ABSTRACT
Since the multicomponent meningococcal B vaccine introduction, the Apulian Regional Health Authority implemented postmarketing surveillance program, as provided by Italian laws.
From National Pharmacovigilance Network, we selected 4CMenB AEFIs reported in Apulia from 01 January 2014 to 31 December 2019, while the number of 4 cMen B doses administered per year was obtained from the regional immunization database (GIAVA).
For each subject who experienced an adverse event following meningococcal B vaccine (AEFIs), a predefined form was filled in.
A total of 214 AEFIs (26.5 × 100.000 doses) were reported after any dose of MenB-4 c vaccination of which 58/214 (27.1%) were classified as serious (7.2 × 100,000 doses), 145/214 (67.8%) as not serious (180 × 100,000 doses), and 11/214 (5.1%) as undefined (1.3 × 100,000 doses).
The average age of subjects who experimented and AEFI was 30 months. The majority of serious AEFIs were reported in 2- to 11-month-old children (44/57; 77.2%). A total of 31/58 (3.8 × 100,000 doses; 53.4%) serious AEFIs were reported as having a ‘consistent causal association’ with vaccination. Of these, fever/hyperpyrexia was reported in 21/31 (2.6 × 100,000 doses; 67.7%); hypotonic-hyporesponsive episode was reported in 7/31 (0.9 × 100,000 doses [add %-age]) and was the most frequent adverse event with neurological symptoms. A total of 13/31 (41.9%) serious AEFIs classified as ‘consistent causal association’ were reported after the first dose of 4cMenB, of these 5/13 (38.5%) children did not complete the vaccination schedule.
Our data seemed to confirm, in a large population, the a good safety profile of the universal mass vaccination with 4CMENB.
KEYWORDS: AEFI, post-marketing surveillance; causality assessment; hesitancy; hypotonic-hyporesponsive episode
Introduction
Neisseria meningitidis is a gram-negative bacterium commensal of the nasopharynx, but also an important exclusive human pathogens, which may cause meningococcal invasive diseases (IMD), such as meningitis and sepsis.1,2 It is classified into 12 serogroups, identified according to the different capsular polysaccharide structure: in particular, A, B, C, W135, Y, and recently also the X group (that in Saharan Africa is responsible for 99% of cases of meningococcal disease) can cause IMD in humans. IMD may occur as sporadic cases, outbreaks and epidemic.3–7
Prevalence changes overtime, and also W135 and C serotypes are causing large outbreaks since serotype A epidemics were eliminated after widespread vaccination with Men ACV.8,9
Because of gaps in surveillance, there are currently no reliable global estimates of IMD burden. Due to the dynamic nature of IMD epidemiology, the global distribution of the different serogroups of Neisseria meningitidis may change over time: the highest incidence rates are usually observed in the Sahel, from Senegal to Ethiopia (the so-called African meningitis belt).2,7–9 Currently, meningococcal serogroup B (MenB) is a major cause of IMD in North America, South America, Australia, North Africa, and Europe, although a decreasing incidence trend is being observed. Meningococcal serogroup C (MenC) was also reported as one of the most prevalent serogroups in Brazil, China, Russia, India, and Niger/Nigeria. In India, the predominant serogroup was meningococcal serogroup A (MenA). In Japan and Southern Africa (Mozambique) meningococcal serogroup Y (MenY), and meningococcal serogroup W (MenW) predominated, respectively. The emergence of MenW and MenY was evident in some countries worldwide.10
Globally, serogroup B caused the highest proportion of cases in all age groups below 65 years and accounted for 70% of IMD in children under the age of five years.11,12 For example, serogroup B has been responsible for an outbreak in France in 2000–2003 and several outbreaks at US university and college campuses from 2013 to 2015.13–16
ECDC reported that notifications of serogroup B infectious diseases decreased from 0.42 cases per 100 000 in 2013 to 0.30 cases in 2017. The decrease was most pronounced in children, where rates diminished from 10.4 to 5.4 per 100 000 in children <1 year of age, and from 2.6 to 1.7 per 100 000 in ones 1–4 years of age.14
In the past decades, effective and safe vaccines against N.meningitidis serogroup A,C,W135 and Y have been developed and licensed on the basis that the conjugation of the respective polysaccharide to a protein is able to induce immunological memory and be immunogenic even in infants and young children. The application of this technique to develop vaccines against serogroup B was not feasible as the polysaccharide is an autoantigen being expressed by some host tissues (16). Until recently, no broadly effective serogroup B meningococcal vaccines were available as the capsular polysaccharide of meningococcal serogroup B is poorly immunogenic in humans.17,18
