Abstract
The development of new, safe, topical microbicides for intravaginal use for the prevention of sexually transmitted diseases is imperative. Previous studies have suggested that bile salts may inhibit human immunodeficiency virus infection; however, their activities against other sexually transmitted pathogens have not been reported. To further explore the potential role of bile salts in preventing sexually transmitted diseases, we examined the in vitro activities and cytotoxicities of select bile salts against Chlamydia trachomatis, herpes simplex virus (types 1 and 2), Neisseria gonorrhoeae, and human immunodeficiency virus in comparison to those of nonoxynol-9 and benzalkonium chloride using both primary cells and cell lines derived from the human female genital tract. We found that taurolithocholic acid 3-sulfate and a combination of glycocholic acid and taurolithocholic acid 3-sulfate showed excellent activity against all of the pathogens assayed. Moreover, taurolithocholic acid 3-sulfate alone or in combination was less cytotoxic than nonoxynol-9 and benzalkonium chloride. Thus, taurolithocholic acid 3-sulfate alone or in combination warrants further evaluation as a candidate topical microbicidal agent.
Novel approaches to the prevention of sexually transmitted diseases (STDs) are clearly warranted. An urgency emerges from the growing pandemic of STDs, estimated at 333 million cases of curable STDs worldwide by the World Health Organization (6, 12, 14). These diseases affect not only sexually active individuals but also newborns, who may become infected perinatally. Male condoms, in theory, remain a highly effective method for the prevention of STDs and contraception, but problems with compliance limit their efficacy. The development of topical microbicides intended for intravaginal use offers several advantages. Intravaginal formulations would enable women to make their own decisions regarding STD prophylaxis. Ideally, intravaginal microbicides would directly inactivate microbes at the portal of entry, preventing the establishment of infection. Optimally, agents that are active against multiple pathogens, that are nontoxic to genital epithelia and normal flora, that are inexpensive to manufacture, and that could thus be made available worldwide could be identified.
One class of intravaginal topical microbicides currently in use is surface-active agents. For example, the cationic quaternary ammonium compound benzalkonium chloride (BZC) is currently used in several vaginal contraceptive preparations. It has been shown to inhibit the reverse transcriptase activity of human immunodeficiency virus (HIV) (31) and to protect mice challenged with Chlamydia trachomatis (22). However, BZC has profound adverse effects on the normal vaginal microflora of pig-tailed macaques (23), and concerns about the cytotoxicity of this detergent have been raised. Similarly, the nonionic surface-active agent nonoxynol-9 (N-9), the most commonly used spermicide currently on the market, has been shown to be active in vitro against a wide array of STD pathogens. These include Neisseria gonorrhoeae, HIV, and herpes simplex virus (HSV) (5, 11, 19, 32, 33). However, N-9 is cytotoxic for most cell lines in vitro, including primary human cervical and vaginal cells (15). Importantly, frequent use of N-9 causes epithelial disruption of the cervix and vagina and may increase the risk for STDs (20, 34). In clinical studies, the effect of N-9 in preventing HIV transmission remains controversial. Improvement in the safety and efficacy of vaginal antimicrobial agents, therefore, is a priority.
Recently, a class of naturally occurring anionic surface-active agents, bile acids, has received attention as potential contraceptive agents. Specifically, cholic acid has been shown to have strong spermicidal activity and has been shown to inhibit sperm motility at a concentration of 6 mM (26). Cholic acid is one of the active ingredients in F-5 gel preparations marketed for use within a vaginal sponge (Protectaid) available in Canada (8, 26). However, concerns have been raised regarding the cytotoxicity of cholic acid. For example, Baba and colleagues (1) examined the effects of a series of cholic acid derivatives on HIV infection and found that sodium cholate (NaC) had a selectivity index of <1.0 for HIV replication in MT-2 cells (1). Similarly, Psychoyos and colleagues (26) found that NaC at a concentration of 2.5 mM (∼1 mg/ml) reduced the viability of normal peripheral blood lymphocytes by 45% (26). The effect of NaC or other bile salts on STD pathogens other than HIV and the cytotoxic effects of this class of compounds on human vaginal or cervical epithelial cells have not been reported.
Therefore, to further explore the potential role of bile salts as intravaginal topical microbicides, we compared select bile salts to N-9 and BZC for their in vitro activities against Chlamydia trachomatis, HSV type 1 (HSV-1), HSV-2, N. gonorrhoeae, and HIV. We also assayed the cellular toxicities of these compounds with primary human vaginal and cervical cells. The results obtained suggest that taurolithocholic acid-3-sulfate (NaTLC-3-SO4) alone or combined with glycocholic acid exhibits excellent activity in vitro against all of the pathogens tested with little or no cytotoxicity for human cervical epithelial cells. Thus, this bile salt, alone or in combination, may be superior to N-9 or BZC and warrants further investigation.
MATERIALS AND METHODS
Primary and permanent cell cultures.
The human cervical epithelial cell lines HeLa 229, and CaSki were purchased from the American Type Culture Collection (Rockville, Md.). HeLa cells were maintained in Eagle’s minimal essential medium containing 10% heat-inactivated fetal bovine serum (FBS), nonessential amino acids, l-glutamine, and gentamicin (50 μg/ml). CaSki cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. A 24th passage of the T-lymphocyte cell line MT-2 was obtained from the AIDS Research and Reference Reagent Program of the National Institutes of Health (Rockville, Md.). The MT-2 cells were maintained in RPMI 1640 with glutamine containing 20% heat-inactivated FBS and 50 μg of gentamicin per ml. Primary human endocervical, ectocervical, and vaginal mucosal cultures were derived from specimens obtained during hysterectomies performed for benign conditions such as uterine leiomyomas and endometriosis as described previously (15, 30). For viral infectivity and cytotoxicity studies, most samples had been subcultured one to two times.
