Abstract
Background
Few studies to date have explored the health-related quality of life (HRQoL) in patients with long COVID.
Methods
The Anticipate Study is a prospective single-centre observational cohort study. Hospitalised and nonhospitalised patients were seen at a dedicated post-COVID clinic at a 2-4 month (Timepoint 1) and 7-14 month follow-up (Timepoint 2). The main objectives of this study are to assess the longitudinal impact of COVID-19 in patients using the 12-item Short Form Survey (SF-12) score, a health-related quality of life tool, and to identify predictors of developing post–COVID-19 syndrome (PoCS). In addition, we aimed to describe symptomatology and identify predictors of PoCS at 1-year.
Results
A total of 155 patients were enrolled, 105 (68%) were female aged 43.3 (31-52) years. In total 149 (96%) and 94 (61%) patients completed follow-up at median 96 (76-118) days and 364 (303-398) days. The overall cohort had significantly reduced physical composite score (PCS) of the SF-12 (45.39 [10.58] vs 50 [10], p = 0.02). Participants with PoCS had significantly lower scores than those without symptoms at 1-year follow-up (37.2 [10.4] v 46.1 [10.9] p <0.001), and scores for these patients did not improve over the 2 Timepoints (PCS 34.95 [10.5] – 37.2 [10.4], p = 0.22). Fatigue was the most common symptom. Those with 5 or more symptoms at initial diagnosis had lower PCS and mental composite score (MCS) at 1-year. Predictors of PoCS at 1-year were lower PCS and higher baseline heart rate (HR) at clinic review median 3 months after COVID-19.
Conclusion
Patients with PoCS have lower PCS scores during follow-up, which did not significantly improve up to a 1-year follow-up. Lower PCS scores and higher HR at rest can be used in the weeks after COVID-19 can help predict those at risk of PoCS at 1 year.
Keywords: SARS-CoV-2, COVID-19, Post–COVID-19 Syndrome, SF-12, long COVID
Introduction
To date (February 2022), over 388 million infections with COVID-19 have been reported by the World Health Organisation (WHO) (WHO, 2022). International vaccination efforts have significantly reduced the risk of hospitalisation and death from COVID-19 by approximately 90% in those vaccinated (Botton et al., 2022), allowing many countries to relax other public health measures.
One less understood consequence of infection is the development of ‘long COVID’, a general term describing symptoms persisting 4 weeks after onset of acute COVID-19. The National Institute for Health and Care Excellence (NICE), the Centres for Disease Control (CDC) and the World Health Organisation) have all released definitions that generally describe the persistence of symptoms after acute COVID-19; ‘Post–COVID-19 Syndrome’ (PoCS), ‘Post–COVID conditions’ and ‘Post–COVID-19 condition’ (CDC, 2021, NICE, 2020, WHO, 2021). All of these definitions can come under the umbrella term of long COVID, Table 1 .
Table 1.
| NICE definitions | ‘long COVID’ is commonly used to describe signs and symptoms that continue or develop after acute COVID-19 | |
| Ongoing symptomatic COVID-19 | Signs and symptoms of COVID-19 from 4 to 12 weeks | |
| Post–COVID-19 syndrome | Signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis | |
| CDC definition | ||
| Post-COVID conditions | A wide range of new, returning, or ongoing health problems people can experience four or more weeks after first being infected with the virus that causes COVID-19 | |
| WHO definition | ||
| Post–COVID-19 condition | A history of probable or confirmed SARS CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms and that last for at least 2 months and cannot be explained by an alternative diagnosis. |
CDC = Centres for Disease Control and Prevention; NICE = National Institute for Health and Care Excellence; WHO = World Health Organisation.
Patients can have multiple symptoms, and it does not appear that the severity of acute COVID-19 is predictive of persistence of symptoms (Lopez-Leon et al., 2021) (Dennis et al., 2020, Townsend et al., 2021, Townsend et al., 2020). The quoted prevalence of long COVID in the literature varies, which may be because of heterogeneous study design and small sample sizes. As many as 76% of patients have been shown to have symptoms at 6 months (Nalbandian et al., 2021) (Huang et al., 2021). A more recent study, Real-Time Assessment of Community Transmission-2 (REACT-2), involved community patients in the United Kingdom (UK) with over 78,000 initially symptomatic participants, which showed 37.7% of patients had 1 or more symptoms beyond 12 weeks after initial infection (Whitaker M, 2021). Few studies describe symptomatology beyond 6 months. A report from a single centre suggest that 24% of patients have ongoing dyspnoea at a median 44 weeks (Nguyen et al.). The scale and level of disability because of long COVID needs to be more clearly defined.
Management of long COVID in most countries is under-resourced and under-developed. In the UK, steps have been taken to mitigate this by establishing over 80 clinics focusing on multidisciplinary integrative care and dedicated studies into long COVID (DoHaSC, 2021). In addition, the United States Congress have pledged $1.15 billion over 4 years to the National Institute of Health to investigate the prolonged health consequences of COVID-19 (NIH, 2021).
