While real-time reverse-transcription polymerase chain reaction (RT-PCR) in respiratory samples remains the primary laboratory method to diagnose coronavirus disease 2019 (COVID-19) associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], COVID-19 testing based on rapid SARS-CoV-2 antigen detection has increasingly been used [2]. Unlike RT-PCR [3], this testing method is prone to yield false-positive results (https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html#anchor_1597523027400). Consequently, positive antigen test results need to be confirmed by RT-PCR [4] whereas benefits of rapid antigen tests (RATs), such as lower turnaround time and greater ease of use than RT-PCR assays, are undefined for specific scenarios/settings [5]. Here, we aimed to assess the rates of RAT positivity before and after RT-PCR confirmation of antigen results in different settings, which included symptomatic and asymptomatic individuals consecutively tested for SARS-CoV-2. This study was presented in part at the 31th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Online, 9–12 July 2021.
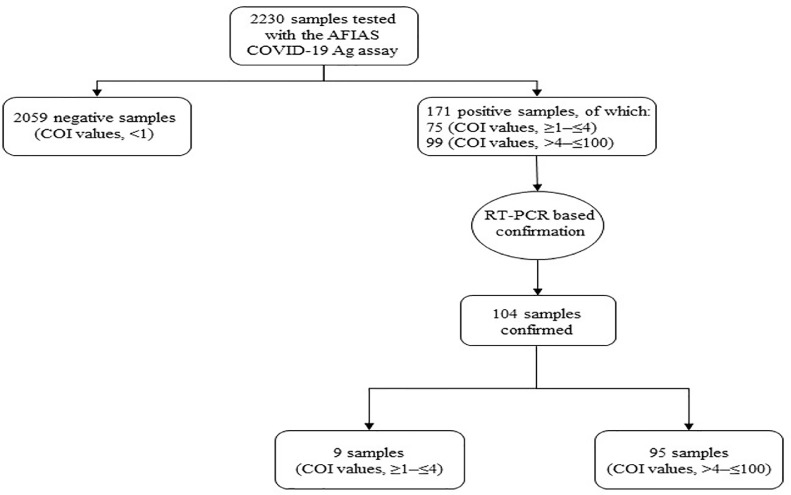
We report on 171 (7.7%) of 2230 individuals (Fig. 1 ) who tested positive with the AFIAS COVID-19 Ag (Boditech Med., Chuncheon-si, Gang-won-do, Republic of Korea) assay—an automated fluorescence immunoassay detecting SARS-CoV-2 nucleoprotein antigen in nasopharyngeal swabs within 12 min from sample collection (https://www.avant-medical.com/products/afias-covid-19-antigen-test/)—during COVID-19 testing at the Ospedale San Carlo GVM of Rome (Italy). The study period was from 01/12/2020 to 01/02/2021. Of these individuals (78/171 had COVID-19–compatible symptoms), 76 were emergency-department (ED) admitted patients (68/76 were symptomatic), 69 were pre-hospitalized patients (0/69 were symptomatic), and 26 were healthcare workers (10/26 were symptomatic). Among individuals’ groups, the median (interquartile range) age was 61 (46–78), 68 (55–81), and 55 (44–70), respectively. Positive antigen results (expressed as values ≥1 cutoff index [COI]) were evaluated in comparison with those obtained by RT-PCR (expressed as RNA detected or undetected, respectively), which was performed using the DiaSorin Simplexa COVID-19 Direct assay [6] on nasopharyngeal swabs of 171 individuals (Table 1 ), who were resampled the same day to confirm antigen positivity as recommended (https://www.salutelazio.it/covid-19- L -offerta-di-test-nel-lazio).
Fig. 1.
COVID-19 diagnostic/screening flowchart of nasopharyngeal swab samples (n = 2230) tested with the AFIAS COVID-19 Ag assay for SARS-CoV-2 antigen. Samples (n = 171) that resulted positive by the assay were submitted to confirmation by the RT-PCR assay. Of 104 confirmed samples, 95 (91.3%) had COI values ranging from >4 to ≤100.
Table 1.
Positive antigen results stratified by those obtained with the RT-PCR assay for SARS-CoV-2 detection among tested individuals’ groups.a
| Positive antigen results expressed as COI valueb | ED admitted patients (n = 76) | Pre-hospitalized patients (n = 69) | Healthcare workers (n = 26) | |||
|---|---|---|---|---|---|---|
| RT-PCR results expressed as RNA | RT-PCR results expressed as RNA | RT-PCR results expressed as RNA | ||||
| Detected | Undetected | Detected | Undetected | Detected | Undetected | |
| ≥1 to ≤4 (n = 75) | 7 | 6 | 1 | 48 | 1 | 12 |
| >4 to ≤100 (n = 96) | 63 | 0 | 19 | 1 | 13 | 0 |
| All (n = 171) | 70 | 6 | 20 | 49 | 14 | 12 |
Abbreviations. RT-PCR, real-time reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COI, cutoff index; ED, emergency department.
Nasopharyngeal swabs from 171 individuals were tested for the presence of SARS-CoV-2 antigen using the AFIAS COVID-19 Ag assay (see text for details). The same individuals were resampled for RT-PCR detection of SARS-CoV-2 RNA, which was performed using the DiaSorin Simplexa COVID-19 Direct assay [6]. A positive result (i.e., a cycle threshold [Ct] less than 40; not shown) for at least one of two viral targets (S [spike] and ORF1ab [open reading frame 1ab]) indicated the presence of SARS-CoV-2 RNA in the individual's nasopharyngeal swab sample.
In five cases, COI values of >100 were rounded to 100 for comparison purposes.
