ABSTRACT
After the Japanese Ministry of Health, Labor and Welfare (MHLW) suspended its recommendation for the human papillomavirus (HPV) vaccine in June 2013, the rate of members of the new target population receiving of at least one of three doses of HPV vaccine declined, reaching 0.3% in 2016. Recently, however, the monthly number of vaccines delivered to healthcare facilities has significantly increased, from 878 doses over December 2016–April 2017 to 35,396 doses over January–March 2021. This may be due to governmental efforts to convey information about the HPV vaccination to the eligible population and their caregivers, as well as local educational programs, despite ongoing suspension of the recommendation. The incidence of reported adverse events per vaccine dose has not increased since 2016. While governmental recommendation of the HPV vaccination remains essential for optimal vaccine coverage, the recent increase in the number of doses delivered to healthcare facilities is promising.
KEYWORDS: human papillomavirus, vaccine, surveillance, adverse event, HPV vaccine
Letter
Government funding for human papillomavirus (HPV) vaccination in Japan began in 2010 for girls aged 12–16 years. While the vaccine has been included in the Japanese national immunization program since April 2013, concerns about vaccine-related adverse events (AEs) were widely reported in the Japanese mass media.1 In June 2013, the Japanese Ministry of Health, Labor and Welfare (MHLW) suspended its proactive recommendation of the HPV vaccine pending clarification of the actual frequency of AEs in order to provide accurate information to the public. Specifically, the government decided to maintain the HPV vaccine program for those eligible for it but suspended sending notifications and vaccination coupons to encourage vaccination. As a consequence, the rate of new target population receiving at least one of three doses of HPV vaccine in Japan declined from a reported > 70% to 0.3% in 2016.2 This has raised concerns about a future increase in the number of patients with HPV-related diseases.3,4
However, the situation concerning HPV vaccine administration has recently been changing. Figure 1 shows the number of HPV vaccine doses delivered to Japanese healthcare facilities using data available from 2016 onward.5 The monthly number of HPV vaccine doses delivered, including bivalent, quadrivalent, and nine-valent HPV vaccines, significantly increased, from 878 doses in December 2016–April 2017 to 35,396 doses during January–March 2021.4 Using the Cochran-Armitage trend test, we found that this increase in demand was significant (p value < .001).
Figure 1.
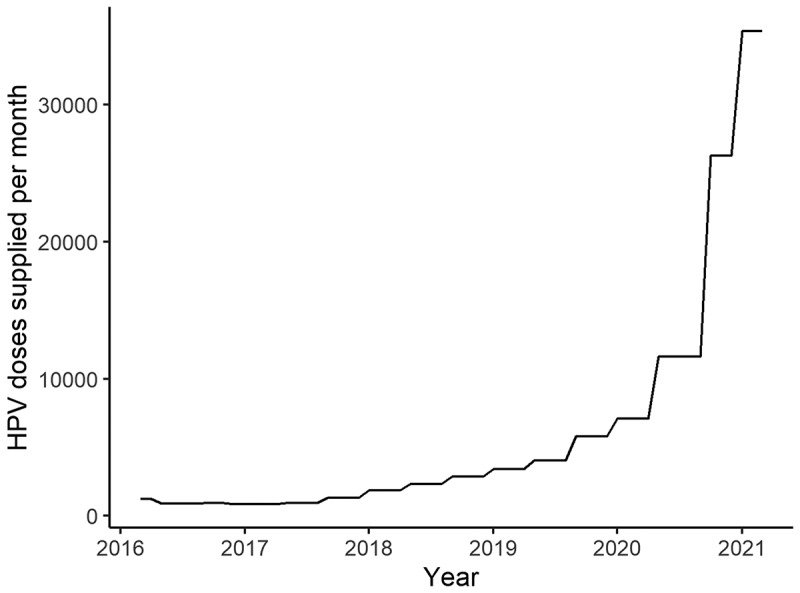
The monthly number of HPV vaccine doses delivered to healthcare facilities.
Abbreviation: HPV, human papillomavirus.
The number of AEs reported by physicians in Japan is presented in Figure 2. Of note, under the Immunization Law, physicians are required to report all designated adverse events or adverse events of concern to the MHLW; in addition, vaccinated persons themselves can report directly if they so desire. The Advisory Committee of Health Science Council on Vaccine Safety is convened to announce and evaluate the number of reports on a regular basis, but because the interval between meetings is irregular, data for two to five months are compiled and reported. Furthermore, reports of AEs are recorded at the time they are reported, and do not take into account the time between the vaccination and the AE, which introduces reporting bias. For this reason, the number of reports of relatively new AEs due to vaccination during the period covered by the report (2–5 months) is published separately. This made it appear that there was a very high number of AE reports from 2016 to 2018; however, the AEs were not actually reported as a result of vaccination during that time period, but rather as a result of females vaccinated in the past with HPV who suspected HPV vaccine AEs.