We aim to add to this knowledge in a number of ways. First, by examining the prolonged impact of COVID-19 on patients using the 12-Item Short Form Survey (SF-12) health-related quality of life (HRQoL) questionnaire at 3 months (Timepoint 1) and 12 months (Timepoint 2) after acute COVID-19 symptom onset (Timepoint 0). Second, to identify factors that may be associated with lower SF-12 scores at 12 months: health care worker (HCW) status, number of initial symptoms, hospitalisation, admission to intensive care, age, sex, and presence of PoCS. Third, to describe symptomatology in this cohort, the presence of PoCS at 12 months, and identify early predictors for the persistence of PoCS at 12 months.
Methods
This is a single-centre prospective observational cohort study carried out in the Mater Misericordiae University Hospital, a 580-bed tertiary hospital and site of the national isolation unit in Ireland. Patients were recruited from the post-COVID clinic managed by the Infectious Diseases Department, between June 2020 and November 2020. Patients were eligible if they had laboratory-confirmed COVID-19 or in those with a clinical diagnosis of COVID-19, which requires patients to have had symptoms of acute COVID-19 including fevers, shortness of breath (SOB), cough, myalgia, anosmia, or other symptoms in returning travellers or local patients at times where community transmission was known in those respective areas. All patients with COVID-19 who were admitted, who were seen and diagnosed in the emergency department and discharged with home monitoring (as they did not meet criteria for admission), and patients referred by general practitioners from the catchment area with COVID-19 were offered a follow-up appointment in the clinic.
The onset of initial symptoms is labelled ‘Timepoint 0’. Patients were retrospectively asked about symptoms at this time, and charts of hospitalised patients were reviewed. HRQoL questionnaires were completed at 2-4 months (Timepoint 1) follow-up after initial symptom onset, and patients were also recruited at this time. A second review was done at 7-14 months (Timepoint 2). The aim was to capture individuals around 1 year after Timepoint 0. A wide range of time was given for follow-up to buffer the potential reduction in clinic availability because of subsequent infectious waves of COVID-19. Data collection included age, sex, date of symptom onset, and confirmatory testing in those with a positive nasopharyngeal polymerase chain reaction (PCR) test, co-morbidities, HCWs status, hospitalisation, complications, length of stay, intensive care unit (ICU) admission, need for mechanical ventilation, and vital signs and were all collected from patient notes. The results of chest x-ray and computerised tomography thoraces were collected from the electronic hospital record. Data collection was performed by members of the research team only. Data were pseudo anonymised and collected on Redcap®, an online password-protected research tool only accessible by members of the research team. Patients were classified as having PoCS at Timepoint 2 according to NICE guidelines ‘Signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis’, as this was the original definition available at the time of the study (NICE, 2020).
SF-12 is an HRQoL measure which is a shortened and validated version of the SF-36 questionnaire (Ware et al., 1996). Two scores are generated from the SF-12, a physical composite score (PCS) and a mental composite score (MCS). These scores are generated using an algorithm that compares patients with a standardised otherwise well population normalised on a scale of 0-100 and mean (SD) PCS or MCS of 50 (10). Scores below or above 50 for patients in the cohort confer a higher or lower impact of long COVID on daily function relative to the standardised population. Although not validated for COVID-19, the score is well-validated in a number of populations, including United States, UK, France, Germany, Sweden, Denmark, and a number of conditions including stroke, patients on dialysis, osteoarthritis, rheumatoid arthritis, acquired immunodeficiency syndrome, amongst other populations and conditions (Gandek et al., 1998; Gandhi et al., 2001; Han et al., 2002; Johnson and Coons, 1998; Lacson et al., 2010; Lim and Fisher, 1999). Patients were characterised as asymptomatic, symptomatic at Timepoint 1 or asymptomatic, or labelled PoCS if symptoms were present at Timepoint 2.
Statistical analysis
Statistical analysis was performed using IBM SPSS Version 24.0 (IBM Corporation, Armonk, New York). Continuous data are presented as median and interquartile ranges (IQR) or means SD where appropriate. Categorical data are presented as frequencies (percentages) with 95% confidence intervals (CI). Normality testing was done using the Shapiro-Wilk test. Associations between factors of interest were assessed using Mann-Whitney U test and Wilcoxon signed rank test. McNemar test was used for paired categorical data. Univariate logistic regression was used to identify potentially significant predictive factors of PoCS. Variables with a p value <0.10 on univariate analysis were considered for a multivariate model. Variables with the lowest p values were added through stepwise forward selection to generate the multivariate logistic regression model. Kruskal-Wallis test was used to explore the significance of the number of symptoms with PCS and MCS scores.