Microbial pathogens.
The C. trachomatis strain used in this study was serotype E/UW-5/CX. The E strain of C. trachomatis is well characterized and has previously been shown to be capable of infecting explants of primate and human fallopian tubes in culture (7, 21, 24, 25). Stocks were maintained through passage in HeLa 229 cells for a minimum of nine passages, concentrated to 108 inclusion-forming units per ml, and stored at −80°C in HEPES-sucrose-calcium (HSC) buffer (3). The wild-type strain HSV-2(G) and strain HSV-1(17)(dUTPase/LAT) were used. The latter strain has been genetically engineered and expresses the β-galactosidase gene under control of the viral immediate-early gene promoter dUTPase (gift of E. Wagner, Stanford University) (29). HSV-1(17)(dUTPase/LAT) behaves like wild-type HSV-1(17) with respect to binding and infectivity (18). The N. gonorrhoeae strain used was MS11. The strain was monitored for transparency and piliation, and inocula of transparent, piliated organisms were used. The gonococci were grown on GC agar (Difco Laboratories, Detroit, Mich.) plates, and stocks were stored in 10% skim milk (Difco Laboratories) at −80°C until use. The HIV strain (G910-SI; zidovudine resistant) was obtained from the AIDS Research and Reference Reagent Program of the National Institutes of Health and is identified as a stable HIV clone which will produce syncytia in MT-2 cells. The virus was expanded by culture in MT-2 cells maintained in RPMI 1640 with glutamine containing 10% heat-inactivated FBS and 50 μg of gentamicin per ml. The final virus stock concentration was 6 × 103 PFU/ml.
Infectivity assays.
To measure the effects of the compounds on C. trachomatis, infectivity assays with HeLa cells were performed as described previously, with modifications (9). Briefly, 24-well plates were seeded at 4 × 105 to 5 × 105 cells/ml and the cells were grown to confluence. The compounds (0 to 10 mg/ml or 0 to 0.1% [vol/vol] for N-9) were diluted in HSC (pH 7.35) and were mixed with a concentration of chlamydia to yield 100 to 300 inclusions per well in untreated wells for 4 h at 4°C. Control organisms were treated with buffer containing no compounds. HeLa cells were then inoculated with chlamydia for 1 h at 37°C. The medium was removed, and the infected cells were incubated in tissue culture with Eagle’s minimal essential medium supplemented with 10% fetal calf serum, 30 μM glucose, 1 μg of cycloheximide per ml, and 50 μg of gentamicin sulfate per ml for 48 h at 37°C. After 48 h of incubation, the HeLa cells were trypsinized, pelleted by centrifugation, fixed, permeabilized, and stained for flow cytometry (Caltag Laboratories, Burlingame, Calif.). The infected cells were stained with a fluorescein-isothiocyanate-conjugated monoclonal antibody to chlamydial lipopolysaccharide protein (Kallestad). Infected cells were enumerated with a FACS Vantage flow cytometer (Becton Dickinson, San Jose, Calif.), and data from 10,000 cells were collected in the list mode for analysis with H-P-LYSYS II software.
The effects of bile salts, N-9, and BZC on N. gonorrhoeae were also determined. Various concentrations of compounds were added to GC agar and the agar was poured into plates. The GC inoculum was made by using a 0.5 McFarland standard. Agar plates were inoculated with serial dilutions of the gonococcal standard and were incubated for 24 h in a 7% CO2 atmosphere at 37°C. The plates were scored for colony growth, and counts from plates containing compound were compared to those from plates containing no compound. A percentage of gonococcal viability was determined.
For studies with HSV-2, plaque assays were conducted (15, 18). Nearly confluent primary human cervical or vaginal cells in 24-well plates or confluent CaSki cells in 25-cm2 flasks were inoculated with virus (50 to 100 PFU/well or 200 to 500 PFU/flask) in phosphate-buffered saline (PBS) in the presence or absence of bile salts (0 to 5 mg/ml). Plaques were counted after 1 day (primary cells) or 3 days (CaSki cells). To visualize the plaques, an immunoassay was performed as described previously with monoclonal antibody 1103 (anti-HSV-1 and HSV-2 gD; Goodwin Institute) (16, 17). For studies with HSV-1, viral infectivity assays were conducted as described previously (15, 18). Briefly, primary cells in 96-well dishes were inoculated with HSV-1(17)(dUTPase/LAT) at a multiplicity of infection of 5 PFU/cell in the absence or presence of bile salts or detergent. After a 5-h period at 37°C, the β-galactosidase expression from infected cells was quantified. To determine if the bile salts were virucidal or cytotoxic, plaque assays were modified. For virucidal activity, HSV-2 at a concentration of about 109 PFU/ml was mixed with various concentrations of bile salt or with PBS as a control. After incubation for 1 h at 37°C, the mixtures were diluted to yield 100 to 500 PFU/flask for controls, with a final concentration of bile salt of 10−6 to 10−7 mg/ml. CaSki cells were inoculated with the mixture to determine whether the compound had irreversible effects on viral infectivity. For cytotoxic activity, the cells were incubated in the presence of bile salts at various concentrations or PBS, as a control, for 1 h at 37°C and were then washed extensively. The cells were then inoculated with virus to determine whether the compounds were cytotoxic, resulting in an inability to support viral infection.
For studies with HIV, MT-2 cells were infected with a syncytium-inducing strain of HIV (G910-SI; zidovudine resistant) in the presence or absence of bile salts with a viral inoculum that produced approximately 30 to 50 syncytia/well by day 3 of incubation. MT-2 cells (5.0 × 105) were aliquoted into each test well, and the wells were inoculated with virus in 100 μl of medium containing the appropriate concentration of bile salt to be assayed (0 to 1 mg/ml). Cultures were incubated in a humidified 37°C incubator containing 5% CO2. The cultures were examined daily. The number of syncytia in each well was quantified on day 3. Each drug concentration was tested in quadruplicate.
