Abstract
Objectives
There are indications that SARS-CoV-2 can trigger new onset or relapses of neuro-immunological disease. We report a patient with relapsing remitting multiple sclerosis (RRMS) under disease-modifying therapy (DMT) who experienced a relapse of RRMS after mild COVID-19.
Case report
The patient is a 27-year-old female with RRMS who developed a third exacerbation of RRMS under DMT two weeks after mild COVID-19. Compared to previous imaging findings, new studies revealed an increase in the lesion load and an enhancing lesion over two segments in the thoracic spine. The patient profited from steroids and replacement of her previous DMT. She tolerated the first SARS-CoV-2 vaccination without side effects 6 months after the SARS-CoV-2 infection.
Conclusions
SARS-CoV-2 infections can be followed by exacerbation of MS and failure of DMT. More arguments in favour than against a causal relation can be raised. Neurologist should remain vigilant for new or relapsing neuro-immunological disease following SARS-CoV-2 infections.
Keywords: SARS-CoV-2, COVID-19, Multiple sclerosis, Central nervous system, Relapse
1. Introduction
SARS-CoV-2 infections not only affect the lungs but generally all organs including the central and peripheral nervous system (CNS, PNS) (neuro-COVID) [1], [2]. The underlying pathophysiology for SARS-CoV-2 associated CNS disease is suspected to be immunogenic in the majority of the cases [3]. Neuro-immunologic CNS disease in association with SARS-CoV-2 infections so far reported include acute, disseminated encephalo-myelitis (ADEM), acute, haemorrhagic, necrotising encephalitis (AHNE), immune encephalitis, hypophysitis, cerebellitis, ventriculitis, transverse myelitis, posterior, reversible encephalopathy syndrome (PRES), cerebral vasculitis, venous sinus thrombosis, neuromyelitis optica spectrum disorders (NMO-SDs), and multiple sclerosis (MS) [4], [5]. There are indications that SARS-CoV-2 infections not only trigger relapses in patients with relapsing remitting MS (RRMS) [6] but also induce new onset MS [7]. Here we report a patient with RRMS under interferon β-1a (IFNβ-1a) who not only experienced a relapse of RRMS after mild COVID-19 but also failure of her previous disease modifying therapy (DMT).
2. Case report
The patient is a 27yo female, who was diagnosed with multiple sclerosis (MS) at age 24 y according to the McDonalds criteria. At this age she had experienced left visual impairment due to a first left optic neuritis. Cerebral MRI at that time showed an enhancing left optic nerve, non-enhancing lesions peri-ventricularly in the left corpus callosum, in both frontal regions, and in the left cerebellum. Cerebro-spinal fluid (CSF) investigations showed mild pleocytosis and positive oligoclonal bands. A spinal MRI with contrast medium revealed a T2-hyperintense lesion at the level C4 with mild enhancement, and two non-enhancing lesions on the levels C6/C7. Under steroids her visual symptoms completely resolved and she was put on IFNβ-1a. Under this regimen she experienced a second exacerbation one year after diagnosis.
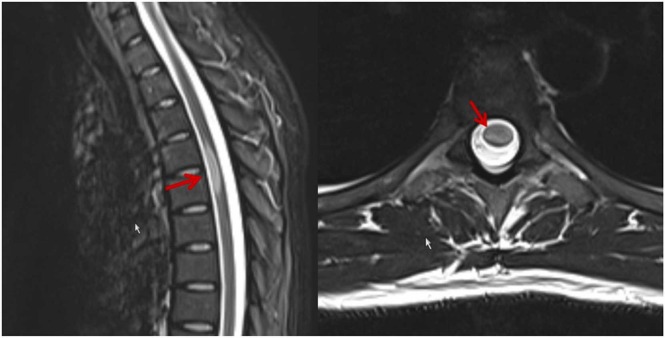
At age 26 years she got infected with SARS-CoV-2 manifesting with mild COVID-19 (fever, coughing, and dysgeusia), which resolved without treatment. She was unvaccinated at that time and negative for neutralising antibodies against SARS-CoV-2 (neutralisation titre (NT) 40). About two weeks later, she experienced bilateral sensory disturbances (dysesthesias and hypoesthesias) over the dermatomes C6/7, the abdomen, and the thighs, the lateral lower legs, the forefoot, and the toes. Additionally, she complained about gait disturbance. The Lhermitte sign was highly positive. After a spinal MRI had shown a new, mildly enhancing, expanding lesion at Th4/5 and non-enhancing lesions at C6 and Th10 ( Fig. 1), she received steroids about two months after onset of the sensory deficits. Antibodies against aquaporin, glycin, MOG, JCV, hepatitis virus, VZV, HIV, and Mycobacterium tuberculosis were negative. Since the relapse occurred under IFNβ-1a and the lesion load had increased, the patient was switched from IFNβ-1a to natalizumab. Under this regimen she slowly recovered. She received her first jab with an mRNA-based SARS-CoV-2 vaccine six months after the SARS-CoV-2 infection.