Results
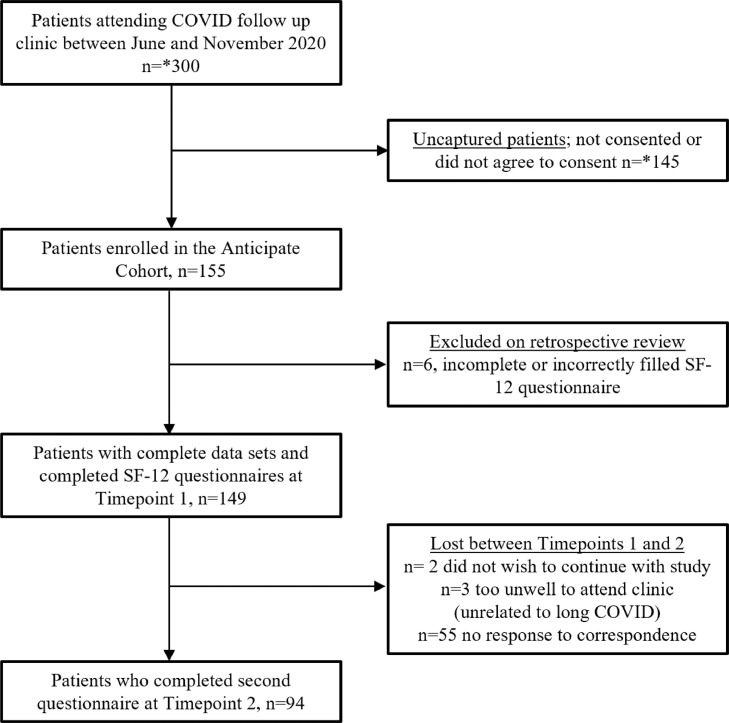
A total of 155 participants were included in the Anticipate Study, 105 (68%) of whom were female with a median age of 43.3 (IQR 31-52) years and 150 (96.8%) of whom were proven SARS-CoV2 PCR positive. Most participants were HCWs, n = 97 (60%), the largest subgroup of whom were student or staff nurses 52 (55.9%). The most common co-morbidity for the cohort was hypertension (16.1%), Table 2 . Between 12 and 15 patients were seen in the clinic weekly, approximating 300 patients attending the clinic during the enrolment period. In total, 94 participants completed follow-up at Timepoint 2, Figure 1 .
Table 2.
Participant demographics
| n(%) | 95%CI | |
|---|---|---|
| Total | 155 | |
| Female | 103(67.7%) | 60-75% |
| Median age (IQR) | 43.1(31-52) | |
| Severity markers | ||
| Hospital admission | 70(45.2%) | 37.3-53% |
| Antiviral medication* | 40(25.8%) | 18.9-32.7% |
| ICU | 9(5.8%) | 2.9.5% |
| MV | 5(3.3%) | 0.4-6% |
| ECMO | 0(0%) | NA |
| Acute symptoms | ||
| Cough | 82(52.9%) | 45-60.8% |
| SOB | 71(45.8%) | 38-53.7% |
| Fever >38°C | 70(45.2%) | 37.3-53% |
| Myalgia | 59(38.1%) | 30.4-45.7% |
| Headache | 44(28.4%) | 21.3-35.5% |
| Anosmia | 40(25.8%) | 18.9-32.7% |
| Sore throat | 24(15.5%) | 9.8-21.2% |
| Chest pain | 21(13.5%) | 8.2-18.9% |
| Nausea/vomiting | 13(8.4%) | 4-12.8% |
| Diarrhoea | 12(7.7%) | 3.5-11.9% |
| Rhinorrhoea | 6(3.9%) | 0.8-6.95 |
| Abdominal pain | 4(2.6%) | 0.1-5.1% |
| Joint pain | 4(2.6%) | 0.1-5.1% |
| Weakness | 3(1.9) | 0-4.1 |
| Co-morbidities | ||
| Hypertension | 25(16.1) | 10.3-21.9 |
| Previous surgery requiring GA | 22(14.2) | 8.7-19.7 |
| Hypercholestolaemia | 18(11.6) | 6.6-16.7 |
| Chronic lung disease | 17(11) | 6-15.9 |
| mood disorder | 15(9.7) | 5-14.3 |
| Diabetes mellitus | 12(7.7) | 3.5-11.9 |
| Gastrointestinal diagnosis | 11(7.1) | 3.1-11.1 |
| Coronary artery disease | 10(6.5) | 2.6-10.3 |
| Neurological diagnosis | 9(5.8) | 2.1-9.5 |
| Rheumatological diagnosis | 9(5.8) | 2.1-9.5 |
| Previous infection requiring hospitalisation | 9(5.8) | 2.1-9.5 |
| Endocrinological diagnosis | 8(5.2) | 1.7-8.6 |
| Malignancy | 6(3.9) | 0.8-6.9 |
| Previous thromboembolism | 5(3.2) | 0-4.6 |
| Chronic haematological condition | 5(3.2) | 0.4-6 |
| Obesity | 4(2.6) | 0.1-5.1 |
| Pregnancy | 2(1.3) | 0-3.1 |
| Chronic kidney disease | 2(1.3) | 0-3.1 |
| Peripheral arterial disease | 2(1.3) | 0-3.1 |
ECMO = extracorporeal membrane oxygenation; GA = general anaesthetic; ICU = intensive care unit; IQR = interquartile range; MV = mechanical ventilation; SOB = shortness of breath.
