate expression of genes required to maintain cellular lipid homeostasis. SREBP-1c is the main isoform in the liver and its upregulation has been implicated in the development of NAFLD 45. SCD is a key enzyme involved in the biosynthesis of unsaturated fatty acids and is regulated by SREBP-1c. SCD1 is the main isoform in the liver and has a key role in the promotion of hepatic de novo triglyceride synthesis 46; 47. Both SREBP-1c and SCD-1 are downregulated in rodent models by leptin administration therapy in concordance with the improvement in hepatic steatosis 43; 44. In addition, there is evidence that serum fatty acid composition is associated with insulin resistance and hepatic steatosis 48–50. Thus, our suggested finding of a decrease in 14:0 fatty acids and slight increases in polyunsaturated fatty acids can be interpreted as a reflection of the overall metabolic improvement.
Previous in vitro studies have linked leptin to the development of hepatic fibrosis through the activation of hepatic stellate cells 51; 52. Some previous rodent studies have also suggested this idea 53; 54. In the RLD study, 5 out of 7 subjects had no change in fibrosis except one showing worsening and another one demonstrating an improvement in fibrosis score. In addition, leptin has previously been associated with increased blood pressure 55. It has been suggested that obesity-associated hypertension is mediated through leptin as it may act centrally to impact sympathetic nervous system overactivity 56. Similar to patients with lipodystrophy treated with metreleptin, the range of leptinemia achieved in this study is consistent with levels seen in obesity although the reliability of leptin levels after metreleptin treatment is questionable due to frequent antibody development that would interfere with measured leptin levels. Nevertheless, metreleptin treatment was reported not to cause an increase in blood pressure in subjects with lipodystrophy 57. Similarly, in this study, we observed no significant changes in blood pressure during metreleptin treatment. It is possible that the careful selection of subjects to those with RLD mitigated the potential of any side effects on doses aimed to reach physiological levels. Because neutralizing antibodies were seen in patients who were treated during an extension open-label phase of obesity trial using metreleptin with pramlintide 21, the development of metreleptin treatment programs for obesity and related disorders were halted while the development of the program for lipodystrophy continued. There are concerns that neutralizing the native leptin signaling could result in worsening metabolic parameters or immune status, though direct evidence for this is very limited. Therefore, studies in populations such as NASH/NAFLD should only move forward after careful risk-benefit analyses and preferably after characterization of the full in vivo effects of the neutralizing antibodies 58. None of the subjects in this study were noted to develop neutralizing antibodies though they all demonstrated some level of anti-drug antibodies that affected the measurement of the leptin levels using the immunoassay.
Our observations in the PL cohort: building upon what is known
In the PL study, we observed an overall favorable effect of metreleptin on PL associated NASH. The primary study endpoint, global NASH scores, as well as NAS scores and levels of transaminases, significantly improved during the study. Global NASH scores improved with reductions in steatosis, inflammation, and hepatic injury components. Overall, there were no significant changes in the fibrosis scores though 5 individual subjects experienced 1 point worsening in the fibrosis scores. As discussed above with the RLD study, leptin had profibrotic effects in previous in vitro and rodent studies 51; 53; 54; 52. Whether this small degree of worsening in the liver fibrosis is of clinical importance is not certain. Natural disease progression despite metreleptin therapy and/or sampling related factors may have played a role in the small changes in serial biopsies. Liver fat content decreased on MR assessment even in 4 of 5 individuals with a slight increase in fibrosis score. Despite the open-label design, our dataset is the largest dataset of systematic biopsies performed after 1 year of therapy in individuals with PL. Previous liver directed reports in lipodystrophy by Javor et al. 15, Safar Zadeh et al. 16, and Brown et al. 59 included fewer individuals with PL and the timing of biopsies showed a wide range with not all first biopsies being performed at baseline and the second biopsies being performed anywhere between 3 months to 5 years after initiation of metreleptin. In our study, we obtained paired biopsies from 18 subjects with PL at baseline and 12 months of treatment.
As reported in previous studies, the metabolic abnormalities of leptin deficiency noted in severe generalized lipodystrophy can be reversed by exogenous leptin therapy 60; 17. As a result of these previous studies, metreleptin is now an FDA approved treatment, indicated as an adjunct to diet to treat the metabolic complications in patients with congenital or acquired generalized lipodystrophy in the United States 61. Metreleptin is also indicated for treatment of patients with partial lipodystrophy who are older than age 12 in Europe when other metabolic therapies fail to achieve metabolic control 62.
Our current PL study differs from previous studies in that we have presented findings on treatment of participants with PL with quite variable leptin levels. Overall, the metabolic improvement seen in this study is in line with previous reports 63. The metabolic effects reported here, are not as robust and are more variable than seen in GL 60; 64; 17 but are consistent with other studies in PL 60; 63. Park et al. 65 reported that long-term leptin replacement was associated with metabolic benefits in subjects with PL, which was later supported by several other studies 66; 67. Diker-Cohen et al. 68 reported that metreleptin effectively reduced HbA1c and triglycerides in subjects with PL who had severe baseline metabolic abnormalities or low leptin.
Our data show that one does not have to observe an improvement in triglyceride or glucose control to see an improvement in liver related parameters. Similar to our findings, Simha et al. 69 reported that both FPLD subjects (caused by pathogenic variants of the LMNA gene) with severe (mean leptin level: 1.9 ng/mL) and moderate (mean leptin level: 5.3 ng/mL) hypoleptinemia could benefit from metreleptin therapy in terms of decreasing triglyceride levels and hepatic steatosis but not glucose and hemoglobin A1c levels. In a recent study, Baykal et al. 70 showed that metreleptin reduced de novo lipogenesis in subjects with lipodystrophy across a broad range of leptinemia (endogenous leptin ranging from 0.5 to 35.7 ng/mL) despite lack of significant improvement in triglycerides. Our previous work also suggested the best candidates for metreleptin treatment were PL subjects with earlier onset of disease, subjects with LMNA pathogenic variants or other clear genetic abnormalities, and those with severe metabolic abnormalities 71. The current dataset expands upon these observations and suggests that some of the PL patients may have important histopathological improvements in their liver related parameters over a 12-month treatment period.
