Abstract
Background
: Quantitative results of SARS-CoV-2 testing reported as viral load copies/mL can provide valuable information, but are rarely used in practice. We analyze whether viral load in the upper respiratory tract is correlated with transmission and disease course and how this information can be used in practice.
Study design
: Municipal Health Service (MHS) and clinical patients ≥18 years tested positive for SARS-CoV-2 with RT-PCR between June 1 and September 25, 2020 were included. Transmission was defined as an index having at least one contact tested positive. Test delay was defined as the time between symptom onset and SARS-CoV-2 testing.
Results
: 683 patients were included (656 MHS and 27 clinical patients). The viral load was considerably lower among clinical patients compared to MHS patients: median log10 copies/mL 2.51 (IQR −1.52 – 6.46) vs 4.92 (IQR −0.54 – 8.26), p < 0.0001. However, the test delay was higher for clinical patients (median 7 [IQR 2 – 19] vs 3 [IQR 0 – 26] days, p < 0.0001). SARS-CoV-2 transmitters showed much higher viral loads than non-transmitters (log10 copies/mL 5.23 [IQR −0.52 – 8.26] vs 4.65 [IQR −0.72 – 8.00], p < 0.0001), but not for those with a test delay > 7 days. Higher viral loads were significantly correlated with older age and with more (severe) COVID-19 related symptoms.
Conclusion
: Indexes that transmitted SARS-CoV-2 had more than three times higher viral loads than non-transmitters. Viral load information can be useful during source and contact tracing to prioritize indexes with highest risk of transmission, taking into account the test delay.
Keywords: SARS-CoV-2, COVID-19, Viral load, Transmission, Test delay
Abbreviations
- MHS
Municipal Health Service
1. Background
Shortly after the emergence of SARS-CoV-2, the first COVID-19 index was confirmed on February 27, 2020 in the Netherlands. The major human transmission route of SARS-CoV-2 is through respiratory droplets, although transmission through aerosols, contact with contaminated surfaces and fecal-oral transmission has also been reported [1]. Proximity and duration are crucial in the risk of transmission, pointing out the importance of droplet transmission rather than aerosol transmission [1].
RT-PCR has been the main method for SARS-CoV-2 diagnosis and results are mainly reported qualitatively as negative or positive. Quantitative test results, i.e. viral load copies/mL, allow for a more detailed study of transmission risk. Viral load tends to be highest in the upper respiratory tract in an early phase after infection and decreases rapidly after symptom onset, in which higher viral loads shift from the upper to the lower respiratory tract [1], [2], [3]. Higher viral loads are associated with more severe symptoms, irrespective of the stage of infection, and with increased risk of hospitalization and mortality [1,[4], [5], [6], [7], [8]]. Higher viral loads make it more likely to isolate infectious virus during the first eight days of disease onset, although this period is extended with severe disease and due to immune compromised status [1], [2], [3]. Recent studies from the UK and Spain showed that patients with higher viral loads are the most infectious [9,10].
In accordance with the national guidelines, for each COVID-19 case extensive source and contact tracing is performed by the 25 Municipal Health Services (MHSs) in the Netherlands to trace and control transmission. However, this was not possible during periods with a high incidence of SARS-CoV-2. In this retrospective study, we investigate in detail whether higher viral loads among adult MHS and clinical patients from June-September 2020 (a period during which extensive source and contact tracing was performed), is correlated with SARS-CoV-2 transmission and disease severity compared to patients with lower viral loads. We also propose how to use this information in practice.
2. Study design
2.1. Study population
The research proposal was submitted to the Privacy Officers of both the MHS Hart voor Brabant and Elisabeth-Tweesteden Hospital for ethical review. As a result, patients < 18 years were excluded as they cannot consent/object the use of their personal data without their parents, making this group extra vulnerable.
From June to mid-August 2020, only persons with COVID-19 related symptoms could be tested. After this period, SARS-CoV-2 testing became also available for travellers from high incidence countries that were asymptomatic. The study population included:
-
1)
MHS patients ≥18 years living in the working area of MHS Hart voor Brabant that tested positive for SARS-CoV-2 between June 1 and September 25, 2020. The samples were taken in a MHS test facility and were then sent for diagnosis and tested positive for SARS-CoV-2 at Microvida Laboratory for Medical Microbiology and Immunology, at the Elisabeth-Tweesteden Hospital in Tilburg, The Netherlands.