Figure 1.
Flow diagram
*Approximation
Timepoint 0 (Initial COVID-19 presentation)
Symptoms at the time of initial infection were present in 152 (98.1%) patients. Symptoms and severity markers can be found in Table 2. The median number of symptoms per patient was 3 (IQR 2-5), and median time from symptom onset to swab positive result was 4 days (IQR 2-7). In total, 11 (7.1%) had radiological changes consistent with pneumonitis on an x-ray or computerised tomography thorax. A total of 70 (45%) patients were admitted to the hospital for a median of 7 days (IQR 3-14 days), 9 (13%, CI 5-21%) of whom required admission to intensive care.
HRQoL outcomes (SF-12)
Timepoint 1 (2-4 month follow-up)
Of 155 patients, 149 completed an SF-12 questionnaire and had complete data from Timepoint 0 in the clinic setting at Timepoint 1 at a median of 96 days (IQR 76-118 days) from diagnosis. The composite scores PCS and MCS for the cohort during this period was significantly lower than a standardised population with mean (SD) PCS and MCS score of 50 (10) (PCS, <0.001, MCS 0.002). PCS scores were significantly lower for older patients (40-59 years and 60+ years) p = 0.001, and those hospitalised with COVID-19 at Timepoint 0, p = 0.008. Overall, MCS scores tended to be higher than PCS scores. Patients in intensive care had significantly higher MCS scores at 3 months than hospitalised patients not admitted to ICU (52 [11.4] vs 44 [10.9], p = 0.5), Appendix, Table 1.
Timepoint 2 (7-14 month follow-up)
In total, 94 patients completed the second follow-up at Timepoint 2. The median time to follow-up was 364 days (IQR 303-398). For the whole cohort, PCS did improve by over 2 points from the score at Timepoint 1, which was significant (42.96 [10.9] – 45.39 [10.6], p = 0.02) but the score was still lower than the mean (50 [10] points) for the standardising population at Timepoint 2 (p = 0.001). MCS scores did not change significantly between Timepoint 1 and Timepoint 2 (p = 0.311), and scores were similar to a standardised well population at Timepoint 2 (p = 0.699), Appendix, Table 2.
Factors associated with lower SF-12 scores at Timepoint 2
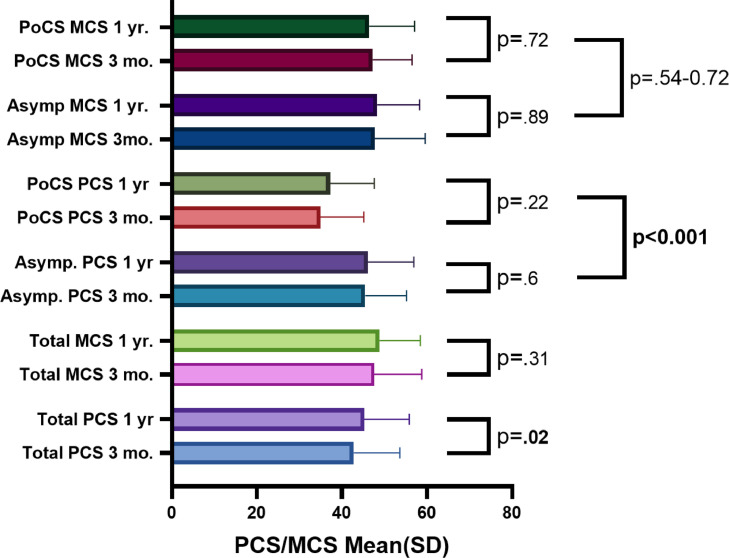
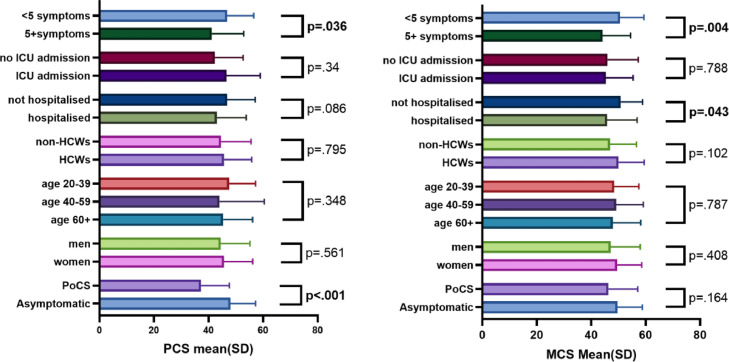
Of 24 (25.5%) patients with ongoing symptoms at Timepoint 2, labelled PoCS, PCS scores were significantly lower at Timepoint 2 compared with those that had no symptoms (37.2 [10.4] vs 46.1 [10.9] p <0.001). On retrospective review of PCS scores of these 24 patients at Timepoint 2, these patients had the lowest PCS scores within the symptomatic cohort (n = 107 [69%]) at Timepoint 1. Furthermore, there was no significant improvement across Timepoints 1 and 2 for this PoCS cohort (PCS 34.95 [10.5] – 37.2 [10.4], p = 0.22). Changes in PCS and MCS for PoCS and asymptomatic groups between Timepoint 1 and Timepoint 2 can be seen in Figure 2 , and data can be found in Appendix Table 2. Patients who required hospitalisation did show lower MCS scores than patients with COVID-19 who were never hospitalised (p = 0.043), and patients who had 5 or more symptoms at initial diagnosis had lower PCS and MCS scores at Timepoint 2 than those who had less than 5 symptoms (p = 0.036, p = 0.004).
