ABSTRACT
COVID-19 is an international public health emergency in need of effective and safe vaccines for SARS-CoV-2. A systematic review has been done to analyze the availability, development and status of new COVID-19 vaccine candidates as well as the status of vaccines for other diseases that might be effective against SARS-CoV-2 infection. PubMed, MEDLINE, EMBASE, Science Direct, Google Scholar, Cochrane library, ClinicalTrials.gov, Web of Science and different trial registries were searched for currently available and probable future vaccines. Articles and ongoing clinical trials are included to ascertain the availability and developmental approaches of new vaccines that could limit the present and future outbreaks. Pharmaceutical companies and institutions are at different stages of developing new vaccines, and extensive studies and clinical trials are still required.
KEYWORDS: SARS-CoV-2, vaccine candidates, coronavirus, covid-19, clinical trial, vaccine development
Introduction
COVID-19 (Coronavirus disease 2019), a communicable illness mainly causing respiratory distress, is caused by the recently identified virus Severe acute respiration syndrome coronavirus 2 (SARS-CoV-2),1 previously called 2019 novel coronavirus (2019-nCoV).2 SARS-CoV-2, a single-stranded positive-sense RNA virus,3 is mainly spread by droplets exhaled by infected persons. To date no widely available drugs or biologics for worldwide use have been shown to be effective for the prevention of COVID-19. Numerous antiviral agents, immunotherapies, and vaccines are being studied and advanced as potential therapies.4 To date COVID-19 has caused tens of millions of confirmed cases and over one million deaths.
The Wuhan strain has been identified as a new strain of Beta-coronavirus from group 2B with ~70% genetic similarity to the previous described SARS-CoV. SARS-CoV-2 has 96% similarity to a bat coronavirus, so it is suspected to originate from bats. Vaccines had been produced for several diseases caused by other coronaviruses, including infectious avian bronchitis virus,5 canine coronavirus6 and feline coronavirus.7 Previous initiatives to produce vaccines against viruses in the family Coronaviridae may provide vaccines that are clinically useful. Vaccines against severe acute respiratory syndrome (SARS)8 and Middle East respiratory syndrome (MERS)9 had been tested in animal models. However, no previous such coronavirus vaccine has been shown to be effective in humans.10 According articles from 2005–6, the identity and development of new SARS vaccines become a concern for public and private health agencies around the world. At the time that MERS was predominant, it was generally believed that studies on SARS could deliver a convenient model for MERS vaccine development.11 Only one MERS vaccine (DNA-based) finished phase I clinical trials;12 the development of three other vaccine candidates is in progress, all as viral-vectored vaccines, two of them are adenoviral (ChAdOx1-MERS, BVRS-GamVac), and one MVA- (MVA-MERS-S) vectored.12
Many organizations have undertaken significant investment and research and development activities to develop a SARS-CoV-2 vaccine used the published genomic sequences.13 The objective of this systematic review is to summarize the availability, development and status of new COVID-19 vaccine candidates as well as the status of vaccines for other diseases that might be effective against SARS-CoV-2 infection.
Coronavirus: at a glance
Coronaviruses are enveloped viruses and carry a positive-sense single-stranded RNA as genome and a nucleocapsid of helical symmetry. The genome size of coronaviruses ranges from approximately 26–32 kilobases, the largest among all RNA viruses, and they cause several diseases in mammals and birds. The symptoms in other species may vary: in chickens, they cause an upper respiratory tract disease, while in cows and pigs they cause diarrhea. Coronaviruses were first discovered in the 1930s as avian viruses during acute respiratory infection of chickens caused by an unknown infectious bronchitis virus at that time. Arthur Schalk and M.C. Hawn described the new avian virus as the possible causal agent of the respiratory infection in chickens in North Dakota in the year 1931.14
In humans, these viruses cause respiratory tract infections, which ranges from mild to lethal infections. Mild illnesses include some cases of the common cold (symptoms observed by the naked eye are similar to those of rhinoviruses). Some lethal varieties are SARS, MERS, and COVID-19. This group of viruses has typical club-shaped spikes (protein or glycoprotein) that project from their outer surface, which in electron micrographs create an image similar to the solar corona or halo, from which their name is derived. The name was given by June Almeida and David Tyrrell, Common Cold Research Unit, Salisbury, Wiltshire, who observed and studied a kind of new coronavirus from a human source. The word was first used in print in 1968 by an ‘informal group of virologists’ to designate the new family of viruses.15 In the book entitled ‘Cold Wars: The Fight against the Common Cold,’ Dr. D.A.J. Tyrrell noted “We looked more closely at the appearance of the new viruses and noticed that they had a kind of halo surrounding them. Recourse to a dictionary produced the Latin equivalent, corona, and so the name coronavirus was born.”16
Infection to multiplication then transmission
Infection by coronaviruses begins when the viral spike protein (S) attaches to its complementary host cell receptor. Upon attachment, a protease of the host cell cleaves and triggers the receptor-attached spike protein. Depending on the host cell protease available, cleavage and activation allow the virus to enter the host cell through endocytosis or direct fusion of the viral envelope with the host membrane. Coronaviruses are multiplied through conventional replication, transcription, and then recombination systems. A number of the non-structural proteins combine to form a multi-protein replicase-transcriptase complex to carry over the process. After completion of the process of viral replication, RNA-dependent RNA polymerase (RdRp) of coronaviruses directly mediates the synthesis of the negative-sense sub-genomic RNA molecules from the positive-sense genomic RNA. This process is followed by the transcription of these negative-sense sub-genomic RNA molecules to their corresponding positive-sense mRNAs. The replicase-transcriptase complex is also capable of genetic recombination when at least two viral genomes are present in the same infected cell.17 RNA recombination appears to be a major driving force in determining the genetic variability within a coronavirus species, the capability of a species of coronavirus to jump from one host to another, and unusually, in determining the emergence of novel coronaviruses.18 The particular method of recombination in coronaviruses is still undiscovered, but it probably involves template switching during genome replication.18 These replicated positive-sense mRNAs are translated by the ribosomes of the host cell into the structural proteins S, E, and M, and a number of accessory proteins. The viral structural proteins transfer along the secretory pathway into the Golgi intermediate section. Progeny viruses are then released from the host cell by exocytosis through secretory vesicles. Once released, the viruses can infect other host cells.
Infected carriers are able to shed the viruses into the environment. The interaction of the coronavirus spike protein with its complementary cell receptor is central in determining the tissue tropism, infectivity, and species range of the released virus. Coronaviruses mainly target epithelial cells. They are transmitted from one host to another host, depending on the coronavirus species, by either aerosol, fomite, or the fecal-oral route. Human coronaviruses infect the epithelial cells of the respiratory tract, while animal coronaviruses generally infect the epithelial cells of the digestive tract. It was reported that SARS infects, via the aerosol route, the human epithelial cells of the lungs by binding to the angiotensin-converting enzyme 2 (ACE2) receptor. Transmissible gastroenteritis coronavirus (TGEV) infects, via the fecal-oral route, the pig epithelial cells of the digestive tract by binding to the alanine aminopeptidase (APN) receptor.19
Methods
Types of studies included
Any study regarding the intervention defined as SARS-CoV-2 vaccine or SARS-CoV vaccine or coronavirus vaccine and used for curative intent either as the only vaccine or as an adjuvant to antiviral SOC or standard of care, which includes distinct prophylactic vaccine candidates, vaccine candidates with numerous adjuvants, several RNA vaccines, DNA vaccines, and technologically altered live vaccines. There was no constraint on the quantity or vaccination plan. Control sets may be present or not; if present, they may be receiving no treatment or placebo (Figure 1). The acceptance criteria have been defined according to the population, interventions, and comparisons including outcomes as well as study design (PICOS) arrangement.20 (View supplementary file)
Figure 1.
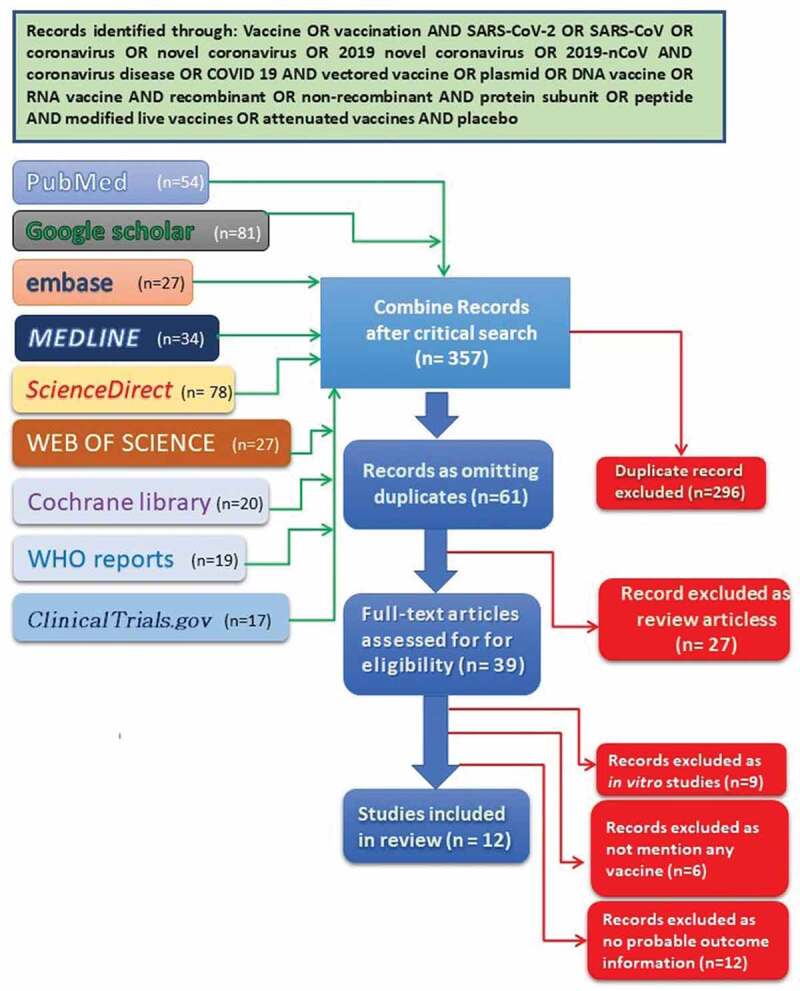
PRISMA diagram showing literature search for eligible articles.