The neutralizing antibodies are noted in patients with lipodystrophy 21 and may attenuate the treatment response to metreleptin. In our PL cohort, none of the patients developed in vitro neutralizing antibodies during the 1-year treatment period, but one patient who continued on the treatment beyond 12 months for clinical benefit developed this at 18-months of therapy with loss of metabolic control 23.
Limitations of study
The major limitations of these studies are the open-label designs, the small sample sizes, and the lack of placebo control. We also followed the designed protocols in the dosing regimen and used the maximum practicable dose as the ultimate ceiling in daily dose. It is possible that higher level of efficacy could be noted if higher doses could be used in both cohorts.
A lack of control group makes it difficult to interpret if the noted improvements were a direct effect of metreleptin or indirect effects due to reduction in food intake and/or weight loss particularly in the RLD study and it is impossible to eliminate the possible effect of other confounding factors. Background medications, and recruitment related factors, etc. may have also contributed additional confounders. Although we endeavored not to increase or change background medications during the 12 months of the studies, this was not always achievable; in the PL study we had to change some of the concomitant metabolic medications (even though the majority of the changes made were down-titrations) and this may have impacted some of our observations.
Keeping in mind that these types of studies are essentially uncontrolled and biased, our findings are still of importance because they lay the foundational groundwork for concepts of RLD and/or “preserved leptin responsiveness” in metabolic diseases. Both RLD and “preserved leptin responsiveness” can be analogous to how the concepts of “relative insulin deficiency” and “adequate therapeutic response to insulin therapy” are generally viewed in garden variety type 2 diabetes.
Overall Conclusions
In conclusion, given the previously and currently noted response of patients with lipodystrophy to metreleptin and the encouraging results we have seen in the RLD study; we believe that it may be time to consider the therapeutic benefit of the leptin pathway more broadly, carefully evaluating efficacy in subpopulations with common metabolic diseases such as NASH who may be amenable to treatment.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Elif A. Oral (eliforal@med.umich.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate any unique code. The datasets obtained during the RLD study including the mRNA profiling data are provided in full. The lipidomics datasets can be provided upon request. We are currently conducting additional analyses from the PL study database but, we provided full data for results reported in the manuscript including liver biopsy images.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Description of overall study designs
Cross-sectional study in NASH
Fifty non-diabetic, non-cirrhotic subjects with biopsy-proven NASH were studied in the context of a cross-sectional study focusing on describing the various metabolic, histopathological, and body composition characteristics of subjects with biopsy-proven NASH. Patients were enrolled between 2005 and 2009. Subjects were excluded from the study for the following reasons: other causes of chronic liver diseases, alcohol use more than 40 g per week (≥ 5 drinks per week) within the past 3 months or a history of long-term alcohol abuse or dependence in the past, use of medications reported to cause steatosis (including amiodarone, steroids, tamoxifen, valproic acid, methotrexate, and IV tetracycline) within the past 3 months or for more than 6 months in the past 2 years, weight reduction surgery within 1 year or jejunoileal bypass in the past, weight loss medication or participation in a weight loss program in the past 3 months, evidence of hepatic decompensation, HIV antibody positive, pregnancy, or breast-feeding. Adults with diabetes were not included in this study because possible changes in anti-diabetic therapy and glycemic control could confound the analysis.
All subjects underwent same-day anthropometric, laboratory, and radiological investigations. Measurements of the waist and hip circumference, weight, height, BMI, and blood pressure were performed. Laboratory testing for metabolic abnormalities including fasting triglyceride, glucose and insulin was obtained. The measurement of insulin resistance was determined by the HOMA index ([(fasting insulin*fasting glucose/18)/22.5]). Whole-body DEXA scanning using a dual X-ray absorptiometry (Lunar, General Electric, Madison, WI) was used for the estimation of fat and lean body mass. A non-contrast abdominal CT (single slice) scan at the level of L4 was performed for the measurement of visceral fat mass and abdominal non-visceral fat mass. Abdominal subcutaneous fat mass was estimated as the difference between abdominal and visceral fat.
Stored fasting sera were obtained on these subjects after a minimum of 8 hours of physical rest and analyzed for circulating leptin levels. These levels were then linked with the existing database on the individuals’ various clinical, biochemical, radiological, and histological characteristics. We classified the subjects studied into three subgroups according to the following criteria: (1) RLD: subjects with level < 25th percentile of NHANES III population based on their sex and BMI; (2) normal leptin levels (NLL): subjects with levels falling between 25th and 75th percentile of NHANES III population based on sex and BMI and (3) relative leptin excess (RLE): subjects with levels > 75th percentile of NHANES III population based on sex and BMI. Table S1 summarizes the cut-offs for the 25th percentile for the National Health and Nutrition Examination Survey (NHANES) III population (complete data courteously provided by Dr. Ruhl).