Cytotoxicity assays.
The cytotoxic effects of the bile salts on both primary cervical cells and CaSki cells were determined by quantitating cell viability through the uptake of neutral red dye as described previously (2, 4, 10, 27, 28) or by quantitating the inhibition of host cell DNA synthesis. For quantitation of DNA synthesis, cells were grown directly in glass scintillation vials to half confluence and were then incubated overnight in medium containing serial dilutions of test compounds and 2.5 μCi of [3H]thymidine per ml. The cells were washed extensively, and the cell-associated radioactivity was quantitated. HeLa cell viability was determined with propidium iodide (PI) by flow cytometry analysis as described previously, with modifications (10). The HeLa cells were exposed to compounds or PBS, as a control, for 20 to 24 h. Cells were trypsinized and incubated with 5 μg of PI per ml in PBS for 5 min at room temperature prior to fluorescence-activated cell sorter analysis. The positive controls were cells permeabilized to damage the cell membrane and allow the DNA to be stained with PI.
Bile salt compounds and detergents.
Sodium salts of glycocholic acid (NaGC), NaTLC-3-SO4, taurocholic acid (NaTC), and cholic acid were purchased from Sigma; BZC and N-9 (Tergitol, NP-9) were also purchased from Sigma. All bile salts and detergents were dissolved in PBS to a stock concentration of 10 mg/ml (1% [vol/vol] for N-9) prior to use.
Statistical analysis.
The data presented are means with standard deviations, as indicated in the figure legends. Student unpaired, two-tailed t tests were performed as indicated.
RESULTS
Effects of bile salts on STD pathogens.
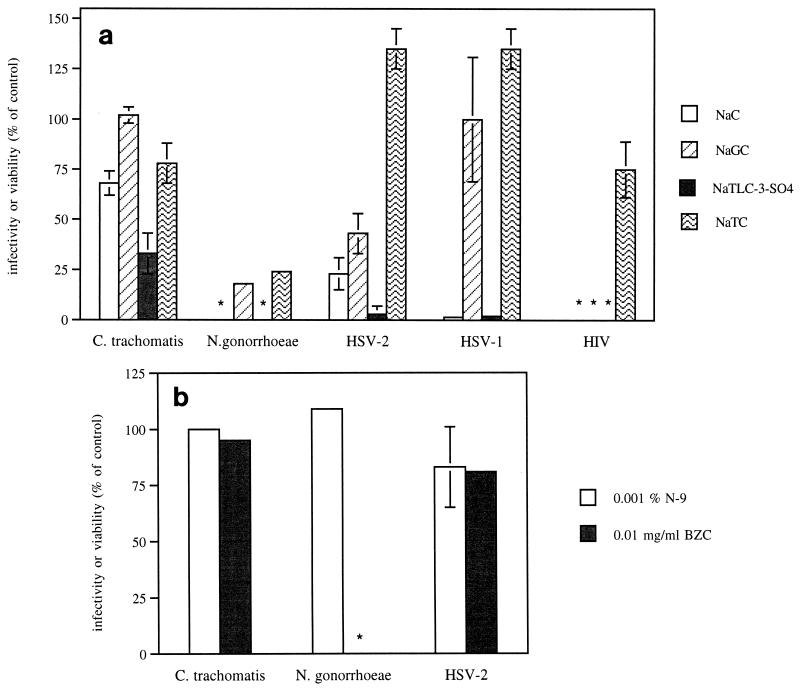
On the basis of pilot studies, we focused on four bile salts as potential topical microbicides (NaC, NaTC, NaTLC-3-SO4, and NaGC) and compared their in vitro activities to those of N-9 and BZC. We first compared their activities against C. trachomatis. Chlamydia were pretreated with various concentrations of the bile salts, BZC, or N-9 for 4 h prior to inoculation onto HeLa cell monolayers. At a concentration of 1 mg/ml, NaTLC-3-SO4 inhibited chlamydial infection by 67% ± 10% (P = 0.0001) (Fig. 1a). None of the other bile salts exhibited significant antimicrobial activity. Notably, neither N-9 nor BZC at concentrations as high as 0.01% or 0.01 mg/ml, respectively, inhibited chlamydial infection, and higher concentrations of either detergent disrupted the HeLa cell monolayer, resulting in a selectivity index of ≤1 for either detergent (Fig. 1b and Table 1).
FIG. 1.
Effects of bile salts at a concentration of 1.0 mg/ml (a), N-9 at a concentration of 0.001% (b), or BZC at a concentration of 0.01 mg/ml (b) against microbial infection. For chlamydia, the results are presented as the number of inclusions formed in the presence of compound as a percentage of the number of inclusions formed in the absence of compound. Each point is the mean of values obtained from at least two independent experiments performed in duplicate. For gonococci, the results are presented as the number of viable gonococci found in the presence of compound as a percentage of the number of gonococci found in the absence of compound. Each point is the mean of values obtained from two independent experiments performed in duplicate. No viable gonococci were observed on the plates containing 0.01 mg of BZC per ml or 1 mg NaC or NaTLC-3-SO4 (∗) per ml. For HSV-2, results are presented as the number of PFU per well in the presence of compound as a percentage of the number of PFU per well in the absence of compound. For HSV-1, results are presented as β-galactosidase expression (absorbance at 410 nm) in the presence of compound as a percentage of β-galactosidase expression in the absence of compound. Each point is the mean of three to five experiments performed in duplicate with primary cells obtained from different patients. For HIV-1, results are presented as syncytia observed on day 3 of infection in the presence of compound as a percentage of syncytia formed in the absence of compound. Each point is the mean of two experiments performed in quadruplicate. ∗∗∗, no syncytia were observed after NaC, NaTLC-3-SO4, or NaGC treatment at concentrations as low as 0.1 mg/ml. The error bars indicate standard deviations.