Search approaches for the identification of studies
A methodical search was conducted in EMBASE, MEDLINE, PubMed, Cochrane Library, Web of Science, Science Direct, WHO reports, Clinical Trials.gov, and Google Scholar from database initiation through an MS-windows operating computer-based search of articles cited by or cited in the selected articles, and relevant published literature or web-based interventions until 18th September, 2020 using the keywords vaccine OR vaccination AND SARS-CoV-2 OR SARS-CoV OR coronavirus OR novel coronavirus OR 2019 novel coronavirus OR 2019-nCoV AND coronavirus disease OR COVID 19 AND vectored vaccine OR plasmid OR DNA vaccine OR RNA vaccine AND recombinant OR non-recombinant AND protein subunit OR peptide AND modified live vaccines OR attenuated vaccines AND placebo. To get an insight into the clinical trials regarding previously available vaccine candidates for other diseases along with newly developed vaccine candidates, which are not published, a high-speed broadband-based Google search was performed. A standard Google search was performed to find out internet-based depictions, which had similar keywords as per the searching strategy (Figure 1).
Exclusion criteria
Studies that did not agree with the present interest and those that were primarily not dealing with SARS-CoV-2 vaccination were excluded.
Findings
Profile of SARS-CoV-2
SARS-CoV-2 belongs to the coronavirus group, which are enveloped viruses containing single-stranded, positive-sense RNA viruses that are well dispersed largely among humans, several other mammals, and bird species, causing neurologic, respiratory, hepatic, and enteric problems.21,22 There are six coronavirus species that cause severe infections in humans. Among them, four viruses, namely 229E, OC43, NL63, and HKU1 coronaviruses are predominant and typically show symptoms of the common cold in immunocompetent people.23,24 The two other strains, namely SARS-CoV and MERS-CoV are probably zoonotic in origin and have sometimes been linked to incurable infections.25 SARS-CoV was the main causative agent of the SARS outbreak in 2002–2003 in China. MERS-CoV is likely to be the main pathogen behind the MERS outbreak in 2012 in different Middle East countries.12 With their high prevalence and worldwide distribution, huge genetic diversity, frequent recombination, and growing human-animal interface activities, coronaviruses are likely to emerge and infect humans periodically because of recurrent cross-species infections. SARS-CoV-2 has three key membrane-bound structural proteins, spike (S), envelope (E), and membrane (M) proteins, and several exclusive glycoproteins with a single structural protein within the virus core, the nucleocapsid (N) protein. Multiple vaccine candidates are being developed along with other therapeutic approaches to combat the present pandemic, and vaccines would likely become a major tool to control the present SARS-CoV-2 outbreak.23 Key to the development of effective vaccine candidates is the generation of neutralizing antibodies, which would act with the S glycoprotein on the viral surface to provide complete protection upon passive transfer and are consistently connected with multiple vaccine formulations.12
Preclinical research
The WHO has issued a statement requesting collaboration from all concerned persons to develop a vaccine against SARS-CoV-2.23 The collaboration with WHO inspires international cooperation between agencies, such as national controlling authorities, policymakers, finance persons, public health associations, and different governmental organizations for the development and manufacture of a new and successful vaccine candidate in large quantities, sufficient to supply all affected regions, mostly in low-resource countries.23 As SARS-CoV-2 is a newly discovered virus, most of the properties are still being revealed, which requires innovative developmental strategies and technologies. The risks related to developing successful vaccine candidates through every step of preclinical and clinical research are high.23 Before the clinical trial for an experimental vaccine on human beings starts, the general tendency is to try the vaccine on laboratory animals showing similar symptoms as humans to determine whether it is safe for humans and effective to prevent the particular disease. Only after conducting successful trials in animal models and being adjusted along the way, can a newly developed formulation be tested in human trials. There are several animals from mice24,25 to golden Syrian hamsters26 to monkeys that are tested as animal models,27 but in a news report,28 at present in most cases, researchers engaged in the vaccine development process rush to test SARS-CoV-2 vaccine on people without knowing how well it works on similar kind of animals.29
Rationales of different candidate vaccines
There are some clinical factors that would be essential in the new vaccine candidate against SARS-CoV-2. They are as follows:
To develop a vaccine candidate, first of all, a vigorous immune response generating long-lasting neutralizing antibodies to SARS-CoV-2 antigens (S and/or N) is needed. When someone is infected with any foreign antigen, they induce simultaneously innate and adaptive immune reactions with a coordinated antigen-presenting cell (APC) attack on the virus and T-helper cell activation, which leads to B-lymphocytes producing antibodies. In the present framework of developing an ideal vaccine against SARS-CoV-2, the antibodies must directly restrict the ability of the virus to adhere to epithelial cells and allow type II pneumocytes, through ACE2, to neutralize the viruses. If an infection occurs, this might restrict the virus from infecting a host cell and make the host cells protective. It is still unknown what comprises a protective immune response against COVID-19 and how long it would stay in human blood.29
The probable vaccine candidate against SARS-CoV-2 may also induce potent T-lymphocyte immunity. It would generally be a well-coordinated T-cell response that includes T-helper and cytotoxic T-cell subsets that identify SARS-CoV-2 infected cells in the host and destroy them to stop viral replication, along with memory T-cells to prevent reinfection after several years.30
This type of candidate vaccine should limit any serious adverse events at the site of injection or systemically. For the respiratory disorders caused by any infectious agents, it is essential that vaccine-associated enhanced respiratory disease, antibody-dependent enhancement, antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, including cytokine storm-inducing effects are completely avoided.31 Complement, inflammation, and coagulation systems are tangled and hyperactive in COVID-19 positive cases and the vaccine should not provoke these host response systems.32,33
Required profile of new vaccine candidates
(i)The candidate vaccine should be oral or nasal. (ii) It should be easy to administer and preferably in the lowest possible quantity. (iii) Feasible and rapid manufacturing must be essential. (iv) For underdeveloped nations with inadequate supply chains and cold chain capacities, long-term storage of the vaccine at room temperature would be ideal.33
Moreover, before the mass application of the vaccines against SARS-CoV-2, we must remember that younger individuals tend to have a higher proportion of asymptomatic people or people with mild symptoms are missing from any statistics in extreme scenarios34 but it is not clear how far they can infect other people. In contrast, elderly people are more vulnerable because of comorbidities to multimorbidities35 along with several chronic diseases. These elderly people must be in the target frame of the vaccine developers.
Diversity of technology platforms
A conspicuous feature of the vaccine development landscape for SARS-CoV-2 is the variety of technology platforms being assessed, which include approaches with non-replicating and replicating viral vectors, nucleic acid (DNA and RNA), recombinant protein, peptide, virus-like particle, live attenuated virus, an inactivated virus (Figure 2) (Table 1). Several of the above-mentioned platforms are presently not approaching licensed vaccines, but a platform such as oncology is encouraging developers to use the opportunities of next-generation sequencing approaches to increase the speed of development and manufacture. It may be possible that some vaccine platforms are appropriate for specific population subtypes, which comprises of elderly people, immunocompromised patients, pregnant women, or children. Considering the probable vaccine candidates depicted in Table 1, the new platforms based on cDNA or mRNA offer antigen manipulation.36 Certainly, clinical trials of the mRNA-based vaccine, mRNA-1273, just started a couple of months after SARS-CoV-2 sequence identification. Another platform based on viral vectors offers a high level of protein expression and long-term steadiness and induces durable immune responses. Moreover, the presence of licensed vaccine platforms for other diseases and these candidates may have the advantage of a prevailing large-scale manufacturing capacity.36
Figure 2.
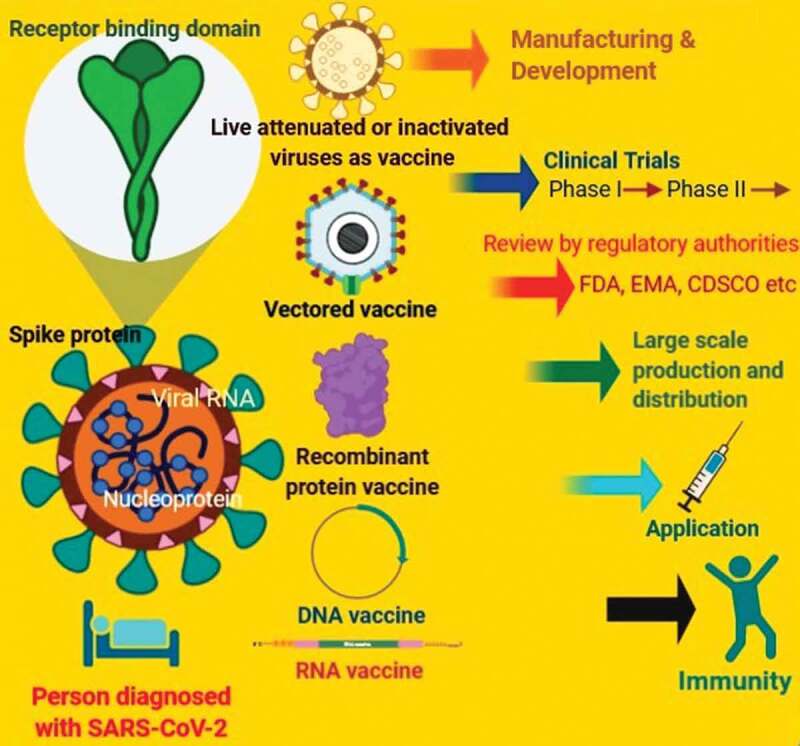
Schematic illustration that depicts the strategies of development of new vaccine candidates against coronavirus or SARS-CoV-2.