Figure 2.
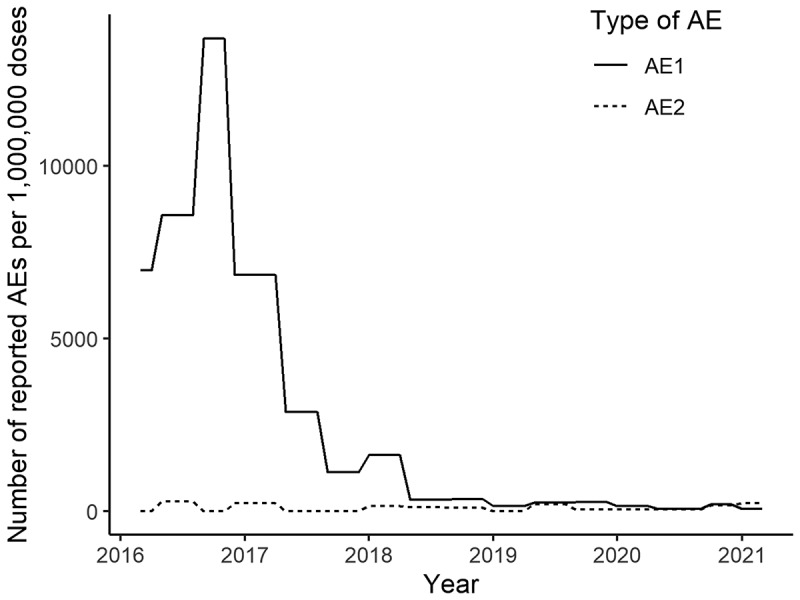
The number of adverse events reported by physicians or patients per million vaccine doses vs. those reported by the national council within 2–5 months of vaccination.
‘AE1ʹ includes all AE reports. ‘AE2ʹ refers to AEs reported by the national council for those who had the vaccination and reported AEs over a 2–5 months period.
Abbreviation: AE, adverse event.
Figure 2 demonstrates the differences associated with the two types of reporting. ‘AE1ʹ includes all AE reports by both physicians and laypersons. ‘AE2ʹ refers to AEs reported by the national council for those vaccinated over the preceding 2–5 months period. It should also be noted that almost all AEs (except 4 cases) reported during March 2016–August 2018 were from vaccinations administered before the period when AEs were reported (i.e., likely more than 2–5 months previously). The monthly number of AEs per million vaccine doses significantly decreased over the entire period of this report (p value < .001). On the other hand, the AEs reported in those who received the HPV vaccination in the 2–5 months investigation period (AE2) per million vaccine doses did not change during the period of this report (p value = .074).
The significant increase in the demand for HPV vaccines in Japan beginning in 2018–2019 is likely ascribable to multiple factors. First, the MHLW made efforts to convey correct information on the HPV vaccine; for example, by creating and releasing a leaflet about the HPV vaccine for those eligible for vaccination, their caregivers, and medical personnel in January 2018.6 In August 2019, in response to findings that the leaflet was not being used effectively, the National Immunization Council initiated a discussion on how to improve the provision of this information to eligible persons. In July 2020, the contents of the leaflet were revised to include information about cervical cancer and the actual use of the HPV vaccine in Japan and abroad. In October 2020, the MHLW requested that local governments send individual notification letters with the revised leaflet so that female students eligible for the national HPV immunization program could decide whether or not to receive the vaccination free of charge. In July 2020, the 9-valent HPV vaccine (Sylgard, Merck Sharp & Dohme Corp., Rahway NJ, USA) was approved for females, and its availability in the national vaccination program is being considered.3 In December 2020, the MHLW approved an additional regulatory indication for the quadrivalent HPV vaccine (Gardasil, Merck Sharp & Dohme) for men. In addition, multiple local education programs about the HPV vaccination have been conducted for the past several years.7–9 In response to these efforts, the attitudes and understanding of people who are eligible for vaccination are also gradually changing.10
The majority of AEs reported between 2016 and 2018 were linked to HPV immunizations administered before the period when the AEs were reported. This suggests that many AEs reported up to 2018 may have occurred more than 2–5 months postvaccine. This may be because of reporting bias in the early period, given that negative information about the HPV vaccine was predominant in Japan until a few years after the governmental withdrawal of the recommendation for the HPV vaccine.1 The number of AEs following HPV immunizations administered in the investigated period per vaccine dose has not increased. This leveling off of reports may encourage the government to resume its proactive recommendation of routine HPV vaccination. In the US, the reported rate of AEs from the quadrivalent HPV vaccine was 327/million doses between 2009 and 2015.11 While the reporting systems of Japan and the US differ, the reported rate of AEs in recent Japanese data (273.1/million doses in January–March 2021) is not high compared with the US data.