-
2)
Clinical patients ≥18 years living in the working area of MHS Hart voor Brabant, that were hospitalized at the Elisabeth-Tweesteden Hospital and tested positive for SARS-CoV-2 between June 1 and September 25, 2020. The samples were taken in the hospital and were then sent for diagnosis and tested positive for SARS-CoV-2 at Microvida Laboratory for Medical Microbiology and Immunology, at the Elisabeth-Tweesteden Hospital in Tilburg, The Netherlands.
In case a patient tested positive twice within eight weeks, the second test was excluded as this was considered the same episode and in this case the MHS performs source and contact tracing only the first time tested positive.
2.2. Viral load analysis method
Nasopharyngeal/throat samples were taken according to national guidelines for SARS-CoV-2 PCR testing. Two separate swabs (one nasopharyngeal and one throat) were collected into one virus transport medium and tested as one sample from MHS patients. One single swab was used to take a throat sample followed by taking the nasopharyngeal sample with the same swab and thereafter collected into virus transport medium from clinical patients. Therefore all PCR results are as detected from one combined nasopharyngeal/throat sample for all patients.
SARS-CoV-2 detection was performed in two ways: 1) Viral RNA was extracted using the QIAsymphony DSP virus/pathogen midi kit and pathogen complex 400 protocol of the QIAsymphony according to manufacturer's instructions (Qiagen, Hilden, Germany). RT-PCR was performed with primers and probes targeting the Betacoronavirus E-gene [11]. 2) Alternatively, SARS-CoV-2 RNA was extracted and detected using the dual target assay for the SARS-CoV-2 RdRp and N genes using the Alinity M automated system (Abbott Molecular, Des Plaines, IL, USA).
Since Ct-values obtained from different platforms are not comparable, Ct- values in both assays were transformed into copies/mL using a standard curve to obtain comparable measurements for both assays (see Supplementary methods for a detailed description).
2.3. Source and contact tracing
Source and contact tracing interviews for all patients was performed by MHS Hart voor Brabant. During these interviews indexes were asked about the places they visited (including date and time) and to list all their contacts, including time, place, distance and duration of contact. Based on this information, the MHS divided contacts in the following contact categories: category 1 (households), category 2a (contact within 1.5 m and ≥ 15 min), category 2b (contact within 1.5 m, but < 15 min) and category 3 (present in the same room but contact at a distance of > 1.5 m and < 15 min). Contacts from all categories were unmasked exposures, as during the study period face masks were not mandatory in the Netherlands. After the study period, less extensive source and contact tracing was performed due to the beginning of the second wave.
2.4. Data collection and analysis
For MHS patients, data regarding SARS-CoV-2 testing was collected from the National Registration System. First day of onset, age, gender, comorbidities, symptoms [12], [13], [14], [15], hospitalization/death and case-contact information was obtained from the MHS Patient Registration System. Data on survival/death was supplemented with information from the Electronic Patient Record system from the Elisabeth-Tweesteden Hospital.
For clinical patients, SARS-CoV-2 testing data was collected from the Laboratory Information System. Data regarding symptoms, comorbidities and hospitalization/death were collected from the Electronic Patient Record system of the Elisabeth-Tweesteden Hospital. Information on the first day of onset and case-contact information were collected from the MHS Patient Registration System.
Severe symptoms were defined as having at least fever (≥ 38 Celsius degrees) and/or shortness of breath [14], all other symptoms were defined as mild/moderate. Transmission was defined as an index having at least one PCR confirmed positive contact. The secondary attack rate was reported in percentages and calculated by dividing the number of positive tested contacts by the total number of contacts of each index.
Chi-square and Fisher's exact tests were performed to analyze differences between MHS and clinical patients. Non-parametric tests were used to investigate the correlation between viral load (presented as median and interquartile range) and patient characteristics, disease severity and transmission. Kruskal Wallis and Mann Whitney U tests were used where appropriate. R Studio version 4.0.3 was used for data processing and statistical analysis. P-values < 0.05 were considered statistically significant.
3. Results
The total study population included 683 patients, of which 656 MHS patients and 27 clinical patients. Clinical patients were significantly older (median age 66 [IQR 35 – 84] vs 30 [IQR 18 – 90]), more likely to have cardiovascular disease (40.7% vs 4.3%), hypertension (33.3% vs 3.2%), diabetes mellitus (29.6% vs 2.4%), immunocompromised (25.9% vs 2.7%), malignancy (7.4% vs 0%), obesity (33.3% vs 0.8%) and severe symptoms (Table 1 ).