Table 1.
Different potential vaccines against COVID-19 (prepared after Milken Institute web page for corona monitoring for development on emerging vaccine candidates scheduled in 2020)
| Category of Probable Vaccine candidates | Descriptions | Phase of Development | Clinical Trials for COVID-19 Identifier ID | Used in other Diseases |
|---|---|---|---|---|
| DNA-vaccines | DNA plasmid; INO-4800 | Started Phase 1 in April 2020; initial data expected June 2020; could enter Phase 2/3 trials as early as summer 2020 | NCT04336410 | Same platform as vaccine candidates for Lassa, Nipah, HIV, Filovirus, HPV, cancer indications, Zika, and Hepatitis B |
| DNA plasmid | Pre-clinical | |||
| DNA plasmid, needle-free delivery | Same platform as vaccine candidates for SARS | |||
| DNA | Same platform as vaccine candidates for cancer | |||
| Inactivated virus | Inactivated (inactivated + alum) | Phase 1/2 started April 2020 | NCT04352608 | Same platform as vaccine candidates for HAV, InfA, ZIKV, FMD, SIV, RSV, DENV |
| Inactivated virus | Phase 1 started in May 2020 | ChiCTR2000031809 | Same platform as vaccine candidates for MERS | |
| Inactivated (inactivated + CpG 1018) | Pre-clinical | |||
| Inactivated virus | Same platform as vaccine candidates for influenza | |||
| VLA2001, Inactivated | Same platform as vaccine candidates for Japanese Encephalitis | |||
| Live attenuated virus | Codon deoptimized live attenuated virus | Pre-clinical | Same platform as vaccine candidates for LASV, EBOV, MARV, HIV Animal data in summer 2020 | |
| Non-replicating viral vector | Non-replicating viral vector; MVA encoded VLP | Pre-clinical | Same platform as vaccine candidates for influenza, TB, Chikungunya, Zika, MenB, plague | |
| Non-replicating viral vector; Ad26 (alone or with MVA boost) | Same platform as vaccine candidates for many pathogens | |||
| Non-replicating viral vector; replication defective simian adenovirus (GRAd) encoding SARS-CoV-2 S | Same platform as vaccine candidates for influenza | |||
| Non-replicating viral vector; Ad5 | Same platform as vaccine candidates for MERS | |||
| Non-replicating viral vector; ChAdOx1 | Phase 1/2 began April 2020, data expected in May 2020; Phase 2/3 trials expected to start by the middle of 2020 | NCT04324606 | Same platform as vaccine candidates for InfA, CHIKV, LASV, NORV, EBOV, RVF, HBV, VEE | |
| Non-replicating viral vector; MVA-S encoded | Pre-clinical | Same platform as vaccine candidates for EBOV | ||
| AdCOVID; single-dose, intranasal vaccine; non-replicating viral vector; adenovirus-based NasoVAX expressing spike protein | Same platform as vaccine candidates for HPV | |||
| Non-replicating viral vector; Ad5 S (GREVAX™ platform) | ||||
| Oral Ad5 S | ||||
| Adenovirus-based + HLA-matched peptides (Pan-Corona) | ||||
| Non-replicating viral vector; Oral Vaccine platform | ||||
| Recombinant deactivated rabies virus containing S1 | ||||
| Non-replicating viral vector, MVA expressing structural proteins | Same platform as vaccine candidates for Influenza | |||
| Non-replicating viral vector; dendritic cell-based vaccine | ||||
| Non-replicating viral vector; parainfluenza virus 5 (PIV5)-based vaccine expressing the spike protein | Same platform as vaccine candidates for MERS | |||
| Non-replicating viral vector; Adenovirus Type 5 vector (Ad5-nCoV) | Phase 2 started April 2020 | NCT04313127 ChiCTR2000030906ChiCTR2000031781 | Same platform as vaccine candidates for HIV, RSV, Influenza | |
| Protein subunit | Protein subunit, capsid-lide particle (CLP) | Pre-clinical | Phase 1 to begin by February 2021 | |
| Protein subunit, drosophila S2 insect cell expression system VLPs | ||||
| Protein subunit; S protein | ||||
| Protein subunit, S protein + adjuvant | ||||
| Protein subunit, VLP-recombinant protein + adjuvant | Same platform as vaccine candidates for HIV, SARS-CoV, Influenza | |||
| Protein subunit, native like trimeric subunit spike protein | Same platform as vaccine candidates for Inf H7N9 | |||
| Protein subunit; peptide | Same platform as vaccine candidates for MERS | |||
| Protein subunit; adjuvanted protein subunit (RBD) | Influenza, Ebola | |||
| Protein subunit; S protein | Same platform as vaccine candidates for HPV | |||
| Protein subunit; recombinant spike protein with Advax adjuvant | Same platform as vaccine candidates for Influenza, SARS-CoV (FDA approved vaccine) | |||
| Protein subunit; Ii-Key peptide | Same platform as vaccine candidates for RSV, CCHF, HPV, VZV, EBOV | |||
| Protein subunit; S protein | Same platform as vaccine candidates for cancer (NSCLC), HIV, malaria, Zika | |||
| PittCoVacc, Protein subunit, microneedle arrays S1 subunit | Same platform as vaccine candidates for Nipah, influenza, Ebola, Lassa | |||
| Protein subunit; S protein | Same platform as vaccine candidates for SARS | |||
| Protein subunit; COVID-19 XWG-03 truncated Spike proteins | ||||
| Protein subunit; S protein, baculovirus production | ||||
| Protein subunit; recombinant protein vaccine, utilizing baculovirus expression vector system technology | ||||
| NVX-CoV2373; Protein subunit; Full length S trimers/nanoparticle + Matrix M | ||||
| Protein subunit (gp-96 backbone) | Same platform as vaccine candidates for Ebola, Marburg, HIV, Zika, Influenza, HPV therapeutic vaccine, Breast Cancer | |||
| Protein subunit; peptide vaccine | Same platform as vaccine candidates for Ebola | |||
| Protein subunit; molecular clamp stabilized Spike protein | ||||
| Protein subunit; S1 or RBD protein | Same platform as vaccine candidates for cancer and infectious diseases, including malaria and anthrax | |||
| Protein subunit, recombinant protein, nanoparticles (based on S-protein and other epitopes) | ||||
| Protein subunit, adjuvanted microsphere peptide | Same platform as vaccine candidates for West nile, CHIKV, Ebola, Lassa, Zika, MERS Animal testing results expected in April 2020 | |||
| Protein subunit, peptide | Same platform as vaccine candidates for Zika, H7N9, CHIKV | |||
| Protein subunit, synthetic long peptide vaccine candidate for S and M proteins | Same platform as vaccine candidates for smallpox, monkeypox | |||
| Protein subunit; oral E. coli-based protein expression system of S and N proteins | Same platform as vaccine candidates for Ebola, Marburg, Lassa | |||
| Protein subunit, nanoparticle vaccine | ||||
| Protein subunit; spike based | ||||
| Protein subunit, recombinant S1-Fc fusion protein | ||||
| Protein subunit, recombinant protein | ||||
| Protein subunit, recombinant S protein in IC-BEVS | ||||
| Protein subunit; DPX-COVID-19, protein subunit, peptide antigens formulated in LNP | Start Phase 1 testing by summer 2020 | |||
| Protein subunit; plant virus nanotechnology formulated as injectable and microneedle patch | ||||
| Replicating viral vector | Replicating viral vector; YF17D Vector | Pre-clinical | ||
| Replicating viral vector; measles vector | Same platform as vaccine candidates for multiple candidates Start animal testing in April 2020 | |||
| Live attenuated virus, measles virus (S, N targets) | Same platform as vaccine candidates for multiple candidates | |||
| Replicating viral vector; horsepox vector; TNX-1800 | Same platform as vaccine candidates for EBOV, LASV, MARV, Inf (H7N9), RABV | |||
| Replicating viral vector, live viral vectored vaccine based on attenuated influenza virus backbone (intranasal) | Same platform as vaccine candidates for RABV, LASV, YFV, MERS, InfA, ZIKV, DengV, NIPV | |||
| Replicating viral vector, recombinant vaccine based on Influenza A virus, for the prevention of COVID-19 (intranasal) | Same platform as vaccine candidates for influenza | |||
| Replicating viral vector, replication-competent VSV chimeric virus technology (VSVdeltaG) delivering the SARS-CoV-2 Spike (S) glycoprotein | ||||
| Replicating viral vector, influenza vector expressing RBD | Same platform as vaccine candidates for cancer | |||
| M2-deficient single replication (M2SR) influenza vector | Same platform as vaccine candidates for influenza | |||
| RNA- vaccine | RNA; LNP-encapsulated mRNA cocktail encoding VLP | Pre-clinical | ||
| RNA; Replicating defective SARS-CoV-2 derived RNAs | Same platform as vaccine candidates for flu, rotavirus, norovirus, West Nile virus, and cancer | |||
| RNA; LNP-encapsulated mRNA | ||||
| RNA; several mRNA candidates | ||||
| RNA; LNP-encapsulated mRNA (mRNA 1273) | Phase 1 started March 2020, study ends June 2021; Phase 2 to start Q2 2020; Phase 3 to start fall 2020 | NCT04283461 | ||
| LUNAR-COV19; RNA; mRNA | Pre-clinical | |||
| RNA; saRNA | ||||
| RNA; mRNA | ||||
| RNA; mRNA; BNT162 | Phase 1/2 started April 2020 | EudraCT 2020-001038-36 | ||
| RNA; liposome-encapsulated mRNA | Pre-clinical | Animal studies begin in April 2020 | ||
| RNA; mRNA in an intranasal delivery system (cross-strain protective COV-2 mRNA) vaccine for high-risk populations | ||||
| RNA; ZIP-1642, vaccine consists of a combination of mRNA molecules, encoding multiple SARS-CoV-2 antigens | ||||
| Virus Like Particle | VLP; virus-like particle, based on RBD displayed on virus-like particle | Pre-clinical | ||
| VLP; plant-derived VLP | ||||
| VLP; ADDomerTM multiepitope display | ||||
| VLP (COVID-19 and SARS1) | ||||
| VLP; eVLP | Same platform as vaccine candidates for malaria | |||
| Enveloped virus-like particle (eVLP): Pan-coronavirus vaccine candidate, targeting COVID-19, SARS, and MERS, spike protein | Same platform as vaccine candidates for glioblastoma, cytomegalovirus, and Zika | |||
| Unknown | Self-assembling vaccine (fusion protein of a heat shock protein and Avidin, with biotinylated immunogenic peptides) | Pre-clinical | Animal study results by October 2020 | |
| LV-SMENP-DC Dendritic cells modified with lentiviral vector expressing synthetic minigene based on domains of selected viral proteins; administered with antigen-specific cytotoxic T lymphocytes | Phase 1/2 started March 2020 | NCT04276896 | ||
| Artificial antigen-presenting cells modified with lentiviral vector expressing synthetic minigene based on domains of selected viral proteins | Phase 1. Started February 2020 | NCT04299724 | ||