Compared to RT-PCR, 49 (71.0%) of 69 pre-hospitalized patients, 12 (46.2%) of 26 healthcare workers, and 6 (7.9%) of 76 ED patients, corresponding to 67 (39.2%) of 171 individuals, were falsely detected as positive for SARS-CoV-2 by the AFIAS COVID-19 Ag assay (Table 1). Using a chi-square test analysis, we found that rates of false positive results differed significantly among individuals’ groups (P < 0.001 for the comparisons of ED patients versus pre-hospitalized patients or versus healthcare workers; and P = 0.02 for the comparison of pre-hospitalized patients versus healthcare workers). Consistently, AFIAS COVID-19 Ag agreed with RT-PCR for 104 (60.8%; 95% CI, 53.1–68.2%) of 171 results (COI values, ≥1 to ≤100) and, excluding nine samples (COI values, ≥1 to ≤4), for 95 (99.0%; 95% CI, 94.3–100.0%) of 96 results (COI values, >4 to ≤100) (Fig. 1), with agreement rates differing significantly from each other (chi-square test; P < 0.001).
We show that false-positive result rates with the AFIAS COVID-19 Ag assay varied depending on different testing scenarios, which in turn reflect the different (low, moderate, or high) pre-test probability levels for SARS-CoV-2 infection currently encountered [4,5]. False positivity mostly occurred when testing individuals with a low pre-test probability of having SARS-CoV-2 infection, such as pre-hospitalized patients or healthcare workers. Using a COI value of >4 to evaluate the positive AFIAS COVID-19 Ag results for all of these individuals allowed to reduce false-positive result rates to negligible percent values. We did not determine the diagnostic accuracy of the AFIAS COVID-19 Ag before routinely implementing RAT for COVID-19 diagnostic/screening purposes, as well as we did not correlate SARS-CoV-2 antigen results with RT-PCR cycle threshold (Ct) values or with the days after symptom onset in this study. However, the Kweon et al.’s findings published only in April 2021, which showed excellent AFIAS COVID-19 Ag's specificity for nasopharyngeal swab samples with higher viral loads and/or collected within seven days after symptom onset [7], did not surprise us. This means that patients with a high probability of testing positive may rapidly benefit from RAT positivity [4] and, importantly, timely management as SARS-CoV-2 infected patients [8]. Our study's nature hampered us to assess the risk of false negativity with the AFIAS COVID-19 Ag assay, which may be mitigated by using RAT in conjunction with RT-PCR assay as Kweon et al. suggested [7]. In conclusion, we believe that careful application of AFIAS COVID-19 Ag assay or similar RATs [9,10] to specific clinical settings may significantly increase the probability that a positive test result for SARS-CoV-2 represents a true positive patient, thereby curbing repeating RT-PCR results for confirmation.
Declarations of Competing Interest
The authors declare that they have no conflicts of interests.
Acknowledgments
We thank Franziska Lohmeyer, PhD (Scientific Direction, FPG IRCCS, Rome, Italy) for English revision of the manuscript.
References
- 1.Islam K.U., Iqbal J. An update on molecular diagnostics for COVID-19. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.560616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2020. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK, 19 November 2020.https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-and-uk [Google Scholar]
- 3.Hadaya J., Schumm M., Livingston E.H. Testing individuals for coronavirus disease 2019 (COVID-19) JAMA. 2020;323:1981. doi: 10.1001/jama.2020.5388. [DOI] [PubMed] [Google Scholar]
- 4.Peeling R.W., Olliaro P.L., Boeras D.I., Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect. Dis. 2021;21(9):e290–e295. doi: 10.1016/S1473-3099(21)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks Z.C., Das S. COVID-19 testing. Am. J. Clin. Pathol. 2020;154:575–584. doi: 10.1093/ajcp/aqaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liotti F.M., Menchinelli G., Marchetti S., Morandotti G.A., Sanguinetti M., Posteraro B., Cattani P. Evaluation of three commercial assays for SARS-CoV-2 molecular detection in upper respiratory tract samples. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(2):269–277. doi: 10.1007/s10096-020-04025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kweon O.J., Lim Y.K., Kim H.R., Choi Y., Kim M.C., Choi S.H., Chung J.W., Lee M.K. Evaluation of rapid SARS-CoV-2 antigen tests, AFIAS COVID-19 Ag and ichroma COVID-19 Ag, with serial nasopharyngeal specimens from COVID-19 patients. PLoS ONE. 2021;16(4) doi: 10.1371/journal.pone.0249972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pekosz A., Parvu V., Li M., Andrews J.C., Manabe Y.C., Kodsi S., Gary D.S., Roger-Dalbert C., Leitch J., Cooper C.K. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin. Infect. Dis. 2021;73(9):e2861–e2866. doi: 10.1093/cid/ciaa1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liotti F.M., Menchinelli G., Lalle E., Palucci I., Marchetti S., Colavita F., Sorda M.La, Sberna G., Bordi L., Sanguinetti M., Cattani P., Capobianchi M.R., Posteraro B. Performance of a novel diagnostic assay for rapid SARS-CoV-2 antigen detection in nasopharynx samples. Clin. Microbiol. Infect. 2021;27(3):487–488. doi: 10.1016/j.cmi.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-García F., Romanyk J., Gómez-Herruz P., Arroyo T., Pérez-Tanoira R., Linares M., Pérez Ranz I., Labrador Ballestero A., Moya Gutiérrez H., Ruiz-Álvarez M.J., Cuadros-González J. Diagnostic performance of CerTest and Panbio antigen rapid diagnostic tests to diagnose SARS-CoV-2 infection. J. Clin. Virol. 2021;137 doi: 10.1016/j.jcv.2021.104781. [DOI] [PMC free article] [PubMed] [Google Scholar]