Table 1.
Baseline characteristics of the study population. Bold values denote statistical significance at the p < 0.05 level.
| Total study population (n = 683) | MHS patients(n = 656) | Clinical patients(n = 27) | P-value | |
|---|---|---|---|---|
| Median age in years (range) | 32 (18 – 90) | 30 (18 – 90) | 66 (35 – 84) | < 0.0001 |
| Age in years 18–29 30–39 40–49 50–59 60–69 70–79 80+ |
319 (46.7%) 96 (14.1%) 92 (13.5%) 105 (15.4%) 36 (5.3%) 22 (3.2%) 13 (1.9%) |
319 (48.6%) 94 (14.3%) 88 (13.4%) 102 (15.5%) 30 (4.6%) 15 (2.3%) 8 (1.2%) |
0 (0.0%) 2 (7.4%) 4 (14.8%) 3 (8.1%) 6 (22.2%) 7 (25.9%) 5 (18.5%) |
< 0.0001 0.40 0.77 0.78 < 0.0001 < 0.0001 < 0.0001 |
| Gender Male Female Not specified |
351 (51.4%) 331 (48.5%) 1 (0.2%) |
337 (51.4%) 318 (48.5%) 1 (0.2%) |
14 (51.9%) 13 (48.1%) 0 (0.0%) |
> 0.99 |
| Hospitalised a Yes No Unknown |
32 (4.7%) 591 (86.5%) 60 (8.8%) |
5 (0.8%) b 591 (90.1%) 60 (9.1%) |
27 (100.0%) 0 (0.0%) 0 (0.0%) |
< 0.0001 |
| Death a Yes No Unknown |
8 (1.2%) 502 (73.5%) 173 (25.3%) |
1 (0.2%) 482 (0.5%) 173 (99.4%) |
7 (25.9%) 20 (74.1%) 0 (0.0%) |
< 0.0001 |
| Comorbid conditions Lung disease Cardiovascular disease Hypertension Immunocompromised Diabetes mellitus Neurological or neuromuscular disease Obese (BMI ≥ 30) Kidney disease Pregnancy Dementia or Alzheimer Liver disease Malignancy |
47 (6.9%) 39 (5.7%) 30 (4.4%) 25 (3.7%) 24 (3.5%) 19 (2.8%) 14 (2.0%) 6 (0.9%) 5 (0.7%) 4 (0.6%) 3 (0.4%) 2 (0.3%) |
43 (6.6%) 28 (4.3%) 21 (3.2%) 18 (2.7%) 16 (2.4%) 16 (2.4%) 5 (0.8%) 5 (0.8%) 5 (0.8%) 3 (0.5%) 2 (0.3%) 0 (0.0%) |
4 (14.8%) 11 (40.7%) 9 (33.3%) 7 (25.9%) 8 (29.6%) 3 (11.1%) 9 (33.3%) 1 (3.7%) 0 (0.0%) 1 (3.7%) 1 (3.7%) 2 (7.4%) |
0.20 < 0.0001 < 0.0001 < 0.0001 < 0.0001 0.048 < 0.0001 0.25 > 0.99 0.19 0.16 0.002 |
| Number of symptoms 0 (asymptomatic) 1 2 3 4 5 6 7 8 9 10 11 12 13 Unknown |
14 (2.0%) 60 (8.8%) 138 (20.2%) 144 (21.1%) 147 (21.5%) 79 (11.6%) 30 (4.4%) 24 (3.5%) 10 (1.5%) 7 (1.0%) 6 (0.9%) 3 (0.4%) 2 (0.3%) 3 (0.4%) 16 (2.3%) |
14 (2.1%) 60 (9.1%) 137 (20.9%) 144 (22.0%) 143 (21.8%) 75 (11.4%) 28 (4.3%) 20 (3.0%) 9 (1.4%) 3 (0.5%) 3 (0.5%) 1 (0.2%) 1 (0.2%) 2 (0.3%) 16 (2.4%) |
0 (0.0%) 0 (0.0%) 1 (3.7%) 0 (0.0%) 4 (14.8%) 4 (14.8%) 2 (7.4%) 4 (14.8%) 1 (3.7%) 4 (14.8%) 3 (11.1%) 2 (7.4%) 1 (3.7%) 1 (3.7%) 0 (0.0%) |
0.0004 |