In January 2013, a novel vaccine against Neisseria meningitidis serogroup B, the multicomponent meningococcal serogroup B vaccine (4CMenB), was approved by the European Medicines Agency. The 4CMenB vaccine consists of subcapsular antigens including New Zealand strain outer membrane vesicles (NZOMV) with PorA 1.4 antigenicity and recombinant antigens which include NadA (neisserial adhesion A), NHBA (Neisseria heparin binding antigen) and fHbp (factor H binding protein).19–22
Based on initial published studies and licensure application, the vaccine has been shown to be immunogenic in infants between two and 5 months of age and has been approved for use in a three-dose primary series followed by a booster between 12 and 15 months of age (3 + 1 dose schedule), and in infants 6 months to 24 months a two-dose primary series followed by a booster; in young children from two years of age, adolescents and adults two doses led to protective antibodies against the vaccine antigens.23–26
However, the successful implementation of vaccine programs depends on many factors including safety and reactogenicity profile of the vaccine that may influence, the acceptability and adherence of the patient/parent. Because vaccines are administered to healthy populations, there is low tolerance for any potential risks as adverse events following immunization (AEFIs).27,28
In the 4CMenB pre-registrative clinical trials, 77% of infants experienced fever of 38 · 5°C or higher after any dose, compared with 45% after routine vaccines alone; also the incidence of vaccine-related serious adverse events in individuals receiving 4CMenB is significantly higher than that of routine vaccines. In addition, prelicensure studies and postmarketing surveillance data suggested that 4CMenB is more reactogenic when coadministered with routine vaccines.29–32
National Regulatory Authorities such as AIFA in Italy) monitor the safety of vaccines in the postmarketing phase by collecting and analyzing reports of adverse events (passive surveillance) or by specific active surveillance programs.33,34
Apulia is a large region in the South of Italy (4,000,000 inhabitants) where meningococcal B vaccine (Bexsero is offered free-of-charge to all newborns since 2014. Since 2017 the vaccine is also used for catch-up strategies that targeted adolescents and adult people affected by some risk condition (e.g. splenectomized patients).35
Since MenB vaccine introduction, the Apulian Regional Health Authority implemented an active post-marketing surveillance program to evaluate safety and effectiveness of antimeningococcal B vaccine administered in infants, young children, adolescents, and adults.
This work reports the results of the regional postmarketing passive surveillance program from 2014 to 2019.
Material and methods
In Apulia Region surveillance of AEFIs is carried out by healthcare workers of Vaccination Centers, Family Pediatricians and Hospital Physicians. Each one has to report every case of AEFIs occurred in their patients to the National Pharmacovigilance Network (RNF), a platform managed by the Italian Drug Authority (AIFA). The report of AEFIs can also be carried out directly by the children’s parents.
The source of information for this study were National Pharmacovigilance Network and the Regional Database of Immunization (GIAVA).
AEFIs after 4CMenB vaccination reported from Apulia Region from 01 January 2014 until 31 December 2019 were selected from the National Pharmacovigilance Network database, the number of 4cMen B doses administered per year in Apulia Region was obtained from the regional immunization database (GIAVA).
For every subject who experienced an adverse event following meningococcal B vaccination (AEFIs), a specific form was built, including information on date of birth, gender, date of vaccine administration, other vaccines administered in the same visit and information about the AEFIs (date of onset and date of computing in National Pharmacovigilance Network, clinical characteristics of the adverse events, case description, duration and treatment, hospitalization or emergency room access, final outcome).
Excel spreadsheet was used to built the database and perform the analyses.
The total reporting rate was calculated as the total number of AEFIs/number of 4cMen B doses administered, while the annual reporting rate was calculated using the number of AEFIs occurred in the year by the number 4cMen B doses administered in the same year.