Fig. 1.
Spinal MRI showing an enhancing, eccentric lesion over the segments Th4/5, being interpreted as acute demyelinating lesion due to a relapse of an RRMS.
3. Discussion
The index patient is interesting for experiencing the third exacerbation of a RRMS two weeks after a mildly symptomatic SARS-CoV-2 infection. The relapse also disclosed that IFNβ-1a was no longer effective and had to be replaced by natalizumab. Whether there was a causal relation between COVID-19 and the relapse remains speculative but there are arguments in favour of and against a causal relation. Arguments in favour of a causal relation are that relapses of MS following an infection with SARS-CoV-2 have been previously reported [6]. Though none of these reports demonstrated a clear-cut causal connection, a pathophysiologic relation cannot be excluded. A second argument for a causal relation is that relapses of MS have been reported in association with other viral infections [8]. A third argument is that even SARS-CoV-2 vaccinations can be associated with relapses of MS [9]. A fourth argument is that retrospective observational studies have shown that the risk of exacerbation of MS has increased during the pandemic [6]. A fifth argument is that SARS-CoV-2 was made responsible for a number of newly onset or flares of immunological disease: Immunologic disorders in addition to MS reported having been triggered by a SARS-CoV-2 infection include myasthenia, NMO-SD, immune hepatitis, myositis, myocarditis, vestibular neuritis, Graves disease, immune thrombocytopenia, rheumatoid arthritis, Sjögren syndrome, juvenile idiopathic arthritis, and autoimmune thyroiditis [10], [11], [12]. A sixth argument is that SARS-CoV-2 triggers a hyper-inflammatory immune reaction, which can be documented by lymphopenia, elevated cytokines, chemokines, and glial markers (cytokine storm) occasionally leading to pulmonary failure, ARDS, and death [13].
Arguments against a causal relation are that only single cases with SARS-CoV-2 associated immunological disease have been reported, that the overall prevalence of immunological disorder did not increase significantly since the outbreak of the pandemic, and that the exact mechanism by which SARS-CoV-2 triggers the onset of any immunological disease has not been elucidated yet. As long as the relation between SARS-CoV-2 and flares or new development of immunological diseases remains speculative, it is not feasible to establish a secure causal relation.
Whether RRMS had a predisposing effect on the acquisition of the SARS-CoV-2 infection in the index patient remains speculative. Whether the interferon therapy was responsible for the only mild disease course also remains speculative. A study of 603 MS patients under IFNβ-1a found that MS patients receiving IFNβ-1a for RRMS had relatively low rates of serious disease and/or severe outcomes of COVID-19 [14]. Another study found that COVID-19 contraction may not increase the risk of acute MS attacks shortly after COVID-19 contraction [15]. It was hypothesised that COVID-19-associated lymphopenia may partly preclude the autoreactive memory cells from expansion and initiating relapses through a so-called bystander effect of COVID-19 infection [15]. There are also indications that withdrawal of DMT (e.g fingolimod) may favour the acquisition of COVID-19 [16]. There are also indications that MS patients under DMTs and experiencing COVID-19 may develop pseudo-exacerbations [17].
4. Conclusions
This case shows that SARS-CoV-2 infections can be followed by exacerbation of MS due to failure of DMT and that a causal relation between the viral infection and the relapse cannot be definitively excluded. Currently, more arguments in favour than against a causal relation can be raised. Neurologist should remain vigilant for new or relapsing neuro-immunological disease following an infection with SARS-CoV-2.
Ethics approval and consent to participate
Obtained.
Consent for Publication
Obtained.
Funding
None received.
CRediT authorship contribution statement
JF: design, literature search, discussion, first draft, critical comments,
Competing interests
None.
Acknowledgements
None.
Data Availability
All data reported are available from the corresponding author.