| Symptoms Nose cold Coughing Throat complaints Fever (≥ 38 Celsius) Headache Loss of taste Loss of smell Sore muscles Fatigue Shortness of breath Sneezing Elevated temperature (up to 38 Celsius) Diarrhoea Malaise Nausea Loss of appetite Chest pain Chills Dizziness Stomach pain Eye complaints Backache Other c |
342 (50.1%) 307 (44.9%) 219 (32.1%) 192 (28.1%) 191 (28.0%) 174 (25.5%) 168 (24.6%) 156 (22.8%) 133 (19.5%) 126 (18.4%) 65 (9.5%) 43 (6.3%) 39 (5.7%) 37 (5.4%) 30 (4.4%) 30 (4.4%) 29 (4.2%) 25 (3.7%) 23 (3.4%) 20 (2.9%) 13 (1.9%) 9 (1.3%) 18 (2.6%) |
338 (51.5%) 286 (43.6%) 219 (33.4%) 167 (25.5%) 186 (28.4%) 170 (25.9%) 163 (24.8%) 149 (22.7%) 122 (18.6%) 107 (16.3%) 63 (9.6%) 42 (6.4%) 28 (4.3%) 17 (2.6%) 23 (3.5%) 13 (2.0%) 20 (3.0%) 16 (2.4%) 19 (2.9%) 14 (2.1%) 13 (2.0%) 8 (1.2%) 8 (1.2%) |
4 (14.8%) 21 (77.8%) 0 (0.0%) 25 (92.6%) 5 (18.5%) 4 (14.8%) 5 (18.5%) 7 (25.9%) 11 (40.7%) 19 (70.4%) 2 (7.4%) 1 (3.7%) 11 (40.8%) 20 (74.1%) 7 (25.9%) 17 (63.0%) 9 (33.3%) 9 (33.3%) 4 (14.8%) 6 (22.2%) 0 (0.0%) 1 (3.7%) 10 (37.0%) |
0.0002 0.002 < 0.0001 < 0.0001 0.31 0.25 0.47 0.62 0.013 < 0.0001 > 0.99 0.78 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 0.016 < 0.0001 > 0.99 0.43 < 0.0001 |
MHS: Municipal Health Service; BMI: Body Mass Index.
Hospitalisation and death are not notifiable and may be underestimated.
Three patients were hospitalised in the Elisabeth-Tweesteden-Hospital, of which only the first positive test was included (MHS group).
Throwing up, confusion, palpitations, skin abnormalities.
3.1. Test delay
One patient was excluded due to missing viral load data, leaving 682 patients for further analysis. The median viral load was 2.51 log10 copies/mL (IQR −1.52 – 6.46) for clinical patients compared to 4.92 log10 copies/mL (IQR −0.54 – 8.26) for MHS patients, p < 0.0001 (Supplementary Figure 1).
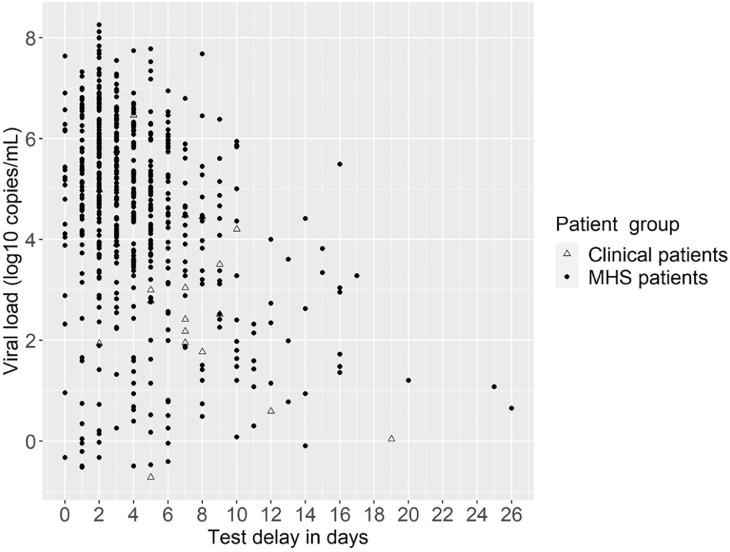
The test delay, i.e. the time between first day of onset and testing date, could be calculated for 653/682 patients, as 19 patients were asymptomatic on the testing date and for ten patients the day of onset was unknown. The median test delay was 3 (IQR 0 – 26) days for MHS patients and 7 (IQR 2 – 19) days for clinical patients, p < 0.0001. In general, higher viral loads were observed for patients with less test delay, and this decreases as the test delay increases (Fig. 1 ).