Of note, the data presented in this letter do not describe actual vaccinations administered but the number of vaccine doses delivered to healthcare facilities. However, given that annual national vaccination rates in Japan are reported after a delay of several years, we believe that the increase in the number of vaccine doses delivered to healthcare facilities is an important sign of an increase in the Japanese HPV vaccination rate. Another limitation is the varying duration of investigation periods, which range from 2 to 5 months. This may lead to differences in sensitivity in the capture of acute AEs. Resumption of the government’s recommendation is essential to further stimulating the behavioral changes needed to reduce future cancer cases. An increase in the number of vaccinees will increase the amount of national information available to confirm the vaccine’s safety and provide a basis for making such decisions.
Supplementary Material
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No conflict of interest to declare.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1986333.
References
- 1.Tsuda K, Yamamoto K, Leppold C, Tanimoto T, Kusumi E, Komatsu T, Kami M.. Trends of media coverage on human papillomavirus vaccination in Japanese newspapers. Clin Infect Dis. 2016;63(12):1634–38. doi: 10.1093/cid/ciw647. [DOI] [PubMed] [Google Scholar]
- 2.Simms KT, Hanley SJB, Smith MA, Keane A, Canfell K. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health. 2020;5(4):e223–e234. doi: 10.1016/S2468-2667(20)30010-4. [DOI] [PubMed] [Google Scholar]
- 3.Yagi A, Ueda Y, Nakagawa S, Ikeda S, Tanaka Y, Sekine M, Miyagi E, Enomoto T, Kimura T. Potential for cervical cancer incidence and death resulting from Japan’s current policy of prolonged suspension of its governmental recommendation of the HPV vaccine. Sci Rep. 2020;10(1):15945. doi: 10.1038/s41598-020-73106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka Y. Time to resume active recommendation of the HPV vaccine in Japan. Lancet Oncol. 2020;21(12):1552–53. doi: 10.1016/S1470-2045(20)30608-2. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health, Labour and Welfare of Japan . HPV Vaccine adverse event reports in the National Committee on Vaccine Safety [accessed 2021 September 3]. https://www.mhlw.go.jp/stf/shingi/shingi-kousei_284075.html.
- 6.Shiomi M, Ueda Y, Abe H, Yagi A, Sakiyama K, Kimura T, Tanaka Y, Ohmichi M, Ichimura T, Sumi T, et al. A survey of Japanese mothers on the effectiveness of the Ministry of Health, Labor and Welfare’s revised HPV vaccine leaflet. Hum Vaccin Immunother. 2020;16(10):2555–58. doi: 10.1080/21645515.2020.1723362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ugumori N, Ueda Y, Yagi A, Abe H, Shiomi M, Nakagawa S, Hiramatsu K, Miyoshi A, Kobayashi E, Kimura T, et al. A potential means to help the HPV vaccine penetrate the Japanese public while under the continued suspension of governmental recommendation. Hum Vaccin Immunother. 2021;17(9):3096–101. doi: 10.1080/21645515.2021.1910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuhara T, Ishikawa H, Okada M, Kato M, Kiuchi T. Persuasiveness of statistics and patients’ and mothers’ narratives in human papillomavirus vaccine recommendation messages: a randomized controlled study in Japan. Front Public Health. 2018;6:105. doi: 10.3389/fpubh.2018.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiwada N, Suzuki C, Hasebe S, Tsuchiya A, Takeuchi N, Hishiki H, Sato Y, Sugita K. The effects of health education on health science teachers’ intention to recommend adolescent HPV vaccine for female students in Japan. Hum Vaccin Immunother. 2020;16(11):2752–57. doi: 10.1080/21645515.2020.1732163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagase Y, Ueda Y, Abe H, Yagi A, Sawada M, Nakagawa S, Hiramatsu K, Egawa-Takata T, Matsuzaki S, Kobayashi E, et al. Changing attitudes in Japan toward HPV vaccination: a 5-year follow-up survey of obstetricians and gynecologists regarding their current opinions about the HPV vaccine. Hum Vaccin Immunother. 2020;16(8):1808–13. doi: 10.1080/21645515.2020.1712173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arana JE, Harrington T, Cano M, Lewis P, Mba-Jonas A, Rongxia L, Stewart B, Markowitz LE, Shimabukuro TT. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36(13):1781–88. doi: 10.1016/j.vaccine.2018.02.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