| ISR-50 | Pre-clinical | Animal study results expected in Q2 2020, Phase 1 begins Q4 2020 | ||
| Unknown | Phase 1 begins as early as September 2020 | |||
| Unknown | Phase 1 to start in summer 2020 | |||
Profile of vaccine developers
Among the established active vaccine candidates, more than 70% are being developed with the resources of private/industry vaccine developers, with the remaining portion comprising academics, institutions, and other nonprofit organizations. Although a good number of big multinational vaccine developers are taking initiatives to be involved in the development of a vaccine against SARS-CoV-2, currently, many of the lead vaccine developers are small and/or inexperienced or from an academic background in large-scale vaccine production.36 Therefore, it is essential to establish convinced coordination of vaccine manufacturing to supply the capability that meets the requirements. To date, US-based developers of the established active vaccine candidates are engaged the most in vaccine development activity against COVID-19, which is related to other vaccine developers around the world. In addition to the above, vaccine development efforts against COVID-19 have also been reported from China. The prime developers of probable SARS-CoV-2 vaccine candidates are distributed abundantly across countries, which together comprise over three-quarters of the people living on earth. However, currently, there is no published data available on vaccine development activity from Africa or Latin America, besides the vaccine manufacturing capacity and regulatory frameworks present in these regions. The epidemiology of COVID-19 may differ geographically, and it is far probable that effective management of the pandemic will require more participation and coordination between vaccine research and development activities.37
In early April, the Coalition for Epidemic Preparedness Innovations (CEPI) observed that a hundred and fifteen vaccine candidates have been in development as either exploratory/preclinical ventures or in phase I safety trials in human participants.37 The data in Table 1 originates from public sources, WHO websites, wikipedia, and the Milken Institute web page for corona monitoring for development on emerging vaccine candidates scheduled in 2020.38
Use of readily available vaccines
The absence of any vaccine against SARS-CoV-2, compelled frontline health workers to use a readily available vaccine, which has heterologous effects or nonspecific effects. It is presumed that vaccines that have nonspecific effects can have benefits beyond the disease they prevent. Keeping this in mind, several country old vaccines were tried as possible stopgap vaccines. Some country old readily available vaccines widely tried include the Bacille Calmette-Guérin (BCG) and the Measles vaccines. Through a prospective study39 to find out among 178 countries, which were suitable for its study, 131 countries had national programs of BCG vaccination still running currently. The study also showed that the death rate has been reducing in the countries with running BCG vaccination compared to the countries devoid of it.40
In March 2020, a randomized trial of BCG vaccine was initiated to reduce the COVID‑1938,39 in the Netherlands with a moderate number of frontline health workers.40 Simultaneously, a similar randomized trial has started in Australia in a similar population of health workers with four times larger numbers.41,42 Healthcare employees from Boston and Houston are going to be recruited in another BCG trial;43 similarly, Cairo University, Egypt has registered healthcare employees for a BCG vaccine trial.44 An added trial in the Netherlands is testing whether this vaccine delivers protection for older people.45 A trial of BCG on healthcare employees in Medellín, Colombia has been registered.46 Other trials of BCG for healthcare givers were registered in May 2020 in Brazil,47 France,48 and Denmark49 (Table 2). However, in April 2020, WHO issued a statement, which indicates that there is still no material evidence that the BCG vaccine protects COVID-19 patients.50–52 Owing to the absence of evidence, WHO declared that currently, they do not endorse BCG vaccination intended for the prevention of COVID-19.53,54
Table 2.
Ongoing trials of readily available vaccines for clinical trials against SARS-CoV-2
| Clinical trials for COVID-19 |
||||||
|---|---|---|---|---|---|---|
| Vaccine | Vaccination method | Identifier ID | Phase | Locations | Participants | Period |
| BCG | BCG vaccine Danish strain 1331 Concentrate and solvent for solution for intradermal injection | EudraCT 2020–000919-69 | Ongoing | Netherlands | 1000 | Ongoing from March, 2020 |
| BCG | multicentre, open label randomized controlled trial | NCT04327206 | Phase-III | Australia | 4170 | March to October, 2020 |
| BCG | randomized to vaccine: placebo in a 1:1 ratio | NCT04348370 | Phase-IV | Boston and Houston, USA | 1800 | April 20, 2020 to May 2021 |
| BCG | intradermal injection of BCG vaccine or placebo normal saline in a 2:1 ratio | NCT04350931 | Phase-III | Cairo, Egypt | 900 | April 20, 2020 to October, 2020 |
| BCG | randomized to vaccine and placebo using a 1: 1 allocation ratio | NCT04362124 | Phase-III | Medellín, Colombia | 1000 | April, 2020 to June, 2021 |
| BCG | randomized parallel study between two groups participants receiving vaccine or placebo | NCT04369794 | Phase-IV | Brazil | 1000 | June 2020 to June 2021 |
| BCG | randomized controlled trial of vaccine with placebo powder and solvent for dispersion for injection | EudraCT 2020–001678-31 | Ongoing | France | 1120 | Ongoing from April 17, 2020 |
| BCG | multi-center randomized placebo-controlled parallel study | NCT04373291 | Phase-III | Denmark | 1500 | May, 2020 to December 2020 |
| Measles-Mumps-Rubella (MMR) Vaccine | randomized parallel assignment | NCT04357028 | Phase-III | Cairo, Egypt | 200 | May 2020 to November, 2020 |
Adjuvants
Adjuvants can boost immunogenicity and make lower doses feasible, thus allowing the vaccination of more people deprived of conceding safety. To date, several vaccine candidate developers have specified strategies to develop adjuvanted vaccines against SARS-CoV-2, and vaccine developers have committed to making licensed adjuvants, AS03, MF59, and CpG 1018, accessible for use with novel coronavirus vaccines.21 However, it must be clear that adjuvants are used only with subunit vaccines. Community data on the detailed SARS-CoV-2 antigen(s) used in vaccine development is inadequate. The maximum candidates for which information is out there aim to induce neutralizing antibodies against the viral spike (S) protein, averting uptake via the human ACE2 receptor. However, it is uncertain how different forms and/or alternatives of the S protein utilized in different vaccine candidates relate to each other.27 Experience with SARS vaccine development indicates the potential for improvement in the immune effects of various antigens, which may be a topic of debate and will be relevant for the development of new vaccine candidates.55,56
Aluminum hydroxide:
Aluminum hydroxide (alum) is the most commonly used agent as an adjuvant. In non-human primates (NHPs), when purified inactivated SARS-CoV vaccine with and without alum was administered to Cynomolgus and Rhesus macaques, it protected the monkeys from the challenge of SARS-CoV without causing any adverse reactions. Inactivated SARS-CoV-2 vaccine with alum as an adjuvant has been tried.55
Matrix-M:
Recombinant Spike Protein Nanoparticle vaccine SARS-CoV-2 rS has been tried with Matrix-M as an adjuvant.55
Delta inulin (Advax™)
Delta inulin (DI) is an isoform of inulin polysaccharide, which is stable at a higher temperature and has better immune potency. When co-administered with antigen it activates the complement system and helps mount a robust antigen-specific adaptive immune response consisting of both antibody and cell-mediated immunity. Vaccine developers use Advax as an adjuvant and recombinant spike protein as vaccine.55
CoVaccine HT
CoVaccine HT is an oil-in-water emulsion consisting of sucrose fatty acid sulfate ester (SFASE) and squalene, which has been reported to induce both humoral and cell-mediated immunity and has been tried as an adjuvant for SARS-CoV-2 vaccine candidates.55,56
MF59
MF59 has been used as an adjuvant by different developers from Germany and Australia, who employ novel molecular-clam technology in their COVID-19 vaccine candidates.56
AS03
AS03 has been used as an adjuvant (squalene-based) in some recombinant protein vaccine candidates.56
Glucopyranosyl lipid adjuvant (GLA)
Glucopyranosyl lipid adjuvant (GLA), a synthetic analogue of the MPL has been utilized as novel adjuvants by the Infectious Disease Research Institute (IDRI, Seattle, USA) for its SARS-CoV-2 VLP vaccine development.55
CpG 1018
CpG 1018 is an adjuvant based on TLR9 agonists, which was developed in the USA and is being used in a recombinant spike protein (S-Trimer) vaccine candidate against COVID-19 in China. The CpG 1018 adjuvant has been used in an FDA (Food and Drug Administration)-approved hepatitis B vaccine.55
Different newly developed potential vaccines have completed phase-I or phase-II trials
The different newly developed vaccine candidates that are thought to have potentials against SARS-CoV-2 are antibodies, antivirals, cell-based, RNA-based, and scanning compounds to be repurposed.39 (Table 1)
Ad5-nCoV vaccine
Ad5-nCoV is the first reported vaccine against SARS-CoV-2, and it is now going through a clinical trial in China. The vaccine candidate Ad5-nCoV is a genetically engineered vaccine candidate with the replication-defective adenovirus type 5 as the vector to express SARS-CoV-2 spike protein. Outcomes from preclinical studies on Ad5-nCoV in animals show that the vaccine candidate induces a strong immune response in animal models. Studies on preclinical safety in animal models also confirmed a decent profile of safety.57 (Table 3)
Table 3.