Fig. 1.
Test delay and associated viral load (log10 copies/mL) for clinical and Municipal Health Service (MHS) patients.
3.2. Disease severity
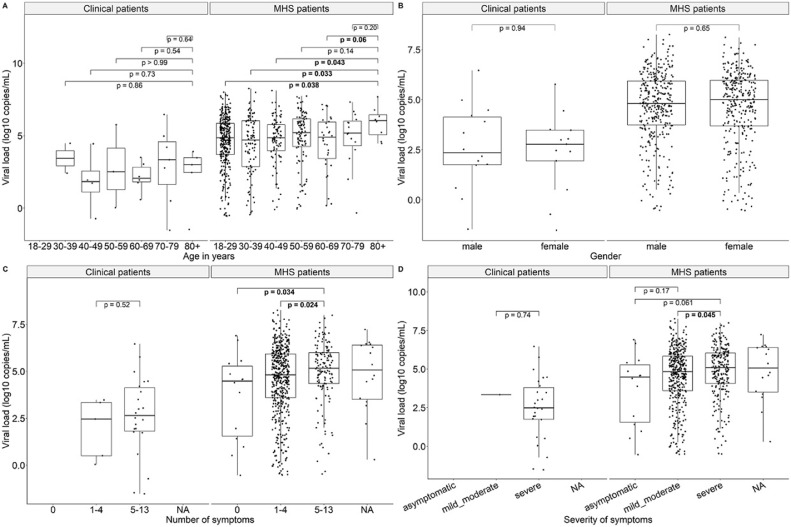
MHS patients aged 80+ showed significantly higher viral loads compared to younger age groups (except 70–79 and 50–59 years), but this was not observed for clinical patients. Significantly higher viral loads were also observed among MHS patients with ≥ 5 symptoms compared to MHS patients with less/no symptoms; this was in line with observations in the clinical group, although not statistically significant. MHS patients with severe symptoms also had significantly higher viral loads compared to those with mild/moderate symptoms; all clinical patients (except one) had severe symptoms. No significant differences in viral loads were observed between males/females in both MHS and clinical patients (Fig. 2 ).
Fig. 2.
Correlation between viral load (log10 copies/mL) and patient characteristics and disease course. MHS: Municipal Health Service. Bold values denote statistical significance at the p < 0.05 level.
3.3. Transmission
In total, 592/682 (86.9%) patients had at least one contact and could be included in the transmission analysis. MHS patients had on average more contacts compared to clinical patients: 5.4 (range 1 – 41) vs 3.4 (range 1 – 10). The majority of contacts were category 1 or category 2a contacts (Table 2 ).
Table 2.
Indexes’ number and type of contact.
| Total (n = 592) | MHS patients (n = 575) | Clinical patients (n = 17) | |
|---|---|---|---|
| Mean number of contacts (range) | 5.4 (1 – 41) | 5.4 (1 – 41) | 3.4 (1 – 10) |
| Number of contacts 1 2 3 4 5 6 7 8 9 10 > 10 |
94 (15.9%) 91 (15.4%) 99 (16.7%) 69 (11.7%) 49 (8.3%) 25 (4.2%) 35 (5.9%) 29 (4.9%) 20 (3.4%) 19 (3.2%) 62 (10.5%) |
90 (15.7%) 88 (15.3%) 96 (16.7%) 67 (11.6%) 45 (7.8%) 25 (4.3%) 35 (6.1%) 29 (5.0%) 20 (3.5%) 18 (3.1%) 62 (10.8%) |
4 (23.5%) 3 (17.6%) 3 (17.6%) 2 (11.8%) 4 (23.5%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 1 (5.9%) 0 (0.0%) |
| Type of contact Category 1 (household) Category 2a (< 1.5 m and ≥ 15 min) Category 2b (< 1.5 m, but < 15 min) Category 3 (> 1.5 m and < 15 min) Category unknown |
1115 (35.0%) 1811 (56.9%) 79 (2.5%) 33 (1.0%) 145 (4.6%) |
1097 (35.1%) 1779 (56.9%) 79 (2.5%) 32 (1.0%) 139 (4.4%) |
18 (31.6%) 32 (56.1%) 0 (0.0%) 1 (1.8%) 6 (10.5%) |
MHS: Municipal Health Service.