TABLE 1.
Antimicrobial and cytotoxic effects of select bile salts and detergents
| Bile salt or detergent |
C. trachomatis, HeLa cells
|
HSV-2, primary cervical cells
|
HIV, MT-2 cells
|
ED50 for N. gonorrhoeae | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ED50a | CD50b | SI50c | ED50 | CD50 | SI50 | ED50 | CD50 | SI50 | ||
| NaC | >1 | 1.5 | NDd | 0.3 | 0.2 | 0.67 | 0.01 | 0.009 | 0.9 | 0.1 |
| NaGC | >1 | >5 | ND | 0.8 | 4 | 5 | 0.01 | 0.007 | 0.7 | 0.5 |
| NaTLC-3-SO4 | 0.4 | 3.5 | 8.75 | 0.2 | 0.5 | 2.5 | 0.02 | >0.1 | >5 | 0.3 |
| NaTC | >1 | >5 | ND | >1 | 3 | ND | >0.1 | >0.1 | ND | 0.4 |
| N-9 | 0.03% | 0.005% | 0.17 | 0.003% | <0.001% | <0.3 | NEe | NE | NE | 0.02% |
| BZC | 0.03 | 0.03 | 1 | 0.06 | 0.02 | 0.33 | NE | NE | NE | <0.01 |
ED50, the dose of bile salt or detergent that inhibited 50% of chlamydial infection of HeLa cells, HSV-2 infection of primary cervical cells, HIV-1 infection of MT-2 cells, or gonococcal viability was determined from a dose-response curve generated from two to three independent experiments conducted at least in duplicate. Doses are in milligrams per milliliter for all compounds unless indicated otherwise.
CD50, the dose of bile salt or detergent that killed 50% of HeLa cells as measured with PI by flow cytometry analysis, primary cervical cells as measured by neutral red dye uptake, or MT-2 cells as measured by trypan blue exclusion. Doses are in milligrams per milliliter for all compounds unless indicated otherwise.
SI50 (selectivity index), ratio of CD50 to ED50.
ND, not determined.
NE, not evaluated.
To examine the microbicidal activities of the bile salts and detergents against N. gonorrhoeae, another major bacterial STD, GC agar cultures were incubated in the presence or absence of various concentrations of compounds, and gonococcal viability was determined. NaTLC-3-SO4 and NaC were the most effective bile salts and at concentrations of 1 mg/ml completely inhibited gonococcal growth (Fig. 1a). Similarly, BZC at 0.01 mg/ml completely inhibited gonococcal growth (Fig. 1b). In contrast, even at cytotoxic concentrations the detergent N-9 only modestly inhibited gonococci (Table 1).
Next, we compared the activities of the same series of compounds against HSV-2 and HSV-1 infection. Virus was mixed with bile salt or detergent immediately prior to inoculating primary or permanent human cervical cells. NaTLC-3-SO4 inhibited 98% ± 1% of HSV-2 infections as measured by the quantitation of plaque formation and 96% ± 4% of HSV-1 infections as measured by quantitation of β-galactosidase expression (P < 0.001; Fig. 1a). NaC also inhibited 77% ± 8% of HSV-2 and 98% ± 2% of HSV-1 infections. NaTC had little or no inhibitory activity against either serotype, and NaGC inhibited HSV-2 plaque formation only by about 50%. Similar results were obtained with permanent cervical cell lines. At a dose of 1 mg/ml, NaTLC-3-SO4 completely inhibited HSV-2 infections and inhibited 98% ± 1% of HSV-1 infections in CaSki cells (data not shown). In contrast, neither N-9 nor BZC inhibited HSV-2 plaque formation at concentrations that did not appear to be cytotoxic for primary cells (0.001% and 0.01 mg/ml, respectively; Fig. 1b). Higher concentrations of either detergent disrupted the cellular monolayer.
The observation that bile salts are surface active and inhibit HSV early gene expression and plaque formation in parallel suggests that they may be virucidal and may directly inactivate HSV. To further explore this, we compared the virucidal and cytotoxic effects of bile salts as described in Materials and Methods. For virucidal assays, HSV-2(G) at a concentration of about 109 PFU/ml was mixed with the bile salts at 1 mg/ml, and the mixture was incubated for 1 h at 37°C. The virus-bile salt mixture was then diluted to yield 102 to 103 PFU/ml and a final concentration of bile salt of 10−6 to 10−7 mg/ml and was plated onto plaque dishes. Conversely, for cytotoxicity assays, CaSki cells were incubated with bile salts at 1 mg/ml for 1 h at 37°C and were then extensively washed prior to inoculation of the cells with virus. The results are presented in Table 2. The results suggest that following a 1-h exposure at a concentration of 1 mg/ml, the bile salts are irreversibly virucidal but not cytotoxic.
TABLE 2.
HSV-2 plaque formation following preincubation of virus or cells with bile salts
| Compound (1 mg/ml) | PFU/dish (% of control)a
|
||
|---|---|---|---|
| Virusb | Cellsc | Virus-cellsd | |
| NaC | 20 ± 13 | 74 ± 21 | 3 ± 4 |
| NaGC | 59 ± 0.7 | 92 ± 13 | 60 ± 1.4 |
| NaTLC-3-SO4 | 0e | 96 | 0e |
For all three experimental methods, the results are presented as the number of PFU in the presence of compound as a percentage of the number of PFU in the presence of PBS. Each of the experiments was performed in duplicate with two different dilutions of virus.