WHO guidelines were used to classify AEFIs as “serious” or “not serious.” An AEFI is considered serious, if: it results in death; it is life-threatening; it requires in-patient hospitalization or prolongation of existing hospitalization; it results in persistent or significant disability/incapacity; it is a congenital anomaly/birth defect, or requires intervention top event permanent impairment or damage. Additionally, in 2016 AIFA published a list of particular health conditions that must be considered as serious AEFIs, if they occur after vaccination. This list is the Italian edition of EMA IME list.36,37
For serious AEFIs, we retrospectively applied the WHO causality assessment algorithm to classify AEFI as ‘consistent causal association,’ ‘inconsistent causal association,’ indeterminate,’ or ‘not-classifiable.’38
For serious AEFIs, 1 month after notification, a follow up was been carried out in order to guarantee a supplemental surveillance of vaccine safety.
For AEFIs that required hospitalization, we reviewed the causality assessment using additional data from the medical record.
Results
From 2014 to 2019 a total of 807.446 doses of 4CMenB vaccine were administered in Apulia Region. During the same period, a total of 214 AEFIs after MenB-4c immunization (reporting rate: 26.5 × 100,000 doses) were reported in Apulia Region: data are similar to Italian ratio as indicated in 2018 in AIFA report (30.8 × 100,000 doses)
In Graph 1, the number and rate of AEFIs reported by year is: the highest number (54/214, 25.2%) was registered in 2017 while the highest reporting rate (47.3 × 100,000 doses) was registered in 2015.
Graph 1.
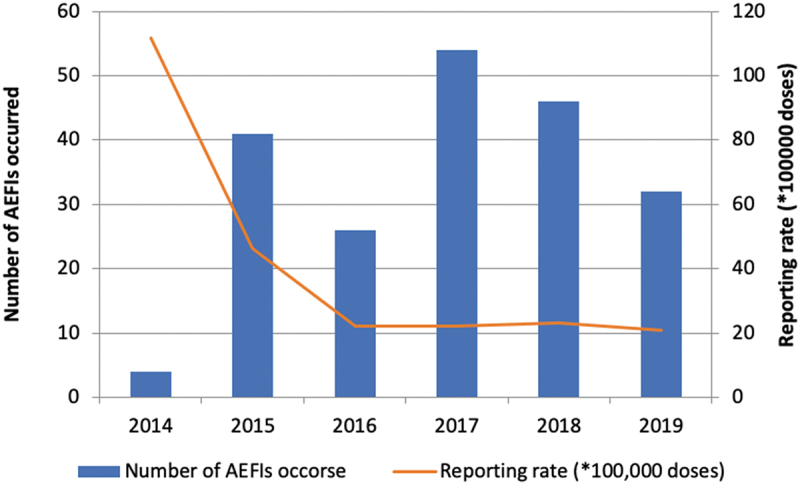
Distribution of 4CMenB AEFIs, per year of onset and annual reporting rate × 100,000 doses. Puglia Region (Italy), 2014–2019.
The average time elapsed between administration of the vaccine and the onset of the adverse event is 1.9 ± 7.6 days (range = 0.0–70.0) while the median time between the onset of the suspected adverse reaction and the report to RNF is 32.0 days (IQR interval = 11.0–60.0; interval = 0.0–833.0).
In several AEFIs report (n = 183; 85.6%), 4CMenB was administered alone while in 11/214 (5.1%) the meningococcal serogroup ACYW vaccine was simultaneously administered, in 7/214 rotavirus vaccine and in 5/214 the meningococcal serogroup C vaccine. The majority of the reports (n = 182; 85.0%) were from healthcare professionals and 25 (11.7%) from consumers and 7 (3.3%) from pharmacists.
The median age of people who had a 4CMENB AEFI reported was 30.0 months (range: 2–394), the age was unknown for 7 subjects, 50.9% (109) were males and 48.1% (103) were females, the gender was unknown for 2 (0,9%).
58/214 (27.1%) AEFIs were classified as serious (reporting rate: 7.2 × 100,000 doses); 145/214 (67.8%) as not serious (reporting rate = 180 × 100,000 doses); and 11/214 (5.1%) as undefined (reporting rate = 1.3 × 100,000 doses).
Out of the serious AEFIs, 30/58 (51.2%) were hospitalized, 1/58 resulted in death (1.7%), 1/58 in life threatening (1.7%), 2/58 in sequelae (3.5%), and 24/58 (41.9%) had particular health conditions (listed by AIFA in 2016) (Table 1).36
Table 1.