Different newly developed potential vaccine candidates against SARS-CoV-2 completed phase-I or phase-II trials
| Clinical trials for COVID-19 |
|||||||
|---|---|---|---|---|---|---|---|
| Vaccine candidates | Technology | Target | Identifier ID | Phase | Participants | Location | Duration |
| Ad5-nCoV | recombinant adenovirus type 5 vector | Spike | NCT04341389 | Phase II interventional trial for dosing and side effects | 500 | China | March 2020 to December 2020 |
| Ad5-nCoV | recombinant adenovirus type 5 vector | Spike | NCT04313127 | Phase I | 108 | China | March 2020 to December 2020 |
| BNT162 (a1, b1, b2, c2) | RNA | 3CLpro, NSP5, Mpro, others | EudraCT No: 2020–001038-36 | Phase I–II of four vaccines, dose escalation, parallel cohorts | 196 | Germany | April 2020 to May 2021 |
| ChAdOx1 nCoV-19/AZD1222 | adenovirus vector | Spike |
NCT04324606 NCT04444674 NCT04536051 |
Phase I–II and III randomized, placebo-controlled, multiple sites | 510 | United Kingdom, South Africa, Brazil | April 2020 to May 2021 |
| INO-4800 | DNA plasmid transported by electroporation | Spike | NCT04336410 | Phase I–II | 140 | United States, South Korea | April 2020 to November 2020 |
| mRNA-1273 | lipid nanoparticle dispersion containing messenger RNA | Spike | NCT04283461 | Phase I | 45 | United States | March 2020 to Spring-Summer 2021 |
| Covid-19/aAPC | lentiviral vector, pathogen-specific artificial antigen presenting dendritic cells | Spike | NCT04299724 | Phase I | 100 | China | March 2020 to 2023 |
| LV-SMENP-DC | lentiviral minigene vaccine, dendritic cells modified with lentiviral vector | Undisclosed | NCT04276896 | Phase I | 100 | China | March 2020 to 2023 |
| bacTRL-Spike | DNA, bacterial medium (oral) | Spike | NCT04334980 | Phase I | 84 | Canada | April 2020 to December 2021 |
| unnamed | inactivated SARS-CoV-2 virus | Whole virus | NCT04352608 | Phase I–II randomized, double-blinded, single-center, placebo-controlled | 744 | China | April 2020 to December 2020 |
| unnamed | inactivated COVID-19 virus (vero cells) | Undisclosed | ChiCTR2000031809 | Phase I | 288 | China | April 2020 to November 2021 |
Clinical trial of Ad5-nCoV vaccine
Phase-I Trial: ChiCTR2000030906. The study is due to complete in December 2021.57
Phase-II Trial: NCT04341389. A randomized, double-blind, placebo-controlled phase II clinical trial, which is ongoing and will assess the safety and immunogenicity of the recombinant SARS-CoV vaccine (adenovirus vector) in healthy adults of more than 18 years.58 The present clinical trial is intended to estimate the immunogenicity and safety of Ad5-nCoV, which codes for a whole spike (S) protein of SARS-CoV-2. The phase-II trial started on 12th April, 2020.58,59 (Table 3)
BNT162 (a1, b1, b2, c2) vaccine:
European Union Trial: EudraCT No: 2020–001038-36
A multi-site phase I/II, two-part, dose-escalated trial for examining the safety and immunogenicity of four prophylactic SARS-CoV-2 RNA vaccines against SARS-CoV-2 using different dosing regimens in healthy adults aged more than 65 years.60 (Table 3)
ChAdOx1 nCoV-19 vaccine
Phase-I/II Trial: NCT432460661
Phase-III Trial: NCT0444467462
ChAdOx1 nCoV-19 is manufactured from the ChAdOx1 virus, which is a debilitating form of the common cold virus (adenovirus) that is contagious to chimpanzees, the ChAdOx1 virus has been genetically modified so that it does not infect humans. The viral genetic material of SARS-CoV-2 has been added to the ChAdOx1 construct that is used to make proteins from the COVID-19 virus (SARS-CoV-2) called spike glycoprotein (S).63 This protein is usually found on the surface of SARS-CoV-2 and has a crucial role in the infection cascade of SARS-CoV-2. SARS-CoV-2 uses its spike protein to bind to the ACE2 receptors on human cells to gain access to the cells to initiate an infection.64 (Table 3)
The clinical trials of the ChAdOx1 nCoV-19 vaccine, now known as Oxford’s COVID-19 vaccine, begun in April 2020. The main focus of the phases I, II, and III studies is to assess the efficacy of the vaccine against COVID-19, its possible side effects, and whether it induces good immune responses.65
Phase I/II
A total of 1,077 participants were recruited at the commencement in April 2020 across multiple study sites in Oxford, Southampton, London, and Bristol. The participants were randomly allocated to receive either the ChAdOx1 nCoV-19 vaccine or a licensed vaccine (MenACWY) that is used as a control for comparison. The dose used in this trial was chosen based on previous experiences with other ChAdOx1-based vaccines. There was also a separate small group of ten volunteers who received two doses of ChAdOx1 nCoV-19 4 weeks apart. The study participants were not aware that they have received the ChAdOx1 nCoV-19 vaccine until the end of the trial. After receiving the vaccine, the participants recorded any symptoms experienced for 7 days. They also recorded how they felt (unwell or well) for the following 3 weeks. Following the vaccination visit, the participants attended a series of follow-up visits to check their conditions, and their blood samples were taken. These blood samples were used to assess the immune response to the vaccine, by testing the neutralizing antibodies and T cell responses.63,64,65
Phase II/III
In total, this study will enroll up to 10,560 adults and children across the UK. The phase II part of the study involves expanding the age range of the participants, to include a small number of adults and children: aged between 56–69 years, over 70 years, and 5–12 years. For these groups, the researchers are assessing the immune responses to the vaccine in people of different ages, to find out whether there is variation in how well the immune system responds in older people or children. The phase III part of the study involves assessing how the vaccine works in a large number of people over the age of 18 years. This group will allow the assessment of how well the vaccine works to prevent people from becoming infected with COVID-19. As of mid-July 2020, over 8,000 people have been recruited. Following the same protocol as above, the adult participants were randomized to receive one or two doses of either the ChAdOx1 nCoV-19 vaccine or a licensed vaccine (MenACWY) that is used as a control for comparison. To recruit the large number of participants needed for this trial, multiple clinical research sites across the UK are involved in delivering the study. This is a collaborative effort led by the University of Oxford and a full list of the study sites is available on the trial website.65
The phase III studies have recently been initiated in South Africa, by the leadership of the University of Witwatersrand in collaboration with the Medical Research Council, South Africa, Bill and Melinda Gates Foundation, and the University of Oxford,62 to assess the vaccine in populations of South African adults with and without HIV-infection. The data is examined collectively across all the trial sites, and any cases of COVID-19 are recorded to assess the effectiveness of the vaccine.65
To assess whether the vaccine works to protect from COVID-19, the statisticians in the team will compare the number of infections in the control group with the number of infections in the vaccinated group. How quickly the trial team led by the University of Oxford will reach the numbers required is dependent upon the levels of virus transmission in the community. It is thought to follow the current low transmission levels in the UK; this could take several months. To increase the chances of achieving an efficacy result sooner, the trial team led by the University of Oxford, prioritized those who have a higher chance of being exposed to SARS-CoV-2, such as frontline healthcare workers, frontline support staff, and public-facing key workers. Conducting studies in multiple locations where transmission levels vary also increases the chances of reaching an efficacy endpoint sooner.64,65 Currently, investigators involved in the ChAdOx1 nCoV-19 trials observed that the vaccine showed signs of priming the immune system of the rhesus macaque monkeys to fend off the deadly virus and showed no indications of adverse effects.