Figure 2.
Change in mental composite score (MCS) and physical composite score (PCS) between Timepoint 1 (median 3) months and Timepoint 2 (median 1 year) after initial infection with COVID-19
MCS = mental composite score; PCS = physical composite score; SD = standard deviation.
Of the 57 HCWs who completed the second questionnaire at Timepoint 2 there was no difference in outcomes compared with other participants (PCS; 45.7 [10.3] vs 44.6 [11] p = 0.795, MCS; 50 [9.4] vs 46.9 [9.7] p = 0.102). In addition, neither age, sex, nor ICU admission was factors that were associated with differences in PCS and MCS scores of participants at Timepoint 2, Figure 3 .
Figure 3.
Baseline variables and significance of association with the physical composite score (PCS) and mental composite score (MCS) at 1-year follow-up
HCW = health care worker; ICU = intensive care unit; MCS = mental composite score; PCS = physical composite score; PoCS = post–COVID-19 syndrome; SD = standard deviation.
Symptoms
A total of 107 (72%) patients had ongoing symptoms at Timepoint 1. The most common symptoms described were SOB (n = 51 [48%]), fatigue (n = 44 [41%]), and chest pain (n = 16 [15%]), Table 3 . The median number of symptoms was 1 per patient (IQR 1-3). Increased number of symptoms was associated with lower PCS scores (but not MCS scores), Appendix, Figure 1.
Table 3.
Frequency of symptoms at Timepoint 1
| Symptoms | N(%) |
|---|---|
| Total | 107(100) |
| Shortness of breath | 51(48) |
| Fatigue | 44(41) |
| Chest pain | 16(15) |
| Headaches | 13(12) |
| Cough | 12(11) |
| General body pain | 10(9) |
| Anosmia | 7(7) |
| Palpitations | 5(5) |
| Myalgia | 5(5) |
| Sleep disturbance | 4(4) |
| Reduced exercise tolerance | 4(4) |
| Joint pain | 3(3) |
| Anxiety | 2(2) |
| Low mood | 2(2) |
| night sweats | 2(2) |
| fevers | 1(1) |
| Diarrhoea/vomiting | 1(1) |
| peripheral neuropathy | 1(1) |
| Dizziness | 1(1) |
| Falls | 1(1) |
Of the 24 (25.5%) participants with PoCS at Timepoint 2, a reduction from 71 (75.5%) at Timepoint 1 was significant, p <.001. In addition, the profile of symptoms differed at Timepoint 2. Neurological issues including headache, anosmia, tinnitus, numbness, and paraesthesia were present in 10 (37.5%) patients. Symptoms of fatigue (n = 10 [42%]) and difficulties with concentration (n = 8 [33%]) were also common, Table 4 . The median number of symptoms was 2 (IQR 1-3) at Timepoint 2. Regarding presence of symptoms in HCWs compared with other participants, 65 (70.7%) and 42 (73.7%) had symptoms at Timepoint 1 p = 0.689, also 18 (31.6%) and 6 (16.2%) had symptoms respectively at Timepoint 2 p = 0.095. Blood tests, including full blood count, kidney and liver profile, C-reactive protein, were done in these patients to screen for other potential causes. Repeat chest x-rays were done in 5 patients who previously had pneumonitis, all of which showed resolution of previous changes.
Table 4.