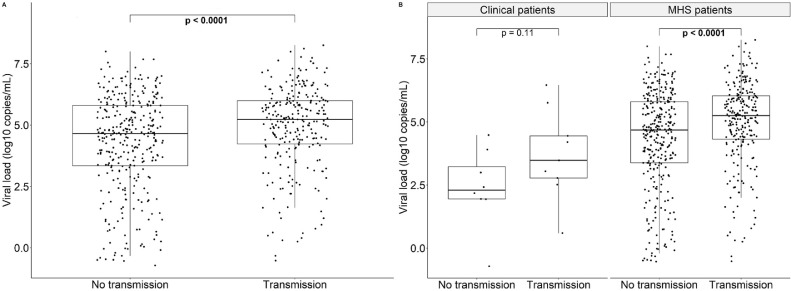
In the total study population, 257/592 (43.4%) indexes transmitted SARS-CoV-2 to at least one contact. The transmission group had > 3 times higher viral loads than the non-transmission group: viral load log10 copies/mL 5.23 (IQR −0.52 – 8.26) vs 4.65 (IQR −0.72 – 8.00), p < 0.0001 (Fig. 3 A). This was in line with observations among MHS patients: 248/575 (43.1%) indexes transmitted SARS-CoV-2 to at least one contact, of which transmitters showed > 3 times higher viral loads (5.25 log10 copies/mL [IQR −0.52 – 8.26] vs 4.68 log10 copies/mL [IQR −0.54 – 8.00], p < 0.0001). Two transmitters were asymptomatic (5.56 and 6.66 log10 copies/mL), while the remaining were symptomatic or symptom information was unknown. Among clinical patients, 9/17 (52.9%) indexes transmitted SARS-CoV-2, of which transmitters (all symptomatic) showed > 14 times higher viral loads, although not statistically significant: 3.48 log10 copies/mL (IQR 0.59 – 6.46) vs 2.30 log10 copies/mL (IQR −0.72 – 4.48), p = 0.11 (Fig. 3B).
Fig. 3.
Viral load (log10 copies/mL) of indexes that transmitted SARS-CoV-2 and indexes that did not among the total study population (A) and the clinical versus Municipal Health Service (MHS) patients (B). Bold values denote statistical significance at the p < 0.05 level.
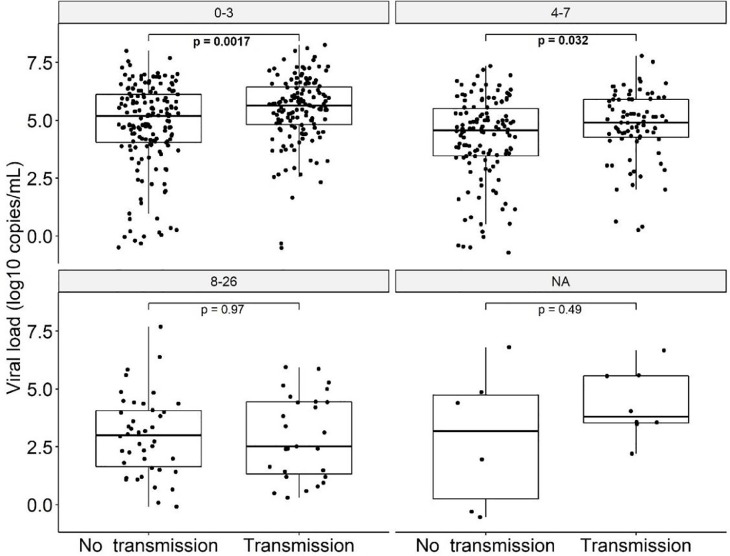
When dividing the transmission and non-transmission group by test delay categories (0–3 vs 4–7 vs 8–26 days), the transmission group showed higher viral loads only for the categories 0–3 days (p = 0.0017) and 4–7 days (p = 0.032), see Fig. 4 .
Fig. 4.
Viral load (log10 copies/mL) of indexes that transmitted SARS-CoV-2 and indexes that did not, presented by test delay categories (0–3 days, 4–7 days, > 7 days). Bold values denote statistical significance at the p < 0.05 level.