INO-4800 vaccine
Phase -I Trial: NCT0433641066
INO-4800 is a DNA vaccine candidate matched to SARS-CoV-2, which causes COVID-19. This one-of-a-kind platform brings optimized DNA into cells, where this optimized DNA is translated into proteins that activate the immune system to generate a robust targeted T cell and antibody response [44]. DNA vaccines are composed of optimized DNA plasmids, which are small circles of double-stranded DNA that are synthesized or reorganized by a computer sequencing technology and designed to produce a specific immune response in the body. Injection of the DNA plasmid into a patient generates robust in vivo monoclonal antibody production.66,67 (Table 3)
The investigational DNA immunotherapy, INO-4800 (GLS-5300), is being developed. It is delivered as a vaccine intramuscularly, using the electroporation delivery device. The vaccine was well-tolerated and demonstrated high immune responses when previously used against MERS-CoV, with high response in 94% of patients in the early-stage clinical trial in July 2019. It also generated broad-based T cell responses in 88% of the subjects. The developers started preclinical testing for SARS-CoV-2. The development is supported by a ten million dollar grant from the Coalition for Epidemic Preparedness Innovations (CEPI). In addition,, the developers have received five million dollars as a grant from the Bill & Melinda Gates Foundation to accelerate the testing and development of their electroporation delivery device for the intradermal delivery of INO-4800.67
In the preclinical animal challenge study, INO-4800 provided full protection against SARS-CoV-2 replication in the lungs in mice challenged with the virus.68
The phase 1 trial started in April 2020 with 40 volunteers in Philadelphia, PA (University of Pennsylvania) and Kansas City, MO. A total of 94% of the phase 1 trial participants demonstrated overall immune responses at 6 weeks after two doses of INO-4800. Through the 8th week, the INO-4800 regimen was deemed safe and well-tolerated with no serious adverse events; all reported adverse events were Grade 1 in severity.
The INO-4800 Phase II/III U.S. clinical trial is preparing to start this summer, according to a report from the developers at the end of April. The developers have agreed to expand their manufacturing partnership with a German-based contract manufacturer, to support large-scale manufacturing of the INOVIO investigational DNA vaccine, with plans to produce one million doses of INO-4800 by the end of 2020.67
A new phase I/II two-stage trial of INO-4800, the first clinical study of COVID-19 vaccine in Korea,67 is aimed to start in June and is funded by the Coalition for Epidemic Preparedness Innovations and supported by the Korea CDC/Korea NIH. Under normal circumstances, it would generally take several years to start clinical trials of a new vaccine. During the COVID-19 pandemic, however, the trial in Korea will be conducted within two months after a similar clinical study began in the USA in early April 2020. Seoul National University Hospital said it administered the first dose of INO-4800 vaccine to a patient in his 40s on the 15th of July, 2020.67,68
mRNA-1273 vaccine
Phase-I Trial: NCT0428346169
The investigational vaccine is going through development on a genetic platform called mRNA (messenger RNA). The investigational vaccine directs the cells of the body to express a virus protein that it is hoped will elicit a robust immune response.69 The mRNA-1273 vaccine has shown promise in animal models, and this is the first trial in humans69 (Table 3). The ongoing trial entitled Phase I, open-label, dose-ranging study of the safety and immunogenicity of 2019-nCoV vaccine (mRNA-1273) in healthy adults under the supervision of the National Institute of Allergy and Infectious Diseases (NIAID), USA.
Novel lipid nanoparticle (LNP)-encapsulated mRNA vaccine (mRNA-1273) against COVID-19 encoding for a prefusion stabilized form of the Spike (S) protein. The developers of the mRNA-1273 have shipped the first batch to the National Institute of Allergy and Infectious Diseases (NIAID) for use in a planned phase I study in the U.S. The primary aim of the Phase I open-label, dose-ranging trial study, which is yet to recruit patients at the deadline, is to evaluate the safety and reactogenicity of a 2-dose vaccination schedule of mRNA-1273, given 28 days apart, across 3 dosages in healthy adults.69 The first patient was dosed on March 16 and phase I is still ongoing and expected to conclude on June 1. The participants will be followed for one year. An unusual aspect is that this vaccine has not been tested in laboratory animals as is the rule. The phase II trials are expected to start this spring since the developers of the mRNA-1273 have received the green light to initiate the trial.69
Covid-19/aAPC vaccine
Phase-I Trial: NCT0429972470
Designed after the complete analysis of the SARS-CoV-2 genome and search for potential immunogenic targets, a synthetic minigene has been engineered on the basis of the conserved domains of the SARS-CoV-2 structural proteins and a polyprotein protease. The infection by SARS-CoV-2 is facilitated through binding of the spike protein (S) to the ACE2 receptor, and the replication of the viral genome depends on the molecular mechanisms of the viral protein. The Covid-19/aAPC vaccine trial aims to develop a universal vaccine and test innovative SARS-CoV-2 minigenes based on multiple viral genes, using an operative lentiviral vector system (NHP/TYF) to express the viral proteins and immune-modulatory genes to modify the artificial antigen-presenting cells (aAPC) and to activate T cells.70 (Table 3)
LV-SMENP-DC vaccine
Phase-I Trial: NCT0427689671
This is strongly based on the genomic sequence of the novel coronavirus or SARS-CoV-2, using the selected, conserved, and critical structural and protease protein domains to manufacture lentiviral SMENP minigenes to express SARS-CoV-2 antigens. The LV-SMENP-DC vaccine is made by modifying DC with lentivirus vectors expressing SARS-CoV-2 minigene SMENP and immune-modulatory genes. The CTLs will be activated by LV-DC presenting SARS-CoV-2 specific antigens.71 (Table 3)
bacTRL-Spike vaccine
Phase-I Trial: NCT0433498072
bacTRL-Spike is an oral vaccine. An individual dose of bacTRL-Spike contains the bacterial medium with either 1 billion (Group 1A), 3 billion (Group 2A), or 10 billion (Group 3A) colony-forming-units (CFU) of live Bifidobacterium longum, which has been modified to deliver plasmids containing synthetic DNA encoding the spike protein from SARS-CoV-2. The placebo will comprise a bacterial medium without bacteria.72 (Table 3)
Inactivated SARS-CoV in some unnamed candidate vaccines
Phase-I Trial: NCT0435260873
The SARS-CoV Z-1 viral strain is used for inactivated SARS-CoV vaccine candidate and was isolated from the blood of the first SARS positive patient from China, during 2003. The vaccine is formaldehyde inactivated whole virus prepared in cultured Vero cells. At present this virus has been used in a phase I clinical trial as a potential vaccine against SARS-CoV-2.73 (Table 3)
Inactivated SARS-CoV-2 virus in vero cells
Phase-I Trial: ChiCTR200003180974
The SARS-CoV Sino3 strain was propagated in Vero cells, obtained from the American type culture collection that was free of adventitious agents inactivated by β-propiolactone. When the total inactivation has been verified, the process of vaccination was initiated.74 The placebo vaccine for the control consisted of only sterile saline and aluminum hydroxide. The safety was assessed and the dose range was selected on the basis of preclinical trials in mice, rats, and rhesus monkeys, which need an aluminum hydroxide adjuvant.74 (Table 3)
Other vaccine approaches
Currently, a vaccine against SARS-CoV-2 is developed by using the technology of the Hyleukin-7 platform.75 Through the fusion of interleukin-7 (IL-7) to hy Fc, the Hyleukin-7 platform improves the immune responses anticipated to hybridize IgD and IgG4 for long-acting effects of Fc fusion proteins.76,77 IgD has a stretchy hinge structure that assists in the biological activity of the Fc-fusion protein. IgG4 has an unexposed junction site that decreases adverse immunogenicity by averting antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).78 In the influenza A virus infection model, it has been described that improved vaccine efficacy shows the accretion of pulmonary T cells and an increase in plasmacytoid dendritic cells by treating with Fc-fused IL-7.79 Along with the vaccine technologies described here, some other identified hurdles must be overcome to develop a successful vaccine. Antibody-dependent enhancement (ADE) of disease is a phenomenon in which virus-specific antibodies allow the virus to enter into the host cell via the Fc receptor pathway, leading to higher viral infection.80 However, antibodies produced by vaccination may promote viral infectivity. Successful administration of ADE has been reported in some vaccines against Dengue and Zika viruses.81 Although the occurrence of ADE of SARS-CoV-2 has not yet been established, potential ADE of MERS-CoV and SARS-CoV has been observed in an in vitro model system.82 Numerous approaches have been introduced in vaccine development to circumvent the antagonistic effects of ADE. For example, the nucleocapsid (N) protein may be an alternative target to overcome ADE. Though the N protein is not a surface protein, antibodies induced by the N protein-based vaccine will not be able to facilitate viral entry into the host cell. DNA vaccine candidates targeting SARS-CoV N protein as an immunogen can produce N-specific humoral and cellular immune responses.83 Another major difficulty for vaccine development is the higher mutation rates of RNA viruses compared with the DNA viruses, consequential in higher genetic diversity.84 Moreover, the RBD of the S protein is the most flexible region in the SARS-CoV-2 genome.85 Therefore, the ADE and the higher genetic diversity should be considered as important factors for the design of new vaccine candidates against SARS-CoV-2.86
Potential limitations
There is a possibility that vaccines in the process of development may not be safe or effective or the effective vaccines may not be marketed or may be marketed but cannot be administered owing to different issues.87 From the preclinical research to the successful administration of a vaccine, at present, it is required to undergo a lengthy and complicated process (Figure 3). From start to finish, a successful vaccine development may take 15–20 years. It was found that between 2006 and 2015, the success rate of obtaining approval for vaccines from phase I to successful phase III trials was 16.2%21 and CEPI indicates a potential success rate of only 10% for the development of vaccine candidates in 2020.87 The rapid development and urgency of creating a vaccine against the COVID‑19 pandemic may increase the risk and failure rates of developing a safe and effective vaccine.88 To assess vaccine efficacy using COVID‑19-specific animal models, such as ACE2-transgenic mice, other laboratory animals, and non-human primates, indicates a necessity for biosafety-level 3 containment measures for manual supervision of living viruses, and able coordination between academics and industrial persons to ensure standardized safety procedures.87 In April 2020 CEPI stated that robust cooperation and coordination between vaccine inventors, controllers, public health bodies, funders, policymakers, and administrations will be needed to confirm that auspicious late-stage vaccine candidates can be manufactured in sufficient quantities and impartially distributed to all affected areas, mainly in low-resource countries.87 The general flu vaccine is largely produced by inserting the virus into the eggs of chickens but this method is not applicable for the SARS-CoV-2 vaccine, as the virus is not able to replicate inside eggs.89 Industrial analysis of vaccine development history shows a failure rate of 84–90%.47,90 To assess the potential for vaccine efficacy, exceptional computer simulations91 and new symptoms specific to animal models showing symptoms similar to humans upon infection by SARS-CoV-2 are being developed. These approaches remain internationally unapproved for the unknown COVID‑19 virus.92 Among the confirmed active vaccine candidates, about 70% are being developed by developers from private industries, with the remaining being academic, government coalitions, and health organizations93 At present, a large number of the COVID-19 vaccine developers are small firms or university research teams with little experience in successful vaccine design and limited capacity for advanced clinical trial costs and manufacturing.94 The general geographic distribution of the SARS-CoV-2 vaccine development involves agencies belonging to the USA and Canada, which together have about 46% of the world’s dynamic vaccine research, relative to 36% in Asian countries, including China, and 18% in European countries.94,95
Figure 3.