Frequency of symptoms at Timepoint 2
| Symptoms | N(%) |
|---|---|
| Total | 24(100) |
| Fatigue | 10(42) |
| Decreased concentration | 8(33) |
| Shortness of breath | 7(29) |
| Cough | 4(17) |
| Pain | 4(17) |
| Neurological issues | 4(17) |
| Headache | 3(13) |
| Anosmia | 3(13) |
| Chest pain | 3(13) |
| Joint pain | 2(8) |
| Low mood | 2(8) |
| Dizziness | 1(4) |
| Lower back pain | 1(4) |
| Myalgia | 1(4) |
Univariate logistic regression was done to explore factors that may predict PoCS at Timepoint 2. Factors analysed, including MCS (p = 0.85), age (p = 0.66), sex (p = 0.76), hospitalisation (p = 0.98), ICU admission (p = 0.99), need for re-admission after initial hospitalisation (p = 0.21), number of co-morbidities (p = 0.59), respiratory rate (p = 0.6, missing 21), and peripheral oxygen saturation (p = 0.193, missing 5) at 3 month follow-up were not significantly associated with PoCS. Predictor variables of PoCS with p value <0.10 on univariate analysis (PCS scores at 3 months, HR at rest at 3 months, and the number of initial symptoms) were considered for multivariate analysis. Multivariate analysis showed significant predictor variables for PoCS at 1-year, including PCS scores and HR at rest at the median 3-month review, p <0.001. The model shows that for every decrease in a point of PCS, the odds of developing post–COVID-19 syndrome increase by 9.3%, conversely an increase in 1 point of HR at rest increases odds by 4.5%, Table 5 . The number of initial symptoms was nonsignificant in multivariable modelling (p = 0.114) and therefore excluded.
Table 5.
Multivariable Model for PoCS
| Variables | Beta(B) | P value | EXP(B) (odds ratio) | 95% CI for OR | |
|---|---|---|---|---|---|
| For every point decrease in PCS | -0.098 | 0.001 | 0.907 | 0.857 | 0.959 |
| Heart rate (per 1 bpm increase) | 0.046 | 0.038 | 1.047 | 1.003 | 1.094 |
bpm = beats per minute; CI = confidence interval; EXP = exponential coefficient; PCS = physical composite score.
Discussion
Overall, patients with PoCS at 1-year had significantly worse PCS scores than those without symptoms. PCS scores did not improve significantly even at a median follow-up of 1 year in the PoCS cohort. A similar study of 96 patients showed symptoms of COVID-19 can persist for over 1 year and is associated with reduced SF-12 component scores compared with those without symptoms (PCS, p = 0.006, MCS, p = 0.031) (Seeßle et al., 2021). Decreased physical function longitudinally has been reported elsewhere. Delbressine et al. found significantly reduced self-reported walking time in 239 patients with PoCS 6 months after the onset of symptoms (Delbressine et al., 2021). In general, studies examining HRQoL in long COVID patients are small. Logue et al. report ongoing symptoms in 24% of 177 patients at 9 months, 29% of whom report worse HRQoL than pre-COVID-19 function (Logue et al., 2021). Another study of 101 patients after hospitalisation with COVID-19 pneumonia showed significant reduction across all domains of SF-36 (excluding bodily pain) 6 weeks after infection (van der Sar-van der Brugge et al., 2021). Although this study showed little improvement in the physical impact of COVID-19 at 1 year in those with ongoing symptoms, others have found a small but significant increase in work productivity, functional status and quality of life despite a large burden of the number of symptoms (median 6(3-8)) at 6 months, which offers some hope (Vaes et al., 2021).
Overall, MCS scores were not as badly impacted as PCS scores. At Timepoint 2, MCS scores were not significantly different from an otherwise well population. Interestingly, patients in the ICU at Timepoint 1 scored higher in MCS than any other sub-group. In a larger study exploring HRQoL in 294 patients with COVID-19, 40 of whom were admitted to ICU, a similar phenomenon was seen at a 3-month follow-up where ICU patients scored higher on a mental HRQoL than their non-ICU counterparts (Vlake et al., 2021). The authors hypothesise that in the context of intubation and sedation the duration of psychological distress because of illness was reduced in these patients. The increase in MCS scores in ICU patients dissipated by 1-year follow-up in this study (ICU vs non-ICU p = 0.788). Overall, hospitalised patients did have lower MCS scores at 1-year than nonhospitalised patients in this study.