Number and reporting rate of symptoms/clinical signs most frequently notified in spontaneous 4cMen B vaccine AEFI-reports. Puglia Region (Italy), 2014–2019
| Symptom/clinical sign | n |
Reporting rate (× 100.000 dosses) |
|---|---|---|
| Injection site reactions (redness, skin rash, swelling, local pain) |
138 | 17.0 |
| Fever, hyperpyrexia | 94 | 12.9 |
| Neurological symptoms | 74 | 8.8 |
|
11 | 1.4 |
|
20 | 2.5 |
|
12 | 1,5 |
|
22 | 2.7 |
|
7 | 0.9 |
|
13 | 1.6 |
|
3 | 0.4 |
| Gastrointestinal diseases | 31 | 3,8 |
| Allergic reaction | 6 | 0.9 |
| Lymphadenitis | 2 | 0.3 |
| Other local signs/symptoms | 50 | 7.7 |
The majority of serious AEFI reports were in children of 2–11 months of age (n = 44/57; 77.2%) (Table 2). For 7 subjects the age in unknown.
Table 2.
Distribution of 4cMenB AEFIs reports per severity and age class. Puglia Region (Italy), 2014–2019
| Age groups | 2–11 months |
11–23 months |
>23 months |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Serious | 44 | 77.2 | 1 | 1.8 | 12 | 21.0 | 57* | 100 |
| Not serious | 97 | 69.3 | 8 | 5.7 | 35 | 25.0 | 140** | 100 |
| Not defined | 10 | 100 | 0 | 0 | 0 | 0 | 10*** | 100 |
*for 1 people age is unknown.
**for 5 people age in unknown.
***for 1 people age is unknown.
Performing causality assessment, 31/58 (53.4%, reporting rate = 3.8 × 100,000 doses) serious AEFIs were classified as ‘consistent causal association’ to the 4cMenB vaccination, while 2/58 (3.4%, reporting rate = 2.5 × 100,000) were indeterminate and 17/58 (29.4%, reporting rate = 2.1 × 100,000) were classified as ‘not consistent causal association’; 8/58 serious AEFIs (13.8%, reporting rate = 3.8 × 100,000 doses) were considered as not classifiable.
Fever/hyperpyrexia was detected in 21/31 (67.7%) ‘consistent causal association’ serious AEFIs reporting rate = 2.6 × 100,000 doses); hypotonic-hyporesponsive episode (7/31, reporting rate 0.9 × 100,000 doses) was the most frequent adverse event of neurological symptoms (Graph 2).
Graph 2.
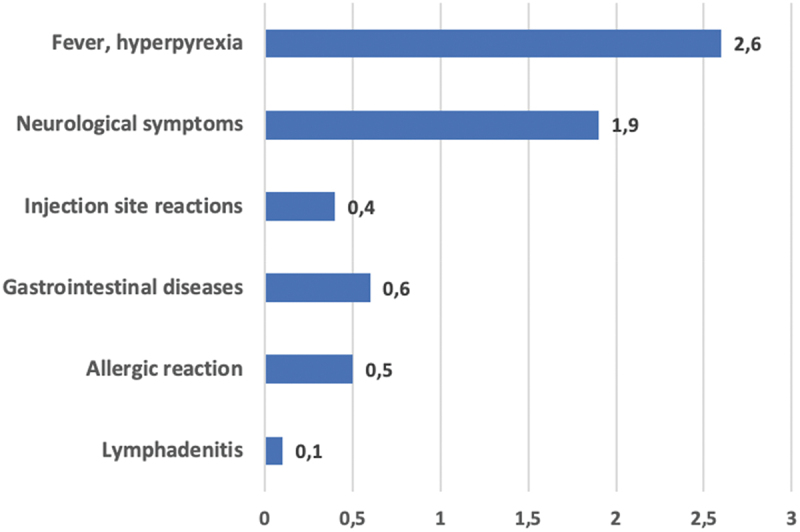
Reporting rate of symptoms/clinical signs most frequently notified in serious 4cMenB AEFIs reports with a consistent causal association with immunization. Puglia Region (Italy), 2014–2019.
At the time of data collection serious AEFIs, classified as ‘consistent causal associated’ to the 4cMenB vaccine were completely resolved without sequelae.
A total of 13/31 (41.9%) serious AEFIs classified as ‘consistent causal association’ were reported after the first dose of 4cMenB and 5/13 (38.5%) of these children did not complete vaccination schedule.
Of 15/31 (48.4%) children who experienced a serious AEFIs classified as ‘consistent causal association’ after the subsequent doses, 5/15 (33.3%) have not completed the vaccine schedule; for 3/31 serious AEFIs classified as ‘consistent causal association’ the information of the dose administered is not available.