Timeline cascade showing the development of two popular vaccines against Polio and Ebola. However, still there are no vaccine available for SARS-CoV-1 for approximately more than 15 years and MERS for more than 6 years of first recognized case. Similar to the other betacoronavirusus, no vaccines are still available for SARS-CoV-2, which is also a betacoronavirusus ‘only’ after 14 months of first recognized case.
Conclusions
Various private companies and public-funded institutions are at different stages of developing a SARS-CoV-2 vaccine. Still, massive funds and extensive studies are required for clinical trials of vaccine development to address the present serious public health risk caused by the COVID-19 pandemic (Figure 3). Profitable marketplaces for vaccines, chiefly against evolving infectious diseases, are quite inadequate because vaccine development requires lots of money with time,93 and the ambiguity in cost-effectiveness. Thus, the development of infrastructure and conducive environment for vaccine research is being hampered because of the massive gap between academic, institutional research, and commercial markets. Therefore, efficient, continuing research programs must be established with universally supported projects for the progress of vaccine development against newly emerged infectious diseases.94,95 Therefore, the cooperation of various institutions, academics, governments, and pharmaceutical companies is inevitably necessary to prevent further spread of current and future outbreaks.
Supplementary Material
Acknowledgments
The authors acknowledge staff of Virology Division, ICMR-National Institute of Cholera and Enteric Diseases, Kolkata, India for help and support.
Biography
MB and MKS conceived and designed the study. MB and SD established the search strategy. MB and SN extracted the data. MB, SD, MKS, and SN wrote the article. All the authors read the manuscript before they have given the final approval for publication.
Funding Statement
No funding was received for this research
Authors’ information (optional)
Dr. Mihir Bhatta [Technical Officer, National HIV Reference Laboratory], Ms. Srijita Nandi [Research Officer, National HIV Reference Laboratory], Dr. Shanta Dutta [Scientist-G & Director, ICMR-National Institute of Cholera & Enteric Diseases] and Dr. Malay Kumar Saha [Scientist-F, Virology Division; In-Charge, National HIV Reference Laboratory; Focal Person, Regional Institute: HIV Surveillance] ICMR-National Institute of Cholera & Enteric Diseases P-33, CIT Road, Scheme - XM, Beliaghata, Kolkata-700 010, West Bengal, India.
Ethics approval and consent to participate
Not applicable as published data were analyzed for this study.
Availability of data and material
All data generated or analyzed are included in this article
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2020.1865774.
References
- 1.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauci AS, Lane HC, Redfield RR.. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382(13):1268–69. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakraborty D, Debnath F, Biswas S, Bhatta M, Ganguly S, Deb AK, Saha MK, Dutta S. Exploring repurposing potential of existing drugs in the management of COVID-19 epidemic: a critical review. J Clin Med Res. 2020;12(8):463–71. doi: 10.14740/jocmr4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32(6):567–82. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggieri A, Di Trani L, Gatto I, Franco M, Vignolo E, Bedini B, Elia G, Buonavoglia C. Canine coronavirus induces apoptosis in cultured cells. Vet Microbiol. 2007;121(1–2):64–72A. doi: 10.1016/j.vetmic.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paltrinieri S, Metzger C, Battilani M, Pocacqua V, Gelain M, Giordano A. Serum α1-acid glycoprotein (AGP) concentration in non-symptomatic cats with feline coronavirus (FCoV) infection. J Feline Med Surg. 2007;9(4):271–7A. doi: 10.1016/j.jfms.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripp RA, Haynes LM, Moore D, Anderson B, Tamin A, Harcourt BH, Jones LP, Yilla M, Babcock GJ, Greenough T, et al. Monoclonal antibodies to SARS-associated coronavirus (SARS-CoV): identification of neutralizing and antibodies reactive to S, N, M and E viral proteins. J Virol Methods. 2005;128(1–2):21–28. doi: 10.1016/j.jviromet.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shehata MM, Gomaa MR, Ali MA, Kayali G. Middle East respiratory syndrome coronavirus: a comprehensive review. Front Med. 2016;10(2):120–36. doi: 10.1007/s11684-016-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang S, Lu L, Du L. Development of SARS vaccines and therapeutics is still needed. Future Virol. 2013;8(1):1–2. doi: 10.2217/fvl.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong CY, Ong HK, Yeap SK, Ho KL, Tan WS. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front Microbiol. 2019;10:1781. doi: 10.3389/fmicb.2019.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modjarrad K, Roberts CC, Mills KT, Castellano AR, Paolino K, Muthumani K, Reuschel EL, Robb ML, Racine T, Oh M-D, et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. The Lancet Infectious Diseases. 2019;19(9):1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler D. SARS veterans tackle coronavirus. Nature. 2012;490(7418):20. Bibcode: 2012 Nature 490-20B. doi: 10.1038/490020a. [DOI] [PubMed] [Google Scholar]
- 14.Estola T. Coronaviruses, a new group of animal RNA viruses. Avian Dis. 1970;14(2):330–36. doi: 10.2307/1588476. [DOI] [PubMed] [Google Scholar]
- 15.Almeida JD, Berry DM, Cunningham CH, Hamre D, Hofstad MS, Mallucci L, McIntosh K, Tyrrell DAJ. Virology: coronaviruses. Nature. 1968;220:650. [Google Scholar]
- 16.Tyrrell DA, Fielder M. Cold Wars: the fight against the common cold. Oxford: Oxford University Press; 2002. p. 96. ISBN 0 19 263285 X [Google Scholar]
- 17.Payne S. ed. Chapter 17 - family coronaviridae, viruses. USA: Academic Press; 2017. p. 149–58. [Google Scholar]
- 18.Su S, Wong G, Shi W, Liu J, Lai AC, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–69. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.McGrail S. Sanofi, GSK partner to develop adjuvanted COVID-19 vaccine. In: Murphy KS. Denvers: Pharma News Intelligence, Xtelligent Healthcare Media; 2020. [Google Scholar]
- 22.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization timeline - COVID-19 . World health organization. 27 April 2020
- 24.Kim E, Okada K, Kenniston T, Raj VS, AlHajri MM, Farag EA, AlHajri F, Osterhaus ADME, Haagmans BL, Gambotto A, et al. Immunogenicity of an adenoviral-based Middle East Respiratory Syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32(45):5975–82. doi: 10.1016/j.vaccine.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenough TC, Babcock GJ, Roberts A, Hernandez HJ, Thomas WD, Coccia JA, Graziano R, Srinivasan M, Lowy I, Finberg R, et al. Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J Infect Dis. 2005;191(4):507–14A. doi: 10.1086/427242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts A, Thomas WD, Guarner J, Lamirande EW, Babcock GJ, Greenough TC, Vogel L, Hayes N, Sullivan J, Zaki S, et al. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis. 2006;193(5):685–92. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao W, Tamin A, Soloff A, D’Aiuto L, Nwanegbo E, Robbins PD, Bellini WJ, Barratt-Boyes S, Gambotto A. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362(9399):1895–96. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boodman E. Researchers rush to test coronavirus vaccine inpeople without knowing how well it works in animals. Boston: STAT. 2020. p. 1. [Google Scholar]
- 29.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–46. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 30.Hotez PJ, Corry DB, Bottazzi ME. COVID-19 vaccine design: the Janus face of immune enhancement. Nat Rev Immunol. 2020;20:347–48. doi: 10.1038/s41577-020-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217:e20200678. doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;5:S1931–S5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funk CD, Laferrière C, Ardakani A. A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. Front Pharmacol. 2020;11:937. doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortis D. On determining the age distribution of COVID-19 pandemic. Front Public Health. 2020;8:202. doi: 10.3389/fpubh.2020.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatta M, Nandi S, Dutta N, Dutta S, Saha MK. HIV care among elderly population: systematic review and meta-analysis. AIDS Res Hum Retroviruses. 2020;36(6):475–89. doi: 10.1089/aid.2019.0098. [DOI] [PubMed] [Google Scholar]
- 36.Le T Thanh, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nature Reviews Drug Discovery. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed]
- 37.Lee CE, Welker K, Perlmutter-Gumbiner E. Health officials eyeing at least one of 14 potential coronavirus vaccines to fast-track. NBC News. 2020.
