ABSTRACT
This study aimed to explore how cold acclimation (CA) modulates cold stress in tobacco leaves and reveal the relationship between CA and cold stress resistance, and the mechanism of CA-induced plant resistance to cold stress. This study examined the effects of CA treatment (at 8–10℃ for 2 d) on the cold tolerance of tobacco leaves under 4°C cold stress treatment using seedlings without CA treatment as the control (NA). In both CA and NA leaves, cold stress treatment resulted in a decrease in maximum photochemical efficiency of PSII (Fv/Fm), increase in relative variable fluorescence (VJ) at 2 ms on the standardized OJIP curve, inhibition of PSII activity, and impairment of electron transfer on the acceptor side. Besides increasing the malondialdehyde (MDA) content and electrolyte leakage rate, the cold stress exacerbated the degree of membrane peroxidation. The CA treatment also induced the accumulation of reactive oxygen species (ROS), including superoxide anion (O2·−) and H2O2, and increased the activities of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbic acid peroxidase (APX). The CA treatment also enhanced the accumulation of soluble sugar (SS) and soluble protein (SP), cyclic electron flow (CEF), and the proportion of regulatory energy dissipation Y(NPQ). Moreover, CA+ cold stress treatment significantly reduced CEF and Y(NPQ) in tobacco leaves than under NA+ cold stress treatment, thus significantly alleviating the degree of PSII photoinhibition. In conclusion, CA treatment significantly alleviated PSII photoinhibition and oxidative damage in tobacco leaves under cold stress treatment. Improvement in cold resistance of tobacco leaves is associated with the induction of antioxidant enzyme activity, accumulation of osmoregulation substances, and initiation of photoprotective mechanisms.
KEYWORDS: Tobacco, cold acclimation, cold stress, osmotic regulation, antioxidant mechanism, photoprotective
1. Introduction
Cold stress is one of the most common meteorological disasters in agricultural production in alpine areas. Exposure of plants and animals to lower suboptimal temperatures prior to cold stress condition modulates their physiological processes inducing cold stress resistance, a process known as cold acclimation (CA).1The CA treatment modulates morphological, anatomical, physiological and biochemical properties of plants, significantly enhancing their stress resistance.2 Plants acclimate to cold stress by accumulating osmotic regulators, such as soluble sugar (SS),3 proline (Pro),4 soluble protein (SP), and polyamines (PAs).5 Plants exhibit enhanced activities of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), glutathione peroxidase (GPX), and ascorbic acid peroxidase (APX) and eliminate excess reactive oxygen species (ROS) to alleviate the oxidative damage in plant cells under cold stress6–9 Plants can also resist cold injury by increasing the proportion of unsaturated fatty acids (oleic acid, linoleic acid, and linolenic acid),10,11 CA is induced by highly complex biochemical mechanisms, including the expression of genes encoding stress proteins (dehydrins), increased levels of sugars, enhanced antioxidant defense mechanisms, and changes in lipid compositions12–14 CA increases the proportion of unsaturated fatty acids in cucumber seedlings, improves fluidity, and alleviates the damage of membrane peroxidation under cold stress by improving the activity of antioxidant enzymes and accumulating antioxidants in plants.15 CA also induces plant cold resistance by regulating the metabolism of hormones, such as gibberellin, cytokinin, and auxin16 or inducing the expression of potential anti-freeze genes or proteins17–19 Cold stress can regulate about 20% of the genes in the genome of the model plant Arabidopsis thaliana.20 Cold stress also induces Ca2+ channels, increases intracellular Ca2+ concentration, regulates the phosphorylation or dephosphorylation of proteins, thus initiating the expression of genes related to cold stress and synthesis of cold-resistant specific proteins, thus enhancing cold resistance of plants.21 Cunningham et al.22 found that 2℃ cold stress treatment can induce the expression of inositol galactoside synthase gene (GaS) in alfalfa and the GaS gene expression in root cap tissues of alfalfa varieties with strong cold resistance. Stockinger et al.23 showed that CBF1 gene induced the expression of the cold regulated/responsive (CORs) genes and enhanced the cold resistance of Arabidopsis thaliana. Similarly, other researchers have also indicated that CBF family genes can induce the overexpression of CORs genes and improve the cold resistance of transgenic plants.24,25 Moreover, the production of endogenous anti-freeze proteins (AFP) in Secale cereale L. through CA can improved the cold resistance of ryegrass (Secale cereale L.).26
Photosynthesis is highly sensitive to cold stress.27 Cold stress decreases the photochemical activities28–30 inhibits carbon assimilation,31,32 causes the imbalance between plant light energy absorption and utilization,33 and perturbs the ROS metabolism, especially for thermophilic plants.34,35 Plants have developed various photosynthetic regulatory mechanisms to adapt to cold stress during the long-term evolution process. For instance, the damage of D1 protein in plant PSII reaction center under cold stress can be reassembled through rapid turnover.36 Plants can dissipate excess light energy through non-chemical quenching (NPQ) and other ways to prevent excessive reduction of QA, thus balancing the absorption and utilization of light energy.37,38 Particularly, the energy quenching component (qE) in NPQ can dissipate the absorbed light energy into heat energy.39 CA can prevent photodamage under cold stress and promote recovery.40,41 For instance, CA can improve the PSII photochemical activity of mulberry under cold stress.42 CA can also increase the activity of many Calvin cycle enzymes, including Rubisco.43 Moreover, CA significantly enhances the synthetic ability of D1 in cucumber seedlings than in the NA plants.44 CA can also improve the photosynthetic ability of plants under cold stress by regulating hormones and redox signals in chloroplasts.45
For tobacco production in high latitude areas, seedlings are raised in a greenhouse, and then transplanted to the field in early spring, when outside temperature may drop to 2–4°C. The transplanted seedlings undergo cold stress in the fields, resulting in growth retardation and poor survival rate. For this reason, part of the greenhouse plastic film is often uncovered before the seedlings are transplanted to ensure the temperature is maintained at about 8–10℃ for CA treatment, thus improving the cold stress resistance of tobacco seedlings. The seedlings are then transplanted into the field after several days of CA treatment, as a measure to prevent the effect of cold stress (2–4℃) after transplantation, and significantly enhancing seedling survival and growth. The seedlings without cold acclimation (NA) may be at risk of chilling and freezing stress. This study assessed the changes of osmotic regulatory substance accumulation and antioxidant enzyme activity in tobacco seedlings under CA treatment (8–10℃) and 4℃ cold stress to reveal the internal mechanism of this phenomenon. The mechanism of cyclic electron flow (CEF) and energy distribution of PSII reaction center in improving the activity of PSII reaction center under cold stress was also studied. This study can provide a theoretical basis for improving the cold stress tolerance of tobacco seedlings and formulating appropriate cultivation measures.
2. Materials and methods
2.1. Plant materials and treatment
Tobacco seeds were sown in peat soil and vermiculite matrix at a fully mixed volume ratio of 1:1. One seedling was retained in each bowl (length, width, and height, 7.5 cm). The seedlings were raised in a greenhouse at 25℃ and a light intensity of 400 μmol·m−2·s−1, photoperiod of 12/12 h (light/dark). Forty seedlings with relatively consistent growth were selected and divided into two groups of 20 seedlings each. Seedlings in one group were put in an artificial climate box at 8–10℃ for cold acclimation treatment (recorded as CA). The seedlings in the other group were grown in the greenhouse without cold acclimation (NA). The CA and NA seedlings were placed in a 4℃ low-temperature artificial climate box for cold stress treatment after 2 d of CA treatment. Except for temperature, other cultural and environmental conditions were the same as normal growth conditions during CA and cold stress treatments. The physiological indexes were measured after CA and cold stress treatments for 2 d.
2.2. Determination parameters and methods
2.2.1. Determination of OJIP curve
The tobacco leaves with different treatments were subjected to 0.5 h dark adaptation using a dark adaptation clamp. A Handy-PEA chlorophyll fluorometer (Hansatech company, UK) was used to assess the OJIP curve of leaves after the dark adaptation. Each treatment had five replicates, and the OJIP curve was drawn using the average value of the five repetitions. The O, J, I, and P points on the OJIP curve were 0.01, 2, 30, and 1000 ms, respectively. The relative fluorescence intensities of O and P points were expressed in Fo and Fm, respectively. The maximum photochemical efficiency of PSII (Fv/Fm) was then calculated as follows: Fv/Fm = (Fm-Fo)/Fm. The OJIP curve was standardized using the formula, VO-P = (Ft-Fo)/(Fm-Fo), to obtain the VO-P curve, and the relative variable fluorescence VJ of point J at 2 ms on the VO-P curve, where Ft represents the relative fluorescence intensity of each time point on the OJIP curve. The difference between the standardized VO-P curve and NA treatment curve of tobacco seedling leaves under different treatments was expressed as ΔVO-P.