The design of the treatment study in RLD (ClinicalTrials.gov identifier: NCT00596934)
Nine subjects from the RLD group agreed to participate in a prospective, open-label pilot study to test the efficacy of metreleptin. Patients were enrolled from 2009 to 2011. The primary outcome was determined as the global NASH score, obtained from histopathological assessments of the liver biopsy samples (Table S2). Changes in individual components of NASH and fibrosis scores were studied in a blinded fashion to assess the role of metreleptin on liver histopathology. Participants were admitted to the Michigan Clinical Research Unit for two days to undergo baseline tests for metabolic state and body composition and a liver biopsy. During this hospital stay, standardized weight maintenance diets of 50 % carbohydrates, 20 % protein, and 30 % fat were served as soon as the tests of the day were completed. Following the completion of all baseline testing, subjects were started on metreleptin at a dose of 0.1 mg/kg/day given once a day subcutaneously. There was no dose adjustment throughout the 12-month period. The maximum dose that was administered was 10 mg/day due to practicability as one vial produced 10 mg to be administered. Metreleptin was provided primarily by Amylin Pharmaceuticals, San Diego, CA, and is currently owned by Amryt Pharmaceuticals, Dublin, Ireland. All medical therapies at baseline in these subjects were noted and remained unchanged for the 12 months of the study. Participants were seen monthly for the first 6 months and then every 2 months to assess the safety and tolerability of metreleptin. Blood was collected for metabolic parameters at each visit. Body composition was assessed using MRI and DEXA at baseline, 6 months, and 12 months. Liver fat using MRI was assessed at baseline, 6 months, and 12 months. A liver biopsy was repeated at 12 months. The 12-month studies were conducted as inpatients over two days using the aforementioned standardized study feeding routines.
The design of the treatment study in PL (ClinicalTrials.gov identifier: NCT01679197)
This next study was designed as an open-label study performed at the University of Michigan, Ann Arbor, MI which was funded by the NIDDK (R01 DK088114) and approved by the University of Michigan IRBMED. Patients were enrolled between 2012 and 2015. Subjects enrolled in the study had the diagnosis of acquired or inherited PL made by physician assessment. The molecular basis of disease was determined using resources described in our previous work 22. The presence of liver disease was assessed by ultrasound or prior liver biopsy demonstrating fatty liver disease. Exclusion criteria included presence of human immunodeficiency virus, advanced liver disease (labs demonstrating abnormal synthetic function), viral hepatitis, alcohol consumption greater than 40 grams per week, end-stage renal disease, active cancer, greater than New York Heart Association class 2 congestive heart failure, known allergy to Escherichia coli derived proteins or hypersensitivity to any component of metreleptin treatment. Informed consent was obtained from all subjects or guardians. History and physical examinations were performed, and previous medical records were examined when possible. The first subject was enrolled on October 8, 2012, and data collection for this study was completed on April 14, 2016.
The primary outcome was determined as the global NASH score, obtained from histopathological assessments of the liver biopsy samples (Table S2). Changes in individual components of NASH and fibrosis scores were studied in a blinded fashion to assess the role of metreleptin on liver histopathology. Secondary outcomes were determined as the impact of metreleptin therapy on hepatic fat percent via proton density fat fraction obtained by magnetic resonance imaging, HbA1c and lipid levels as well as energy expenditure.
Following baseline study procedures, participants began metreleptin (recombinant human leptin) therapy. Metreleptin was provided by Amylin Pharmaceuticals (San Diego, CA), later manufactured by Bristol Myers Squibb and Aegerion Pharmaceuticals, and currently Amryt Pharmaceuticals. Participants self-administered subcutaneous injections after reconstitution once a day. Metreleptin doses were started at a dose of 2.5 mg per day in males and 5 mg per day in females. The dose was increased to a maximum of 10 mg daily (in single or divided doses based on individual’s preference) after 2 weeks to 6 months at investigator discretion. We aimed for this higher dose as this was the dose used in the RLD pilot study evaluating the leptin effects in the liver. The dose increases were made at 1.25 to 2.5 mg increments depending on the principal investigator’s opinion on the tolerability of such an increase. Concomitant glucose-lowering treatments were reduced to avoid hypoglycemia. In some patients, minor changes in the glucose-lowering treatments were noted at their follow-up visits, initiated by their local physicians. We made every attempt to keep these treatments as stable as possible. Intercurrent medical events were treated as required. Participants were instructed to keep symptom sheets and safety visits were conducted by phone. Study visits were scheduled at 3 months, 6 months 9 months, and 12 months after the baseline visit and study drug initiation.
Study approval
All study protocols were approved by the University of Michigan Medical School Institutional Review Board and all participants gave informed consent.
METHOD DETAILS
Study Assessment methods
Energy intake and resting energy expenditure
Energy intake was assessed using 3-day food records in the PL study. Subjects were asked to record the type and amount of food and beverage consumed for two consecutive weekdays and one weekend day using standardized measures. Records were reviewed by study dieticians. Food intake data were analyzed, and energy and nutrient intake were calculated using the Nutritionist V Diet Analysis software (First DataBank Inc., San Bruno, CA). A diet aimed at weight maintenance was prescribed by a study dietician. This diet was well-balanced (50% carbohydrate, 20% protein and 30% fat) consisting of calculated weight maintenance calories per day. Alcohol intake was limited to 1 serving of alcoholic drink per week and participants were asked to avoid alcohol consumption 72 hours before scheduled study visits. They were instructed to consume a standardized dinner meal prior to the evening before study visits (750 calories, same composition as previously noted).
Resting energy expenditure (REE) was measured after 30 minutes of rest using a microprocessor-controlled indirect calorimetry device (Sensormedics, Yorba Linda, CA) that measures oxygen consumption and carbon dioxide production 72. Respiratory quotient (RQ) was the ratio of oxygen consumed over carbon dioxide produced.