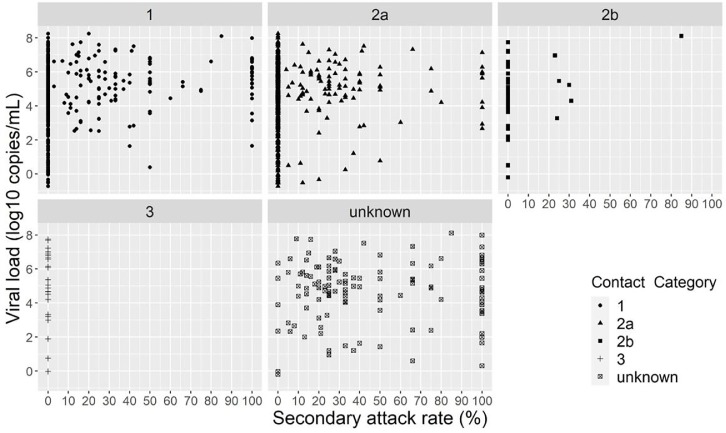
From the 437 contacts that tested positive, 150 (34.3%) were category 2a contacts, 143 (32.7%) category 1, six (1.4%) category 2b, and for 138 (31.6%) the contact category was unknown (Fig. 5 ). None of the category 3 contacts tested positive. Two category 2a contacts that tested positive had indexes with very low viral loads: −0.52 and −0.33 log10 copies/mL with associated test delay ≤ 2 days. There were 48 indexes with 100% secondary attack rate, however 35/48 had only one contact and the remaining indexes had two or three contacts. See Supplementary Table 1 for the secondary attack rate for each index with associated viral load and number of (positive) contacts.
Fig. 5.
Viral load (log10 copies/mL) by secondary attack rate and contact category.
4. Discussion
In the present study we found that persons who transmitted SARS-CoV-2 had > 3 times higher viral loads compared to patients that did not transmit the virus, but not for those with a test delay of > 7 days. Higher viral loads were also significantly correlated with older age and with having more (severe) COVID-19 related symptoms.
As previous studies showed that higher viral loads increase the risk for hospitalization/death [[6], [7], [8],16], it was unexpected that our study showed lower viral loads for clinical patients. However, we also showed greater test delays for this group, which might explain the lower viral loads as they are shown to decline rapidly within one week [1,2]. Also, information bias might have occurred among clinical patients when reporting their first day of onset, as they might be less likely to complain or remember when mild symptoms occurred due to having (severe) underlying conditions, meaning the test delay could in fact be even higher than reported. Another explanation could be the difference in sample collection between clinical patients (one swab was used for sampling throat and nasopharynx) and MHS patients (two separate swabs were used to take a throat and nasopharyngeal sample respectively, and tested as one combined sample). So although slightly different sampling methods were applied, eventually combined nasopharyngeal/throat samples were used for all included patients and therefore we are confident that the quality of specimens is also equally distributed between all patients.
A limitation of our study is that it solely includes SARS-CoV-2 samples of the wild type strain, as variants were not yet detected in the Netherlands [17]. Patients infected with the Alpha variant showed up to 10x higher viral loads and a longer duration of the persistence of SARS-CoV-2 RNA in the respiratory tract [8,18]. The Delta variant also showed 10x higher viral loads than the wild type and 2x higher viral loads compared to Alpha and Beta variants [19]. In addition, strains of the P.1 (Gamma) variant had higher viral loads and 1.7–2.4x more transmissibility [20].
It takes the MHS 8–12 h to perform extensive source and contact tracing for one index. During peak periods, viral load and test delay information can be helpful to prioritize which indexes are more likely to transmit the virus. For example, if an index has a test delay of 0–3 days, the MHS can decide to perform extensive source and contact tracing solely on indexes with viral loads that belong to the upper 50% or 25% of the transmission group. The MHS can alert on the greater risk of transmission among this group and can emphasize on the importance of adherence of isolation (and quarantine for their contacts). MHSs can also decide to not perform source and contact tracing on indexes with a test delay >7 days, as after this point the risk of transmission is very low [1,2,21]. Even without the need to prioritize, viral load data can be used to focus on high-risk groups and their adherence to isolation measures. Due to the low sample size of clinical patients and subsequently insufficient power for statistical significance, it is difficult to describe whether a similar strategy can be applied for clinical patients. Despite this, higher viral loads were observed for those with more symptoms and those that transmitted SARS-CoV-2, which is in line with observations among MHS patients.