2.2.2. Determination of PSII energy distribution parameters
The tobacco leaves with different treatments were subjected to 0.5 h dark adaptation using a dark adaptation clamp. The FMS-2 chlorophyll fluorometer (Hansatech company, UK) was used to measure the initial fluorescence (Fo) and maximum fluorescence (Fm) after dark adaptation. The steady-state fluorescence (Fs) was also measured under the light adaptation of 1000 μmol·m−2·s−1. The PSII effective quantum yield Y(II), PSII non-regulated energy dissipation Y(NO), and PSII regulated energy dissipation yield Y(NPQ) were calculated using the following formulae: Y(II) = (Fm-Fs)/Fm, Y(NO) = Fs/Fm, and Y(NPQ) = 1-Y(II)-Y(NO).46
2.2.3. Proton Gradient Regulation (PGR5)-dependent cyclic electron transfer (PGR5-CEF) measurement
The treated leaves were adapted to the dark for 30 min, treated with 20% weak far-red light treatment for 60 s to oxidize P700. The far-red light was switched off to reduce P700 in the dark, then the same intensity of weak far-red light was given for 60 s. Finally, 100% saturated far-red light (1000 μmol·m−2·s−1) was irradiated for 1 s, while the saturated actinic light (5000 μmol·m−2·s−1) was on. The maximum signal of P700 was then measured. The M-PEA (Hansatech, UK) was then used to measure the redox kinetics of the pigment molecule of the PSI reaction center (P700). Only the proton gradient promotes ATP formation during far-red light treatment, thus significantly reducing the P700 signal. The P700 signal increases when the far-red light is turned off due to the presence of cyclic electron transfer, and the rising gap indicates the level of PGR5-CEF.47 Each treatment had three biological replicates.
2.2.4. NDH-dependent cyclic electron transfer (NDH-CEF) measurement
FMS-2 portable modulated fluorometer (Hansatech, UK) was used to measure the change of chlorophyll fluorescence signal. The leaves were adapted to dark for 30 min, then irradiated with 54 μmol·m−2·s−1 light. The light was turned off at 300 ms. The chlorophyll fluorescence signal increased due to the NDH-dependent CEF. The difference between the lowest and highest points was used to qualitatively describe NDH-CEF.48
2.2.5. Determination of physiological indexes (ROS metabolism and osmoregulation substance content)
The generation rate of O2·− and H2O2 content were determined as described by Yang et al. (2021).49 A conductivity meter (DDS-11 C) was used to determine the electrolyte leakage rate, expressed as relative conductivity. The content of malondialdehyde (MDA), soluble sugar (SS), and soluble protein (SP) was determined as described by Wang.50 Superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX) activities were measured using the appropriate kits (Suzhou Comin Biotechnology Co., Ltd. (Jiangsu, China)). The activity (1 U) of SOD was defined as the amount of enzymes required to reduce nitrotetrazolium blue chloride (NBT) to half of that of the control group. The activity (1 U) of CAT was defined as a 0.1 absorbance reduction at 240 nm. POD activity (1 U) was defined as a 0.01 absorbance reduction at 470 nm. The activity of APX was defined as the amount of ascorbic acid (AsA) (μmol) oxidized per g of fresh sample per min. The activity of dehydroascorbate reductase (DHAR) was defined as the amount of AsA (μmol) per g of fresh sample per min.
2.3. Data processing and statistical methods
Excel and SPSS (12.0) software were used for all data analysis. The data of each index in the figure were expressed as the mean ± standard deviation (SE) of the three replicated samples. ANOVA followed by the LSD test was used to compare the differences among the different treatments.
3. Results and analysis
3.1. Plant phenotype
The CA treatment caused slight wilt in tobacco leaves, but it did not show significant symptoms of chilling injury compared with the NA group (Figure 1). The NA+ cold stress treatment significantly wilted and shrunk the tobacco leaves. However, the CA+ cold stress treatment had significantly less severe symptoms of chilling injury in tobacco, and the leaves maintained an upright position.
Figure 1.

Effects of cold acclimation on plant phenotype of tobacco seedlings under 4℃ cold stress.
3.2. OJIP curve and PSII photochemical activity
In general, cold stress reduced the relative fluorescence of OJIP curves in both CA and NA plants. The differences between the CA and NA group under stressed and unstressed treatments widened significantly over time. The OJIP curve of tobacco seedling leaves treated with CA had no significant change in the relative fluorescence intensity at points O and J compared with NA (P > .05) (Figure 2a). In contrast, the relative fluorescence intensity at points I and P was significantly lower in the CA group than in the NA group (P < .05). CA increased the relative variable fluorescence VJ of point J on the VO-P curve based on the standardization of OJIP curve (VO-P) of tobacco seedling leaves under different treatments (Figure 2b). The cold stress treatment significantly decreased the relative fluorescence intensity at each point on the OJIP curve, while it increased VJ (Figure 2d). However, the changes in OJIP curve and VJ were less in the CA+cold stress group than in the NA+cold stress group. Their Fv/Fm was not significantly different between the CA and NA groups without stress treatment, but the CA leaves had significantly higher Fv/Fm than the NA (P < .05) after the cold stress treatment (Figure 2c). However, VJ increased by 21.22% (P < .05) in the CA group compared with the NA group. The cold stress treatment significantly decreased Fv/Fm while it increased VJ in NA plants. However, Fv/Fm was 33.71% higher in the CA+ cold stress group than in the NA+4°C group (P < .05), while VJ was 10.50% lower in the CA+ cold stress group than in the NA+4°C group (P < .05) (Figure 2c,d).
Figure 2.
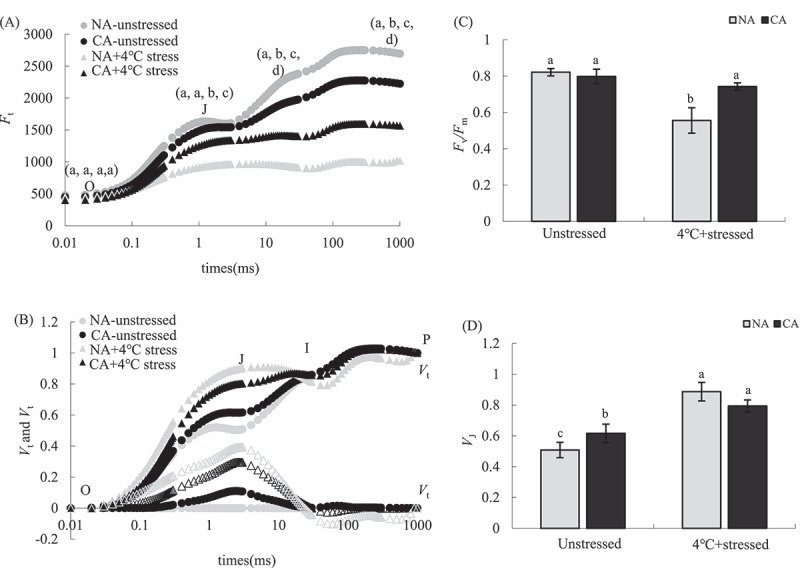
Effects of cold acclimation on OJIP curve (a), standardized OJIP curve (b), Fv/Fm(c) and VJ (d) of tobacco seedling leaves under 4℃ cold stress.
Note. The data in the figure is the average value of three replicated samples. Different letters indicate significant differences among different treatments at P < .05.