Leptin measurements
In the cross-sectional study, leptin levels were measured from a single fasting sample after 10 hours of fasting using radioimmunoassay (RIA), originally manufactured by Linco (St. Charles, MO) and then Millipore. Subjects with RLD were defined based on criteria in Table S1. The levels on the RLD trial were measured all together using the ELISA assay (EMDMilipore, Billerica, MA, USA). Leptin levels were measured by the latter ELISA kit in the PL study. In both of the treatment studies, three samples were drawn 30 minutes apart and averaged to compensate for the pulsatility seen in serum leptin levels across the intervention study periods.
Adipokine measurements
Adiponectin levels were measured as previously described using a commercially available kit by Linco Inc (St Charles, MO). Other cytokines and soluble receptors, as well as soluble leptin receptor levels, were measured with the Quantikine ELISA (R&D Systems, Minneapolis, MN).
Determination of other circulating hormone levels
In order to understand the pathways by which leptin effect can be mediated, the following circulating factors were measured using standardized commercially available kits (Millipore, Billerica, MA): ghrelin, glucagon-like peptide-1, and GIP using samples pretreated with protease and DPPIV inhibitor. The tubes for the incretin hormones contained Pefabloc SC (Sigma-Aldrich, St. Louis, MO) and DPP-IV inhibitor (EMD Millipore, Billerica, MA) to inhibit DPP-IV and other proteases.
Assessment of body composition
Body composition was evaluated using skin thickness measurements and waist and hip circumferences using standardized techniques. Dual X-ray absorptiometry (DEXA) (GE Lunar Prodigy, model PA +41744, Madison, WI) was used to estimate fat and lean body mass 73. Data from DEXA evaluation was used to calculate FMR, a ratio of the percentage of the trunk fat mass to the percentage of the lower limb fat mass. Fat shadow images were generated by processing the DEXA scan files (.dfb) and analyzed by using enCore v14.10 as described previously 74.
MRI for measurement of hepatic fat content
To evaluate hepatic fat content, MR imaging using quantitative multi-echo Dixon method and multi-echo MR-spectroscopy was utilized and this method has been described previously 22; 75. Briefly, three-dimensional volumes were acquired with geometry: nominal field-of-view (FOV) = 400mm × 350mm; in-plane acquired spatial resolution = 2.5mm reconstructed to 2mm resolution; and 56 axial 5mm thick slices centered mid-liver. Six gradient-recalled-echoes within echo times (TE) = 0.96ms – 4.46ms, short repetition time (TR) = 5.6ms, flip-angle = 3o and parallel imaging acceleration = 2 afforded single breath-hold acquisitions. Quantitative proton density fat-fraction (PDFF) maps were reconstructed on the MRI system using a 6-peak complex signal decay model.
Liver biopsies
Percutaneous liver biopsy specimens were obtained at baseline and 12 months of therapy. Histological features of NAFLD/NASH were studied using the validated NASH-clinical research network (CRN) scoring system in a blinded fashion. This scoring system comprises 14 histologic features, 4 of which (steatosis [0–3], lobular inflammation [0–2], hepatocellular ballooning [0–3], and fibrosis [0–4]) are evaluated semi-quantitatively (Table S2). NAFLD activity score (NAS) is the unweighted sum of steatosis, lobular inflammation, and hepatocellular ballooning scores, and this, together with the fibrosis score, constitutes the total NASH score.
Transcriptional profiling
RNA from 5–20 mg of flash-frozen liver biopsy samples were isolated using a Qiagen RNeasy kit followed by treatment with RNase-free DNase (Ambion). The RNA concentration was assessed by Nanodrop (Thermo Fisher Scientific). RNA was then hybridized to the Human HT‐12 BeadChip (Illumina), which interrogates 47 323 transcripts. The microarray hybridizations and normalizations were performed by the DNA sequencing Core at the University of Michigan according to the manufacturer’s instructions. Raw data were background corrected using a normal‐exponential convolution model based on negative control probes, quantile normalized, and log‐2 transformed. Pathway enrichment between paired biopsies was performed using LRpath 19 genes, using p-values and fold-change comparing baseline and post-treatment samples. We tested for enrichment of Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for humans.
RT-PCR analyses
RNA was extracted as above. A 2-μg aliquot of total RNA was reverse-transcribed to generate single-strand complementary DNA (cDNA). Real-time PCR was carried out using an Opticon Cycler (MJ Research) using conditions specific for each primer set. Two microliters of cDNA were then amplified in a final volume of 25 μL using the Qiagen CyberGreen Quantitec system. After an initial denaturation (95°C for 15 min), amplification was performed for 35 cycles. Amplification of 18S RNA was used to normalize each sample for reverse transcription efficiency. In general, each primer set was chosen to amplify a 3’ domain of the mRNA of interest and, where possible, the PCR product would cross a predicted intron to examine the genomic contamination of the PCR reaction. Duplicate samples of RNA from each biopsy were individually quantified.
Measurement of plasma fatty acids
Fatty acid analyses were done in the Metabolomics Core of the Michigan Metabolomics and Obesity Center. Plasma samples were collected in EDTA-containing tubes and immediately centrifuged at 3500 rpm for 5 minutes; plasma was subsequently stored at –20 °C until analysis was performed. Total lipids were extracted from the plasma according to the method of Bligh and Dyer 76. Heptadecanoic acid was added as an internal standard for the quantization of fatty acids. Subclasses of lipids were then isolated by thin layer chromatography and the fatty acid composition analyzed by gas chromatography on an Omega Wax 250 capillary column (Supelco) following methylation. Column temperature was increased from 60º to 240ºC at a rate of 30º per minute. The column was kept at 240º for 8 minutes for a total run time of 14 minutes. Relative abundance of 18 different fatty acid species was done by comparison of retention times with known standards obtained from Nu-Chek Prep (Elysian, MN).