Of the 94 patients who completed median 96-day and 364-day follow-up, 71 (75.5%) and 24 (25.5%) patients had PoCS, respectively. This indicates that most symptomatic patients have a resolution of symptoms by 1-year follow-up after infection. Of these 24 patients with PoCS, symptoms tended to persist from the time of acute illness. Just 2 (16.7%) patients who initially reported no symptoms in the follow-up clinic at a mean of 80 days after initial symptoms went on to report symptoms of difficulties concentrating or forgetfulness, subjectively described as ‘brain fog’ at Timepoint 2 follow-up. The symptomatic profile of patients with PoCS also changed over time from having predominately SOB and fatigue to fatigue and neurological sequelae, including difficulties concentrating. This highlights the nature of long COVID and that symptomatology is not dominated by single organ dysfunction. Fatigue was the most consistent symptom across both Timepoints 1 and 2 and has been reported elsewhere as a very common symptom of long COVID; 79% of individuals at 23 weeks reported severe fatigue in 1 cohort of a long COVID support group, albeit this was improved from 87% at 10 weeks (Van Herck et al., 2021). Underlying mechanisms of persistent immune activation may be at the core of long COVID. Significantly higher ANA levels have been seen in patients with neurocognitive dysfunction compared with well counterparts over 1 year after the onset of symptoms (Seeßle et al., 2021). Aberrant mast cell activation and autoimmune antibody production have been proposed as contributory inflammatory mechanisms to long COVID (Arthur et al., 2021, Bertin et al., 2021, Weinstock et al.).
Lower PCS scores and higher HR at rest levels at Timepoint 1 were predictive of PoCS at Timepoint 2. Early recognition of patients who may have a protracted course with PoCS is important to provide appropriate support and early referral to rehabilitation services to try to mitigate these effects. Large studies exploring potential predictors of long COVID have been done. Sudre CH et al. report female sex, increased BMI and having 5 or more initial symptoms as predictors in a cohort of over 4000 patients with self-reporting through a mobile application (Sudre et al., 2021). They also show that simple modelling involving clustering of symptoms within 7 days can also predict the development of PoCS. Although having 5 or more symptoms was not predictive of PoCS in this study, it was shown that patients certainly had lower PCS scores at 1-year.
Although studies exploring long COVID have relatively small sample sizes and are of short duration, there is clear and growing evidence that a certain proportion of those infected will go on to develop a potentially prolonged and debilitating illness irrespective of initial severity and baseline wellbeing. A very small number of studies, including this study, show that symptoms can persist, and the physical and mental impact of long COVID on daily living can be impaired for over 1 year in these patients.
Limitations
The study is limited by a small sample size from a single centre. In addition, the cohort of 155 who were initially enrolled may not be representative of all patients after acute infection with COVID-19. For instance, many patients who did not have persistent symptoms may have felt there was no need to attend an outpatient service and were not contactable for follow-up at Timepoint 2. In addition, the cohort may be over-representative of patients with persistent symptoms. Furthermore, most of the cohort were HCWs, who may have experienced a greater amount of mental and physical stress than the general population, meaning that final outcomes may be an over-estimate of the impact of PoCS alone, although statistical significance was not found between HCWs and other patients in this study. Potentially, a larger sample size would have identified a significant difference between the 2 groups. Similarly, a number of variables showed a trend towards significance as predictor variables for the development of PoCS, such as the number of symptoms during acute COVID. A larger sample size may have also found these variables to be predictive, as larger studies have shown. In addition, there were no baseline SF-12 questionnaires before COVID-19; some individuals may have had lower scores at baseline because of co-morbidities or other factors.
Conclusion
In summary, patients with PoCS did not show significant improvement in PCS over 1 year and had significantly lower scores than study participants who were asymptomatic at that time. In the weeks after acute infection, higher HR and lower PCS scores may identify those that will have PoCS at 1-year, and these patients should be targeted for prompt multidisciplinary intervention. The authors advise that these tools be implemented for early screening of patients in the weeks after COVID-19 infection.
Competing interests
The authors have no competing interests to declare.
Acknowledgments
Funding
This work was supported by the Health Research Board (HRB) [COV19-2020-123]. In addition, BOK receives a University College Dublin Newman Fellowship sponsored by Gilead, Pfizer and GSK. Gilead, Pfizer, and GSK had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Gilead, Pfizer, or GSK.
Acknowledgements
The authors would like to thank the patients for their continued participation throughout this study. BOK would like to acknowledge the support provided by Gilead, Pfizer and GSK through the UCD Newman Fellowship. The Anticipate research team would like to acknowledge the support provided by the Health Research Board [COV19-2020-123].
Ethical approval
The study was approved by the Mater Misericordiae University Hospital Research Ethics Committee (reference number: 1/378/2141).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.03.013.
Appendix. Supplementary materials
REFERENCES
- Arthur JM, Forrest JC, Boehme KW, Kennedy JL, Owens S, Herzog C, et al. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PloS one. 2021;16(9) doi: 10.1371/journal.pone.0257016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin D, Kaphan E, Weber S, Babacci B, Arcani R, Faucher B, et al. Persistent IgG anti-cardiolipin autoantibodies are associated with post-covid syndrome. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2021 doi: 10.1016/j.ijid.2021.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton J , Dray-Spira R, Baricault B, Drouin J, Bertrand M, Jabagi MJ, et al. Reduced risk of severe COVID-19 in more than 1.4 million elderly people aged 75 years and older vaccinated with mRNA-based vaccines. Vaccine. 2022;40(3):414–417. doi: 10.1016/j.vaccine.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Post-COVID conditions. https://wwwcdcgov/coronavirus/2019-ncov/long-term-effects/indexhtml 2021.