3.3. Energy distribution parameters of PSII reaction center
The leaf Y(II) of the CA group showed a significant decreasing trend compared with that of NA under un-stressed condition (Figure 3), mainly due to the increased Y(NPQ) (increased by 10.41% compared with the NA group). The Y(NO) only increased by 1.91% in the CA group compared with the NA group without cold stress treatment. The cold stress treatment significantly decreased Y(II) (P < .05), while it increased Y(NO) (P < .05). However, Y(II) was significantly higher in the CA+ cold stress group than in the NA+ cold stress group, while Y(NO) was significantly lower in the CA+ cold stress group than in the NA+4°C. Moreover, the Y(NPQ) was slightly higher in the CA+ cold stress group than in the NA+ cold stress group by 6.74%.
Figure 3.
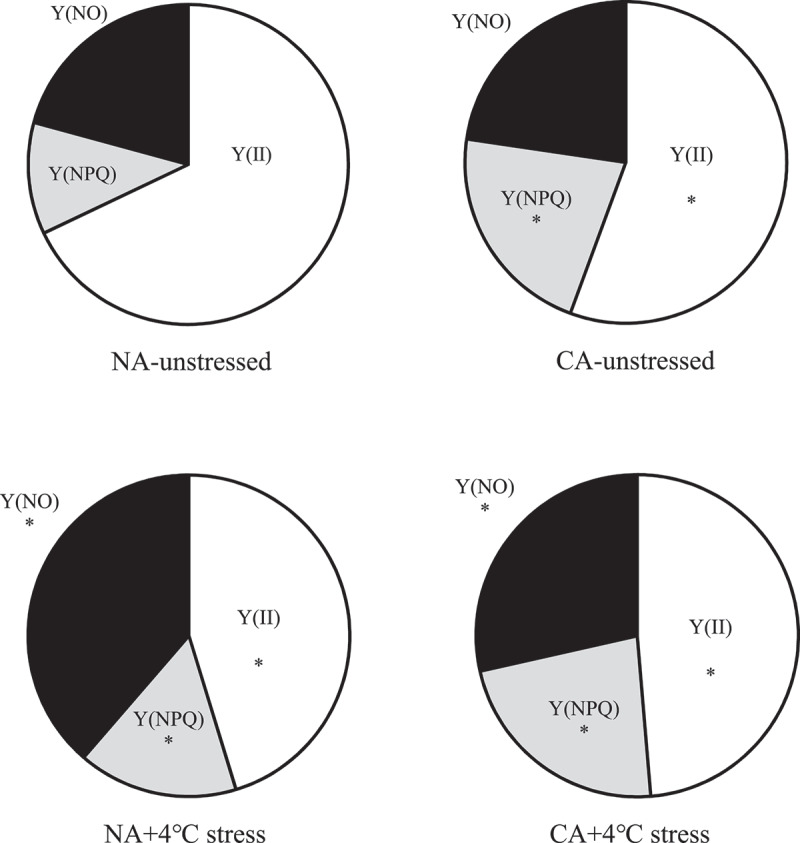
Cold acclimation on Y(II), Y(NPQ) and Y(NO) with and without cold stress in tobacco leaves.
Note. The data in the figure is an average of three replicated samples. *Showed significant difference between treatment and NA-unstressed (P < .05), ns showed no significant difference between treatment and NA-unstressed (P > .05).
3.4. Cyclic electron flow
CA treatment significantly increased the rapid rise of chlorophyll fluorescence (NDH-pathway CEF) and the rise drop of the signal after turning off far-red light (PGR5-pathway CEF) compared with the NA group (Figure 4). The cold stress treatment decreased the CEF of both NDH-pathway and PGR5-pathway in all treatment groups. However, the magnitude of decrease of CEF in both pathways was less in the CA+ cold stress group than in the NA+ cold stress group.
Figure 4.
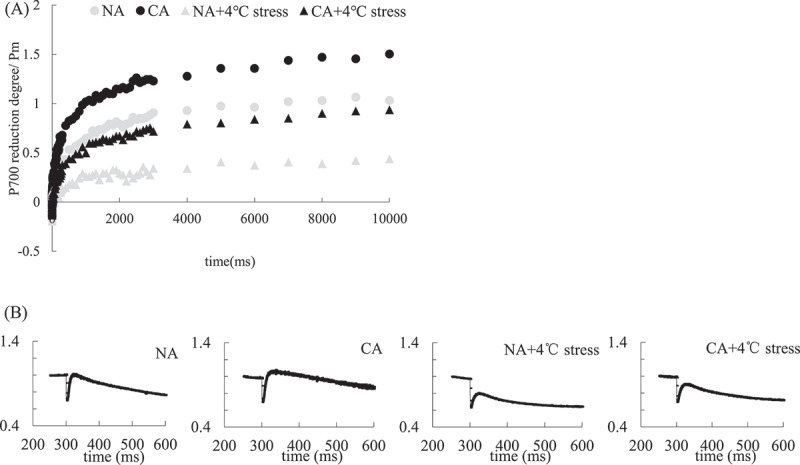
Effect of cold acclimation on PGR5-passway (a) and NDH-passway (b) CEF under cold stress in tobacco leaves.
Note. The data in the figure is the average of three replicated samples.
3.5. ROS content and membrane peroxidation
CA slightly increased the generation rate of O2·− and H2O2 content in the tobacco leaves (Figure 5). However, the generation rate of O2·− and H2O2 content were not significantly different between the NA and CA groups prior to cold stress treatment. Moreover, CA slightly increased the MDA content and electrolyte leakage rate compared with the NA group prior to exposure to cold stress. The cold stress significantly increased ROS content and the degree of membrane peroxidation in both CA and NA plants. However, the generation rate of O2·−, H2O2 and MDA contents and electrolyte leakage rate were lower in the CA+ cold stress group than in the NA+ cold stress group by 30.09% (P < .05), 17.43% (P > .05), 17.02% (P < .05), and 12.92% (P > .05), respectively.
Figure 5.
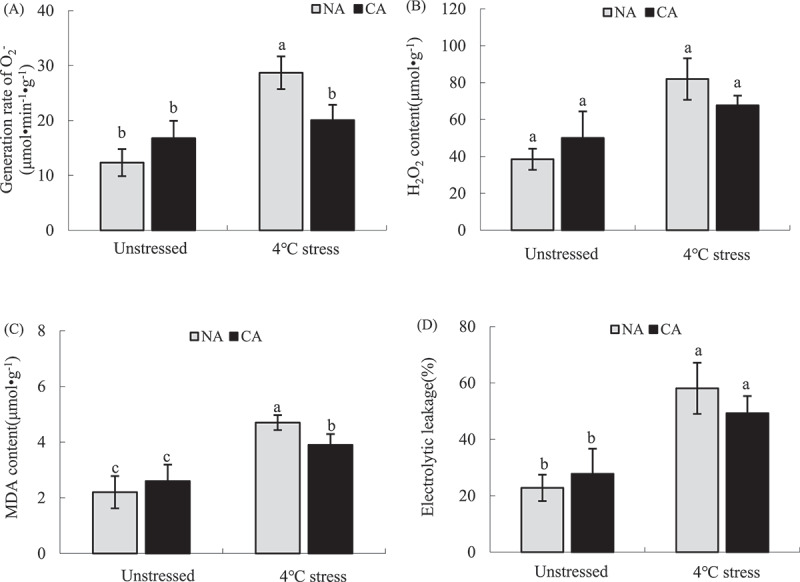
Effect of cold acclimation on generation rate of O2− (a), H2O2 content (b), MDA content (c) and electrolytic leakage (d) with and without cold stress in tobacco leaves.
Note. The data in the figure is the average value of three replicated samples. Different letters indicate significant differences among different treatments at P < .05.
3.6. Antioxidant enzyme activity
CA significantly increased the activities of SOD, POD, and APX in tobacco leaves, compared with the NA group without cold stress treatment (Figure 6). The cold stress treatment significantly increased SOD and POD activities, while it decreased APX activities and CAT showed relatively stable in both CA and NA plants. However, SOD, CAT, and APX activities were significantly higher in the CA+ cold stress group than in the NA+ cold stress.
Figure 6.