Discussion and conclusion
Post-marketing surveillance of adverse events following immunization (AEFIs) is routinely carried out by a passive system, based on the spontaneous notification by Health Care Workers and patients: this model is badly affected by the risk of underreporting. In our study we tried to provide a picture of 4cMenB vaccine safety, even if limited by data from spontaneous reporting (e.g. missing or incomplete data).39,40
Using the AIFA database, all adverse events following 4cMenB vaccination notified in the Apulia region since vaccine authorization were analyzed. In general, the AEFI reports are consistent with the known safety profile of 4CMenB as reflected in the Summary of Product Characteristics and other postmarketing findings.21,41
The reported events were mostly evident in the first and second day after the vaccination: 145/214 regarded local reactions with rapid and spontaneous resolution, while 58/214 (27.1%) were classified as serious (reporting rate: 7.2 × 100,000 doses).
2/58 (3.4%) serious AEFI were related with a death or life threatening, but performing causality assessment they were classified as ‘not consistent causal association. In particular, one of the two events regards a case of B meningitis and meningococcemia happened 7 days after the first dose of 4CMENB (according to temporal pattern, it could not be classified as a vaccine failure and there is not plausibility of a causing role of vaccination in the onset of infection).
Fever and hyperpyrexia are the adverse events most frequently detected in serious AEFI reports (21/31, 67.7%) classified as consistent with immunization (reporting rate 2.6 × 100,000 doses): data are similar to prelicensure findings.
The fever after 4CMenB vaccine remains one of the most important problem about its acceptability and the risk of missing the competition of vaccination schedule. Murdoch et al. demonstrated that fever and hyperpyrexia were connected to an increased risk of hospital admission within 3 days of the vaccine administration and suggested use of prophylactic paracetamol.42,43
Because of fever (≥ 38.5°C) in young children aged less than 2 years is a common and expected adverse event following 4CMenB administration, Government of South Australia recommended Paracetamol with every dose of this vaccine for those aged less than 2 years.44
NHS also recently published a Protocol for the supply or administration of paracetamol oral suspension 120 mg/5 mL to infants under 12 months of age receiving primary doses of MenB vaccination.45
Even if the role of paracetamol in the 4CMenB immunization has been studied also in prelicensure trial, in Italy there is no a formal recommendation about its prophylactic use and postmarketing evaluation is crucial to assess its role in the prevention of serious AEFIs and the risk/benefit balance.
In 7/31 serious AEFI classified as consistent with immunization a hypotonic-hyporesponsive episode was notified: our findings are in line with the previously published literature regarding the benignity of the episodes, as they mostly resolved briefly and all the infants returned to the prevaccination status with no alteration in neuropsychomotor development.46
An emerging issue must be studied in future research: the experience of an AEFI as determinants of missing vaccination schedule. In our report, we documented that 19/28 people with an history of serious AEFIs missed the competition of vaccination cycle and this phenomenon could be very important for Public Health. Because of spontaneous reporting surveillance system are badly affected by underreporting, the impact of the experience of serious AEFIS in vaccination compliance could be, in principle, very notable and requires specific studies on large population.
Acknowledgments
We are very grateful to physicians, pharmacists, health visitors and nurses from Puglia AEFIs working group for the important support in implanting this surveillance program: Vito Bavaro, Maria Cristina Carbonara, Sharon Natasha Cox, Roberta Lupoli, Stella Saponaro, Letizia Rizzo, Giulia Calabrese, Antonio Falco, Francesca Rizzi, Michele Terlizzi, Marisa Ferraro, Marilena Nesta, Giuseppe Palamà, Leda Schirinzi, Marcello De Simone, Giovanni Caputi, Francesco Desiante, and Carmela Nanula.