HSV-2(G) at a concentration of about 109 PFU/ml was mixed with 1 mg of the indicated bile salt per ml or PBS as a control. After incubation for 1 h at 37°C, the mixtures were diluted 6 to 7 logs to yield noninhibitory levels of bile salt and 100 to 500 PFU/flask on control flasks and were plated on CaSki cells to determine whether the compound had irreversible effects on viral infectivity. Plaques were counted after 2 days in culture.
For the cytotoxicity assay, CaSki cells were incubated with 1 mg of the indicated bile salt per ml or PBS as a control for 1 h at 37°C and were then washed extensively prior to inoculation of the cells with virus in a standard plaque assay.
Bile salts were mixed with virus (100 to 500 PFU/ml) at a final concentration of 1 mg/ml immediately prior to inoculation of CaSki cells for a standard plaque assay.
No plaques were visualized on dishes inoculated with the same viral dilution.
Because previous studies with bile salts and HIV infection have yielded variable results, we also examined the activities of the bile salts against HIV using MT-2 cells. The results from three different experiments conducted in quadruplicate are summarized in Fig. 1a and Table 1. NaC, NaTLC-3-SO4, and NaGC at concentrations of 0.1 mg/ml completely inhibited syncytium formation, with limited inhibitory effects at lower concentrations. NaTC had little or no inhibitory effect.
Cytotoxicities of bile salts.
To further assess the potential role of bile salts as topical microbicides, we examined their cytotoxicities using several different cell lines (HeLa, CaSki, or primary human cervical cells) and several different types of assays (neutral red dye uptake, PI uptake, or inhibition of host cellular DNA synthesis). The results are summarized in Tables 1 and 3. Several observations are noteworthy. First, although the trends were parallel, cytotoxicity was dependent on both cell type and assay conditions. For example, bile salts and detergents exhibited more cytotoxicity for primary human cervical cells than for permanent cell lines. Moreover, cytotoxicity became more evident when quantitation of cellular DNA synthesis was compared to cell viability. For example, none of the bile salts would be considered cytotoxic if one measured cell viability with CaSki cells, but some cytotoxicity was evident when cellular DNA synthesis was quantitated.
TABLE 3.
Cytotoxicity following exposure to bile salts or detergents
| Bile salt or detergent (concn) | Viability (% of control)
|
||||
|---|---|---|---|---|---|
| HeLa cells, PIa | CaSki cells, neutral red uptakea | CaSki cells, DNA synthesisb | Primary cells, neutral red uptakea | Primary cells, DNA synthesisb | |
| NaC (1 mg/ml) | 95 ± 1.2 | 84 ± 7 | 28 ± 1 | 35 ± 10 | 22 ± 4 |
| NaTLC-3-SO4 (1 mg/ml) | 98 ± 0.4 | 84 ± 33 | 41 ± 2 | 32 ± 19 | 50 ± 2 |
| NaGC (1 mg/ml) | 99 ± 0.2 | 120 ± 3 | 95 ± 5 | 95 ± 19 | 90 ± 39 |
| BZC (0.1 mg/ml) | 3 ± 2 | 22 ± 19 | 5 ± 6 | 12 ± 10 | 2 ± 0.1 |
| N-9 (0.01%) | 29 ± 1 | 33 ± 5 | 5 ± 0 | 13 ± 2 | 2 ± 0.3 |
Cells were exposed to bile salts, BZC, or N-9 at the indicated concentrations overnight, and cell viability was determined with PI by flow cytometry for HeLa cells or neutral red dye uptake for CaSki and primary cervical cells. Results are presented as the number of viable cells following exposure to compound as a percentage of viable cells exposed to PBS as a control. Each value is the mean ± standard deviation of two experiments performed in duplicate.
For DNA synthesis, half-confluent CaSki or primary cells were exposed to the same series of compounds at the indicated concentrations in medium containing 2.5 μCi of [3H]thymidine per ml overnight. The cells were washed extensively, and the cell-associated radioactivity was quantitated. Results are presented as cell-associated radioactivity in the presence of compound as a percentage of cell-associated radioactivity in the presence of PBS as a control. Each value is the mean ± standard deviation of two experiments performed in duplicate.
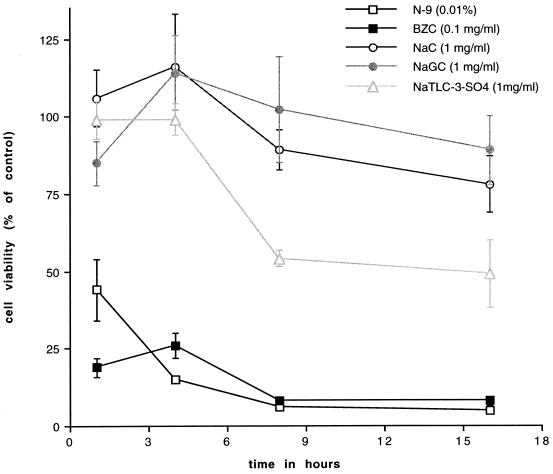
The duration of exposure to compounds also appeared to influence their cytotoxicity. To explore this further, we exposed CaSki cells to compounds at a single concentration, one that inhibited HSV infection, for increasing time periods and quantitated cell viability through the uptake of neutral red dye. The results are shown in Fig. 2. Little or no cytotoxicity was observed for up to 4 h for the bile salts; however, cytotoxicity increased following prolonged exposure. In contrast, both N-9 and BZC were cytotoxic within 1 h of exposure.
FIG. 2.