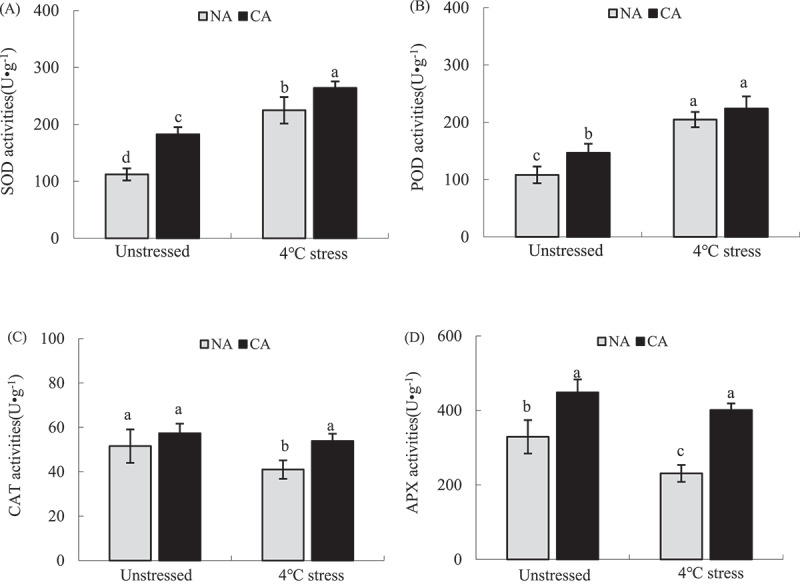
Effect of cold acclimation on SOD (a) POD (b), CAT (c) and APX (d) activity under cold stress in tobacco leaves.
Note. The data in the figure is the average value of three replicated samples. Different letters indicate significant differences among different treatments at P < .05.
3.7. Osmoregulation substances
CA increased SS and SP contents in tobacco leaves by 73.81% (P < .05) and 60.49% (P < .05), respectively, higher than those in NA treatment (Figure 7). Moreover, the cold stress significantly increased SS and SP contents in both NA and CA plants. However, the contents of SS and SP were higher in the CA+ cold stress group than in the NA+ cold stress group by 30.48% (P < .05) and 25.09% (P < .05), respectively. Figure 8
Figure 7.
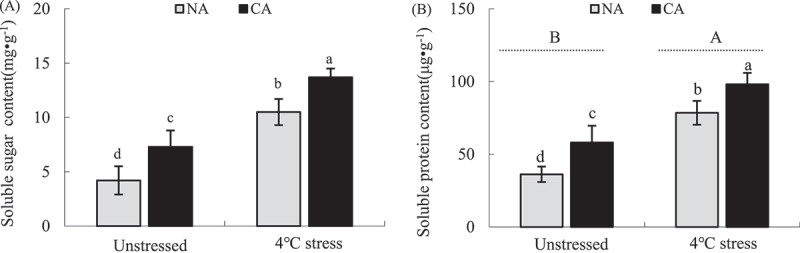
Effect of cold acclimation on soluble sugar (a) and soluble protein (b) content under cold stress in tobacco leaves.
Note. The data in the figure is the average value of three replicated samples. Different letters indicate significant differences among different treatments at P < .05.
Figure 8.
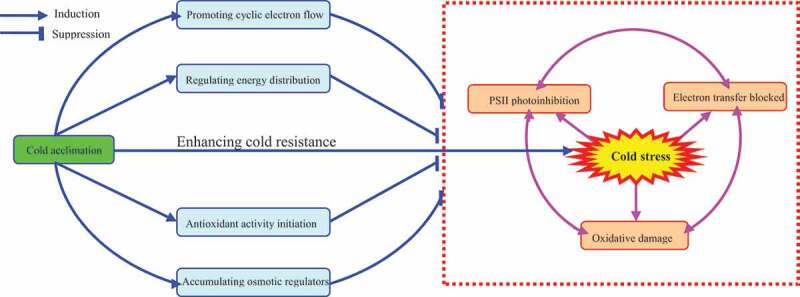
Mechanisms of cold acclimation on enhancing cold tolerance of tobacco leaves.
3.8. Correlation between photosynthetic and physiological parameters
As can be seen from, Fv/Fm was negatively correlated with VJ, O2−, H2O2, MDA and EL (P < .05), and positively correlated with CAT activity (P < .05). The VJ parameter was positively correlated with O2−, H2O2, MDA, EL, SOD, POD, SS, and SP (P < .05), and negatively correlated with CAT and APX (P < .05).Table 1
Table 1.
Correlation between photosynthetic and physiological parameters of tobacco leaves under different treatments
| Fv/Fm | VJ | O2− | H2O2 | MDA | EL | SOD | POD | CAT | APX | SS | SP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fv/Fm | 1.00 | |||||||||||
| VJ | −0.88* | 1.00 | ||||||||||
| O2− | −0.97* | 0.95* | 1.00 | |||||||||
| H2O2 | −0.92* | 1.00* | 0.97* | 1.00 | ||||||||
| MDA | −0.92* | 0.99* | 0.96* | 0.99* | 1.00 | |||||||
| EL | −0.89* | 0.99* | 0.93* | 0.99* | 1.00 | 1.00 | ||||||
| SOD | −0.54 | 0.87* | 0.70 | 0.83 | 0.81* | 0.84* | 1.00 | |||||
| POD | −0.66 | 0.93* | 0.78 | 0.90* | 0.90* | 0.92* | 0.98* | 1.00 | ||||
| CAT | 0.89* | −0.62* | −0.76 | −0.67 | −0.71 | −0.67 | −0.16 | −0.33 | 1.00 | |||
| APX | 0.77 | −0.45* | −0.60 | −0.51 | −0.56 | −0.52 | 0.03 | −0.16 | 0.98* | 1.00 | ||
| SS | −0.51 | 0.85* | 0.65 | 0.80 | 0.80 | 0.84* | 0.99* | 0.98* | −0.17 | 0.12 | 1.00 | |
| SP | −0.52 | 0.86* | 0.67 | 0.81* | 0.81* | 0.84* | 0.99* | 0.99* | −0.17 | 0.25 | 0.99* | 1.00 |
* indicates a significant correlation (P < 0.05) between the two parameters.
4. Discussion
Cold stress inhibits PSII photochemical activity in plant leaves.51,52 The degradation of D1 protein blocks the electron transfer on the PSII receptor side under cold stress, causing excessive reduction.53 Studies have shown that the degree of PSII photoinhibition linearly decreases with decreasing temperature (25–4°C).54 The relative variable fluorescence VJ at point J at 2 ms on the standardized OJIP curve represents QA− accumulation of the reduced primary electron receptor.55 Increased VJ indicates that the blocking of electron transfer flow from QA− to QB leads to the excessive reduction of QA−, and thus is an important index of blocked electron transfer on the PSII receptor side.56,57 In our study, CA treatment increased VJ in tobacco leaves, but it did not significantly decrease Fv/Fm. The Fv/Fm indicates the activity of the PSII reaction center.58 These results show that CA treatment did not cause PSII photoinhibition in tobacco seedling leaves. However, the cold stress treatment decreased Fv/Fm. At the same time, it significantly increased VJ, thus significantly blocking electron transfer on the PSII receptor side and leading to substantial photoinhibition of the PSII reaction center. However, the degree of PSII photoinhibition was significantly lower in the CA+ cold stress group than in the NA+ cold stress group. These results indicate that CA can significantly alleviate PSII photoinhibition in tobacco leaves under 4°C cold stress and promote the electron transfer from the PSII reaction center.
Plants produce excess energy than their utilization capacity under stress.59,60 This results in a decrease in the photosynthetic rate and electron transport capacity, thereby undergoing photoinhibition.61 Enhanced non-chemical quenching (NPQ) is essential in dissipating the excess energy in PSII, thus alleviating PSII photoinhibition.62–64 The PSII-regulated energy dissipation yield Y(NPQ) reflects the ability of PSII to convert excess excitation energy into heat through regulatory energy dissipation (xanthophyll cycle-related energy dissipation).65 Herein, CA treatment significantly decreased Y(II) and increased Y(NPQ) and Y(NO) compared with the NA group, especially for Y(NPQ). These results show that although CA treatment can reduce PSII photochemical activity in tobacco leaves, the seedlings can adapt to CA through regulatory energy dissipation, preventing excessive reduction of plastoquinone pool leading to photoinhibition of the PSII. However, severe stress can gradually aggravate PSII photoinhibition if the excess excitation energy is not consumed through the NPQ pathway and other electron sinks in time, thus producing more ROS that destroy the photosynthetic mechanism.66,67 Moreover, the adaptive mechanism of regulatory energy dissipation is often destroyed under severe oxidative damage.68 The Y(NO) represents the non-regulatory energy dissipation of PSII, which refers to the ability of PSII to passively dissipate excitation energy by closing the reaction center of PSII due to photoinhibitory damage. It can also reflect the photooxidative damage of PSII.69,70 Herein, cold stress reduced the ability of tobacco leaves to dissipate excess energy through the NPQ pathway compared with the CA treatment. However, Y(NPQ) was significantly higher in the CA+ cold stress group than in the NA+4°C group. Moreover, Y(II) was significantly higher in the CA+ cold stress group than in the NA+4°C group. In contrast, Y(NO) was significantly lower in the CA+ cold stress group than in the NA+ cold stress group. These results show that CA treatment can improve resistance to cold stress by regulating the energy distribution of the PSII reaction center.