Funding Statement
This research is part of activities of the regional surveillance programfounded by National Drug Authority (AIFA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
References
- 1.Gianchecchi E, Torelli A, Piccini G, Piccirella S, Montomoli E.. Neisseria meningitidis infection: who, when and where? Expert Rev Anti Infect Ther. 2015;13(10):1249–63. PMID: 26190347. doi: 10.1586/14787210.2015.1070096. [DOI] [PubMed] [Google Scholar]
- 2.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–210. PMID: 17604802. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 3.Gianchecchi E, Piccini G, Torelli A, Rappuoli R, Montomoli E. An unwanted guest: neisseria meningitidis – carriage, risk for invasive disease and the impact of vaccination with insight on Italy incidence. Expert Rev Anti Infect Ther. 2017;15(7):689–701. PMID: 28524748. doi: 10.1080/14787210.2017.1333422. [DOI] [PubMed] [Google Scholar]
- 4.Bosis S, Mayer A, Esposito S. Meningococcal disease in childhood: epidemiology, clinical features and prevention. J Prev Med Hyg. 2015;56(3):E121–4. PMID: 26788732. [PMC free article] [PubMed] [Google Scholar]
- 5.Caugant DA, Maiden MCJ. Meningococcal carriage and disease–population biology and evolution. Vaccine. 2009;27(Suppl 2):B64–70. PMID: 19464092. doi: 10.1016/j.vaccine.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwilow R, Fanella S. Invasive meningococcal disease in the 21st century-an update for the clinician. Curr Neurol Neurosci Rep. 2015;15(3):2. PMID: 25637287. doi: 10.1007/s11910-015-0524-6. [DOI] [PubMed] [Google Scholar]
- 7.Bories S, Slaterus KW, Faucon R, Audiffren P, Vandederdove M. Peut-on individualiser deux nouveaux groupes serologiques de Neisseria meningitidis? Med Trop. 1966;26:603–16. [Google Scholar]
- 8.Evans JR, Artenstein MS, Hunter DH. Prevalence of meningococcal serogroups and description of three new groups. Am J Epidemiol. 1968;87:643–46. PMID: 4968345. doi: 10.1093/oxfordjournals.aje.a120854. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Meningococcal meningitidis - Key Facts. February 2018. [Accessed 2021 January 23]. https://www.who.int/news-room/fact-sheets/detail/meningococcal-meningitis.
- 10.Borrow R, Alarcón P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–28. PMID: 27820969. doi: 10.1080/14760584.2017.1258308. [DOI] [PubMed] [Google Scholar]
- 11.Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–36. PMID: 22178525. doi: 10.1016/j.vaccine.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Lewis R, Nathan N, Diarra L, Belanger F, Paquet C. Timely detection of meningococcal meningitis epidemics in Africa. The Lancet. 2001;358(9278):287–93. PMID: 11498215. doi: 10.1016/S0140-6736(01)05484-8. [DOI] [PubMed] [Google Scholar]
- 13.Balmer P, Burman C, Serra L, York L. Impact of meningococcal vaccination on carriage and disease transmission: a review of the literature. Human Vaccines & Immunotherapeutics. 2018;14(5):1118–30. PMID: 29565712. doi: 10.1080/21645515.2018.1454570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ECDC . Surveillance Report - Invasive meningococcal disease - Annual epidemiological report for 2017. [accessed 2021 Jan 27]. https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2017-invasive-meningococcal-disease.pdf.
- 15.Grodet C, Dequin PF, Watt S, Lanotte P, de Gialluly C, Taha MK, Alonso JM, Quentin R, Goudeau A, Mereghetti L. Outbreak in France of Neisseria meningitidis B:15: P1.12belonging to sequence type 1403. Clin Microbiol Infect. 2004;10(9):845–48. PMID: 15355418. doi: 10.1111/j.1469-0691.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- 16.Caron F, du Châtelet I, Leroy J, Ruckly C, Blanchard M, Bohic N, Massy N, Morer I, Floret D, Delbos V, et al. From tailor-made to ready-to-wear meningococcal B vaccines: longitudinal study of a clonal meningococcal B outbreak. Lancet Infect Dis. 2011;11(6):455–63. PMID: 21489881. doi: 10.1016/S1473-3099(11)70027-5. [DOI] [PubMed] [Google Scholar]
- 17.Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(22):608–12. PMID: 26068564. [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson B, Gandhi A, Balmer P. History of meningococcal outbreaks in the United States: implications for vaccination and disease prevention. Pharmacotherapy. 2016;36(8):880–92. PMID: 27332671. doi: 10.1002/phar.1790. [DOI] [PubMed] [Google Scholar]
- 19.Granoff DM. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50((s2)Suppl 2):S54–65. PMID: 20144017. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison LH. Vaccines for prevention of group B meningococcal disease: not your father’s vaccines. Vaccine. 2015;33(Suppl.4):D32–8. doi: 10.1016/j.vaccine.2015.05.101. [DOI] [PubMed] [Google Scholar]
- 21.European Medicine Agency (EMA) . Bexsero (meningococcal group B vaccine [rDNA, component, adsorbed]). [accessed 2021 Jan 26]. https://www.ema.europa.eu/en/documents/overview/bexsero-epar-summary-public_en.pdf.