In conclusion, our study shows that indexes that transmitted SARS-CoV-2 had viral loads that were > 3 times higher than indexes that did not cause secondary infections. Source and contact tracing can be prioritized on patients with a test delay up to seven days, and within this group further prioritization can be done based on patients viral load data. These results are based on the wild type strain and additional research is needed to analyze whether the same viral load threshold applies to SARS-CoV-2 variants. If sampling methods and viral load quantification are standardized, viral loads could be used to prioritize source and contact tracing on an (inter)national level.
CRediT authorship contribution statement
R. Jajou: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Project administration. AJG Mutsaers- van Oudheusden: Conceptualization, Investigation, Writing – review & editing. J.J. Verweij: Conceptualization, Resources, Investigation, Writing – review & editing. A. Rietveld: Conceptualization, Writing – review & editing. J.L. Murk: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
Acknowledgments
We would like to thank Ines Figaroa and Jenske Leendertse for helping with the data collection of the MHS patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105131.
Appendix. Supplementary materials
References
- 1.Meyerowitz E.A., Richterman A., Gandhi R.T., Sax P.E. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann. Intern. Med. 2021;174:69–79. doi: 10.7326/M20-5008. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Kampen J.J.A., van de Vijver D.A.M.C., Fraaij P.L.A., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat. Commun. 2021;12:267. doi: 10.1038/s41467-020-20568-4. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmans B.J.M., Reusken C.B.E.M., van Oudheusden A.J.G., et al. Test, trace, isolate: evidence for declining SARS-CoV-2 PCR sensitivity in a clinical cohort. Diagn. Microbiol. Infect. Dis. 2021 doi: 10.1016/j.diagmicrobio.2021.115392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh K.A., Jordan K., Clyne B., et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.06701. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng S., Fan J., Yu F., Feng B., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pujadas E., Chaudhry F., McBride R., et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir. Med. 2020;8:e70. doi: 10.1016/S2213-2600(20)30354-4. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westblade L.F., Brar G., Pinheiro L.C., et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell. 2020;38:661–671. doi: 10.1016/j.ccell.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones T.C., Biele G., Mühlemann B., et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 2021;373:eabi5273. doi: 10.1126/science.abi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y.W.L., Rozmanowsk S., Pang M., et al. SARS-CoV-2 infectivity by viral load, S gene variants and demographic factors and the utility of lateral flow devices to prevent transmission. Clin. Infect. Dis. 2021:ciab421. doi: 10.1093/cid/ciab421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks M., Millat-Martinez P., Ouchi D., et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect. Dis. 2021;21:629–636. doi: 10.1016/S1473-3099(20)30985-3. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corman V.M., Olfert L., Marco K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.RIVM. Wekelijkse update epidemiologische situatie van SARS-CoV-2 in Nederland. 2021. Available at: https://www.rivm.nl/coronavirus-covid-19/actueel/wekelijkse-update-epidemiologische-situatie-covid-19-in-nederland. Accessed March 11, 2021.
- 13.ECDC. Risk factors and risk groups. 2021. Available at: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/risk-factors-risk-groups. Accessed March 11, 2021.
- 14.RIVM. Coronavirus disease COVID-19. 2021. Available at: https://www.rivm.nl/en/coronavirus-covid-19/coronavirus-disease-covid-19. Accessed March 11, 2021.
- 15.WHO. Coronavirus disease (COVID-19). 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19#:∼:text=symptoms. Accessed March 11, 2021.
- 16.Fajnzylber J., Regan J., Coxen K., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RIVM. Variants of the coronavirus SARS-CoV-2. 2021. Available at: https://www.rivm.nl/en/coronavirus-covid-19/virus-sars-cov-2/variants. Accessed August 5, 2021.
- 18.Calistri P., Amato A., Puglia I., et al. Infection sustained by lineage B.1.1.7 of SARS-CoV-2 is characterised by longer persistence and higher viral RNA loads in nasopharyngeal swabs. Int. J. Infect. Dis. 2021;105:753–755. doi: 10.1016/j.ijid.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teyssou E., Delagrèverie H., Visseaux B., Lambert-Niclot S., et al. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J. Infect. 2021 doi: 10.1016/j.jinf.2021.08.027. S0163-4453(21)00416-3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faria N.R., Mellan T.A., Whittaker C., et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372:815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.