The CEF is an important photoprotection mechanism in plants when under stress.71,72 Increased CEF rate under stress can inhibit the attack of excess electrons on O2, thus reducing oxidative damage.73 Although CEF cannot produce NADPH, it can be produced by a proton pump. The ΔpH drives ATP synthesis and increases ATP/NADPH ratio.74,75 The additional ATP can also resist various adversity76 and prevents PSI and PSII photoinhibition.77,78 Moreover, the CEF activity in plants caused by cold stress is known.79,80 CEF pathway mainly involves NAD(P)H dehydrogenase (NDH) and Proton Gradient Regulation 5/Proton Gradient Regulation-Like 1 (PGR5/PGRL1) pathways.81,82 Studies have shown that plant CEF is mainly mediated by the PGR5 pathway,76 while the protective effect of the NDH pathway is weak under cold stress.83 However, this study showed that CA treatment increased PGR5 and NDH pathways to varying degrees. CEF can also favor the repair of core protein D1 of PSII under stress, promote the linear electron transfer process,84 increase NPQ and alleviate the photoinhibition of PSII.85–87 Therefore, CA treatment can promote PSII receptor side electron transfer (Figure 2) and NPQ dependent regulatory energy dissipation mechanism (Figure 3) in tobacco leaves under cold stress, possibly because CA can promote CEF in tobacco leaves.
Soluble sugar (SS) and soluble protein (SP), key osmoregulation substances during adverse conditions, can resist dehydration and reduce the damage of cold stress to cells.88,89 Studies have shown that CA can improve the cold tolerance of mulberry leaves by promoting the accumulation of organic substances, such as SS and proline,90 similar to this study. Herein, CA significantly increased SS and SP contents in tobacco leaves than in the NA group. However, SS and SP contents were significantly higher in the CA+ cold stress group than in the NA+ cold stress group (Figure 7). CA promoted the accumulation of SS and SP in tobacco leaves, thus improving cold stress tolerance.
Cold stress and other stresses significantly inhibit the physiological processes, such as photosynthesis and respiration of plants. Excess energy and electrons can mediate the ROS production, thus intensifying the cell membrane lipid peroxidation91–93 As a result, plants often alleviate the peroxidation damage caused by ROS by increasing antioxidant enzymes or accumulating antioxidants.94,95Excess O2·− in cells can be reduced through SOD,96,97 CAT, and POD scavenge excess H2O2in cells.98–100 However, H2O2 in chloroplasts mainly depends on APX clearance in the AsA-GSH cycle.101,102 CA slightly increased O2·− and H2O2 contents in tobacco leaves compared with the NA group. Moreover, CA had a less inhibitory effect on PSII activity in tobacco leaves (Figure 2). CA also increased SOD, POD, CAT, and APX activities (Figure 6). In addition, the correlation analysis showed that the change of CAT activity was the main positive factor affecting Fv/Fm. Cold stress significantly increased SOD and POD activities in tobacco leaves, while it inhibited APX and CAT activities, increasing ROS content and the accumulation of membrane lipid peroxidation product MDA. Studies have shown that although enzymes in plant cells can remove excess ROS, they are vulnerable to ROS attack and lose their activity under severe stress,103,104 Therefore, the cell oxidative damage and PSII photoinhibition in tobacco leaves under the cold stress could be due to the inhibition of antioxidant enzyme activities, such as CAT and APX. Herein, SOD, POD, CAT, and APX activities were higher in the CA+ cold stress group than in the NA+ cold stress group, indicating that CA enhances antioxidant enzyme activity and prevents inactivation under severe stresss, thus improving cold tolerance.
The mechanism of how CA improves the cold tolerance of tobacco leaves is summarized in Figure 7Briefly, CA can alleviate the PSII photoinhibition and oxidative damage of tobacco leaves under 4℃ cold stress by promoting the accumulation of osmotic regulators, improving the activity of antioxidant enzymes, promoting cyclic electron transfer, and optimizing the energy distribution of PSII reaction center.
5. Conclusion
The cold stress leads to the inhibition of PSII activity and the obstruction of electron transfer on the receptor side. Besides increasing the MDA content and electrolyte leakage rate, the cold stress exacerbated the degree of membrane peroxidation. CA treatment increased CEF and Y(NPQ) in tobacco leaves, reducing excess excitation energy and alleviating PSII photoinhibition under 4°C cold stress. In conclusion, CA treatment (8–10°C for 2 d) can significantly reduce the degree of PSII photoinhibition and oxidative damage in tobacco leaves under 4°C cold stress. Our study can contribute to further elucidation of cold acclimation and cold stress response mechanism in tobacco and other mesophilic plants, and provide a theoretical basis for the growth of tobacco in cold areas.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Rinalducci S, Egidi MG, Karimzadeh G, Jazii FR, Zolla L.. Proteomic analysis of a spring wheat cultivar in response to prolonged cold stress. Electrophoresis. 2011;32(14):1807–11. doi: 10.1002/elps.201000663. [DOI] [PubMed] [Google Scholar]
- 2.Borochov A, Walker MA, Pauls KP. Effects of cold acclimation on the morphological and physiological properties of Alfalfa (Medicago sativa) suspension culture cells. J Plant Physiol. 1989;133(6):671–677. doi: 10.1016/S0176-1617(89)80071-9. [DOI] [Google Scholar]
- 3.Pociecha E, Dziurka M. Trichoderma interferes with cold acclimation by lowering soluble sugars accumulation resulting in reduced pink snow mould (Microdochium nivale) resistance of winter rye. Environ Exp Bot. 2015;109:193–200. doi: 10.1016/j.envexpbot.2014.07.009. [DOI] [Google Scholar]
- 4.Zuo ZF, Kang HG, Park MY, Jeong H, Sun H-J, Yang D-H, Lee Y-E, Song P-S, Lee H-Y. Overexpression of ICE1, a regulator of cold-induced transcriptome, confers cold tolerance to transgenic Zoysia japonica. J Plant Biol. 2019;62(2):137–146. doi: 10.1007/s12374-018-0330-1. [DOI] [Google Scholar]
- 5.Shen W, Nada K, Tachibana S. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol. 2000;124(1):431–439. doi: 10.1104/pp.124.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu DF, Wang LY, Duan M, Deng Y-S, Meng Q-W. Antisense-mediated depletion of tomato chloroplast glutathione reductase enhances susceptibility to chilling stress. Plant Physiol Biochem. 2011;49(10):1228–1237. doi: 10.1016/j.plaphy.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Ding S, Lei M, Lu Q, Zhang A, Yin Y, Wen X, Zhang L, Lu C. Enhanced sensitivity and characterization of photosystem II in transgenic tobacco plants with decreased chloroplast glutathione reductase under chilling stress. Biochimica et Biophysica Acta-Bioenergetics. 2012;1817(11):1979–1991. doi: 10.1016/j.bbabio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Duan M, Feng HL, Wang LY, Li D, Meng Q-W. Overexpression of thylakoidal ascorbate peroxidase shows enhanced resistance to chilling stress in tomato. J Plant Physiol. 2012;169(9):8677. doi: 10.1016/j.jplph.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Routaboul JM, Fischer SF, Browse J. Trienoic fatty acid are required to maintain chloroplast function at low temperatures. Plant Physiol. 2000;124(4):1697–1705. doi: 10.1104/pp.124.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iii AHM, Peet MM, Sionit N, Kramer, PJ, et al. Low temperature acclimation of root fatty acid composition, leaf water potential, gas exchange and growth of soybean seedlings. Plant Cell Environ. 2006;3(6):435–441. [Google Scholar]