- 22.ECDC . Expert opinion on the introduction of the meningococcal B (4CMenB) vaccine in the EU/EEA. December 2017. [accessed 2021 Jan 31]. https://www.ecdc.europa.eu/sites/portal/files/documents/Introduction-of-4CMenB-vaccine.pdf.
- 23.Martin NG, Snape MD. A multicomponent serogroup B meningococcal vaccine is licensed for use in Europe: what do we know, and what are we yet to learn? Expert Rev Vaccines. 2013;12(8):837–58. PMID: 23984957. doi: 10.1586/14760584.2013.814862. [DOI] [PubMed] [Google Scholar]
- 24.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2):B87–97. PMID: 22607904. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Block S, Shepard J, Garfield H, Xie F, Han L, Dull P, Smolenov I. Immunogenicity and safety of a 3- and 4-dose vaccination series of a meningococcal ACWY conjugate vaccine in infants: results of a phase 3b, randomized, open-label trial. Pediatr Infect Dis J. 2016. Feb;35(2):e48–59. PMID: 26479973. doi: 10.1097/INF.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 26.Santolaya M, O’Ryan M, Valenzuela M, Prado V, Vergara R, Muñoz A, Toneatto D, Graña G, Wang H, Dull P. Persistence of antibodies in adolescents 18−24 months after immunization with one, two, or three doses of 4CMenB meningococcal serogroup B vaccine. Human Vaccines & Immunotherapeutics. 2013;9(11):2304–10. doi: 10.4161/hv.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santolaya M, O’Ryan M, Valenzuela M, Prado V, Vergara R, Muñoz A, Toneatto D, Graña G, Wang H, Clemens R, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. The Lancet. 2012;379(9816):617–24. PMID: 22260988. doi: 10.1016/S0140-6736(11)61713-3. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Choe Y, Hong Y, Kim K, Park S, Kim Y, Oh C, Lee H, Song H, Bock H, et al. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine in healthy adolescents in Korea—A randomised trial. Vaccine. 2016;34(9):1180–86. PMID: 26826544. doi: 10.1016/j.vaccine.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Di Pasquale A, Bonanni P, Garçon N, Stanberry L, El-Hodhod M, Tavares Da Silva F. Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine. 2016;34:6672–80. PMID: 27836435. doi: 10.1016/j.vaccine.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Zafack J, Bureau A, Skowronski D, de Serres G. Adverse events following immunisation with four-component meningococcal serogroup B vaccine (4CMenB): interaction with co-administration of routine infant vaccines and risk of recurrence in European randomised controlled trials. BMJ Open. 2019;9(5):e026953. PMID: 31110098. doi: 10.1136/bmjopen-2018-026953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A; EU Meningococcal B Infant Vaccine Study group . Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013. Mar 9;381(9869):825–35. PMID: 23324563. doi: 10.1016/S0140-6736(12)61961-8. [DOI] [PubMed] [Google Scholar]
- 32.Flacco ME, Manzoli L, Rosso A, Marzuillo C, Bergamini M, Stefanati A, Cultrera R, Villari P, Ricciardi W, Ioannidis JPA, et al. Immunogenicity and safety of the multicomponent meningococcal B vaccine (4CMenB) in children and adolescents: a systematic review and meta-analysis. Lancet Infect Dis. 2018. Apr;18(4):461–72. PMID: 29371070. doi: 10.1016/S1473-3099(18)30048-3. [DOI] [PubMed] [Google Scholar]
- 33.AIFA . Rapporto sulla Sorveglianza post-marketing dei Vaccini in Italia Anno 2019. [accessed 2021 Feb 4]. https://www.aifa.gov.it/documents/20142/241052/Rapporto_Vaccini_2019.pdf.