Cytotoxic effects of bile salts, BZC, or N-9 on CaSki cells following exposure for increasing lengths of time. CaSki cells were incubated in medium containing the compound at the indicated concentrations for from 1 to 16 h. At each time point, the number of viable cells was quantitated by exclusion of neutral red dye. Results are presented as the number of viable cells in the presence of compound as a percentage of the number of viable cells in the absence of compound. Each point is the mean of two experiments performed in duplicate. The error bars indicate standard deviations.
Combinations of bile salts.
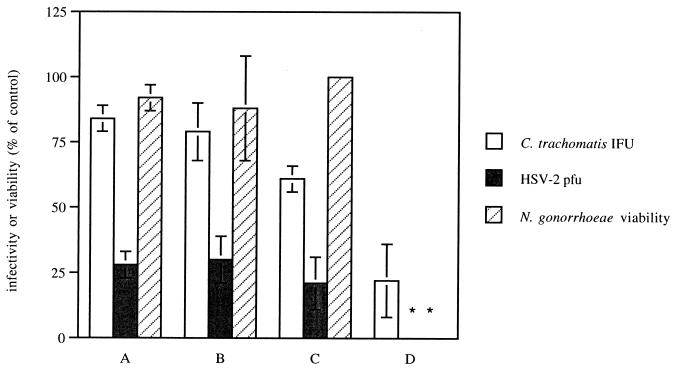
The observation that at 1 mg/ml NaTLC-3-SO4 inhibited all of the STD pathogens but exhibited some cytotoxicity for primary cells prompted us to explore the possibility that a combination of bile salts that would retain antimicrobial activity with less cytotoxicity might be identified. Therefore, we compared the antimicrobial activities of various combinations of NaC, NaGC, and NaTLC-3-SO4 and their cytotoxic effects. The results for select combinations are summarized in Fig. 3. The addition of 0.1 mg of NaC per ml, 0.1 mg of NaGC per ml, or 1.0 mg of NaGC per ml to 0.1 mg of NaTLC-3-SO4 per ml (combinations A, B, and C, respectively) markedly enhanced the anti-HSV activities of the bile salts and produced little or no cytotoxicity. However, no additive effect was observed for chlamydia or gonococci. The combination of 1.0 mg of NaGC per ml with 1.0 mg of NaTLC-3-SO4 per ml (combination D) completely inhibited HSV infection and gonococcal viability and markedly reduced chlamydial infectivity. This combination was not cytotoxic for HeLa cells; 95% ± 0.1% of HeLa cells were viable following a 24-h exposure to the bile salt combination, as determined with PI by flow cytometry. However, this combination did inhibit cellular DNA synthesis to 53% ± 25% of that for the controls following 16 h of exposure for primary human cervical cells. Notably, the concentrations of bile salts in this combination are well above the 50% effective dose for HIV infection of MT-2 cells. Thus, either alone or in combination, NaTLC-3-SO4 appears to be superior to either N-9 or BZC because of its broad range of anti-STD activity with limited cytotoxicity.
FIG. 3.
Antimicrobial effects of combinations of bile salts. Chlamydial organisms were preincubated with 0.1 mg of NaC per ml plus 0.1 mg of NaTLC-3-SO4 per ml (A), 0.1 mg of NaGC per ml plus 0.1 mg of NaTLC-3-SO4 per ml (B), 1.0 mg of NaGC per ml plus 0.1 mg of NaTLC-3-SO4 per ml (C), or 1.0 mg of NaGC per ml plus 1.0 mg of NaTLC-3-SO4 per ml (D) prior to infection of HeLa cells. Results are presented as the number of inclusions formed in the presence of the combination as a percentage of the number of inclusions formed in the absence of compound. Each value is the mean of values obtained from at least two independent experiments performed in duplicate. For HSV-2 infection, primary human cervical cells were inoculated with ∼100 PFU of HSV-2(G) per well in the absence or presence of the indicated combination, and the number of PFU was quantitated at 24 h. Results are presented as the number of PFU formed in the presence of the combination as a percentage of the number of PFU formed in the presence of PBS as a control. Each value is the mean of values obtained from three independent experiments performed in duplicate with cells from different patients. For N. gonorrhoeae, combinations of bile salts were added to GC agar; and the mixture was poured into plates, inoculated with serial dilutions of the gonococcal standard, and incubated for 24 h. Results are means of two experiments performed in duplicate. The error bars indicate standard deviations. IFU, inclusion-forming units.
DISCUSSION
The studies described here were designed to explore the potential role of select bile salts as topical microbicides for the prevention of several STDs. We found that NaTLC-3-SO4 was highly effective against HSV-1, HSV-2, HIV, N. gonorrhoeae, and C. trachomatis. In contrast, although BZC inhibited gonococcal viability, it failed to inhibit HSV or chlamydial infection except at concentrations that were toxic and that completely disrupted the cellular monolayer. Moreover, N-9 also exhibited little or no activity except at cytotoxic concentrations against any of the pathogens assayed. Thus, in vitro, NaTLC-3-SO4 exhibits a more favorable selectivity index than N-9 or BZC for multiple STD pathogens.
The optimal in vitro assay for predicting the in vivo cytotoxicities of candidate topical microbicides is not known. The results of our studies demonstrate that primary cells tend to be more susceptible than cell lines to cytotoxic effects. Bile salts and detergents inhibit cellular DNA synthesis at concentrations lower than those required to kill cells. Presumably, this reflects the fact that any change in the cellular membrane or cellular architecture results in a reduction in cellular DNA synthesis (13). Cytotoxicity is also dependent on the duration of exposure. Many of the bile salts exhibited little or no cytotoxicity when primary or permanent cells were exposed for up to 4 h but did begin to exhibit cytotoxicity 8 to 16 h following exposure. In contrast, BZC and N-9 were cytotoxic within 1 h of exposure at the concentrations that inhibited HSV or chlamydial infection.