- 12.Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50(1):571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 13.Cansev A, Gulen H, Erıs A. Cold-hardiness of olive (Olea europaea L.) cultivars in cold-acclimated and non-acclimated stages: seasonal alteration of antioxidative enzymes and dehydrin-like proteins. J Agric Sci. 2009;147(1):51–61. doi: 10.1017/S0021859608008058. [DOI] [Google Scholar]
- 14.Theocharis A, Clément C, Barka EA. Physiological and molecular changes in plants grown at low temperatures. Planta. 2012;235(6):1091–1105. doi: 10.1007/s00425-012-1641-y. [DOI] [PubMed] [Google Scholar]
- 15.Yang L. Effects of different low temperature treatments on compositions of polar lipids in cucumber cotyledons. Acta Horticulturae Sinica. 2001;28:36–40. [Google Scholar]
- 16.Vanková R, Kosová K, Dobrev P, Vítámvás P, Trávníčková A, Cvikrová M, Pešek B, Gaudinová A, Prerostová S, Musilová J, et al. Dynamics of cold acclimation and complex phytohormone responses in Triticum monococcum lines G3116 and DV92 differing in vernalization and frost tolerance level. Environ Exp Bot. 2014;101:12–25. doi: 10.1016/j.envexpbot.2014.01.002. [DOI] [Google Scholar]
- 17.Trischuk RG, Schilling BS, Low NH, Gray GR, Gusta LV. Cold acclimation, de-acclimation and re-acclimation of spring canola, winter canola and winter wheat: the role of carbohydrates, cold-induced stress proteins and vernalization. Environ Exp Bot. 2014;106(1):156–163. doi: 10.1016/j.envexpbot.2014.02.013. [DOI] [Google Scholar]
- 18.Die JV, Rowland LJ. Elucidating cold acclimation pathway in blueberry by transcriptome profiling. Environ Exp Bot. 2014;106:87–98. doi: 10.1016/j.envexpbot.2013.12.017. [DOI] [Google Scholar]
- 19.Malyshev AV, Henry HAL, Kreyling J. Relative effects of temperature vs. photoperiod on growth and cold acclimation of northern and southern ecotypes of the grass Arrhenatherum elatius. Environ Exp Bot. 2014;106(1):189–196. doi: 10.1016/j.envexpbot.2014.02.007. [DOI] [Google Scholar]
- 20.Lee BH, Henderson DA, Zhu JK. The Arabidopsi cold-responsive transcriptome and its regulation by ICE1. Plant Cell. 2005;17(11):3155–3175. doi: 10.1105/tpc.105.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vergnolle C, Vaultier MN, Taconnt L, Renou J-P, Kader J-C, Zachowski A, Ruelland E. The cold-induced early activation of phospholipase C and D pathways determines the response of two distinct clusters of genes in Arabidopsis cell suspensions. Plant Physiol. 2005;139(3):1217–1233. doi: 10.1104/pp.105.068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham SM, Nadeau P, Castonguay Y, Laberge S, Volenec JJ. Raffinose and stachyose accumulation, galactinol synthase expression and winter injury of contrasting alfalfa germplasms. Crop Sci. 2003;43(2):562–570. doi: 10.2135/cropsci2003.0562. [DOI] [Google Scholar]
- 23.Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that bind to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proceedings of the National Academy of Sciences of the United States of America, Washington State University, Pullman, 1997, 94(3):1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaglo-Ottosen KR, Glmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280(5360):104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- 25.Jeknić Z, Pillman KA, Dhillon T, Skinner JS, Veisz O, Cuesta-Marcos A, Hayes PM, Jacobs AK, Chen THH, Stockinger EJ, et al. Hv-CBF2A overexpression in barley accelerates COR gene transcript accumulation and acquisition of freezing tolerance during cold acclimation. Plant Mol Biol. 2014;84(1–2):67–82. doi: 10.1007/s11103-013-0119-z. [DOI] [PubMed] [Google Scholar]
- 26.Hon WC, Griffith M, Chong P, Yang D. Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves. Plant Physiol. 1994;104(3):971–980. doi: 10.1104/pp.104.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Scheller HV. Photoinhibition of photosystem I at chilling temperature and subsequent recovery in Arabidopsis thaliana. Plant Cell Physiol. 2004;45(11):1595–1602. doi: 10.1093/pcp/pch180. [DOI] [PubMed] [Google Scholar]
- 28.Shen JR, Terashima I, Katoh S. Cause for dark, chilling-induced inactivation of photosynthetic oxygen evolving system in cucumber leaves. Plant Physiol. 1990;93(4):1354–1357. doi: 10.1104/pp.93.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Zhang SB, Cao KF. The different effects of chilling stress under moderate light intensity on photosystem II compared with photosystem I and subsequent recovery in tropical tree species. Photosynth Res. 2010;103(3):175–182. doi: 10.1007/s11120-010-9539-7. [DOI] [PubMed] [Google Scholar]
- 30.Guo SJ, Zhou HY, Zhang XS, Li X-G, Meng Q-W. Overexpression of CaHSP26 in transgenic tobacco alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. J Plant Physiol. 2007;164(2):126–136. doi: 10.1016/j.jplph.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Strauss AJ, Ghj K, Strasser RJ, van Heerden PDR. The role of low soil temperature in the inhibition of growth and PSII function during dark chilling in soybean genotypes of contrasting tolerance. Physiol Plant. 2010;131(1):89–105. doi: 10.1111/j.1399-3054.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 32.Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y. Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot. 2005;57(2):291–302. doi: 10.1093/jxb/erj049. [DOI] [PubMed] [Google Scholar]
- 33.Nityananda K, Geoffrey B, Anna G, Moffatt B, Gray G. Differential mechanisms of photosynthetic acclimation to light and low temperature in Arabidopsis and the Extremophile Eutrema salsugineum. Plants. 2017;6(4):32. doi: 10.3390/plants6030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trzcinska-Danielewicz J, Bilska A, Fronk J, Zielenkiewicz P, Jarochowska E, Roszczyk M, Jończyk M, Axentowicz E, Skoneczny M, Sowiński P, et al. Global analysis of gene expression in maize leaves treated with low temperature. I. Moderate chilling (14 °C). Plant Sci. 2009;177(6):648–658. doi: 10.1016/j.plantsci.2009.09.001. [DOI] [Google Scholar]
- 35.Allen DJ, Ort DR. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001;6(1):36–42. doi: 10.1016/S1360-1385(00)01808-2. [DOI] [PubMed] [Google Scholar]
- 36.He J, Chow WS. The rate coefficient of repair of photosystem II after photoinactivation. Physiol Plant. 2003;118(2):297–304. doi: 10.1034/j.1399-3054.2003.00107.x. [DOI] [Google Scholar]
- 37.Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008;13(4):178–182. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Murchie EH, Niyogi KK. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011;155(1):86–92. doi: 10.1104/pp.110.168831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XP, Björkman O, Shih C, Grossman, AR, Rosenquist, M, Jansson, S, Niyogi, KK, et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403(6768):391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- 40.Huner NPA, Öquist G, Hurry V, Krol M, Falk S, Griffith M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth Res. 1993;37(1):19–39. doi: 10.1007/BF02185436. [DOI] [PubMed] [Google Scholar]
- 41.Bascuñán-Godoy L, Sanhueza C, Cuba M, Zuniga, GE, Corcuera, LJ, Bravo, LA, et al. Cold-acclimation limits low temperature induced photoinhibition by promoting a higher photochemical quantum yield and a more effective PSII restoration in darkness in the Antarctic rather than the Andean ecotype of Colobanthus quitensisKunt Bartl (Cariophyllaceae). BMC Plant Biol. 2012;12(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu XJ, Xu N, Wu YN, Li, JB, Zhong, HXZhang, HH, et al. Photosynthesis, chilling acclimation and the response of antioxidant enzymes to chilling stress in mulberry seedlings. J Forestry Res, 30 . 2018;(1):1–9. [Google Scholar]