Lipidomic profiling of plasma
Liquid chromatography-mass spectrometry-based shotgun lipidomics using a TripleTOF 5600 was applied for lipid identification. Detailed methods can be found in Afshinia et al. 77. Missing values for lipids were imputed using the K nearest-neighbor method 78. The data were log2 transformed and normalization a cross-contribution compensating multiple internal standard normalization method 79. A compound-by-compound t-test was applied to identify differentially regulated lipids that passed the nominal threshold P value of < 0.05, followed by the Benjamini-Hochberg correction for false discovery rate (FDR) to account for multiple comparisons 79.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analysis methods
Statistical analyses were performed using GraphPad Prism version 8 (La Jolla, CA), SAS version 9.2 (Cary, NC), and SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). The primary outcome variable of the open-label RLD clinical trial was the change in global NASH scores. Two-tailed P value of < 0.05 was considered significant as was decided a priori. A chi-square test was used to compare categorical variables. The independent samples t-test was used to compare independent groups. The repeated-measures ANOVA was used to compare variables that were based on repeated observations. Differences in each collected parameter were evaluated using a paired test (compared to baseline). P values are marked if they are significant after multiplicity correction. Paired t-test was used to compare month-12 values to baseline (without multiplicity correction) as the change at 12 months vs. baseline was a prespecified endpoint. Different components of the NASH score before and after metreleptin treatment were compared by Wilcoxon matched-pairs signed rank test. Log transformation was applied if needed. If data were skewed, nonparametric tests were used as needed.
Missing data
The last observation carried forward (LOCF) imputation method was used as repeated measures were taken per subject by longitudinal time points. In the RLD study, the last observed non-missing values (month 6 and month 10, respectively) were used to fill in missing values in two subjects who dropped out of the study before the final visit was completed. For liver-biopsy-related parameters, the analyses were completed only in completers due to the potential confounding effects of the underlying reasons for drop-out. For the PL study, the last observed non-missing values (month 6) were used to fill in missing values at month 9 visit in one subject (subject ID: 21) who missed month 9 visit but completed the study protocol.
Data presentation
Data were presented as mean ± standard deviation (SD) or frequency unless otherwise stated. Triglycerides were reported as geometric mean with 95% confidence intervals for the PL study because the distribution was far more skewed than what was observed in the RLD study.
ADDITIONAL RESOURCES
The RLD study: ClinicalTrials.gov identifier: NCT00596934. https://clinicaltrials.gov/ct2/show/NCT00596934
The PL study: ClinicalTrials.gov identifier: NCT01679197.https://clinicaltrials.gov/ct2/show/NCT01679197
Supplementary Material
Data S1: The RLD study full trial clinical database. Related to Figures 1 and 2.
Data S2: Full dataset of mRNA expression in liver biopsy specimens. Related to Figure 2.
Supplementary Data File: Additional supplementary data for the partial lipodystrophy (PL) study. Related to Figures 3 and 4.
Highlights.
Some patients with nonalcoholic steatohepatitis have relatively low leptin levels.
In this subgroup, leptin therapy leads to reduced hepatic fat and lower NASH scores.
Similar improvements are observed in a group of subjects with partial lipodystrophy.
The therapeutic benefit of leptin may go beyond severe leptin deficiency.
Context and Significance.
Fat goes to the liver when the body has limited fat storage capacity and damages liver cells. Fat stores are communicated to the rest of the body through the fat cell hormone leptin. We have been interested in identifying groups of patients with fatty liver disease and relatively low leptin levels in order to test if exogenous administration of recombinant leptin would impact the amount of fat and the degree of cell damage and scarring in the liver. In this report, we describe the improvement observed in two of these subgroups with less fat and cellular injury after a year of therapy with leptin, suggesting that there may be a role for boosting leptin levels or leptin signal for fatty liver disease in select circumstances.
Acknowledgments
We dedicate this manuscript to Dr. Phillip Gorden, Director Emeritus of NIDDK and a Senior investigator at the NIDDK intramural Program for his long-term leadership and vision in lipodystrophy and leptin studies as well as his mentorship. We also wish to express our gratitude to the participants for volunteering for these complicated studies. We are indebted to the Lipodystrophy United Patient Foundation for help in recruitment for the PL study. We also thank the following sources of philanthropic gifts for Lipodystrophy Research at the University of Michigan: Mr. and Mrs. James Sopha, the White Point Foundation of Turkey (Istanbul, Turkey), and Ionis Pharmaceuticals (Carlsbad, CA). We thank Frank DiPaola for performing liver biopsies of one subject in the PL study. We also thank Peedikayil E. Thomas for assistance in sequencing. We are also indebted to Alex M. DePaoli for his overall advice and efforts in getting the first drug supply to our site before metreleptin changed hands, allowing the launch of the study. The authors also acknowledge Meri D. Pozo and Robert Schupp from Springer inScience Communications for their editorial assistance at the earlier stages of the manuscript with support provided by Aegerion Pharmaceuticals. Metreleptin was provided by Amgen Inc (first supply) and subsequently by Amylin Pharmaceuticals (the majority of the drug supply). During the execution of the study and the writing of the manuscript, metreleptin was owned by 7 different entities and is now owned by Amryt Pharmaceuticals (Dublin, Ireland).
Other than the main funding sources reported in the abstract, there were additional sources of funding to maintain and complete these translational studies: The cross-sectional RLD study support was provided via P&F grants awarded to EAO from the Michigan Diabetes Research Center P60DK020572, the Michigan Nutrition Obesity Research Center (P30DK089503), and Michigan Gastrointestinal Research Center P30DK034933. The RLD Studies received infrastructure support from UL1TR002240, R24DK097153, and P30DK089503. Infrastructure and data management support for the PL studies were provided by the NIH Clinical and Translational Science Awards grant UL1TR000433, the Nutrition Obesity Research Centers grant P30DK089503, and NIH institutional grant DK034933.