- Department of Health and Social Care (DoHaSC). New research into treatment and diagnosis of long COVID. https://wwwgovuk/government/news/new-research-into-treatment-and-diagnosis-of-long-covid 2021. (Last accessed 28.03.2022).
- Delbressine JM, Machado FVC, Goërtz YMJ, Van Herck M, Meys R, Houben-Wilke S, et al. The Impact of Post-COVID-19 Syndrome on Self-Reported Physical Activity. Int J Environ Res Public Health. 2021;18(11) doi: 10.3390/ijerph18116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis A, Wamil M, Kapur S, Alberts J, Badley AD, Decker GA, et al. Multi-organ impairment in low-risk individuals with long COVID. medRxiv 2020:2020.10.14.20212555.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. Journal of clinical epidemiology. 1998;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- Gandhi SK, Warren Salmon J, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item short-form health survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clinical Therapeutics. 2001;23(7):1080–1098. doi: 10.1016/s0149-2918(01)80093-x. [DOI] [PubMed] [Google Scholar]
- Han C, Pulling CC, Telke SE. Huppler Hullsiek K, for the Terry Beirn Community Programs for Clinical Research on A. Assessing the utility of five domains in SF-12 Health Status Questionnaire in an AIDS clinical trial. AIDS (London, England) 2002;16(3) doi: 10.1097/00002030-200202150-00015. [DOI] [PubMed] [Google Scholar]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (London, England) 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Coons SJ. Comparison of the EQ-5D and SF-12 in an adult US sample. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 1998;7(2):155–166. doi: 10.1023/a:1008809610703. [DOI] [PubMed] [Google Scholar]
- Lacson E, Xu J, Lin S-F, Dean SG, Lazarus JM, Hakim RM. A Comparison of SF-36 and SF-12 Composite Scores and Subsequent Hospitalization and Mortality Risks in Long-Term Dialysis Patients. Clinical Journal of the American Society of Nephrology. 2010;5(2):252–260. doi: 10.2215/CJN.07231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LLY, Fisher JD. Use of the 12-item Short-Form (SF-12) Health Survey in an Australian heart and stroke population. Quality of Life Research. 1999;8(1):1–8. doi: 10.1023/a:1026409226544. [DOI] [PubMed] [Google Scholar]
- Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nature Medicine. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NN, Hoang VT, Dao TL, Meddeb L, Lagier J-C, Million M, et al. Long-term persistence of symptoms of dyspnoea in COVID-19 patients. International Journal of Infectious Diseases. 2022 doi: 10.1016/j.ijid.2021.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE Copyright © 2020; 2020.
- NIH. NIH launches new initiative to study “Long COVID”. https://wwwnihgov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid 2021.
- Seeßle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nature Medicine. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L, Dowds J, O'Brien K, Sheill G, Dyer AH, O'Kelly B, et al. Persistent Poor Health after COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Annals of the American Thoracic Society. 2021;18(6):997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS one. 2020;15(11) doi: 10.1371/journal.pone.0240784. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes AW, Goërtz YMJ, Van Herck M, Machado FVC, Meys R, Delbressine JM, et al. Recovery from COVID-19: a sprint or marathon? 6 months follow-up data of online long COVID-19 support group members. ERJ Open Research. 2021:00141–02021. doi: 10.1183/23120541.00141-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sar-van der Brugge S, Talman S, Boonman-de Winter L, de Mol M, Hoefman E, van Etten RW, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respiratory medicine. 2021;176 doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herck M, Goërtz YMJ, Houben-Wilke S, Machado FVC, Meys R, Delbressine JM, et al. Severe Fatigue in Long COVID: Web-Based Quantitative Follow-up Study in Members of Online Long COVID Support Groups. Journal of medical Internet research. 2021;23(9):e30274. doi: 10.2196/30274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlake JH, Wesselius S, van Genderen ME, van Bommel J, Boxma-de Klerk B, Wils EJ. Psychological distress and health-related quality of life in patients after hospitalization during the COVID-19 pandemic: A single-center, observational study. PloS one. 2021;16(8) doi: 10.1371/journal.pone.0255774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. International Journal of Infectious Diseases. 2022 doi: 10.1016/j.ijid.2021.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M EJ, Chadeau-Hyam M, Riley S, Darzi A, Cooke G, Ward H, Elliott P. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people. Imperial College London. 2021 https://spiral.imperial.ac.uk/handle/10044/1/89844 [Google Scholar]
- WHO. Post COVID-19 condition (Long COVID). https://wwwwhoint/srilanka/news/detail/16-10-2021-post-covid-19-condition 2021.
- WHO. WHO Coronavirus (COVID-19) Dashboard 2022. https://covid19whoint/2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.