Brief exposure (1 h) of HSV to bile salts was sufficient to induce irreversible, virucidal effects. This suggests that the viral envelope is more susceptible than the cellular plasma membrane to the damaging effects of bile salts. Similarly, gonococcal growth is markedly reduced in the presence of bile salts at concentrations that fail to disrupt the human cervical cell membranes. This suggests that the gonococcal cell wall is also more susceptible than human cellular plasma membranes to the damaging effects of bile salts.
Although NaTLC-3-SO4 was also microbicidal for C. trachomatis at noncytotoxic concentrations, of all the pathogens assayed chlamydia were the most resistant to any of the surface-active agents. For example, neither N-9 nor BZC at noncytotoxic concentrations inhibited chlamydial infection. While NaTLC-3-SO4 was the most effective of the compounds assayed, its inhibitory effects were dependent on time of exposure. Following a 4-h incubation of elementary bodies with 1 mg of NaTLC-3-SO4 per ml, chlamydial infectivity was reduced by 67% ± 10%, whereas following a 1-h incubation, infectivity was reduced by 50% ± 4% (data not shown). Presumably, challenge of the vaginal mucosa by STD pathogens will occur continuously and simultaneously with exposure to the topical antimicrobial agent. Whether the preincubation time required in vitro will affect the efficacy of this compound in vivo remains to be determined. The differences in susceptibility between C. trachomatis and N. gonorrhoeae, the other bacterial pathogen assayed, may reflect differences between chlamydia and other gram-negative bacteria with respect to cell wall composition. For example, the chlamydial cell wall lacks peptidoglycan. The mechanism of microbicidal activity for surface-active detergents, at a molecular level, has not been determined.
Taken together, the results of these studies suggest that NaTLC-3-SO4 alone or in combination with NaGC exhibits excellent activity in vitro against multiple STD pathogens. It appears to be microbicidal and thus would directly inactivate STD microbes at their portal of entry and prevent the establishment of any infection. Moreover, NaTLC-3-SO4 alone or in combination exhibits little or no cytotoxicity for permanent cervical cells and is less cytotoxic than N-9 or BZC for primary human cervical cells. Thus, this bile salt may be superior to N-9 or BZC and warrants further investigation as a candidate topical intravaginal microbicide for the prevention of the transmission of STDs. Future studies will be directed at formulating bile salts for intravaginal use and determining their spermicidal activities and effects on normal vaginal flora. Studies with animal models are in progress.
ACKNOWLEDGMENTS
We are grateful to Ed Wagner for providing viral strain HSV-1(17)dUTPase/LAT. We thank Ching-yu Sun, Alicia Siston, and Ernest Winkfield for technical support and Larry Stanberry and George Wilbanks for advice.
This work was supported by Public Health Service grant AI37940.
REFERENCES
- 1.Baba M, Schols D, Nakashima H, Pauwels R, Parmentier G, Meijer D K D, De Clercq E. Selective activity of several cholic acid derivatives against human immunodeficiency virus replication in vitro. J Acquired Immune Defic Syndr. 1989;2:264–271. [PubMed] [Google Scholar]
- 2.Babich H, Zuckerbraun H L, Wurzburger B J, Rubin Y L, Borenfreund E, Blau L. Benzoyl peroxide cytotoxicity evaluated in vitro with the human keratinocyte cell line, RHEK-1. Toxicology. 1996;106:187–196. doi: 10.1016/0300-483x(95)03189-m. [DOI] [PubMed] [Google Scholar]
- 3.Bird B R, Forrester F T. Laboratory diagnosis of chlamydia trachomatis infections. Atlanta, Ga: U.S. Department of Health and Human Services; 1981. pp. 33–71. [Google Scholar]
- 4.Borenfreund E, Puerner J A. Cytotoxicity of metals, metal-metal and metal-chelator combinations assayed in vitro. Toxicology. 1986;39:121–134. doi: 10.1016/0300-483x(86)90130-7. [DOI] [PubMed] [Google Scholar]
- 5.Bourinbaiar A S, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9, and gossypol. Contraception. 1994;49:131–137. doi: 10.1016/0010-7824(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 6.Cohen M S. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351(Suppl. III):5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 7.Cooper M D, Rapp J, Jeffrey-Wiseman C, Barnes R C, Stephens D S. Chlamydia trachomatis infection of human fallopian tube organ cultures. J Gen Microbiol. 1990;136:1109–1115. doi: 10.1099/00221287-136-6-1109. [DOI] [PubMed] [Google Scholar]
- 8.Courtot A M, Nikas G, Gravanis A, Psychoyos A. Effects of cholic acid and “Protectaid” formulations on human sperm motility and ultrastructure. Hum Reprod. 1994;9:1999–2005. doi: 10.1093/oxfordjournals.humrep.a138382. [DOI] [PubMed] [Google Scholar]
- 9.Dessus-Babus S, Belloc F, Bebear C M, Poutiers F, Lacombe F, Bebear C, de Barbeyrac B. Antibiotic susceptibility testing for Chlamydia trachomatis using flow cytometry. Cytometry. 1998;31:37–44. [PubMed] [Google Scholar]
- 10.Diva C, Watson J V, Workman P. Multiparametric analysis of cell membrane permeability by two colour flow cytometry with complementary fluorescent probes. Cytometry. 1990;11:244–252. doi: 10.1002/cyto.990110205. [DOI] [PubMed] [Google Scholar]