FUNDING. NIH Grants R03 DK074488 (EAO) and R01 DK088114 (EAO and HC).
Declaration of interests
Drug support was provided by mainly Amylin Pharmaceuticals Inc (later Ltd), the manufacturer of metreleptin at the time of the study, currently owned by Amryt Pharmaceuticals The earlier writing support was provided by Aegerion Pharmaceuticals that owned metreleptin from 2015 to 2019. The following individual level author conflicts are also reported: EAO reports the following conflicts: Grant support: Aegerion Pharmaceuticals (now Amryt Pharmaceuticals), Ionis Pharmaceuticals, Akcea Therapeutics, Gemphire Therapeutics, GI Dynamics (current), AstraZeneca (2015-2017). Consultant or Advisor: AstraZeneca, Thera Therapeutics, and BMS (past), Aegerion Pharmaceuticals (now Amryt Pharmaceuticals), Akcea Therapeutics, Ionis Pharmaceuticals, Regeneron Pharmaceuticals (current). Drug support: Aegerion Pharmaceuticals (now Amryt Pharmaceuticals), Akcea Therapeutics, Rhythm Pharmaceuticals (all current). Other support: Aegerion Pharmaceuticals (now Amryt Pharmaceuticals), Regeneron Pharmaceuticals (current). BA has attended Scientific Advisory Board Meetings organized by Aegerion Pharmaceuticals (now Amryt Pharmaceuticals) and Regeneron Pharmaceuticals and has received honoraria as a speaker from AstraZeneca, Lilly, MSD, Novartis, Novo Nordisk, Boehringer-Ingelheim, Servier, and Sanofi-Aventis. CSM has served as a consultant for Coherus, Servier, Aegerion (now Amryt Pharmaceuticals), Regeneron, Genfit, and NovoNordisk, has received grants through his Institution by Coherus, Novo Nordisk, and Esai, and is shareholder of Coherus and Pangea Inc.
Diversity Statement
We worked to ensure sex balance in the selection of human subjects as much as scientifically applicable. The RLD study focused only on male subjects for scientific reasons mentioned in the paper. One or more authors of the paper self-identify as belonging to a minority group underrepresented in science. One or more of the authors of this paper received support from a program designed to increase minority representation in science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lucas C, Lucas G, Lucas N, Krzowska-Firych J, and Tomasiewicz K (2018). A systematic review of the present and future of non-alcoholic fatty liver disease. Clinical and experimental hepatology 4, 165–174. 10.5114/ceh.2018.78120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, and Clark JM (2013). Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 178, 38–45. 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, and Younossi ZM (2011). Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34, 274–285. 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, and Wymer M (2016). Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md.) 64, 73–84. 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, and Anstee QM (2015). Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 62, 1148–1155. 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH, et al. (2013). A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis 230, 258–267. 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 7.Bellentani S, Dalle Grave R, Suppini A, Marchesini G, and Fatty Liver Italian N (2008). Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology (Baltimore, Md.) 47, 746–754. 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 8.Musso G, Gambino R, Cassader M, and Pagano G (2010). A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 52, 79–104. 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 9.Bjelakovic G, Nikolova D, and Gluud C (2013). Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One 8, e74558. 10.1371/journal.pone.0074558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, et al. (2015). Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965. 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefan N, Haring HU, and Cusi K (2019). Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 7, 313–324. 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, and Friedman JM (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432. 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 13.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, and Friedman JM (1995). Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546. [DOI] [PubMed] [Google Scholar]
- 14.Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, Magkos F, Paruthi J, and Mantzoros CS (2013). Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev 34, 377–412. 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, and Gorden P (2005). Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 41, 753–760. 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 16.Safar Zadeh E, Lungu AO, Cochran EK, Brown RJ, Ghany MG, Heller T, Kleiner DE, and Gorden P (2013). The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol 59, 131–137. 10.1016/j.jhep.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, et al. (2002). Leptin-replacement therapy for lipodystrophy. N Engl J Med 346, 570–578. 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 18.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, and McCamish M (1999). Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282, 1568–1575. 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 19.Sartor MA, Leikauf GD, and Medvedovic M (2009). LRpath: a logistic regression approach for identifying enriched biological groups in gene expression data. Bioinformatics 25, 211–217. 10.1093/bioinformatics/btn592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang CP, and Tall AR (2001). Transcriptional profiling reveals global defects in energy metabolism, lipoprotein, and bile acid synthesis and transport with reversal by leptin treatment in ob/ob mouse liver. J Biol Chem 276, 49066–49076. 10.1074/jbc.M107250200. [DOI] [PubMed] [Google Scholar]
- 21.Chan JL, Koda J, Heilig JS, Cochran EK, Gorden P, Oral EA, and Brown RJ (2016). Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf) 85, 137–149. 10.1111/cen.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajluni N, Meral R, Neidert AH, Brady GF, Buras E, McKenna B, DiPaola F, Chenevert TL, Horowitz JF, Buggs-Saxton C, et al. (2017). Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf) 86, 698–707. 10.1111/cen.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akinci B, Meral R, Rus D, Hench R, Neidert AH, DiPaola F, Westerhoff M, Taylor SI, and Oral EA (2020). The complicated clinical course in a case of atypical lipodystrophy after development of neutralizing antibody to metreleptin: treatment with setmelanotide. Endocrinol Diabetes Metab Case Rep 2020. 10.1530/EDM-19-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, and Lonardo A (2017). NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv Ther 34, 1291–1326. 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, et al. (2016). The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J Clin Endocrinol Metab 101, 4500–4511. 10.1210/jc.2016-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handelsman Y, Oral EA, Bloomgarden ZT, Brown RJ, Chan JL, Einhorn D, Garber AJ, Garg A, Garvey WT, Grunberger G, et al. (2013). The clinical approach to the detection of lipodystrophy - an AACE consensus statement. Endocr Pract 19, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, and Singal AG (2018). Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 16, 198–210 e192. 10.1016/j.cgh.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Depaoli A, Long A, Fine GM, Stewart M, and O’Rahilly S (2018). Efficacy of Metreleptin for Weight Loss in Overweight and Obese Adults with Low Leptin Levels. Diabetes 67. 10.2337/db18-296-LB. [DOI] [Google Scholar]