- 43.Hurry VM, Strand A, Tobiaeson M, Gardestrom P, Oquist G. Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiol. 1995;109(2):697–706. doi: 10.1104/pp.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng JQ, Liu HX, Wang YR, et al. Photoinhibition and recovery of cucumber seedling cotyledons under low temperatures. Acta Photophysiologica Sinica. 1997;23(1):15–20. [Google Scholar]
- 45.Kurepin L, Dahal K, Savitch L, Singh J, Bode R, Ivanov A, Hurry V, Hüner N. Role of CBFs as integrators of chloroplast redox, phytochrome and plant hormone signaling during cold acclimation. Int J Mol Sci. 2013;14(6):12729–12763. doi: 10.3390/ijms140612729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer DM, Johnson G, Kiirats O, Edwards GE. New fluorescence parameters for the Determination of QA redox state and excitation energy fluxes. Photosynth Res. 2004;79(2):209–218. doi: 10.1023/B:PRES.0000015391.99477.0d. [DOI] [PubMed] [Google Scholar]
- 47.Zhong X, Li YT, Che XK, Zhang, ZS, Liu, BB, Li, QM, Gao, HY, et al. The relationship between the activities of photosynthetic apparatus and the expressions of photosynthetic core proteins during the leaf expansion of Cucumis sativus. Plant Physiol J. 2018;54:1045–1054. [Google Scholar]
- 48.Han X, Sun N, Xu M, Mi H. Co-ordination of NDH and cup proteins in CO2 uptake in cyanobacterium Synechocystis sp. PCC 6803. J Exp Bot. 2017;68(14):3869–3877. doi: 10.1093/jxb/erx129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang FW, Zhang HB, Wang Y, He G, Wang J, Guo D, Li T, Sun G, Zhang H. The role of antioxidant mechanism in photosynthesis under heavy metals Cd or Zn exposure in tobacco leaves. J Plant Interact. 2021;16(1):344–354. doi: 10.1080/17429145.2021.1961886. [DOI] [Google Scholar]
- 50.Wang JY, Ao H, Zhang J. The echnology and Experiment Principle of Plant Physiology. Haerbin: Northeast Forestry University press; 2003. [Google Scholar]
- 51.Ma X, Chen C, Yang M, Dong X, Lv W, Meng Q. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol Biochem. 2018;124:29–39. doi: 10.1016/j.plaphy.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Miura K, Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci. 2014;5(2):4. doi: 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta. 2007;1767(6):414–421. doi: 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Sonoike K. Photoinhibition of photosystem I. Physiol Plant. 2011;142(1):56–64. doi: 10.1111/j.1399-3054.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang HH, Xu ZS, Huo YZ, Guo, KW, Wang, Y, He, GQ, Sun, HW, Li, MB, Li, Xin, Xu, N, Sun, GY, et al. Overexpression of Trx CDSP32 gene promotes chlorophyll synthesis and photosynthetic electron transfer and alleviates cadmium-induced photoinhibition of PSII and PSI in tobacco leaves. J Hazard Mater. 2020;397:12289. [DOI] [PubMed] [Google Scholar]
- 56.Gao Y, Liu W, Wang XX, Yang, LH, Han, S, Chen, SG, Strasser, RJ, Valverde, BE, Qiang, S, et al. Comparative phytotoxicity of usnic acid, salicylic acid, cinnamic acid and benzoic acid on photosynthetic apparatus of Chlamydomonas reinhardtii. Plant Physiol Biochem, 128 . 2018;S0981942818301955. [DOI] [PubMed] [Google Scholar]
- 57.Szilvia Z T, Schansker G, Garab G, Strasser RJ. Photosynthetic electron transport activity in heat-treated barley leaves: the role of internal alternative electron donors to photosystem II. Biochim Biophys Acta. 2007;1767(4):295–305. doi: 10.1016/j.bbabio.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 58.Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI, Brestic M, Bussotti F, Calatayud A, Dąbrowski P, et al. Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res. 2014;122(2):121–158. doi: 10.1007/s11120-014-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paredes M, Quiles MJ. Chilling stress and hydrogen peroxide accumulation in Chrysanthemum morifolium and, Spathiphyllum lanceifolium. Involvement of chlororespiration. J Plant Physiol. 2017;211:36–41. doi: 10.1016/j.jplph.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Wang JC, Guo DD, Zhang H, Che Y, Li Y, Tian B, Wang Z, Sun G, Zhang H, et al. Physiological and comparative transcriptome analysis of leaf response and physiological adaption to saline alkali stress across pH values in alfalfa (Medicago sativa). Plant Physiol Biochem. 2021;167:140–152. doi: 10.1016/j.plaphy.2021.07.040. [DOI] [PubMed] [Google Scholar]
- 61.Zhang HH, Liu XQ, Zhang HB, Wang Y, Li T, Che Y, Wang J, Guo D, Sun G, Li X, et al. Thioredoxin-like protein CDSP32 alleviates Cd-induced photosynthetic inhibition of tobacco leaves by regulating cyclic electron flow and excess energy dissipation. Plant Physiol Biochem. 2021;167:831–839. doi: 10.1016/j.plaphy.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 62.Da Q, Sun T, Wang ML, Jin H, Li M, Feng D, Wang J, Wang H-B, Liu B. M-type thioredoxins are involved in the xanthophyll cycle and proton motive force to alter NPQ under low-light conditions in Arabidopsis. Plant Cell Rep. 2018;37(2):279–291. doi: 10.1007/s00299-017-2229-6. [DOI] [PubMed] [Google Scholar]
- 63.Porcel R, Redondo-Gómez S, Mateos-Naranjo E, Aroca R, Garcia R, Ruiz-Lozano JM. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J Plant Physiol. 2015;185(2):75–83. doi: 10.1016/j.jplph.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Xu N, Zhang HH, Zhong HX, Wu, YN, Li, JB, Li, X, Yin, ZP, Zhu, WX, Qu, Yi, Sun, GY, et al. The response of photosynthetic functions of F1 cutting seedlings from Physocarpus amurensis Maxim (♀) × Physocarpus opulifolius “Diabolo” (♂) and the parental leaves to salt stress. Front Plant Sci. 2018;9:71. doi: 10.3389/fpls.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou RH, Kan X, Chen JJ, Hua, HL, Li, Y, Ren, JJ, Feng, K, Liu, HH, Deng, DX, Yin, ZT, et al. Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ Exp Bot. 2019;158:51–62. [Google Scholar]
- 66.Zhang HH, Xu N, Li X, Jin, WW, Tian, Q, Gu, SY, Sun, GY, et al. Overexpression of 2-Cys Prx Increased salt tolerance of photosystem II in tobacco. Int J Agric Biol. 2017;19(4):735–745. doi: 10.17957/IJAB/15.0348. [DOI] [Google Scholar]
- 67.Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50(1):601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 68.Zhang HH, Feng P, Yang W, Sui X, Li X, Li W, Zhang R, Gu S, Xu N. Effects of flooding stress on the photosynthetic apparatus of leaves of two Physocarpus cultivars. J Forest Res. 2018;39(4):1049–1059. doi: 10.1007/s11676-017-0496-2. [DOI] [Google Scholar]
- 69.Liu YF, Qi MF, Li TL. Photosynthesis, photoinhibition, and antioxidant system in tomato leaves stressed by low night temperature and their subsequent recovery. Plant Sci. 2012;196(11):8–17. doi: 10.1016/j.plantsci.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Yang N, Wang CL, He WP, Qu YZ, Li YS. Photosynthetic characteristics and effects of exogenous glycine of Chorispora bungeana under drought stress. Photosynthetica. 2015;54(3):459–467. doi: 10.1007/s11099-016-0187-9. [DOI] [Google Scholar]
- 71.Johnson GN. Physiology of PSI cyclic electron transport in higher plants. Biochimica et Biophysica Acta-Bioenergetics. 2011;1807(3):384–389. doi: 10.1016/j.bbabio.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Miyake C. Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol. 2010;51(12):1951–1963. doi: 10.1093/pcp/pcq173. [DOI] [PubMed] [Google Scholar]
- 73.Chow WS, Hope AB. Electron fluxes through photosystem I in cucumber leaf discs probed by far-red Light. Photosynth Res. 2004;81(1):77–89. doi: 10.1023/B:PRES.0000028396.83954.36. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto H, Takahashi S, Badger MR, Shikanai T. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat Plants. 2016;2(3):16012. doi: 10.1038/nplants.2016.12. [DOI] [PubMed] [Google Scholar]