- 11.Feldblum P J, Weir S S. The protective effect of nonoxynol-9 against HIV infection. Am J Public Health. 1994;8:1032–1034. doi: 10.2105/ajph.84.6.1032-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming D T, McQuillan G M, Johnson R E, Nahmias A J, Aral S O, Lee F K, St. Louis M E. Herpes simplex virus type 2 in the United States, 1976–1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 14.Gerbase A C, Rowley J T, Mertens T E. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351(Suppl. III):2–4. doi: 10.1016/s0140-6736(98)90001-0. [DOI] [PubMed] [Google Scholar]
- 15.Herold B C, Siston A, Bremer J, Kirkpatrick R, Wilbanks G, Fugedi P, Peto C, Cooper M. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob Agents Chemother. 1997;41:2776–2780. doi: 10.1128/aac.41.12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;63:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland T, Sandri-Goldin R, Holland L, Marlin L S, Levine M, Glorioso J. Physical mapping of the mutation in the antigenic variant of herpes simplex virus type 1 by use of an immunoreactive plaque assay. J Virol. 1983;46:649–652. doi: 10.1128/jvi.46.2.649-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Immergluck L C, Domowicz M S, Schwartz N B, Herold B C. Viral and cellular requirements for entry of herpes simplex virus type 1 into primary neuronal cells. J Gen Virol. 1998;79:549–559. doi: 10.1099/0022-1317-79-3-549. [DOI] [PubMed] [Google Scholar]
- 19.Jennings R, Clegg A. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in vitro. J Antimicrob Chemother. 1993;32:71–82. doi: 10.1093/jac/32.1.71. [DOI] [PubMed] [Google Scholar]
- 20.Kreiss J E, Ngugi E, Holmes K K, et al. Efficacy of nonoxynol-9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 21.Kuo C, Wang S, Grayston J T. Differentiation of TRIC and LGV organisms based on enhancement of infectivity by DEAE-dextran in cell culture. J Infect Dis. 1972;125:313–317. doi: 10.1093/infdis/125.3.313. [DOI] [PubMed] [Google Scholar]
- 22.Lyons J M, Io J L. Reducing the risk of Chlamydia trachomatis genital tract infection by evaluating the prophylactic potential of vaginally applied chemicals. Clin Infect Dis. 1995;21(Suppl. 2):S174–S177. doi: 10.1093/clinids/21.supplement_2.s174. [DOI] [PubMed] [Google Scholar]
- 23.Patton D L, Sweeney Y C, Rabe L K, Hillier S L. The vaginal microflora of pig-tailed macaques and the effects of chlorhexidine and benzalkonium on this ecosystem. Sex Transm Dis. 1996;23:489–493. doi: 10.1097/00007435-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Patton D L, Kuo C C, Wang S P, Halbert S A. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infections in pig-tailed macaques. J Infect Dis. 1987;155:1292–1299. doi: 10.1093/infdis/155.6.1292. [DOI] [PubMed] [Google Scholar]
- 25.Patton D L, Halbert S A, Kuo C C, Wang S P, Holmes K K. Host response to primary Chlamydia trachomatis infection of the fallopian tube in pig-tailed monkeys. Fertil Steril. 1983;40:829–840. [PubMed] [Google Scholar]
- 26.Psychoyos A, Creatsas G, Hassan E, Georgoulias V, Gravanis A. Spermicidal and antiviral properties of cholic acid: contraceptive efficacy of a new vaginal sponge (ProtectaidR) containing sodium cholate. Hum Reprod. 1993;8:866–869. doi: 10.1093/oxfordjournals.humrep.a138156. [DOI] [PubMed] [Google Scholar]
- 27.Quinn T C, Gaydos C, Shepherd M, Bob L, Hook E W, Viscidi R, Rompalo A. Epidemiologic and microbiologic correlates of Chlamydia trachomatis infection in sexual partnerships. JAMA. 1996;276:1737–1742. [PubMed] [Google Scholar]
- 28.Rosenthal S L, Stanberry L R, Biro F M, et al. Seroprevalence of herpes simplex virus types 1 and 2 and cytomegalovirus in adolescents. Clin Infect Dis. 1997;24:135–139. doi: 10.1093/clinids/24.2.135. [DOI] [PubMed] [Google Scholar]
- 29.Singh J, Wagner E K. Herpes simplex virus recombination vectors designed to allow insertion of modified promoters into transcriptionally “neutral” segments of the viral genome. Virus Genes. 1995;10:127–136. doi: 10.1007/BF01702593. [DOI] [PubMed] [Google Scholar]
- 30.Turyk M E, Golub T R, Wood N B, Hawkins J L, Wilbanks G D. Growth and characterization of epithelial cells from normal human uterine ectocervix and endocervix. In Vitro Cell Dev Biol. 1989;25:544–556. doi: 10.1007/BF02623567. [DOI] [PubMed] [Google Scholar]
- 31.Wainberg M A, Spira B, Bleau G, Thomas R. Inactivation of human immunodeficiency virus type 1 in tissue culture fluid and in genital secretions by the spermicide benzalkonium chloride. J Clin Microbiol. 1990;28:156–158. doi: 10.1128/jcm.28.1.156-158.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir S S, Feldblum P J, Zekeng L, Roddy R E. The use of nonoxynol-9 for protection against cervical gonorrhea. Am J Public Health. 1994;84:910–914. doi: 10.2105/ajph.84.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whaley K J, Barrett R A, Zeitlin L, Hoen T E, Cone R A. Nonoxynol-9 protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1993;168:1009–1111. doi: 10.1093/infdis/168.4.1009. [DOI] [PubMed] [Google Scholar]
- 34.Zekeng L, Feldblum P J, Oliver R M, Kaptue L. Barrier contraceptive use and HIV infection among high-risk women in Cameroon. AIDS. 1993;7:725–731. doi: 10.1097/00002030-199305000-00018. [DOI] [PubMed] [Google Scholar]