- 29.Fogteloo AJ, Pijl H, Frolich M, McCamish M, and Meinders AE (2003). Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr Metab 16, 109–114. [PubMed] [Google Scholar]
- 30.Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, and Campfield LA (2000). Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab 85, 4003–4009. 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 31.Moon HS, Matarese G, Brennan AM, Chamberland JP, Liu X, Fiorenza CG, Mylvaganam GH, Abanni L, Carbone F, Williams CJ, et al. (2011). Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes 60, 1647–1656. 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, and Weyer C (2009). Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 17, 1736–1743. 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agrawal A, Lourenco EV, Gupta S, and La Cava A (2008). Gender-Based Differences in Leptinemia in Healthy Aging, Non-obese Individuals Associate with Increased Marker of Oxidative Stress. Int J Clin Exp Med 1, 305–309. [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, Li Z, Gabrielsen JS, Simcox JA, Lee SH, Jones D, Cooksey B, Stoddard G, Cefalu WT, and McClain DA (2015). Adipocyte iron regulates leptin and food intake. J Clin Invest 125, 3681–3691. 10.1172/JCI81860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocha N, Bulger DA, Frontini A, Titheradge H, Gribsholt SB, Knox R, Page M, Harris J, Payne F, Adams C, et al. (2017). Human biallelic MFN2 mutations induce mitochondrial dysfunction, upper body adipose hyperplasia, and suppression of leptin expression. Elife 6. 10.7554/eLife.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrann CD, and Rosen ED (2012). New insights into adipocyte-specific leptin gene expression. Adipocyte 1, 168–172. 10.4161/adip.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K, Haddad A, Osme A, Kim C, Borzou A, Ilchenko S, Allende D, Dasarathy S, McCullough A, Sadygov RG, and Kasumov T (2018). Hepatic Mitochondrial Defects in a Nonalcoholic Fatty Liver Disease Mouse Model Are Associated with Increased Degradation of Oxidative Phosphorylation Subunits. Mol Cell Proteomics 17, 2371–2386. 10.1074/mcp.RA118.000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato S, Ding J, Pisck E, Jhala US, and Du K (2008). COP1 functions as a FoxO1 ubiquitin E3 ligase to regulate FoxO1-mediated gene expression. J Biol Chem 283, 35464–35473. 10.1074/jbc.M801011200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, et al. (2006). TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 312, 1763–1766. 10.1126/science.1123374. [DOI] [PubMed] [Google Scholar]
- 40.BasuRay S, Smagris E, Cohen JC, and Hobbs HH (2017). The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 66, 1111–1124. 10.1002/hep.29273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C, Hwang SH, Jia Y, Choi J, Kim YJ, Choi D, Pathiraja D, Choi IG, Koo SH, and Lee SJ (2017). Olfactory receptor 544 reduces adiposity by steering fuel preference toward fats. J Clin Invest 127, 4118–4123. 10.1172/JCI89344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SJ, Depoortere I, and Hatt H (2019). Therapeutic potential of ectopic olfactory and taste receptors. Nat Rev Drug Discov 18, 116–138. 10.1038/s41573-018-0002-3. [DOI] [PubMed] [Google Scholar]
- 43.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, and Friedman JM (2004). Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest 113, 414–424. 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimomura I, Hammer RE, Ikemoto S, Brown MS, and Goldstein JL (1999). Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401, 73–76. 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 45.Moon YA (2017). The SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis. Endocrinol Metab (Seoul). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flowers MT, and Ntambi JM (2008). Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol 19, 248–256. 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lounis MA, Escoula Q, Veillette C, Bergeron KF, Ntambi JM, and Mounier C (2016). SCD1 deficiency protects mice against ethanol-induced liver injury. Biochim Biophys Acta 1861, 1662–1670. 10.1016/j.bbalip.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Ebbesson SO, Tejero ME, Lopez-Alvarenga JC, Harris WS, Ebbesson LO, Devereux RB, MacCluer JW, Wenger C, Laston S, Fabsitz RR, et al. (2010). Individual saturated fatty acids are associated with different components of insulin resistance and glucose metabolism: the GOCADAN study. Int J Circumpolar Health 69, 344–351. 10.3402/ijch.v69i4.17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotronen A, Velagapudi VR, Yetukuri L, Westerbacka J, Bergholm R, Ekroos K, Makkonen J, Taskinen MR, Oresic M, and Yki-Jarvinen H (2009). Serum saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations. Diabetologia 52, 684–690. 10.1007/s00125-009-1282-2. [DOI] [PubMed] [Google Scholar]
- 50.Petersson H, Arnlov J, Zethelius B, and Riserus U (2010). Serum fatty acid composition and insulin resistance are independently associated with liver fat markers in elderly men. Diabetes Res Clin Pract 87, 379–384. 10.1016/j.diabres.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Ikejima K, Okumura K, Kon K, Takei Y, and Sato N (2007). Role of adipocytokines in hepatic fibrogenesis. J Gastroenterol Hepatol 22 Suppl 1, S87–92. 10.1111/j.1440-1746.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- 52.Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, and Anania FA (2004). Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J 18, 1612–1614. 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potter JJ, Rennie-Tankesley L, and Mezey E (2003). Influence of leptin in the development of hepatic fibrosis produced in mice by Schistosoma mansoni infection and by chronic carbon tetrachloride administration. J Hepatol 38, 281–288. 10.1016/s0168-8278(02)00414-2. [DOI] [PubMed] [Google Scholar]
- 54.Saxena NK, Ikeda K, Rockey DC, Friedman SL, and Anania FA (2002). Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology 35, 762–771. 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, et al. (2000). Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest 105, 1243–1252. 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, et al. (2014). Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416. 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown RJ, Meehan CA, and Gorden P (2015). Leptin Does Not Mediate Hypertension Associated With Human Obesity. Cell 162, 465–466. 10.1016/j.cell.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polyzos SA, Kountouras J, and Mantzoros CS (2015). Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism 64, 60–78. 10.1016/j.metabol.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Brown RJ, Meehan CA, Cochran E, Rother KI, Kleiner DE, Walter M, and Gorden P (2017). Effects of Metreleptin in Pediatric Patients With Lipodystrophy. J Clin Endocrinol Metab 102, 1511–1519. 10.1210/jc.2016-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown RJ, Oral EA, Cochran E, Araujo-Vilar D, Savage DB, Long A, Fine G, Salinardi T, and Gorden P (2018). Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine 60, 479–489. 10.1007/s12020-018-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aegerion (2015). MYALEPT PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125390s000lbl.pdf.