References
- 1.Elrobaa I.H., New K.J. COVID-19: pulmonary and extra pulmonary manifestations. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.711616. (Sep 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finsterer J., Scorza F.A., Scorza C.A., Fiorini A.C. Extrapulmonary onset manifestations of COVID-19. Clinics. 2021;76 doi: 10.6061/clinics/2021/e2900. (Jul 5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel P.B., Bearden D. Neuropathogenesis of severe acute respiratory syndrome coronavirus 2. Curr. Opin. Pediatr. 2021;33(6):597–602. doi: 10.1097/MOP.0000000000001068. (Dec 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García S., Cuatepotzo-Burgos F.M., Toledo-Lozano C.G., Balderrama-Soto A., Alcaraz-Estrada S.L., Montiel-López L., De la Vega-Bravo A.H., Mondragón-Terán P., Santosbeña-Lagunes M., Escarela-Serrano M., Rodríguez-Martínez C.M., Méndez-Vidrio M.D.C., Muñoz-López S., Merino-Rajme J.A., Rodríguez-Briseño R.A., Cerda-Téllez F., Coral-Vázquez R.M., Sauri-Suárez S., Quiñonez-Aguilar S., Pineda-Juárez J.A., Suárez-Cuenca J.A. 2021. Neurological manifestations and outcomes in a retrospective cohort of Mexican Inpatients with SARS-CoV-2 pneumonia: design of a risk profile; p. 1501. (Healthcare). (Nov 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finsterer J. Presentation of neuro-COVID is broad and pathogenesis diverse. West J. Emerg. Med. 2021;22(3):799–800. doi: 10.5811/westjem.2021.1.50893. (Apr 8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barzegar M., Vaheb S., Mirmosayyeb O., Afshari-Safavi A., Nehzat N., Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult Scler Relat Disord. 2021;52 doi: 10.1016/j.msard.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarwar S., Rogers S., Mohamed A.S., Ogula E., Ayantayo R.A., Ahmed A., Shahzadi I., Kataria S., Singh R. Multiple Sclerosis Following SARS-CoV-2 Infection: A Case Report and Literature Review. Cureus. 2021 25;13(10) doi: 10.7759/cureus.19036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotelo J., Ordoñez G., Pineda B., Flores J. The participation of varicella zoster virus in relapses of multiple sclerosis. Clin Neurol Neurosurg. 2014;119:44–48. doi: 10.1016/j.clineuro.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Etemadifar M., Sigari A.A., Sedaghat N., Salari M., Nouri H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum Vaccin Immunother. 2021 3;17(10):3481–3483. doi: 10.1080/21645515.2021.1928463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lui D.T.W., Lee K.K., Lee C.H., Lee A.C.H., Hung I.F.N., Tan K.C.B. Development of Graves' Disease After SARS-CoV-2 mRNA Vaccination: A Case Report and Literature Review. Front Public Health. 2021 23;9 doi: 10.3389/fpubh.2021.778964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hügle B., Krumrey-Langkammerer M., Haas J.P. Infection with SARS-CoV-2 causes flares in patients with juvenile idiopathic arthritis in remission or inactive disease on medication. Pediatr Rheumatol Online J. 2021 29;19(1):163. doi: 10.1186/s12969-021-00653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards K., Hussain I. Two Cases of Severe Autoimmune Thyrotoxicosis Following SARS-CoV-2 Infection. J Investig Med High Impact Case Rep. 2021 -;9 doi: 10.1177/23247096211056497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslani M., Mortazavi-Jahromi S.S., Mirshafiey A. Cytokine storm in the pathophysiology of COVID-19: Possible functional disturbances of miRNAs. Int Immunopharmacol. 2021;101(Pt A) doi: 10.1016/j.intimp.2021.108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman M.S., Jack D., Murgašová Z., Todorović M., Seitzinger A. Outcomes of COVID-19 among patients treated with subcutaneous interferon beta-1a for multiple sclerosis. Mult Scler Relat Disord. 2021;56 doi: 10.1016/j.msard.2021.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etemadifar M., Sedaghat N., Aghababaee A., Kargaran P.K., Maracy M.R., Ganjalikhani-Hakemi M., Rayani M., Abhari A.P., Khorvash R., Salari M., Nouri H. COVID-19 and the Risk of Relapse in Multiple Sclerosis Patients: A Fight with No Bystander Effect? Mult Scler Relat Disord. 2021;51 doi: 10.1016/j.msard.2021.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Mayordomo V., Montero-Escribano P., Matías-Guiu J.A., González-García N., Porta-Etessam J., Matías-Guiu J. Clinical exacerbation of SARS-CoV2 infection after fingolimod withdrawal. J Med Virol. 2021;93(1):546–549. doi: 10.1002/jmv.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataria S., Tandon M., Melnic V., Sriwastava S. A case series and literature review of multiple sclerosis and COVID-19: Clinical characteristics, outcomes and a brief review of immunotherapies. eNeurologicalSci. 2020;21 doi: 10.1016/j.ensci.2020.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported are available from the corresponding author.