- 38.COVID-19 treatment and vaccine tracker (PDF). Milken Institute. [Google Scholar]
- 39.Hegarty P, Kamat A, Zafirakis H, Dinardo A. BCG vaccination may be protective against Covid-19. Researchgate preprint. 2020. doi: 10.13140/RG.2.2.35948.10880 [DOI]
- 40.Kleinnijenhuis J, van Crevel R, Netea MG. Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):29–35. doi: 10.1093/trstmh/tru168. [DOI] [PubMed] [Google Scholar]
- 41.de Vrieze J. Can a century-old TB vaccine steel the immune system against the new coronavirus? Science. 2020;370:8297. doi: 10.1126/science.370.6519.895. [DOI] [Google Scholar]
- 42.Bacille Calmette-Guérin (BCG) vaccination and COVID-19 . World health organization (WHO). April 2020.
- 43.EudraCT 2020-000919-69 . EU clinical trials register.
- 44.Murdoch Children’s Research Institute to trial preventative vaccine for COVID-19 healthcare workers . Murdoch children’s research institute.
- 45.BCG Vaccination to Protect Healthcare Workers Against COVID-19 . ClinicalTrials.gov.
- 46.BCG Vaccine for Health Care Workers as Defence Against SARS-COV2 . ClinicalTrials.gov.
- 47.Application of BCG Vaccine for Immune-prophylaxis Among Egyptian Healthcare Workers During the Pandemic of COVID-19 . ClinicalTrials.gov.
- 48.EudraCT Number 2020-001591-15 . EU Clinical Trials Register.
- 49.Performance Evaluation of BCG vs COVID-19 . ClinicalTrials.gov.
- 50.COVID-19: BCG As Therapeutic Vaccine, Transmission Limitation, and Immunoglobulin Enhancement - Full Text View - ClinicalTrials.gov . US National Library of Medicine, National Institutes of Health. clinicaltrials.gov. [Google Scholar]
- 51.EudraCT Number 2020-001678-31 . EU clinical trials register. EU. [Google Scholar]
- 52.Using BCG Vaccine to Protect Health Care Workers in the COVID-19 Pandemic - Full Text View - ClinicalTrials.gov . US National Library of Medicine, National Institutes of Health. clinicaltrials.gov [Google Scholar]
- 53.WHO . Bacille Calmette-Guérin (BCG) vaccination and COVID-19-Scientific Brief. 2020.
- 54. https://www.who.int/news-room/commentaries/detail/bacille-calmette-gu%C3%A9rin-(bcg)-vaccination-and-covid-19
- 55.Gupta T, Gupta SK. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int Immunopharmacol. 2020;86:106717. doi: 10.1016/j.intimp.2020.106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, Nkolola JP, Liu J, Li Z, Chandrashekar A, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–11. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu A. China’s CanSino Bio advances COVID-19 vaccine into phase 2 on preliminary safety data. FiercePharma. 2020. Accessed 13 April 2020. “‘CanSino and its collaborators at the Academy of Military Medical Sciences’ Institute of Biotechnology plan to move their adenovirus type-5 vector-based recombinant COVID-19 vaccine, Ad5-nCoV, into phase 2 clinical trial in China “soon”,’ the company said in a disclosure (PDF) to the Hong Kong Stock Exchange on Thursday. Archived from the original on 30 April 2020.
- 58.Clinical trial number NCT04341389 for “A Phase II Clinical Trial to Evaluate the Recombinant Novel Coronavirus Vaccine (Adenovirus Vector) at ClinicalTrials.gov .
- 59.Clinical trial number NCT04313127 for “A Phase I Clinical Trial in 18-60 Adults at clinicalTrials.gov .
- 60.EudraCT Number 2020-001038-36 . EU Clinical Trials Register. European Union. [Google Scholar]
- 61.Clinical trial number NCT04324606 for A Study of a Candidate COVID-19 Vaccine (COV001) at ClinicalTrials.gov .
- 62.Clinical trial number NCT04444674 for A Study of a Candidate COVID-19 Vaccine (ChAdOx1 nCoV-19) Trial in South African adults with and without HIV-infection at ClinicalTrials.gov .
- 63.University of Oxford commences clinical trial for vaccine candidate (ChAdOx1 nCoV-19) Targeting COVID-19. Trial Site News. 31 March 2020.
- 64.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modjarrad K, Roberts CC, Mills KT, Castellano AR, Paolino K, Muthumani K, Reuschel EL, Robb ML, Racine T, Oh MD, et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19(9):1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clinical trial number NCT04336410 for Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers at ClinicalTrials.gov .
- 67.IVI, INOVIO, and KNIH to partner with CEPI in a Phase I/II clinical trial of INOVIO’s COVID-19 DNA vaccine in South Korea. International Vaccine Institute. 16 April 2020. [Google Scholar]
- 68.NIH clinical trial of investigational vaccine for COVID-19 begins . US national institutes of health. 16 March 2020.
- 69.Clinical trial number NCT04283461 for Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection at ClinicalTrials.gov .
- 70.Clinical trial number NCT04299724 for Safety and Immunity of Covid-19 aAPC Vaccine at ClinicalTrials.gov .
- 71.Clinical trial number NCT04276896 for Immunity and Safety of Covid-19 Synthetic Minigene Vaccine at ClinicalTrials.gov .
- 72.Clinical trial number NCT04334980 for Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19 at ClinicalTrials.gov . US National Library of Medicine, NIH. [Google Scholar]
- 73.Clinical trial number NCT04352608 for Safety and Immunogenicity Study of 2019-nCoV Vaccine (Inactivated) for Prophylaxis SARS CoV-2 Infection (COVID-19) at ClinicalTrials.gov .
- 74.A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated novel coronavirus pneumonia vaccine (Vero cells) ”. Chinese Clinical Trial Registry. April 2020
- 75.hyFc platform . [Accessed 2020 Apr 30]. http://www.genexine.com/m21.php
- 76.Seo YB, Im SJ, Namkoong H, Kim SW, Choi YW, Kang MC, Lim HS, Jin HT, Yang SH, Cho ML, et al. Crucial roles of interleukin-7 in the development of T follicular helper cells and in the induction of humoral immunity. J Virol. 2014;88:8998–9009. doi: 10.1128/JVI.00534-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee JH, Cho JH, Yeo J, Lee SH, Yang SH, Sung YC, Kang J-H, Park C-S. The pharmacology study of a new recombinant TNF receptor-hyFc fusion protein. Biologicals. 2013;41:77–83. doi: 10.1016/j.biologicals.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Loset GA, Roux KH, Zhu P, Michaelsen TE, Sandlie I. Differential segmental flexibility and reach dictate the antigen binding mode of chimeric IgD and IgM: implications for the function of the B cell receptor. J Immunol. 2004;172:2925–34. doi: 10.4049/jimmunol.172.5.2925. [DOI] [PubMed] [Google Scholar]
- 79.Kang MC, Park HW, Choi DH, Choi YW, Park Y, Sung YC, Lee S-W. Plasmacytoid dendritic cells contribute to the protective immunity induced by intranasal treatment with Fc-fused interleukin-7 against lethal influenza virus infection. Immune Netw. 2017;17:343–51. doi: 10.4110/in.2017.17.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tirado SM, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 81.Khandia R, Munjal A, Dhama K, Karthik K, Tiwari R, Malik YS, Singh RK, Chaicumpa W. Modulation of dengue/zika virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in Zika Virus infection. Front Immunol. 2018;9:597. doi: 10.3389/fimmu.2018.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, He L, Chen Y, Wu J, Shi Z, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020;94(5):pii: e02015-19.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang SF, Tseng SP, Yen CH, Yang JY, Tsao CH, Shen CW, Chen K-H, Liu F-T, Liu W-T, Chen YMA, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451:208–14. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16:e3000003. doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak as sociated with a new coronavirus of probable bat origin. Nature. 2020;579:270–73. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andre FE. The future of vaccines, immunization concepts and practice. Vaccine. 2001;19:2206–09. doi: 10.1016/S0264-410X(00)00546-6. [DOI] [PubMed] [Google Scholar]
- 87.CEPI . Our vaccine and platform portfolio. Coalition for Epidemic Preparedness Innovation (CEPI). 30 April 2020.
- 88.CEPI collaborates with the Institute Pasteur in a consortium to develop COVID-19 vaccine . Coalition for Epidemic Preparedness Innovations. 19 March 2020
- 89.Yeung J. Millions of chickens are used to make vaccines each year. But that won’t work for coronavirus. CNN. 2020.
- 90.Di Masi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs”. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Lee BY, Brown ST, Korch GW, Cooley PC, Zimmerman RK, Wheaton WD, Zimmer SM, Grefenstette JJ, Bailey RR, Assi TM, et al. A computer simulation of vaccine prioritization, allocation, and rationing during the 2009 H1N1 influenza pandemic. Vaccine. 2010;28(31):4875–79. doi: 10.1016/j.vaccine.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gouglas D, Le TT, Henderson K, Kaloudis A, Danielsen T, Hammersland NC, Robinson JM, Heaton PM, Røttingen JA. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Global Health. 2018;6(12):e1386–e1396. doi: 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strovel J, Sittampalam S, Coussens NP, Hughes M, Inglese J, Kurtz A, Andalibi A, Patton L, Austin C, Baltezor M, et al. Early drug discovery and development guidelines: for academic researchers, collaborators, and start-up companies. In: Markossian, Sittampalam S, editors. Assay guidance manual [Internet]. Eli Lilly & Company and the National Center for Advancing Translational Sciences. National Institute of Helth; 2020 (revised edition). p. 3–37. [PubMed]
- 94.WHO . Public statement for collaboration on COVID-19 vaccine development. World Health Organization. 13 April 2020
- 95.Jef A. COVID-19 vaccine frontrunners. The Scientist Magazine, April, 7, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed are included in this article