- 34.Di Pietro A, Visalli G, Antonuccio GM, Facciolà A. Today’s vaccination policies in Italy: the national plan for vaccine prevention 2017-2019 and the Law 119/2017 on the mandatory vaccinations. Ann Ig. 2019;31(2 Supple 1):54–64. PMID: 30994164. doi: 10.7416/ai.2019.2277. [DOI] [PubMed] [Google Scholar]
- 35.Regione Puglia . Bollettino ufficiale della Regione Puglia n. 74 del 11/06/2014 – DGR 958 del 20/ 05/2014.Modifica Calendario Regionale per la vita 2012 - DGR 241/2013. Approvazione nuovo Calendario Vaccinale per la vita 2014. [accessed 2021 Feb 3]. http://www.regione.puglia.it/documents/10192/4808761/DELIBERAZIONE+DELLA+GIUNTA+REGIONALE+20+maggio+2014%2C%20n.+958+%28id+4808942%29/268b2b1a-7a73-4653-a758-a10a6706a410;jsessionid=861CA1EEE44809BE84188569E51A8684.
- 36.AIFA – gruppo di Lavoro sull’analisi dei segnali dei vaccini. Guida alla valutazione delle reazioni avverse osservabili dopo vaccinazione. 2016. [accessed 2021 Jan 28]. http://www.aifa.gov.it/sites/default/files/Guida_valutazione_reazioni_avverse_osservabili_dopo_vaccinazione_2.pdf.
- 37.European Medical Agency . Important medical event terms list version (MedDRA) – version 24.0. [accessed 2021 Mar 25]. https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/eudravigilance-system-overview.
- 38.WHO . Causality Assessment of an adverse event following immunization (AEFI). User manual for the revised WHO classification. [accessed 2021 Feb 26]. http://apps.who.int/iris/bitstream/handle/10665/259959/9789241513654-eng.pdf?sequence=1.
- 39.Stefanizzi P, Calabrese G, Infantino V, Del Matto G, Tafuri S, Quarto M. Systematic use of causality assessment in AEFI surveillance: a 2013-2016 pilot study in Puglia. EBMJ. 2017;12(33):154–58. doi: 10.3269/1970-5492.2017.12.33. [DOI] [Google Scholar]
- 40.Varallo F, Guimarães S, Abjaude S, Mastroianni P. Causes for the underreporting of adverse drug events by health professionals: a systematic review. Rev Escola Enfermagem USP. 2014;48(4):739–47. PMID: 25338257. doi: 10.1590/S0080-623420140000400023. [DOI] [PubMed] [Google Scholar]
- 41.Mentzer D, Oberle D, Keller-Stanislawski B. Adverse events following immunisation with a meningococcal serogroup B vaccine: report from post-marketing surveillance, Germany, 2013 to 2016. Eurosurveillance. 2018;23(17). PMID: 29717697. doi: 10.2807/1560-7917.ES.2018.23.17.17-00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puliyel J, Naik P. Revised World Health Organization (WHO)’s causality assessment of adverse events following immunization—a critique. F1000 Research. 2018;7:243. PMID: 30026925. doi: 10.12688/f1000research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murdoch H, Wallace L, Bishop J, Robertson C, Claire Cameron J. Risk of hospitalisation with fever following MenB vaccination: self-controlled case series analysis. Arch Dis Child. 2017;102(10):894–98. PMID: 28931535. doi: 10.1136/archdischild-2017-313079. [DOI] [PubMed] [Google Scholar]
- 44.Government of South Australia . Managing possible fever after meningococcal B vaccine (Bexsero) administration. [accessed 2021 Feb 17]. https://www.sahealth.sa.gov.au/wps/wcm/connect/d4c91180-7abb-470c-ab5b-954e47c90d20/3.+Managing+possible+fever+after+Bexsero+vaccine+%28A5+pad%29+%28WEB+READY%29.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-d4c91180-7abb-470c-ab5b-954e47c90d20-nwLvY6M.
- 45.Public Health England . Publications gateway number: GW-1249. Protocol for the supply or administration of paracetamol oral suspension 120mg/5mL to infants under 12 months of age receiving primary doses of MenB vaccination. [accessed 2021 Mar 2]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881402/20200427PHEParacetamolProtocol__1_.pdf.
- 46.Vigo A, Costagliola G, Ferrero E, Noce S. Hypotonic-hyporesponsive episodes after administration of hexavalent DTP-based combination vaccine: a description of 12 cases. Hum Vaccin Immunother. 2017;13(6):1375–78. PMID: 28301267. doi: 10.1080/21645515.2017.1287642. [DOI] [PMC free article] [PubMed] [Google Scholar]