- 62.Aegerion (2018). https://www.ema.europa.eu/en/medicines/human/EPAR/myalepta.
- 63.Oral EA, Gorden P, Cochran E, Araujo-Vilar D, Savage DB, Long A, Fine G, Salinardi T, and Brown RJ (2019). Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine 64, 500–511. 10.1007/s12020-019-01862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oral EA, and Chan JL (2010). Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract 16, 324–333. 10.4158/EP09155.RA. [DOI] [PubMed] [Google Scholar]
- 65.Park JY, Javor ED, Cochran EK, DePaoli AM, and Gorden P (2007). Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism 56, 508–516. 10.1016/j.metabol.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan JL, Lutz K, Cochran E, Huang W, Peters Y, Weyer C, and Gorden P (2011). Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract 17, 922–932. 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chong AY, Lupsa BC, Cochran EK, and Gorden P (2010). Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia 53, 27–35. 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 68.Diker-Cohen T, Cochran E, Gorden P, and Brown RJ (2015). Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab 100, 1802–1810. 10.1210/jc.2014-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simha V, Subramanyam L, Szczepaniak L, Quittner C, Adams-Huet B, Snell P, and Garg A (2012). Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J Clin Endocrinol Metab 97, 785–792. 10.1210/jc.2011-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baykal AP, Parks EJ, Shamburek R, Syed-Abdul MM, Chacko S, Cochran E, Startzell M, Gharib AM, Ouwerkerk R, Abd-Elmoniem KZ, et al. (2020). Leptin decreases de novo lipogenesis in patients with lipodystrophy. JCI Insight 5. 10.1172/jci.insight.137180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ajluni N, Dar M, Xu J, Neidert AH, and Oral EA (2016). Efficacy and Safety of Metreleptin in Patients with Partial Lipodystrophy: Lessons from an Expanded Access Program. J Diabetes Metab 7. 10.4172/2155-6156.1000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sparti A, DeLany JP, de la Bretonne JA, Sander GE, and Bray GA (1997). Relationship between resting metabolic rate and the composition of the fat-free mass. Metabolism 46, 1225–1230. [DOI] [PubMed] [Google Scholar]
- 73.Haarbo J, Gotfredsen A, Hassager C, and Christiansen C (1991). Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clin Physiol 11, 331–341. [DOI] [PubMed] [Google Scholar]
- 74.Meral R, Ryan BJ, Malandrino N, Jalal A, Neidert AH, Muniyappa R, Akinci B, Horowitz JF, Brown RJ, and Oral EA (2018). “Fat Shadows” From DXA for the Qualitative Assessment of Lipodystrophy: When a Picture Is Worth a Thousand Numbers. Diabetes Care 41, 2255–2258. 10.2337/dc18-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, and Dobbins RL (2005). Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288, E462–468. 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 76.Bligh EG, and Dyer WJ (1959). A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37, 911–917. 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 77.Afshinnia F, Rajendiran TM, Karnovsky A, Soni T, Wang X, Xie D, Yang W, Shafi T, Weir MR, He J, et al. (2016). Lipidomic Signature of Progression of Chronic Kidney Disease in the Chronic Renal Insufficiency Cohort. Kidney Int Rep 1, 256–268. 10.1016/j.ekir.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sas KM, Karnovsky A, Michailidis G, and Pennathur S (2015). Metabolomics and diabetes: analytical and computational approaches. Diabetes 64, 718–732. 10.2337/db14-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Redestig H, Fukushima A, Stenlund H, Moritz T, Arita M, Saito K, and Kusano M (2009). Compensation for systematic cross-contribution improves normalization of mass spectrometry based metabolomics data. Anal Chem 81, 7974–7980. 10.1021/ac901143w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: The RLD study full trial clinical database. Related to Figures 1 and 2.
Data S2: Full dataset of mRNA expression in liver biopsy specimens. Related to Figure 2.
Supplementary Data File: Additional supplementary data for the partial lipodystrophy (PL) study. Related to Figures 3 and 4.
Data Availability Statement
This study did not generate any unique code. The datasets obtained during the RLD study including the mRNA profiling data are provided in full. The lipidomics datasets can be provided upon request. We are currently conducting additional analyses from the PL study database but, we provided full data for results reported in the manuscript including liver biopsy images.