- 75.Walker BJ, Strand DD, Kramer DM, Cousins AB. The response of cyclic electron flow around photosystem I to changes in photorespiration and nitrate assimilation. Plant Physiol. 2014;165(1):453–462. doi: 10.1104/pp.114.238238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye J-Y, Mi H. Chloroplastic NAD(P)H Dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 2006;141(2):465–474. doi: 10.1104/pp.105.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi S, Milward SE, Fan DY, Chow WS, Badger MR. How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol. 2009;149(3):1560–1567. doi: 10.1104/pp.108.134122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tikkanen M, Grieco M, Aro KM, Aro E-M. Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol. 2010;152(2):723–735. doi: 10.1104/pp.109.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamori W, Sakata N, Suzuki Y. Cyclic electron flow around photosystem I via chloroplast NAD(P)H dehydrogenase (NDH) complex performs a signifcant physiological role during photosynthesis and plant growth at low temperature in rice. Plant J. 2011;68:966–976. [DOI] [PubMed] [Google Scholar]
- 80.Pérez-Torres E, Bravo LA, Corcuera LJ, Johnson GN. Is electron transport to oxygen an important mechanism in photoprotection? Contrasting responses from Antarctic vascular plants. Physiol Plant. 2007;130(2):185–194. doi: 10.1111/j.1399-3054.2007.00899.x. [DOI] [Google Scholar]
- 81.Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell. 2002;110(3):361–371. doi: 10.1016/S0092-8674(02)00867-X. [DOI] [PubMed] [Google Scholar]
- 82.Munekage Y, Hashimoto M, Miyake C, Tomizawa K-I, Endo T, Tasaka M, Shikanai T. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature. 2004;429(6991):579–582. doi: 10.1038/nature02598. [DOI] [PubMed] [Google Scholar]
- 83.Barth C, Krause HG, Krause CBH. Study of tobacco transformants to assess the role of chloroplastic NAD(P)H dehydrogenase in photoprotection of photosystems I and II. Planta. 2002;216(2):273–279. doi: 10.1007/s00425-002-0843-0. [DOI] [PubMed] [Google Scholar]
- 84.Allakhverdiev SI, Nishiyama Y, Takahashi S, Miyairi S, Suzuki I, Murata N. Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in synechocystis. Plant Physiol. 2005;137(1):263–273. doi: 10.1104/pp.104.054478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyake C, Shinzaki Y, Miyata M, Tomizawa K-I. Enhancement of cyclic electron flow around PSI at high light and its contribution to the induction of non-photochemical quenching of chl fluorescence in intact leaves of tobacco plants. Plant Cell Physiol. 2004;45(10):1426–1433. doi: 10.1093/pcp/pch163. [DOI] [PubMed] [Google Scholar]
- 86.Kadakia SC, Cassaday M, Shaffer RT, Tomizawa K-I. CO2 Response of Cyclic Electron Flow around PSI (CEF-PSI) in Tobacco Leaves—Relative Electron fluxes through PSI and PSII Determine the Magnitude of Non-photochemical Quenching (NPQ) of Chl Fluorescence. Plant Cell Physiol. 2005;46(4):629–637. doi: 10.1093/pcp/pci067. [DOI] [PubMed] [Google Scholar]
- 87.Munekage YN, Genty B, Peitier G. Effect of PGR5 impairment on photosynthesis and growth in Arabidopsis thaliana. Plant Cell Physiol. 2008;49(11):1688–1698. doi: 10.1093/pcp/pcn140. [DOI] [PubMed] [Google Scholar]
- 88.Antikainen M, Pihakaski S. Early developments in RNA, protein, and sugar levels during cold stress in winter rye (Secale cereale) leaves. Ann Bot. 1994;74(4):335–341. doi: 10.1006/anbo.1994.1126. [DOI] [Google Scholar]
- 89.Döll S, Lippmann R, Mock HP. Proteomic approaches to identify cold-regulated soluble rroteins. Methods Mol Biol. 2014;1166:139–158. [DOI] [PubMed] [Google Scholar]
- 90.Xu N, Sun GY. Responses of mulberry seedlings photosynthesis and antioxidant enzymes to chilling stress after low-temperature acclimation. Chin J Appl Ecol. 2009;20:761–766. [PubMed] [Google Scholar]
- 91.Zhang HH, Li X, Guan YP, Mabo L, Yue W, Meijun A, Yuehui Z, Guanjun L, Nan X, Guangyu S, et al. Physiological and proteomic responses of reactive oxygen species and antioxidant machinery in leaves of mulberry (Morus alba L.) to NaCl and NaHCO3 stress. Ecotoxicol Environ Saf. 2020;193:110259. doi: 10.1016/j.ecoenv.2020.110259. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Wei JP, Scott ER, Liu J-W, Guo S, Li Y, Zhang L, Han W-Y. Exogenous Melatonin Alleviates Cold Stress by Promoting Antioxidant Defense and Redox Homeostasis in Camellia sinensis L. Molecules. 2018;23(1):165. doi: 10.3390/molecules23010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren J, Liu CP, Zhao D, Fu, J, et al. The role of heat shock protein 70 in oxidant stress and inflammatory injury in quail spleen induced by cold stress. Environ Sci Pollut Res 25 21 . 2018;1–13. [DOI] [PubMed] [Google Scholar]
- 94.Fan P, Feng J, Jiang P, Chen X, Bao H, Nie L, Jiang D, Lv S, Kuang T, Li Y, et al. Coordination of carbon fixation and nitrogen metabolism in Salicornia europaea under salinity: comparative proteomic analysis on chloroplast proteins. Proteomics. 2011;11(22):4346–4367. doi: 10.1002/pmic.201100054. [DOI] [PubMed] [Google Scholar]
- 95.Foyer CH, Ruban AV, Noctor G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem J. 2017;474(6):877–883. doi: 10.1042/BCJ20160814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foyer CH. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot. 2018;154:134–142. doi: 10.1016/j.envexpbot.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noctor G, Reichheld JP, Foyer CH. ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol. 2018;8:3–12. doi: 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 98.Nito K, Kamigaki A, Kondo M, Hayashi M, Nishimura M. Functional classification of Arabidopsis peroxisome biogenesis factors proposed from analyses of knockdown mutants. Plant Cell Physiol. 2007;48(6):763–774. doi: 10.1093/pcp/pcm053. [DOI] [PubMed] [Google Scholar]
- 99.Zhang L, Tian LH, Zhao JF, Song Y, Zhang C-J, Guo Y. Identification of an apoplastic protein involved in the initial phase of salt stress response in rice root by two-dimensional electrophoresis. Plant Physiol. 2009;149(2):916–928. doi: 10.1104/pp.108.131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manaa A, Ben Ahmed H, Valot B, Bouchet J-P, Aschi-Smiti S, Causse M, Faurobert M. Salt and genotype impact on plant physiology and root proteome variations in tomato. J Exp Bot. 2011;62(8):2797–2813. doi: 10.1093/jxb/erq460. [DOI] [PubMed] [Google Scholar]
- 101.Shafi A, Pal AK, Sharma V, Kalia S, Kumar S, Ahuja PS, Singh AK. Transgenic potato plants overexpressing SOD and APX exhibit enhanced lignification and starch biosynthesis with improved salt stress tolerance. Plant Mol Biol Rep. 2017;35(5):504–518. doi: 10.1007/s11105-017-1041-3. [DOI] [Google Scholar]
- 102.Zhang HH, Xu N, Teng ZY, Wang J-R, Ma S, Wu X, Li X, Sun G-Y. 2-Cys Prx plays a critical role in scavenging H2O2 and protecting photosynthetic function in leaves of tobacco seedlings under drought stress. J Plant Interact. 2019;14(1):119–128. doi: 10.1080/17429145.2018.1562111. [DOI] [Google Scholar]
- 103.Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G, Molassiotis A, Job D. Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J. 2009;60(5):795–804. doi: 10.1111/j.1365-313X.2009.04000.x. [DOI] [PubMed] [Google Scholar]
- 104.Du CX, Fan HF, Guo SR, Tezuka T, Li J. Proteomic analysis of cucumber seedling roots subjected to salt stress. Phytochemistry. 2010;71(13):1450–1459. doi: 10.1016/j.phytochem.2010.05.020. [DOI] [PubMed] [Google Scholar]


